Activated Carbon from Stipa tenacissima for the Adsorption of Atenolol
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Activated Carbon
2.2. Adsorption Equipment and Procedures
2.3. Characterization
3. Results and Discussion
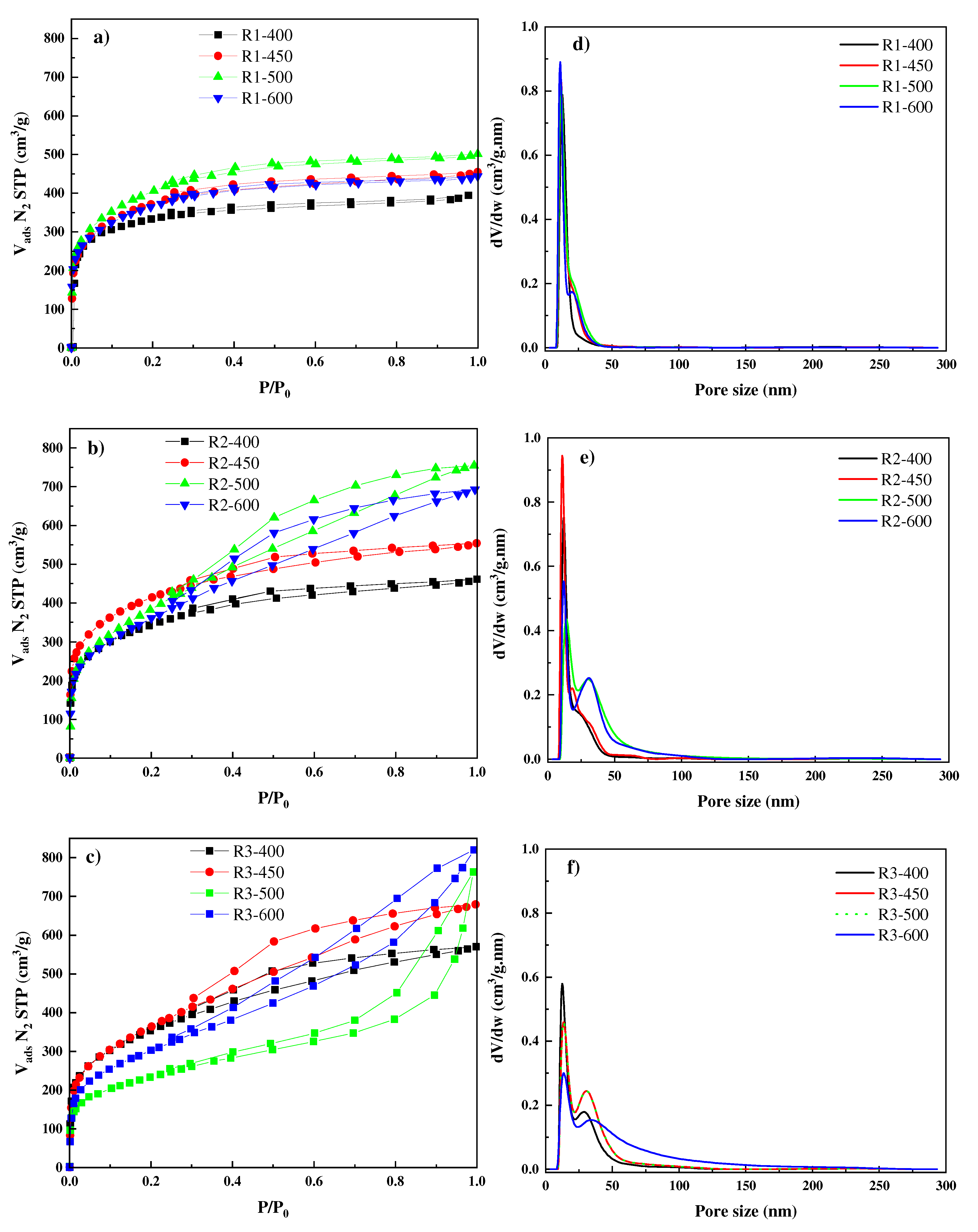
3.1. Characterization
3.2. Adsorption of Atenolol Drug
| Samples | SBET (m2/g) | Vtotal (cm3/g) | Vµp (cm3/g) | Vmeso (cm3/g) |
|---|---|---|---|---|
| R1-500 | 1478 | 0.78 | 0.57 | 0.13 |
| R2-500 | 1387 | 1.17 | 0.53 | 0.53 |
| R3-500 | 1100 | 1.27 | 0.43 | 0.59 |
| CAC | 909 | 0.76 | 0.36 | 0.40 |
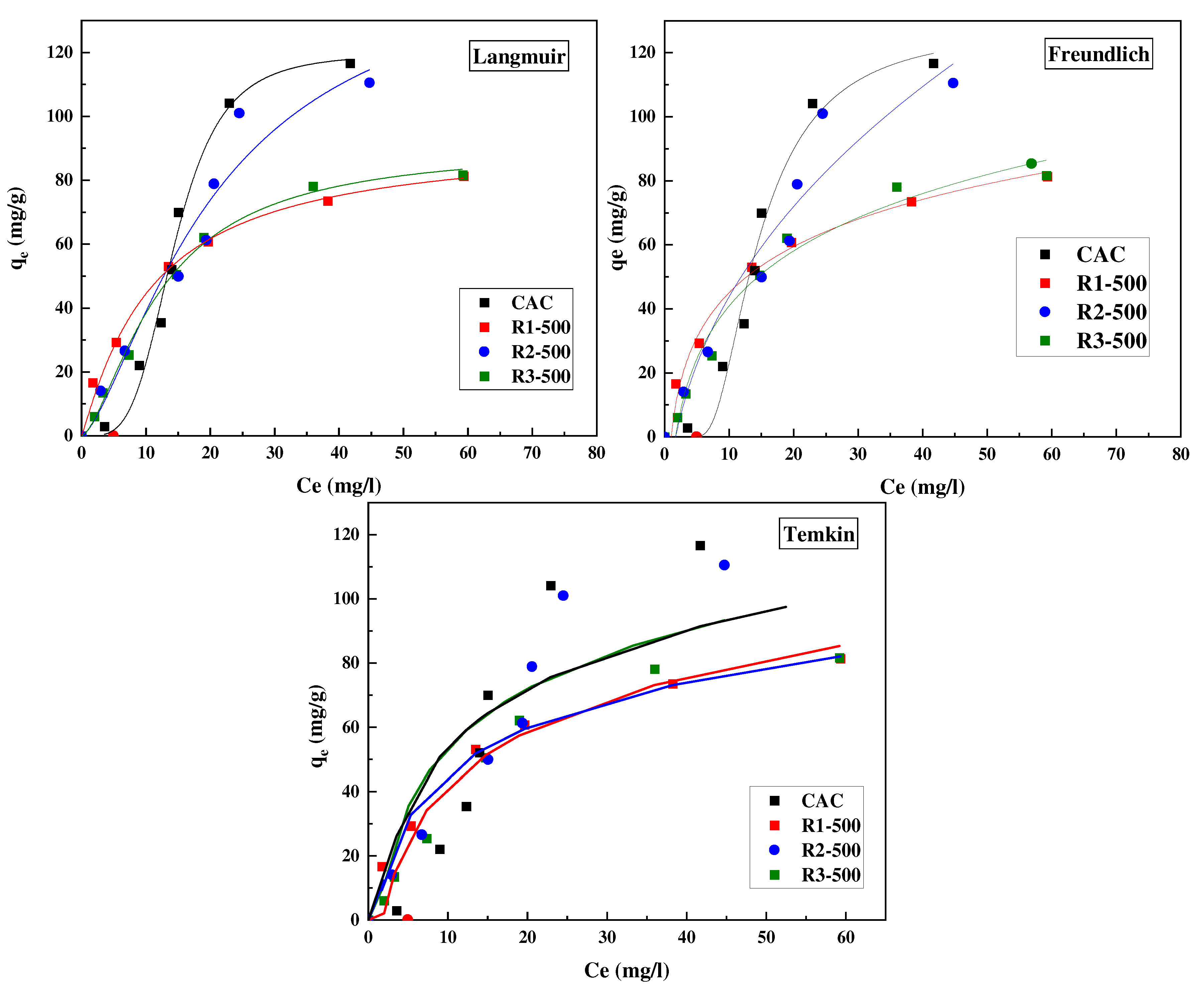
3.2.1. Equilibrium Adsorption
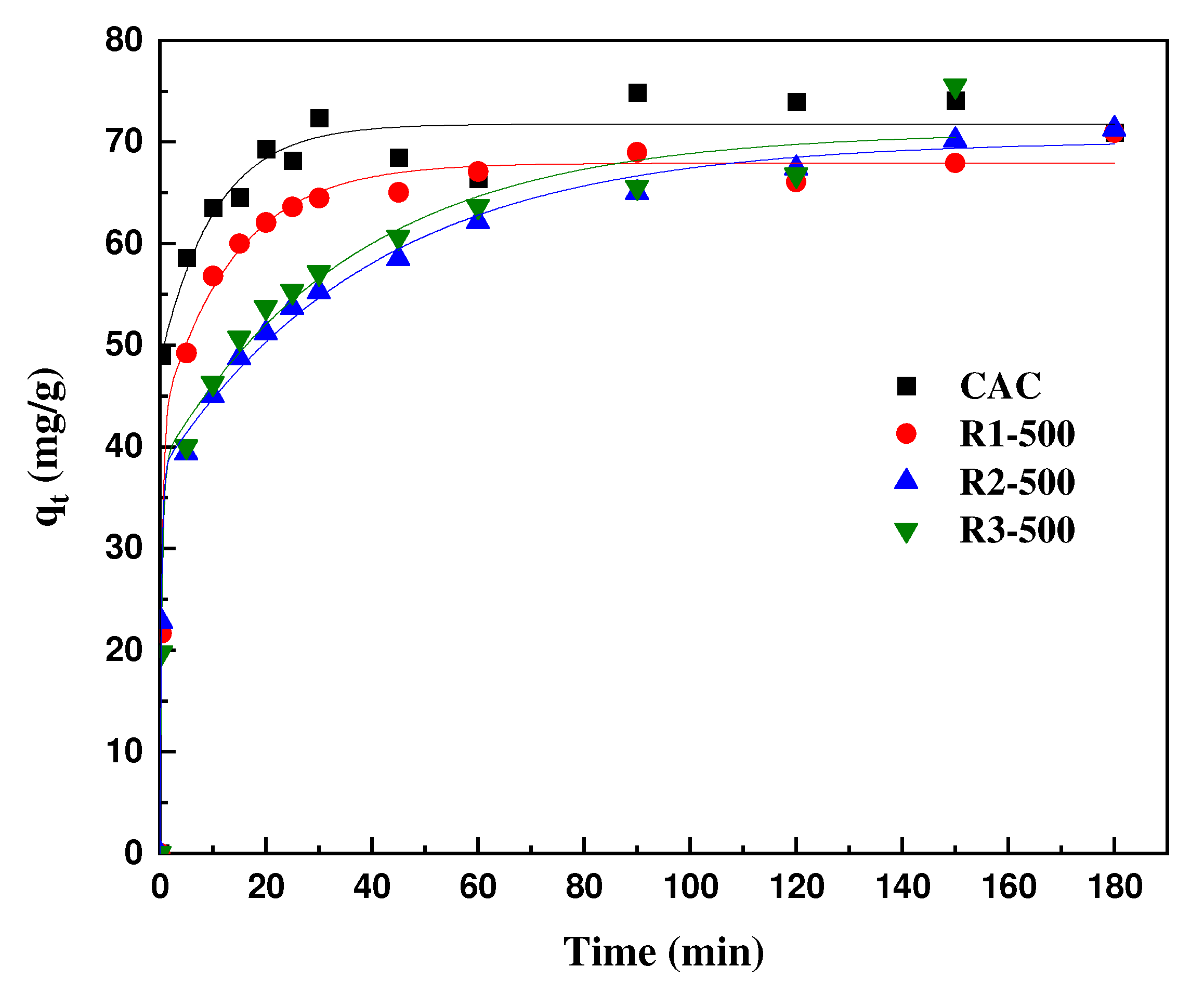
3.2.2. Kinetic Study
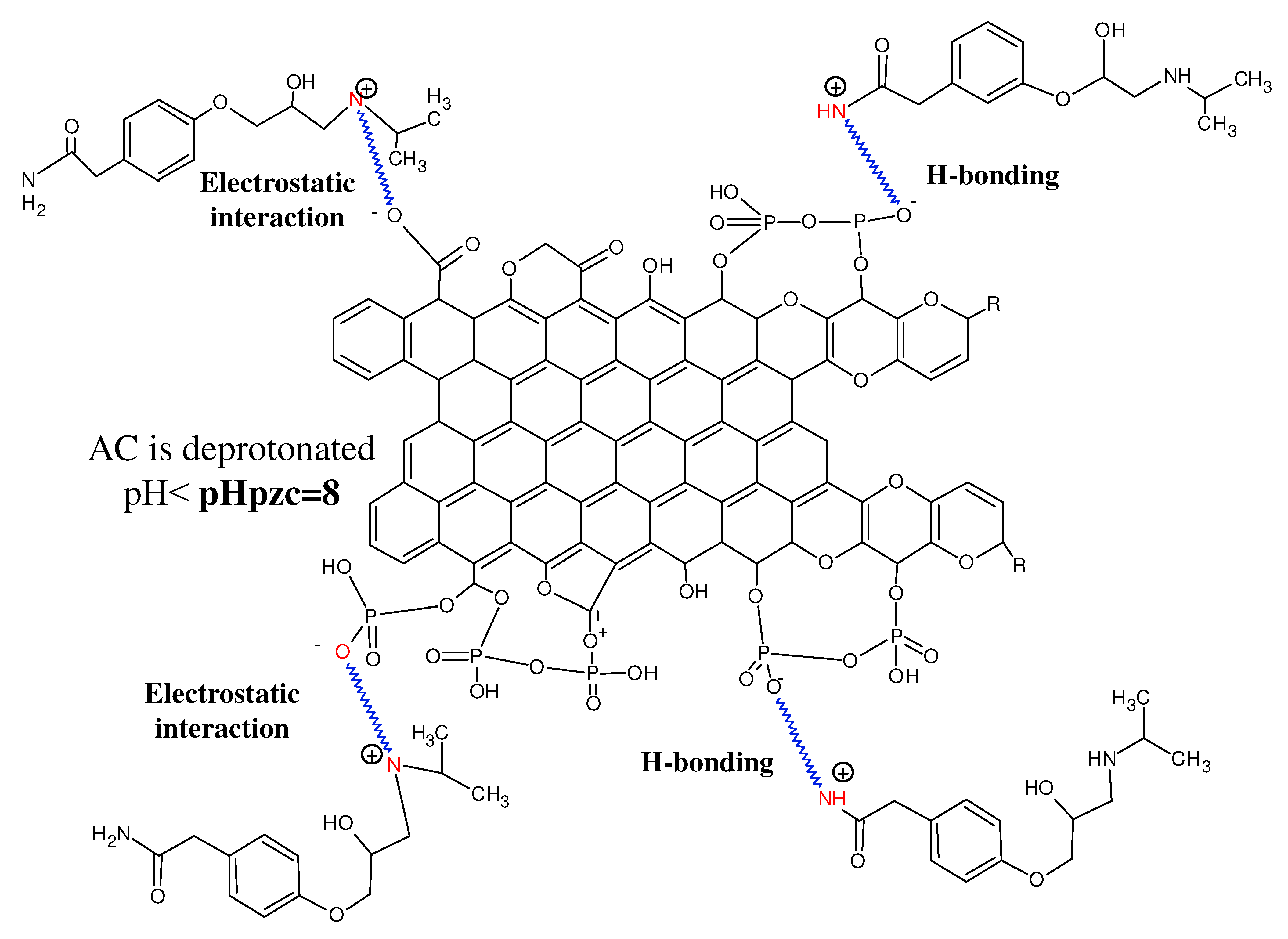
3.2.3. Mechanism of Atenolol Adsorption
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Azwar, E.; Mahari, W.A.W.; Chuah, J.H.; Vo, D.-V.N.; Ma, N.L.; Lam, W.H.; Lam, S.S. Transformation of biomass into carbon nanofiber for supercapacitor application—A review. Int. J. Hydrogen Energy 2018, 43, 20811–20821. [Google Scholar] [CrossRef]
- Moulefera, I.; Trabelsi, M.; Mamun, A.; Sabantina, L. Electrospun Carbon Nanofibers from Biomass and Biomass Blends—Current Trends. Polymers 2021, 13, 1071. [Google Scholar] [CrossRef]
- Nor, N.M.; Lau, L.C.; Lee, K.T.; Mohamed, A.R. Synthesis of activated carbon from lignocellulosic biomass and its applications in air pollution control—A review. J. Environ. Chem. Eng. 2013, 1, 658–666. [Google Scholar] [CrossRef]
- Vargas, A.M.; Cazetta, A.L.; Garcia, C.A.; Moraes, J.C.; Nogami, E.M.; Lenzi, E.; Costa, W.F.; Almeida, V.C. Preparation and characterization of activated carbon from a new raw lignocellulosic material: Flamboyant (Delonix regia) pods. J. Environ. Manag. 2011, 92, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Z.-Y.; Yang, Q.; Li, X.-M.; Luo, K.; Liu, Y.; Zeng, G.-M. Preparation of peanut hull-based activated carbon by microwave-induced phosphoric acid activation and its application in Remazol Brilliant Blue R adsorption. Ind. Crops Prod. 2012, 37, 178–185. [Google Scholar] [CrossRef]
- Liou, T.-H. Development of mesoporous structure and high adsorption capacity of biomass-based activated carbon by phosphoric acid and zinc chloride activation. Chem. Eng. J. 2010, 158, 129–142. [Google Scholar] [CrossRef]
- Yahya, M.A.; Al-Qodah, Z.; Zanariah Ngah, C.W. Agricultural bio-waste materials as potential sustainable precursors used for activated carbon production: A review. Renew. Sustain. Energy Rev. 2015, 46, 218–235. [Google Scholar] [CrossRef]
- Allied Market Research. Activated Carbon Market by Product Type (Powdered, Granular), Application (Liquid, Gaseous) and End Use (Water Treatment, Food & Beverage Processing, Air purification)—Global Opportunity Analysis and Industry Forecast, 2014–202. Allied Mark. Res. 2022. Available online: https://www.alliedmarketresearch.com/activated-carbon-market (accessed on 4 August 2022).
- Acharya, J.; Sahu, J.N.; Mohanty, C.; Meikap, B. Removal of lead(II) from wastewater by activated carbon developed from Tamarind wood by zinc chloride activation. Chem. Eng. J. 2009, 149, 249–262. [Google Scholar] [CrossRef]
- Tsai, W.-T.; Lai, C.-W.; Su, T.-Y. Adsorption of bisphenol-A from aqueous solution onto minerals and carbon adsorbents. J. Hazard. Mater. 2006, 134, 169–175. [Google Scholar] [CrossRef]
- García-Mateos, F.; Ruiz-Rosas, R.; Marqués, M.; Cotoruelo, L.; Rodríguez-Mirasol, J.; Cordero, T. Removal of paracetamol on biomass-derived activated carbon: Modeling the fixed bed breakthrough curves using batch adsorption experiments. Chem. Eng. J. 2015, 279, 18–30. [Google Scholar] [CrossRef]
- Kalkan, Ç.; Yapsakli, K.; Mertoglu, B.; Tufan, D.; Saatci, A. Evaluation of Biological Activated Carbon (BAC) process in wastewater treatment secondary effluent for reclamation purposes. Desalination 2011, 265, 266–273. [Google Scholar] [CrossRef]
- Guo, J.; Xu, W.S.; Chen, Y.L.; Lua, A.C. Adsorption of NH3 onto activated carbon prepared from palm shells impregnated with H2SO4. J. Colloid Interface Sci. 2004, 281, 285–290. [Google Scholar] [CrossRef]
- Sun, Y.; Yang, G.; Zhang, J.; Wang, Y.; Yao, M. Activated Carbon Preparation from Lignin by H3PO4 Activation and Its Application to Gas Separation. Chem. Eng. Technol. 2011, 35, 309–316. [Google Scholar] [CrossRef]
- Dolores, L.-C.; Juan, P.M.-L.; Falco, C.; Titirici, M.-M.; Diego, C.-A. Porous Biomass-Derived Carbons: Activated Carbons. In Sustainable Carbon Materials from Hydrothermal Processes; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2013; pp. 75–100. [Google Scholar] [CrossRef]
- Calvo-Muñoz, E.M.; García-Mateos, F.J.; Rosas, J.; Rodríguez-Mirasol, J.; Cordero, T. Biomass Waste Carbon Materials as adsorbents for CO2 Capture under Post-Combustion Conditions. Front. Mater. 2016, 3, 1–14. [Google Scholar] [CrossRef]
- Ozdemir, S.; Ozdemir, S.; Yetilmezsoy, K. Poultry abattoir sludge as bio-nutrient source for walnut plantation in low-fertility soil. Environ. Prog. Sustain. Energy 2019, 38, e13066. [Google Scholar] [CrossRef]
- García-Mateos, F.J.; Moulefera, I.; Rosas, J.M.; Benyoucef, A.; Rodríguez-Mirasol, J.; Cordero, T. Alcohol Dehydrogenation on Kraft Lignin-Derived Chars with Surface Basicity. Catalysts 2017, 7, 308. [Google Scholar] [CrossRef]
- Matos, I.; Bernardo, M.; Fonseca, I. Porous carbon: A versatile material for catalysis. Catal. Today 2017, 285, 194–203. [Google Scholar] [CrossRef]
- Zhang, S.; Yin, H.; Wang, J.; Zhu, S.; Xiong, Y. Catalytic cracking of biomass tar using Ni nanoparticles embedded carbon nanofiber/porous carbon catalysts. Energy 2020, 216, 119285. [Google Scholar] [CrossRef]
- Szymański, G.; Rychlicki, G. Catalytic conversion of propan-2-ol on carbon catalysts. Carbon 1993, 31, 247–257. [Google Scholar] [CrossRef]
- Arampatzidou, A.C.; Deliyanni, E.A. Comparison of activation media and pyrolysis temperature for activated carbons development by pyrolysis of potato peels for effective adsorption of endocrine disruptor bisphenol-A. J. Colloid Interface Sci. 2016, 466, 101–112. [Google Scholar] [CrossRef]
- Moulefera, I.; García-Mateos, F.J.; Benyoucef, A.; Rosas, J.M.; Rodríguez-Mirasol, J.; Cordero, T. Effect of Co-solution of Carbon Precursor and Activating Agent on the Textural Properties of Highly Porous Activated Carbon Obtained by Chemical Activation of Lignin with H3PO4. Front. Mater. 2020, 7, 1–14. [Google Scholar] [CrossRef]
- Xu, J.; Chen, L.; Qu, H.; Jiao, Y.; Xie, J.; Xing, G. Preparation and characterization of activated carbon from reedy grass leaves by chemical activation with H3PO4. Appl. Surf. Sci. 2014, 320, 674–680. [Google Scholar] [CrossRef]
- Kan, Y.; Yue, Q.; Li, D.; Wu, Y.; Gao, B. Preparation and characterization of activated carbons from waste tea by H 3 PO 4 activation in different atmospheres for oxytetracycline removal. J. Taiwan Inst. Chem. Eng. 2017, 71, 494–500. [Google Scholar] [CrossRef]
- Romero, A.; Lillo-Rodenas, M.A.; de Lecea, C.S.-M.; Linares-Solano, A. Hydrothermal and conventional H3PO4 activation of two natural bio-fibers. Carbon 2012, 50, 3158–3169. [Google Scholar] [CrossRef]
- Bouchenafa-Saïb, N.; Mekarzia, A.; Bouzid, B.; Mohammedi, O.; Khelifa, A.; Benrachedi, K.; Belhaneche-Bensemra, N. Removal of malathion from polluted water by adsorption onto chemically activated carbons produced from coffee grounds. Desalin. Water Treat. 2013, 52, 4920–4927. [Google Scholar] [CrossRef]
- Marsh, H.; Rodríguez-Reinoso, F. Preface. In Activated Carbon; Marsh, H., Rodríguez-Reinoso, F., Eds.; Elsevier Science Ltd.: Oxford, UK, 2006. [Google Scholar] [CrossRef]
- Rodríguez-Reinoso, F.; Molina-Sabio, M. Textural and chemical characterization of microporous carbons. Adv. Colloid Interface Sci. 1998, 76–77, 271–294. [Google Scholar] [CrossRef]
- Nielsen, L.; Biggs, M.J.; Skinner, W.; Bandosz, T.J. The effects of activated carbon surface features on the reactive adsorption of carbamazepine and sulfamethoxazole. Carbon 2014, 80, 419–432. [Google Scholar] [CrossRef]
- Zhang, J.P.; Sun, Y.; Woo, M.W.; Zhang, L.; Xu, K.Z. Preparation of steam activated carbon from black liquor by flue gas precipitation and its performance in hydrogen sulfide removal: Experimental and simulation works. J. Taiwan Inst. Chem. Eng. 2016, 59, 395–404. [Google Scholar] [CrossRef]
- Vernersson, T.; Bonelli, P.R.; Cerrella, E.G.; Cukierman, A.L. Arundo donax cane as a precursor for activated carbons preparation by phosphoric acid activation. Bioresour. Technol. 2002, 83, 95–104. [Google Scholar] [CrossRef]
- Jagtoyen, M.; Groppo, J.; Derbyshire, F. Activated carbons from bituminous coals by reaction with H3PO4: The influence of coal cleaning. Fuel Process. Technol. 1993, 34, 85–96. [Google Scholar] [CrossRef]
- Nabais, J.V.; Laginhas, C.; Carrott, M.R.; Carrott, P.; Amorós, J.C.; Gisbert, A.N. Surface and porous characterisation of activated carbons made from a novel biomass precursor, the esparto grass. Appl. Surf. Sci. 2013, 265, 919–924. [Google Scholar] [CrossRef]
- Fierro, V.; Torné-Fernández, V.; Celzard, A.; Montané, D. Influence of the demineralisation on the chemical activation of Kraft lignin with orthophosphoric acid. J. Hazard. Mater. 2007, 149, 126–133. [Google Scholar] [CrossRef]
- Tancredi, N.; Cordero, T.; Mirasol, J.R.; Rodríguez, J.J. Activated carbons from Uruguayan eucalyptus wood. Fuel 1996, 75, 1701–1706. [Google Scholar] [CrossRef]
- Hernández, V. Lignocellulosic Precursors Used in the Synthesis of Activated Carbon-Characterization Techniques and Applications in the Wastewater Treatment; InTech: Houston, TX, USA, 2012. [Google Scholar] [CrossRef]
- González-García, P. Activated carbon from lignocellulosics precursors: A review of the synthesis methods, characterization techniques and applications, Renew. Sustain. Energy Rev. 2018, 82, 1393–1414. [Google Scholar] [CrossRef]
- Lin, Z.; Wang, D.; Zhang, H.; Li, L.; Huang, Z.; Shen, J.; Lin, Y. Extraction and determination of malachite green from aquatic products based on molecularly imprinted polymers. Sep. Sci. Technol. 2016, 51, 1684–1689. [Google Scholar] [CrossRef]
- Das, A.K.; Saha, S.; Pal, A.; Maji, S.K. Surfactant-modified alumina: An efficient adsorbent for malachite green removal from water environment. J. Environ. Sci. Health Part A 2009, 44, 896–905. [Google Scholar] [CrossRef]
- Farooqi, Z.H.; Sultana, H.; Begum, R.; Usman, M.; Ajmal, M.; Nisar, J.; Irfan, A.; Azam, M. Catalytic degradation of malachite green using a crosslinked colloidal polymeric system loaded with silver nanoparticles. Int. J. Environ. Anal. Chem. 2020. [Google Scholar] [CrossRef]
- Zaccariello, G.; Moretti, E.; Storaro, L.; Riello, P.; Canton, P.; Gombac, V.; Montini, T.; Rodríguez-Castellón, E.; Benedetti, A. TiO2–mesoporous silica nanocomposites: Cooperative effect in the photocatalytic degradation of dyes and drugs. RSC Adv. 2014, 4, 37826–37837. [Google Scholar] [CrossRef]
- Al Qarni, H.; Collier, P.; O’Keeffe, J.; Akunna, J. Investigating the removal of some pharmaceutical compounds in hospital wastewater treatment plants operating in Saudi Arabia. Environ. Sci. Pollut. Res. 2016, 23, 13003–13014. [Google Scholar] [CrossRef]
- Rezaei, R.; Aghapour, A.A.; Khorsandi, H. Investigating the biological degradation of the drug β-blocker atenolol from wastewater using the SBR. Biogeochemistry 2022, 33, 267–281. [Google Scholar] [CrossRef]
- Marques, S.C.; Mestre, A.S.; Machuqueiro, M.; Gotvajn, A.; Marinšek, M.; Carvalho, A.P. Apple tree branches derived activated carbons for the removal of β-blocker atenolol. Chem. Eng. J. 2018, 345, 669–678. [Google Scholar] [CrossRef]
- Haro, N.K.; Del Vecchio, P.; Marcilio, N.R.; Feris, L. Removal of atenolol by adsorption—Study of kinetics and equilibrium. J. Clean. Prod. 2017, 154, 214–219. [Google Scholar] [CrossRef]
- Ahmad, A.A.; Din, A.T.M.; Yahaya, N.K.E.; Karim, J.; Ahmad, M.A. Atenolol sequestration using activated carbon derived from gasified Glyricidia sepium. Arab. J. Chem. 2020, 13, 7544–7557. [Google Scholar] [CrossRef]
- Madani, N.; Bouchenafa-Saib, N.; Mohammedi, O.; Varela-Gandía, F.; Cazorla-Amorós, D.; Hamada, B.; Cherifi, O. Removal of heavy metal ions by adsorption onto activated carbon prepared from Stipa tenacissima leaves. Desalin. Water Treat. 2017, 64, 179–188. [Google Scholar] [CrossRef]
- Miroslav, F.; Radka, K.; Oksana, G.; Zuzana, S.; Aleš, K.; Antonín, N.; Martin, K.; Roman, G. Sorption of atenolol, sulfamethoxazole and carbamazepine onto soil aggregates from the illuvial horizon of the Haplic Luvisol on loess. Soil Water Res. 2018, 13, 177–183. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of Gases in Multimolecular Layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Dubinin, M.M.; Zaverina, E.D.; Timofeyev, D.P. Sorption and structure of active carbons. I. Adsorption of organic vapors. Zhurnal Fiz. Khimii 1947, 21, 1352–1362. [Google Scholar]
- Sayğılı, H.; Güzel, F.; Önal, Y. Conversion of grape industrial processing waste to activated carbon sorbent and its performance in cationic and anionic dyes adsorption. J. Clean. Prod. 2015, 93, 84–93. [Google Scholar] [CrossRef]
- Demiral, I.; Şamdan, C.A. Preparation and Characterisation of Activated Carbon from Pumpkin Seed Shell Using H3PO4. Anadolu Univ. J. Sci. Technol. Appl. Sci. Eng. 2016, 17, 125–138. [Google Scholar] [CrossRef]
- Tadda, M.A.; Ahsan, A.; Shitu, A.; ElSergany, M.; Arunkumar, T.; Jose, B.; Daud, N.N. A review on activated carbon: Process, application and prospects. J. Adv. Civ. Eng. Pract. Res. 2016, 2, 7–13. [Google Scholar]
- Das, D.; Samal, D.P.; Bc, M. Preparation of Activated Carbon from Green Coconut Shell and its Characterization. J. Biosens. Bioelectron. 2015, 6, 100248. [Google Scholar] [CrossRef]
- Durán-Valle, C.J. Techniques Employed in the Physicochemical Characterization of Activated Carbons. In Lignocellulosic Precursors Used in the Synthesis of Activated Carbon-Characterization Techniques and Applications in the Wastewater Treatment; Montoya, V.H., Petriciolet, A.B., Eds.; IntechOpen: London, UK, 2012; pp. 38–58. Available online: https://books.google.dz/books?id=DnmszQEACAAJ (accessed on 4 August 2022).
- Yakout, S.; El-Deen, G.S. Characterization of activated carbon prepared by phosphoric acid activation of olive stones. Arab. J. Chem. 2016, 9, S1155–S1162. [Google Scholar] [CrossRef]
- Angin, D. Production and characterization of activated carbon from sour cherry stones by zinc chloride. Fuel 2014, 115, 804–811. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Jagtoyen, M.; Thwaites, M.; Stencel, J.; McEnaney, B.; Derbyshire, F. Adsorbent carbon synthesis from coals by phosphoric acid activation. Carbon 1992, 30, 1089–1096. [Google Scholar] [CrossRef]
- Kriaa, A.; Hamdi, N.; Srasra, E. Removal of Cu (II) from water pollutant with Tunisian activated lignin prepared by phosphoric acid activation. Desalination 2010, 250, 179–187. [Google Scholar] [CrossRef]
- Girgis, B.S.; Yunis, S.S.; Soliman, A.M. Characteristics of activated carbon from peanut hulls in relation to conditions of preparation. Mater. Lett. 2002, 57, 164–172. [Google Scholar] [CrossRef]
- Jagtoyen, M.; Derbyshire, F. Activated carbons from yellow poplar and white oak by H3PO4 activation. Carbon 1998, 36, 1085–1097. [Google Scholar] [CrossRef]
- Prahas, D.; Kartika, Y.; Indraswati, N.; Ismadji, S. Activated carbon from jackfruit peel waste by H3PO4 chemical activation: Pore structure and surface chemistry characterization. Chem. Eng. J. 2008, 140, 32–42. [Google Scholar] [CrossRef]
- Jun, T.Y.; Latip, N.H.A.; Abdullah, A.M.; Latif, P.A. Effect of Activation Temperature and Heating Duration on Physical Characteristics of Activated Carbon Prepared from Agriculture Waste. Environ. Asia 2010, 3, 143–148. [Google Scholar]
- Donald, J.; Ohtsuka, Y.; Xu, C. Effects of activation agents and intrinsic minerals on pore development in activated carbons derived from a Canadian peat. Mater. Lett. 2011, 65, 744–747. [Google Scholar] [CrossRef]
- Giles, C.H.; Smith, D.; Huitson, A. A general treatment and classification of the solute adsorption isotherm. I. Theoretical. J. Colloid Interface Sci. 1974, 47, 755–765. [Google Scholar] [CrossRef]
- Qu, W.; Yuan, T.; Yin, G.; Xu, S.; Zhang, Q.; Su, H. Effect of properties of activated carbon on malachite green adsorption. Fuel 2019, 249, 45–53. [Google Scholar] [CrossRef]
- Duan, C.; Wang, J.; Liu, Q.; Zhou, Y.; Zhou, Y. Efficient removal of Salbutamol and Atenolol by an electronegative silanized β-cyclodextrin adsorbent. Sep. Purif. Technol. 2021, 282, 120013. [Google Scholar] [CrossRef]
- García-Rosero, H.; Romero-Cano, L.A.; Aguilar-Aguilar, A.; Bailón-García, E.; Carvalho, A.P.; Pérez-Cadenas, A.F.; Carrasco-Marín, F. Adsorption and thermal degradation of Atenolol using carbon materials: Towards an advanced and sustainable drinking water treatment. J. Water Process. Eng. 2022, 49, 102978. [Google Scholar] [CrossRef]

| Proximate Analysis | Weight (%) |
|---|---|
| Ash | 1.19 |
| Fixed carbon | 24.50 |
| Volatile matter | 62.81 |
| Moisture | 11.50 |
| Samples | N | C | H | O * | O/C × 102 | H/C × 102 |
|---|---|---|---|---|---|---|
| STLs | 1.10 | 47.70 | 6.40 | 44.80 | 93.92 | 13.42 |
| R1-400 | 2.50 | 76.50 | 2.40 | 18.60 | 24.31 | 3.14 |
| R1-450 | 0.50 | 81.60 | 2.10 | 15.80 | 19.36 | 2.57 |
| R1-500 | 0.50 | 82.60 | 1.90 | 15.00 | 18.16 | 2.30 |
| R1-600 | 0.40 | 91.00 | 1.60 | 7.00 | 7.69 | 1.76 |
| R2-400 | 2.30 | 76.20 | 2.00 | 19.50 | 25.59 | 2.62 |
| R2-450 | 0.40 | 78.40 | 1.70 | 19.50 | 24.87 | 2.17 |
| R2-500 | 0.40 | 79.10 | 1.40 | 19.10 | 24.15 | 1.77 |
| R2-600 | 0.30 | 81.60 | 1.40 | 16.70 | 20.47 | 1.72 |
| R3-400 | 1.50 | 70.30 | 1.80 | 26.40 | 37.55 | 2.56 |
| R3-450 | 0.20 | 72.00 | 1.50 | 26.30 | 36.53 | 2.08 |
| R3-500 | 0.20 | 75.20 | 1.30 | 23.30 | 30.98 | 1.73 |
| R3-600 | 0.20 | 78.30 | 1.30 | 20.20 | 25.80 | 1.66 |
| Samples | SBET (m2/g) | Vtotal (cm3/g) | Vµp (cm3/g) | Vmeso (cm3/g) | Vµp/Vtot | Dp (nm) |
|---|---|---|---|---|---|---|
| R1-400 | 1204 | 0.61 | 0.53 | 0.07 | 86.88 | 2.03 |
| R1-450 | 1371 | 0.69 | 0.54 | 0.10 | 78.26 | 2.01 |
| R1-500 | 1478 | 0.78 | 0.57 | 0.13 | 73.08 | 2.11 |
| R1-600 | 1340 | 0.69 | 0.52 | 0.11 | 75.36 | 2.06 |
| R2-400 | 1258 | 0.71 | 0.49 | 0.16 | 69.01 | 2.26 |
| R2-450 | 1503 | 0.86 | 0.59 | 0.21 | 68.60 | 2.29 |
| R2-500 | 1387 | 1.17 | 0.53 | 0.53 | 45.29 | 3.37 |
| R2-600 | 1340 | 1.07 | 0.50 | 0.43 | 46.73 | 3.19 |
| R3-400 | 1286 | 0.88 | 0.50 | 0.11 | 56.81 | 2.73 |
| R3-450 | 1317 | 1.05 | 0.47 | 0.45 | 44.76 | 3.18 |
| R3-500 | 1100 | 1.27 | 0.43 | 0.59 | 33.86 | 4.62 |
| R3-600 | 838 | 1.18 | 0.33 | 0.33 | 27.97 | 5.63 |
| ACs | R1-500 | R2-500 | R3-500 | CAC |
|---|---|---|---|---|
| Langmuir isotherm parameters | ||||
| qL (mg/g) | 98.65 | 169.69 | 115.69 | 126.52 |
| KL (L/mg) | 0.08 | 0.04 | 0.05 | 0.06 |
| R2 | 0.99 | 0.95 | 0.99 | 0.99 |
| Freundlich isotherm parameters | ||||
| KF (m2/g) | 14.24 | 13.66 | 11.09 | 12.54 |
| 1/n | 0.44 | 0.56 | 0.51 | 0.62 |
| R2 | 0.98 | 0.91 | 0.97 | 0.98 |
| Temkin isotherm parameters | ||||
| K1 (J/mol) | 121.06 | 93.45 | 101.34 | 47.98 |
| K2 (L/mg) | 0.93 | 0.75 | 0.55 | 0.23 |
| R2 | 0.81 | 0.81 | 0.83 | 0.87 |
| Acs | First-Order Model | Experiment | Second-Order Model | ||||
|---|---|---|---|---|---|---|---|
| qm (mg/g) | K·102 (L/mg) | R2 | qexp (mg/g) | qm (mg/g) | K·102 (L/mg) | R2 | |
| R1-500 | 18.77 | 1.63 | 0.68 | 70.92 | 69.01 | 6.63 | 0.99 |
| R2-500 | 36.51 | 2.12 | 0.94 | 71.27 | 69.75 | 2.16 | 0.99 |
| R3-500 | 41.61 | 1.78 | 0.84 | 75.53 | 71.53 | 2.07 | 0.99 |
| CAC | 19.74 | 0.20 | 0.68 | 74.06 | 70.45 | 7.62 | 0.99 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Madani, N.; Moulefera, I.; Boumad, S.; Cazorla-Amorós, D.; Gandía, F.J.V.; Cherifi, O.; Bouchenafa-Saib, N. Activated Carbon from Stipa tenacissima for the Adsorption of Atenolol. C 2022, 8, 66. https://doi.org/10.3390/c8040066
Madani N, Moulefera I, Boumad S, Cazorla-Amorós D, Gandía FJV, Cherifi O, Bouchenafa-Saib N. Activated Carbon from Stipa tenacissima for the Adsorption of Atenolol. C. 2022; 8(4):66. https://doi.org/10.3390/c8040066
Chicago/Turabian StyleMadani, Nesrine, Imane Moulefera, Souad Boumad, Diego Cazorla-Amorós, Francisco José Varela Gandía, Ouiza Cherifi, and Naima Bouchenafa-Saib. 2022. "Activated Carbon from Stipa tenacissima for the Adsorption of Atenolol" C 8, no. 4: 66. https://doi.org/10.3390/c8040066
APA StyleMadani, N., Moulefera, I., Boumad, S., Cazorla-Amorós, D., Gandía, F. J. V., Cherifi, O., & Bouchenafa-Saib, N. (2022). Activated Carbon from Stipa tenacissima for the Adsorption of Atenolol. C, 8(4), 66. https://doi.org/10.3390/c8040066










