Effect of Absorption Time for the Preparation of Activated Carbon from Wasted Tree Leaves of Quercus alba and Investigating Life Cycle Assessment
Abstract
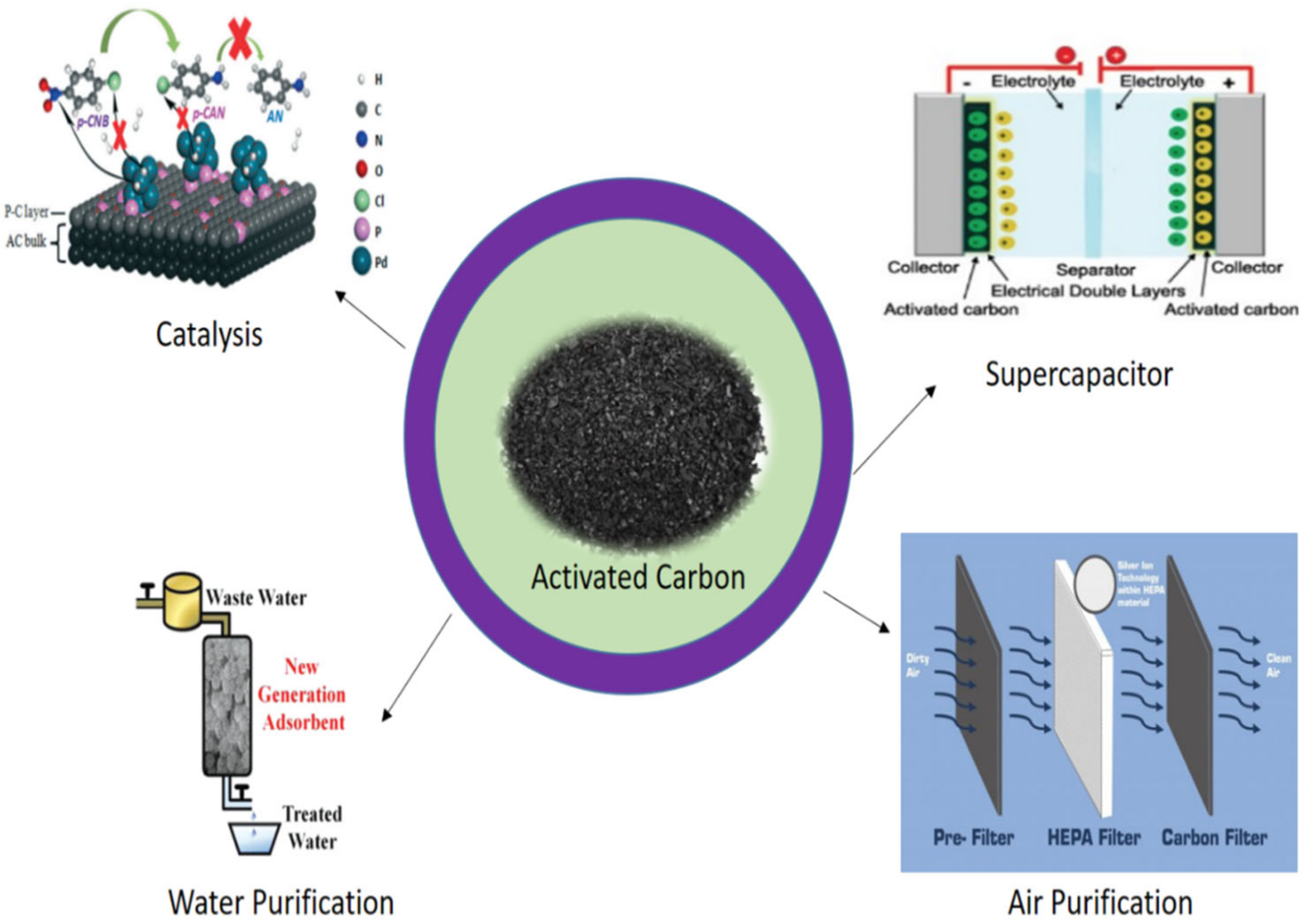
:1. Introduction
2. Materials and Methods
2.1. Chemicals
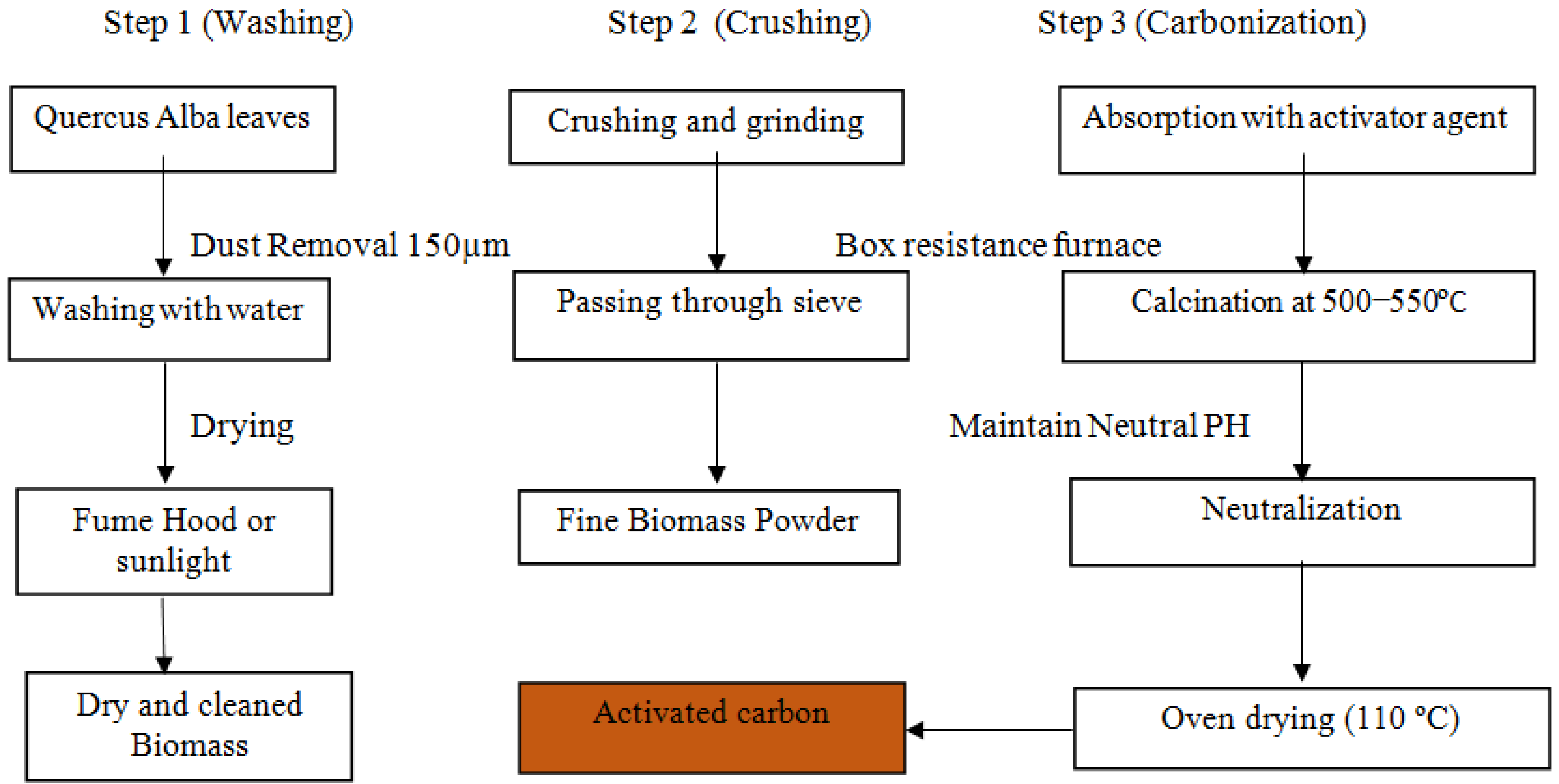
2.2. Activated Carbon Preparation Procedure
2.3. Characterization of Activated Carbon
2.4. Life Cycle Assessment Compartments
2.5. Data Origin
2.6. Goal and Scope Definition
2.7. System Boundary
2.8. Life Cycle Assessment
3. Results and Discussion
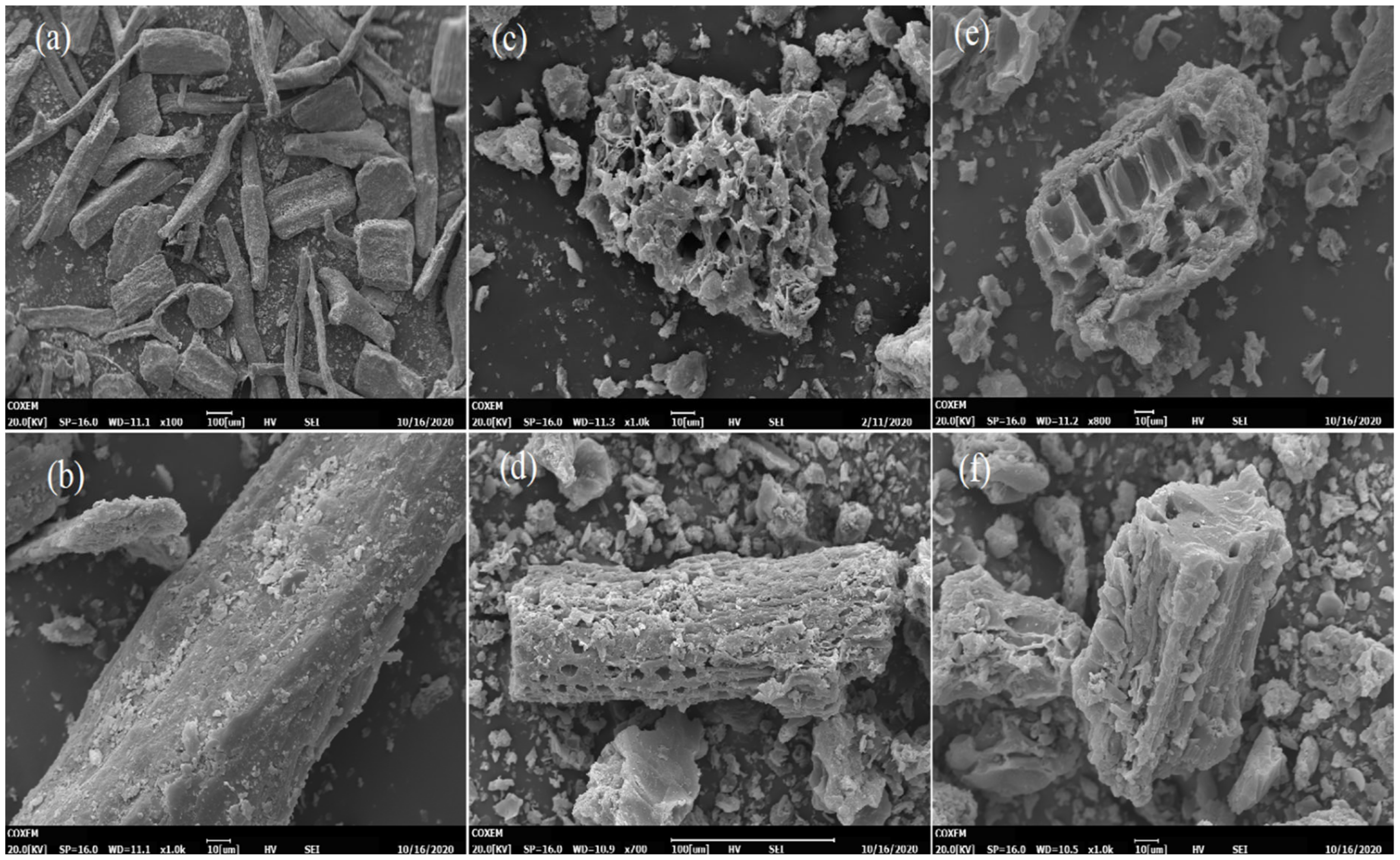
3.1. Scanning Electron Microscopy Analysis (SEM)
3.2. EDX and BET Analysis
3.3. X-Ray Diffraction Analysis
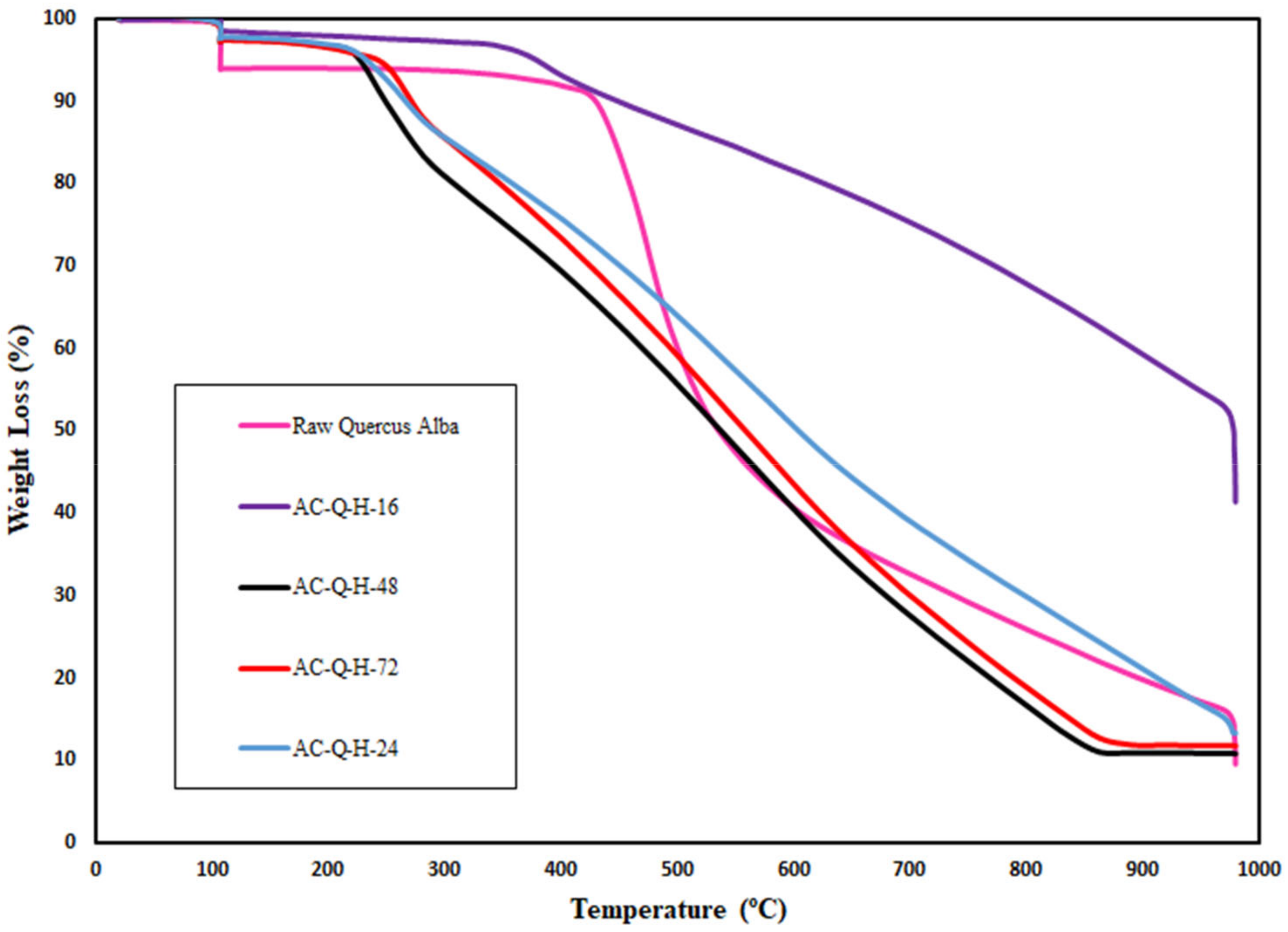
3.4. Thermogravimetric Analysis
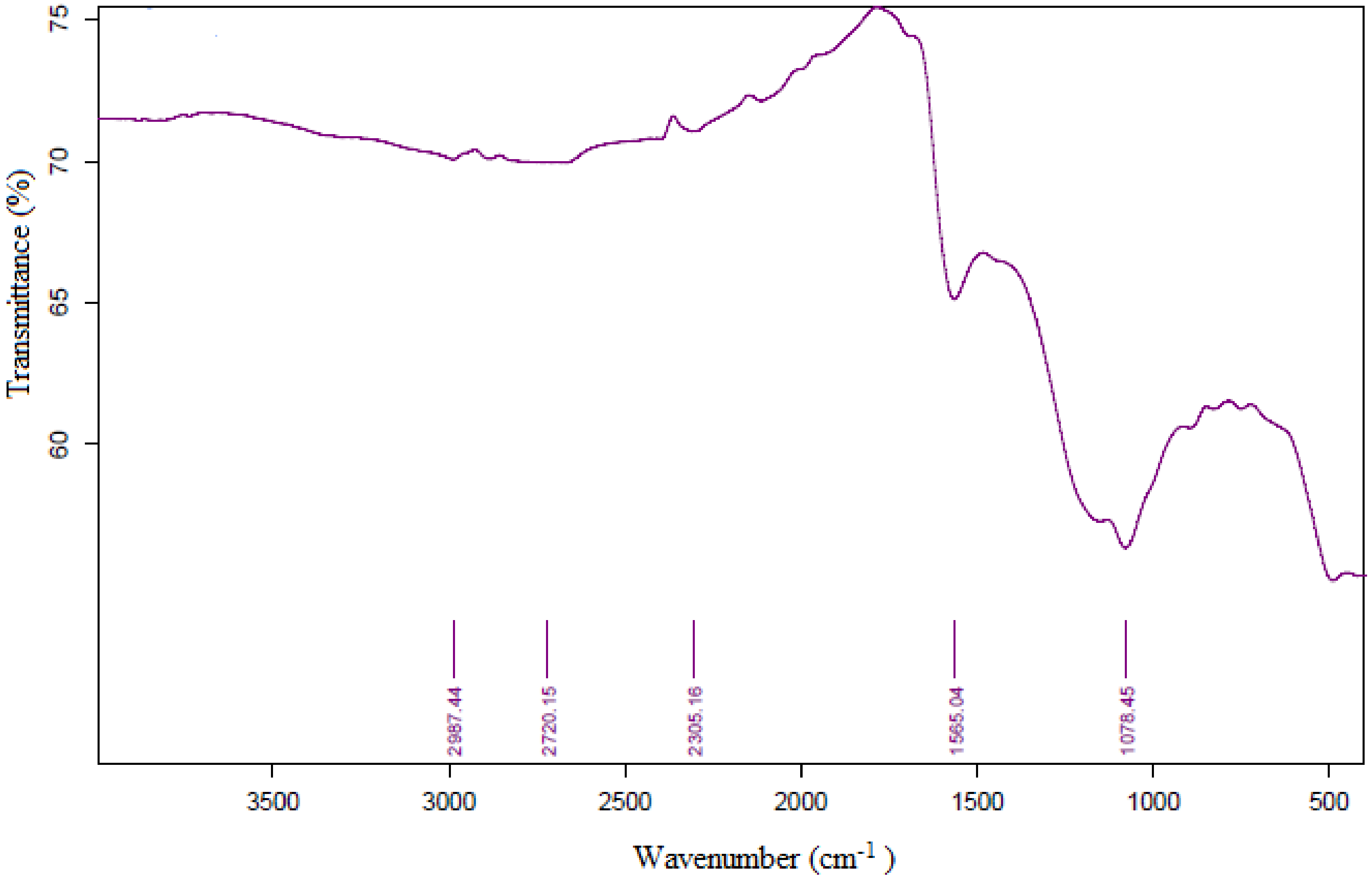
3.5. FT-IR Analysis
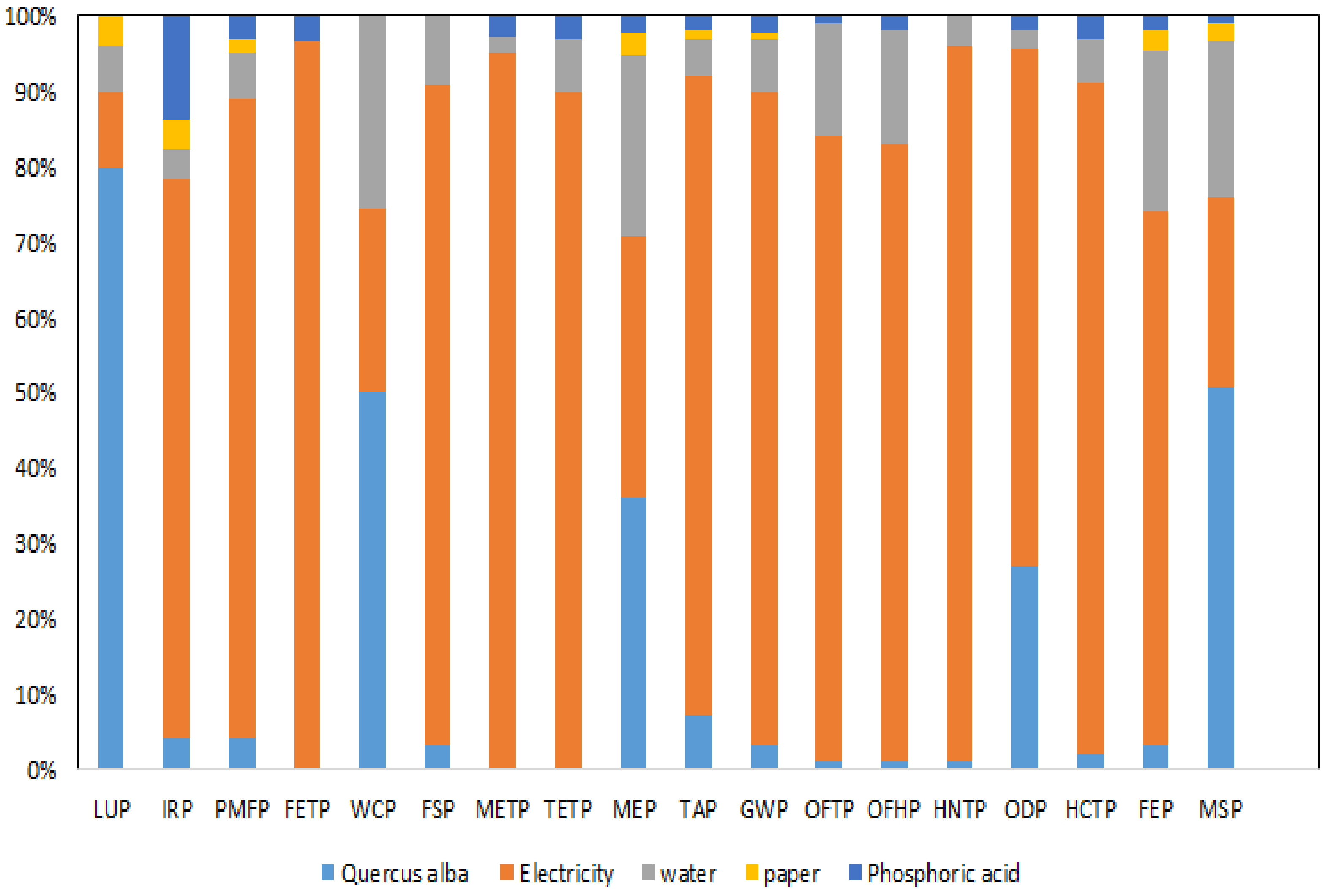
3.6. Environmental Impact Assessment
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tirmenstein, D.A. Quercus alba. In Fire Effects Information System. Available online: https://www.fs.usda.gov/database/feis/plants/tree/quealb/all.html (accessed on 22 October 2022).
- Rogers, R. Quercus alba L. Available online: https://www.srs.fs.usda.gov/pubs/misc/ag_654/volume_2/quercus/alba.htm (accessed on 22 October 2022).
- Thakur, A. Oak of India—Regenaration and Management. Available online: https://ifsa.net/oaks-of-india-regeneration-management/ (accessed on 22 October 2022).
- Zhang, L.; Ding, W.; Zhou, G.; Wen, S.; Yin, J.; Liu, C.; Fu, Y. Two-Dimensional Activated Carbon Nanosheets from Natural Corn Straw Piths and its Rapid Removal of Tetracycline Via Strong Π-Π Electron Donor Receptor Interactions. SSRN Electron. J. 2022, 360, 127544. [Google Scholar] [CrossRef]
- Hu, H.; Qu, J.; Yang, K.-R.; Hao, J.; Yang, J.; Song, R.-F.; Zhang, L.-Y.; Li, Z.-X. Tuning Carbon Contents and Further Capacitances of Coordination Polymer-Derived Carbonaceous Composites by Annealing Temperatures. Cryst. Growth Des. 2022, 22, 4503–4512. [Google Scholar] [CrossRef]
- Bicil, Z.; Doğan, M. Characterization of Activated Carbons Prepared from Almond Shells and Their Hydrogen Storage Properties. Energy Fuels 2021, 35, 10227–10240. [Google Scholar] [CrossRef]
- Elanthamilan, E.; Catherin Meena, B.; Renuka, N.; Santhiya, M.; George, J.; Kanimozhi, E.P.; Christy Ezhilarasi, J.; Princy Merlin, J. Walnut shell derived mesoporous activated carbon for high performance electrical double layer capacitors. J. Electroanal. Chem. 2021, 901, 115762. [Google Scholar] [CrossRef]
- Keppetipola, N.M.; Dissanayake, M.; Dissanayake, P.; Karunarathne, B.; Dourges, M.A.; Talaga, D.; Servant, L.; Olivier, C.; Toupance, T.; Uchida, S.; et al. Graphite-type activated carbon from coconut shell: A natural source for eco-friendly non-volatile storage devices. RSC Adv. 2021, 11, 2854–2865. [Google Scholar] [CrossRef] [PubMed]
- Ouzzine, M.; Serafin, J.; Sreńscek-Nazzal, J. Single step preparation of activated biocarbons derived from pomegranate peels and their CO2 adsorption performance. J. Anal. Appl. Pyrolysis 2021, 160, 11–17. [Google Scholar] [CrossRef]
- Khader, E.H.; Mohammed, T.J.; Albayati, T.M. Comparative performance between rice husk and granular activated carbon for the removal of azo tartrazine dye from aqueous solution. Desalin. Water Treat. 2021, 229, 372–383. [Google Scholar] [CrossRef]
- Amin, M.; Shah, H.H.; Iqbal, A.; Farooqi, Z.U.R.; Krawczuk, M.; Zia, A. Conversion of Waste Biomass into Activated Carbon and Evaluation of Environmental Consequences Using Life Cycle Assessment. Appl. Sci. 2022, 12, 5741. [Google Scholar] [CrossRef]
- Daiem, M.M.A.; Rashad, A.M.; Said, N.; Abdel-Gawwad, H.A. An initial study about the effect of activated carbon nano-sheets from residual biomass of olive trees pellets on the properties of alkali-activated slag pastes. J. Build. Eng. 2021, 44, 102661. [Google Scholar] [CrossRef]
- Eddy, N.O.; Ibok, U.J.; Garg, R.; Garg, R.; Iqbal, A.; Amin, M.; Mustafa, F.; Egilmez, M.; Galal, A.M. A Brief Review on Fruit and Vegetable Extracts as Corrosion Inhibitors in Acidic Environments. Molecules 2022, 27, 2991. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, L.C.A.; Pereira, E.; Guimaraes, I.R.; Vallone, A.; Pereira, M.; Mesquita, J.P.; Sapag, K. Preparation of activated carbons from coffee husks utilizing FeCl3 and ZnCl2 as activating agents. J. Hazard. Mater. 2009, 165, 87–94. [Google Scholar] [CrossRef]
- Amin, M.; Munir, S.; Iqbal, N.; Wabaidur, S.M.; Iqbal, A. The Conversion of Waste Biomass into Carbon-Supported Iron Catalyst for Syngas to Clean Liquid Fuel Production. Catalysts 2022, 12, 1234. [Google Scholar] [CrossRef]
- Rashidi, N.A.; Chai, Y.H.; Ismail, I.S.; Othman, M.F.H.; Yusup, S. Biomass as activated carbon precursor and potential in supercapacitor applications. Biomass Convers. Biorefinery 2022, 913, 0123456789. [Google Scholar] [CrossRef]
- Sieradzka, M.; Kirczuk, C.; Kalemba-rec, I.; Mlonka-mędrala, A.; Magdziarz, A. Pyrolysis of Biomass Wastes into Carbon Materials. Energies 2022, 15, 1941. [Google Scholar] [CrossRef]
- Phainuphong, S.; Taweekun, J.; Maliwan, K.; Theppaya, T.; Reza, S.; Azad, A.K. Synthesis and Characterization of Activated Carbon Derived from Rubberwood Sawdust via Carbonization and Chemical Activation as Electrode Material for Supercapacitor. J. Adv. Res. Fluid Mech. Therm. Sci. 2022, 94, 61–76. [Google Scholar] [CrossRef]
- Mokrzycki, J.; Magdziarz, A.; Rutkowski, P. Biomass and Bioenergy The influence of the Miscanthus giganteus pyrolysis temperature on the application of obtained biochars as solid biofuels and precursors of high surface area activated carbons. Biomass Bioenergy 2021, 164, 106550. [Google Scholar] [CrossRef]
- Heidarinejad, Z.; Dehghani, M.H.; Heidari, M.; Javedan, G.; Ali, I.; Sillanpää, M. Methods for preparation and activation of activated carbon: A review. Environ. Chem. Lett. 2020, 18, 393–415. [Google Scholar] [CrossRef]
- Zou, R.; Qian, M.; Wang, C.; Mateo, W.; Wang, Y.; Dai, L.; Lin, X.; Zhao, Y.; Huo, E.; Wang, L.; et al. Biochar: From by-products of agro-industrial lignocellulosic waste to tailored carbon-based catalysts for biomass thermochemical conversions. Chem. Eng. J. 2021, 441, 135972. [Google Scholar] [CrossRef]
- Khalil, K.M.S.; Elhamdy, W.A.; Mohammed, K.M.H.; Said, A.E.A.A. Nanostructured P-doped activated carbon with improved mesoporous texture derived from biomass for enhanced adsorption of industrial cationic dye contaminants. Mater. Chem. Phys. 2022, 282, 125881. [Google Scholar] [CrossRef]
- Ahmad, A.A.; Al-Raggad, M.; Shareef, N. Production of activated carbon derived from agricultural by-products via microwave-induced chemical activation: A review. Carbon Lett. 2021, 31, 957–971. [Google Scholar] [CrossRef]
- Arena, N.; Lee, J.; Clift, R. Life Cycle Assessment of activated carbon production from coconut shells. J. Clean. Prod. 2016, 125, 68–77. [Google Scholar] [CrossRef] [Green Version]
- Tiegam, R.F.T.; Tchuifon, D.R.T.; Santagata, R.; Nanssou, P.A.K.; Anagho, S.G.; Ionel, I.; Ulgiati, S. Production of activated carbon from cocoa pods: Investigating benefits and environmental impacts through analytical chemistry techniques and life cycle assessment. J. Clean. Prod. 2021, 288, 125464. [Google Scholar] [CrossRef]
- Ur, Z.; Farooqi, R.; Ahmad, I.; Ditta, A.; Ilic, P.; Amin, M. Types, sources, socioeconomic impacts, and control strategies of environmental noise: A review. Environ. Sci. Pollut. Res. 2022, 94, 0123456789. [Google Scholar] [CrossRef]
- Sepúlveda-Cervantes, C.V.; Soto-Regalado, E.; Rivas-García, P.; Loredo-Cancino, M.; Cerino-Córdova, F.d.J.; Reyes, R.B.G. Technical-environmental optimisation of the activated carbon production of an agroindustrial waste by means response surface and life cycle assessment. Waste Manag. Res. 2018, 36, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.; Chung, E.; Shah, H.H. Effect of different activation agents for activated carbon preparation through characterization and life cycle assessen. Int. J. Environ. Sci. Technol. 2022, 78, 0123456789. [Google Scholar] [CrossRef]
- Yu, S.; Tao, J. Simulation based life cycle assessment of airborne emissions of biomass-based ethanol products from different feedstock planting areas in China. J. Clean. Prod. 2009, 17, 501–506. [Google Scholar] [CrossRef]
- Lahmer, K. Numerical investigation of thermal and electrical management during hydrogen reversible solid- state storage using a novel heat exchanger based on thermoelectric modules. Int. J. Hydrogen Energy 2022, 47, 30580–30591. [Google Scholar] [CrossRef]
- Gombau, J.; Cabanillas, P.; Mena, A.; Pérez-Navarro, J.; Ramos, J.; Torner, A.; Fort, F.; Gómez-Alonso, S.; García-Romero, E.; Canals, J.M.; et al. Comparative study of volatile substances and ellagitannins released into wine by Quercus pyrenaica, Quercus petraea and Quercus alba barrels. OENO One 2022, 56, 5551. [Google Scholar]
- Xu, J.; Chen, L.; Qu, H.; Jiao, Y.; Xie, J.; Xing, G. Preparation and characterization of activated carbon from reedy grass leaves by chemical activation with H3PO4. Appl. Surf. Sci. 2014, 320, 674–680. [Google Scholar] [CrossRef]
- Mahmood, S.; Iqbal, A.; Rafi-u-Din; Wadood, A.; Mateen, A.; Amin, M.; Yahia, I.S.; Zahran, H.Y. Influence of Homogenizing Methodology on Mechanical and Tribological Performance of Powder Metallurgy Processed Titanium Composites Reinforced by Graphene Nanoplatelets. Molecules 2022, 27, 2666. [Google Scholar] [CrossRef]
- Gao, Y.; Yue, Q.; Gao, B.; Li, A. Insight into activated carbon from different kinds of chemical activating agents: A review. Sci. Total Environ. 2020, 746, 141094. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yap, X.; Wang, Z.; Li, H.; Shen, X.; Yao, Z.; Qian, F. Synthesis of Activated Carbon from Citric Acid Residue by Phosphoric Acid Activation for the Removal of Chemical Oxygen Demand from Sugar-Containing Wastewater. Environ. Eng. Sci. 2019, 36, 656–666. [Google Scholar] [CrossRef] [Green Version]
- Shi, Y.; Liu, G.; Wang, L.; Zhang, H. Activated carbons derived from hydrothermal impregnation of sucrose with phosphoric acid: Remarkable adsorbents for sulfamethoxazole removal. RSC Adv. 2019, 31, 17841–17851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hjaila, K.; Baccar, R.; Sarrà, M.; Gasol, C.M.; Bláquezn, P. Environmental impact associated with activated carbon preparation from olive-waste cake via life cycle assessment from olive-waste cake via life cycle assessment. J. Environ. Manag. 2013, 130, 242–247. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.; Shah, H.H.; Fareed, A.G.; Khan, W.U.; Chung, E.; Zia, A.; Rahman Farooqi, Z.U.; Lee, C. Hydrogen production through renewable and non-renewable energy processes and their impact on climate change. Int. J. Hydrogen Energy 2022, 47, 33112–33134. [Google Scholar] [CrossRef]
- UNEP. Global Guidance Principles for Life Cycle Assessment Databases. Shonan Guid. Princ. 2010, p. 160. Available online: www.unep.org (accessed on 22 October 2022).
- Huijbregts, M.A.J.; Gilijamse, W.; Ragas, A.M.J.; Reijnders, L. Evaluating uncertainty in environmental life-cycle assessment. A case study comparing two insulation options for a Dutch one-family dwelling. Environ. Sci. Technol. 2003, 37, 2600–2608. [Google Scholar] [CrossRef]
- Hong, J.; Shaked, S.; Rosenbaum, R.K.; Jolliet, O. Analytical uncertainty propagation in life cycle inventory and impact assessment: Application to an automobile front panel. Int. J. Life Cycle Assess. 2010, 15, 499–510. [Google Scholar] [CrossRef]

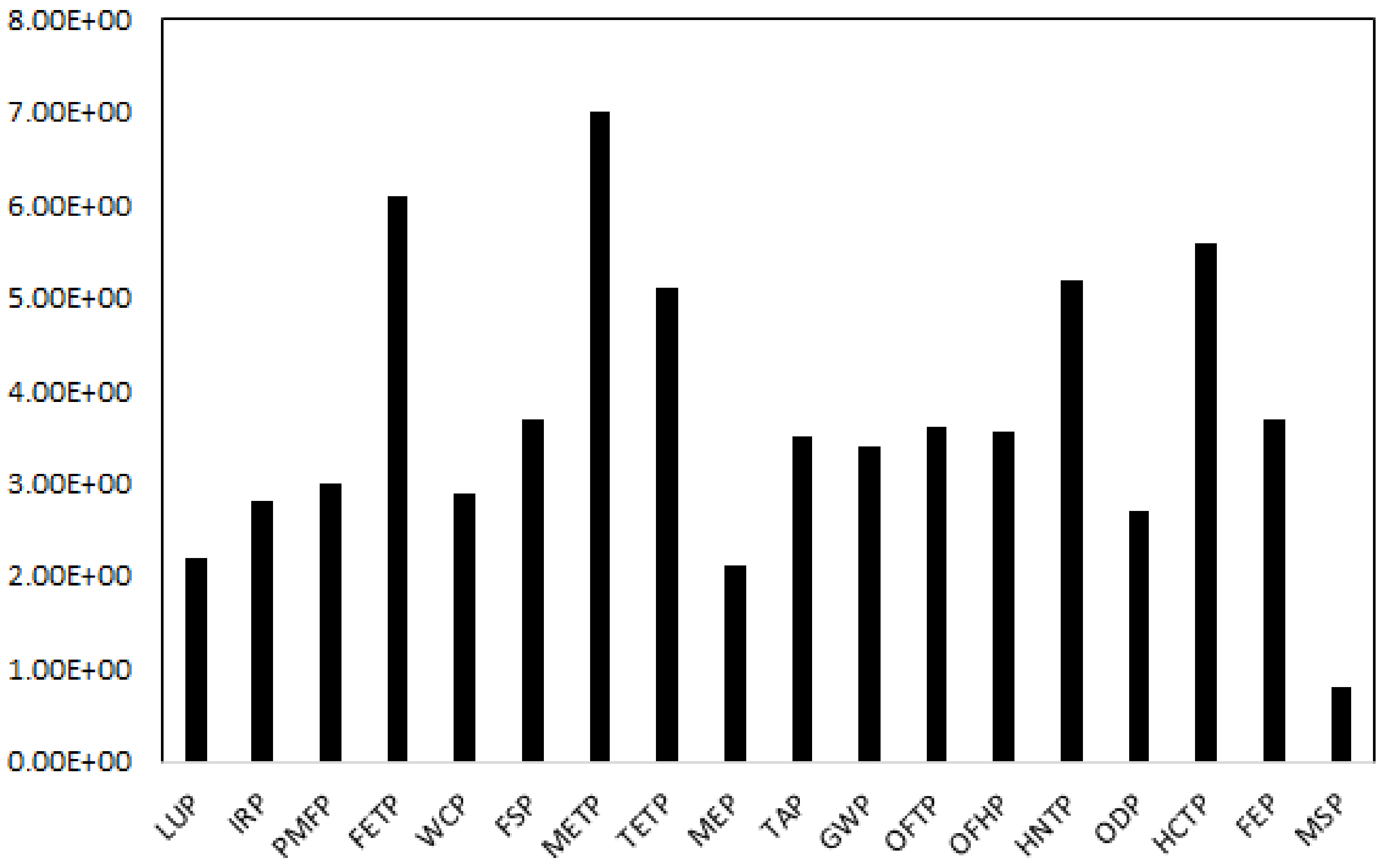
| Sample ID | Initial Biomass (Grams) | Grinding/Sieving | Chemical Activation | Washing/Oven Drying | Final AC (Grams) | |||
|---|---|---|---|---|---|---|---|---|
| ACQ-H-16 | 6 | Power (kWh) | 0.3 | H3PO4 (mL) | 3 | H2O (mL) | 1752 | 1 |
| H2O (mL) | 800 | Power (kWh) | 11.9 | Filter paper | 2 | |||
| H2O (mL) | 5.2 | Power (kWh) | 6 | |||||
| ACQ-H-24 | 6 | Power (kWh) | 0.3 | H3PO4 (mL) | 3 | H2O (mL) | 1841 | 1 |
| H2O (mL) | 800 | Power (kWh) | 11.9 | Filter paper | 2 | |||
| H2O (mL) | 5.2 | Power (kWh) | 6 | |||||
| ACQ-H-48 | 6 | Power (kWh) | 0.3 | H3PO4 (mL) | 3 | H2O (mL) | 2153 | 1 |
| H2O (mL) | 800 | Power (kWh) | 11.9 | Filter paper | 3 | |||
| H2O (mL) | 5.2 | Power (kWh) | 6 | |||||
| ACQ-H-72 | 6 | Power (kWh) | 0.3 | H3PO4 (mL) | 3 | H2O (mL) | 2756 | 1 |
| H2O (mL) | 800 | Power (kWh) | 11.9 | Filter paper | 4 | |||
| H2O (mL) | 5.2 | Power (kWh) | 6 | |||||
| Sample ID | Activation Time (h) | Pyrolysis Temperature (°C) | Pyrolysis Time (h) | Elements Presence (Weight %) | BET Surface Area m2/g | |||
|---|---|---|---|---|---|---|---|---|
| Carbon | Oxygen | Phosphor | Calcium | |||||
| Quercus alba | 34.76 | 58.32 | 0.00 | 6.92 | ||||
| ACQ-H-16 | 16 | 500 | 3 | 60.19 | 26.45 | 11.06 | 2.30 | 787.7 |
| ACQ-H-24 | 24 | 500 | 3 | 53.32 | 29.41 | 13.15 | 4.12 | 890.2 |
| ACQ-H-48 | 48 | 500 | 3 | 52.76 | 28.92 | 12.51 | 4.71 | 943.2 |
| ACQ-H-72 | 72 | 500 | 3 | 54.56 | 30.02 | 12.00 | 3.43 | 896.4 |
| Impact Category | Value | Unit |
|---|---|---|
| (PMFP) Fine particulate matter formation potential | 0.01 | kg PM2.5 eq |
| (FETP) Freshwater ecotoxicity potential | 1.10 | kg 1,4-DCB |
| (WCP) Water consumption potential | 0.03 | m3 |
| (IRP) Ionizing radiation potential | 0.80 | kBq Co-60 eq |
| (ODP) Stratospheric ozone depletion potential | 0.00 | kg CFC11 eq |
| (LUP) Land use potential | 0.30 | m2 a crop eq |
| (METP) Marine ecotoxicity potential | 1.35 | kg 1,4-DCB |
| (MEP) Marine eutrophication potential | 0.00 | kg N eq |
| (TAP) Terrestrial acidification potential | 0.02 | kg SO2 eq |
| (OFTP) Ozone formation, Terrestrial ecosystem potential | 0.01 | kg NOx eq |
| (TETP) Terrestrial ecotoxicity potential | 30.12 | kg 1,4-DCB |
| (FSP) Fossil resource scarcity potential | 1.18 | kg oil eq |
| (GWP) Global warming potential | 3.61 | kg CO2 eq |
| (FEP) Freshwater eutrophication potential | 0.00 | kg P eq |
| (MSP) Mineral resource scarcity potential | 0.81 | kg Cu eq |
| (OFHP) Ozone formation, Human health potential | 0.01 | kg NOx eq |
| (HNTP) Human non-carcinogenic toxicity potential | 4.30 | kg 1,4-DCB |
| (HCTP) Human carcinogenic toxicity potential | 0.21 | kg 1,4-DCB |
| Raw Material | Impact Categories | Value | Unit | References |
|---|---|---|---|---|
| Cocoa pods | Global warming potential | 4.63 | kg CO2 eq | [25] |
| Acidification Potential | 0.03 | kg SO2 eq | ||
| Human Toxicity Potential | 0.27 (carcinogenic) | kg 1,4-DCB | ||
| 6.35 (non-carcinogenic) | ||||
| Coconut shells | Global warming potential | 2.1 × 10−11 (normalized) | kg CO2 eq | [24] |
| Acidification Potential | 4.1 × 10−11 (normalized) | kg SO2 eq | ||
| Human Toxicity Potential | 1.2 × 10−10 (normalized) | kg 1,4-DCB | ||
| Olive tree | Global warming potential | 5.210 | kg CO2 eq | [11] |
| Acidification Potential | 0.100 | kg SO2 eq | ||
| Human Toxicity Potential | 3.262 | kg 1,4-DCB | ||
| Quercus alba | Global warming potential | 3.61 | kg CO2 eq | This work |
| Acidification Potential | 0.02 | kg SO2 eq | ||
| Human Toxicity Potential | 0.21 (carcinogenic) | kg 1,4-DCB | ||
| 4.30 (non-carcinogenic) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amin, M.; Shah, H.H. Effect of Absorption Time for the Preparation of Activated Carbon from Wasted Tree Leaves of Quercus alba and Investigating Life Cycle Assessment. C 2022, 8, 57. https://doi.org/10.3390/c8040057
Amin M, Shah HH. Effect of Absorption Time for the Preparation of Activated Carbon from Wasted Tree Leaves of Quercus alba and Investigating Life Cycle Assessment. C. 2022; 8(4):57. https://doi.org/10.3390/c8040057
Chicago/Turabian StyleAmin, Muhammad, and Hamad Hussain Shah. 2022. "Effect of Absorption Time for the Preparation of Activated Carbon from Wasted Tree Leaves of Quercus alba and Investigating Life Cycle Assessment" C 8, no. 4: 57. https://doi.org/10.3390/c8040057
APA StyleAmin, M., & Shah, H. H. (2022). Effect of Absorption Time for the Preparation of Activated Carbon from Wasted Tree Leaves of Quercus alba and Investigating Life Cycle Assessment. C, 8(4), 57. https://doi.org/10.3390/c8040057






