Activated Carbon from Stipa tenacissima for the Adsorption of Atenolol
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Activated Carbon
2.2. Adsorption Equipment and Procedures
2.3. Characterization
3. Results and Discussion
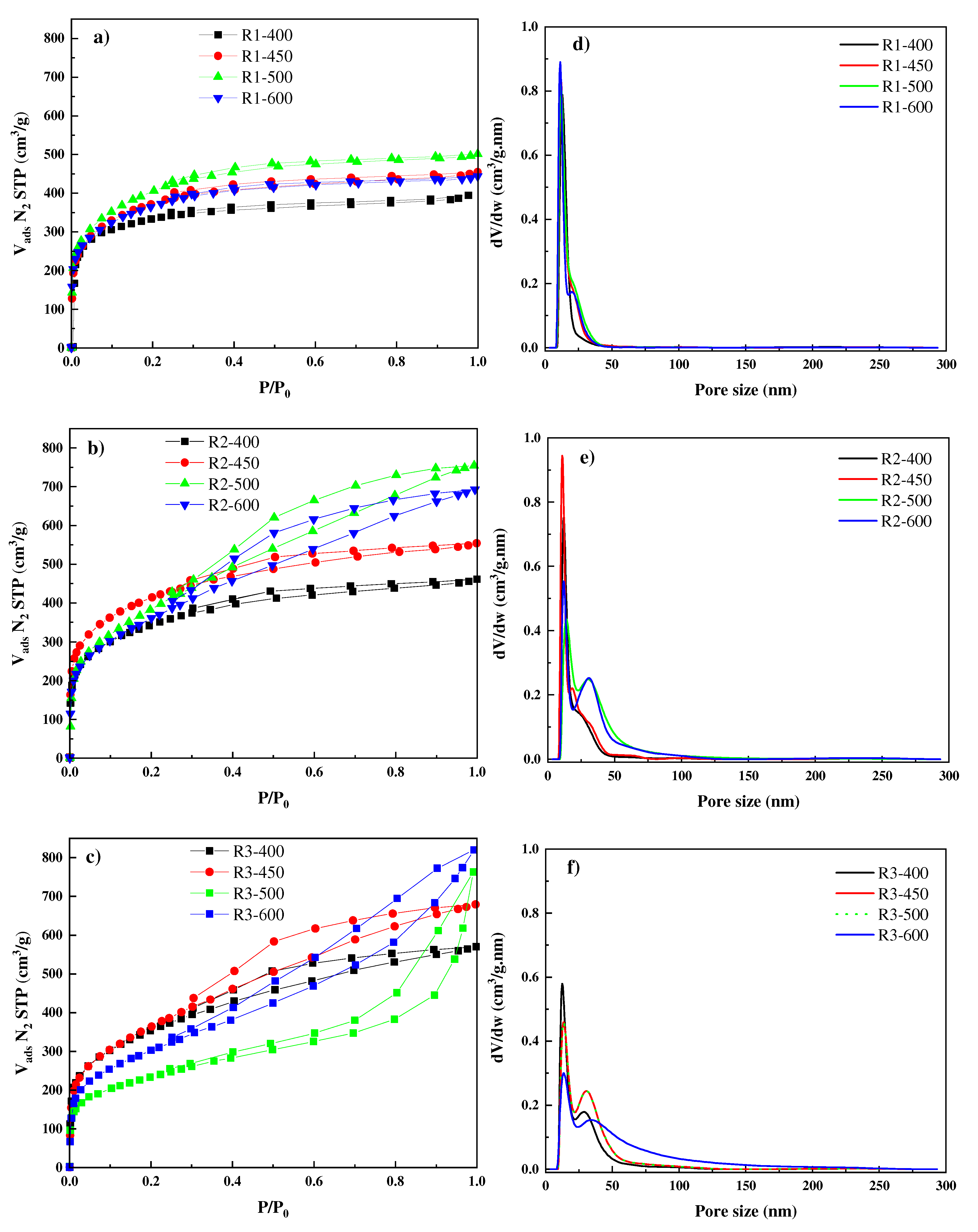
3.1. Characterization
3.2. Adsorption of Atenolol Drug
| Samples | SBET (m2/g) | Vtotal (cm3/g) | Vµp (cm3/g) | Vmeso (cm3/g) |
|---|---|---|---|---|
| R1-500 | 1478 | 0.78 | 0.57 | 0.13 |
| R2-500 | 1387 | 1.17 | 0.53 | 0.53 |
| R3-500 | 1100 | 1.27 | 0.43 | 0.59 |
| CAC | 909 | 0.76 | 0.36 | 0.40 |
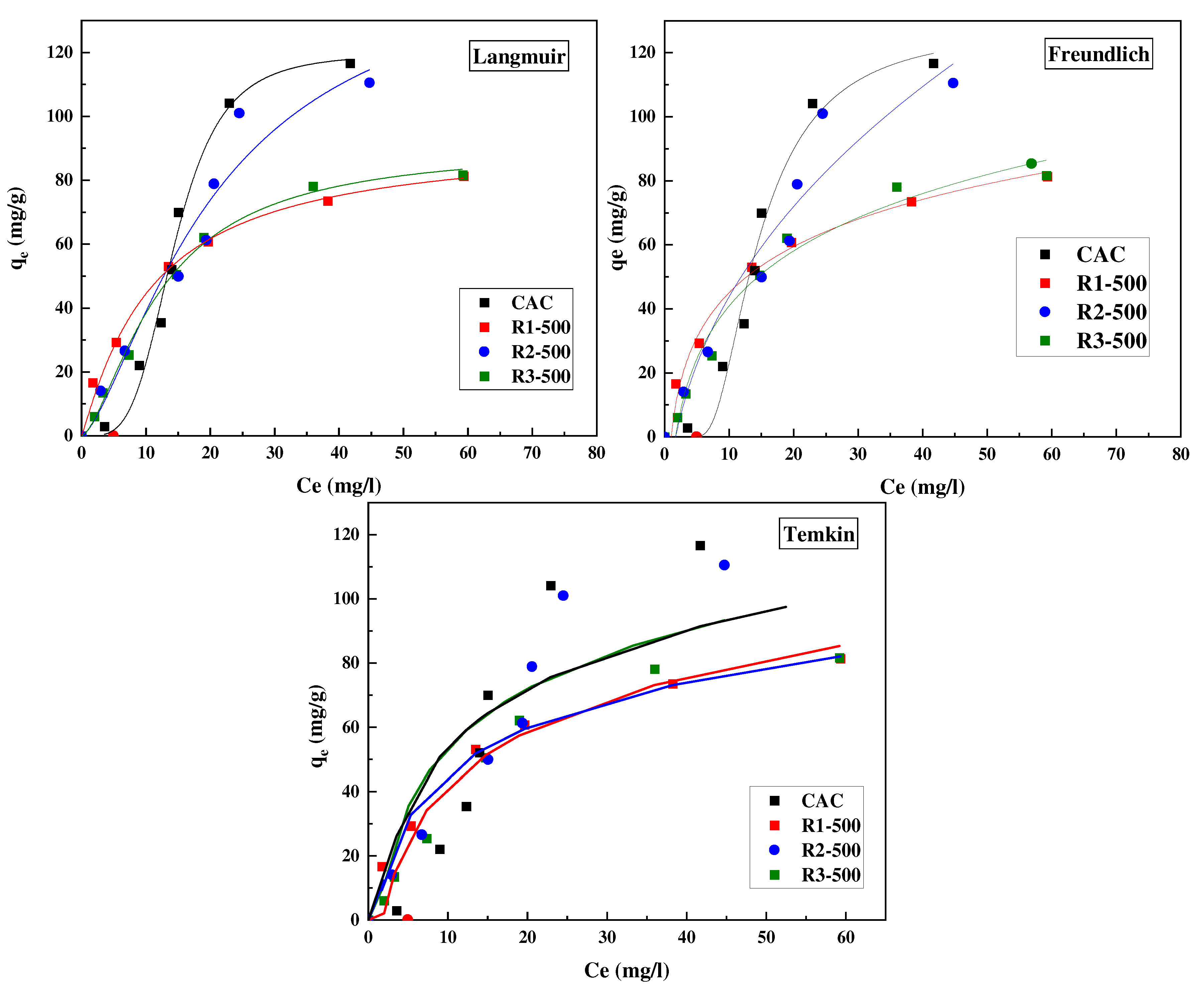
3.2.1. Equilibrium Adsorption
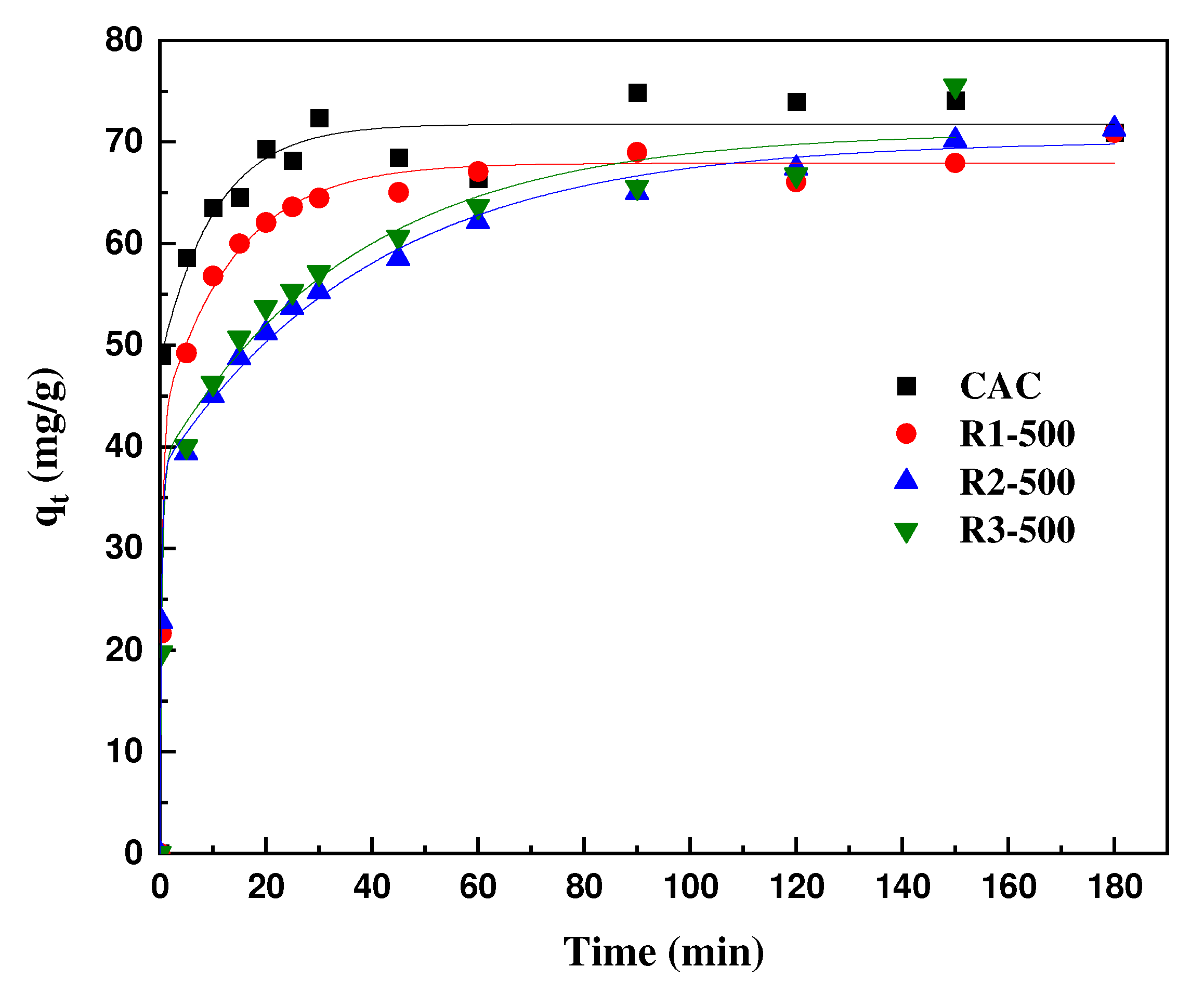
3.2.2. Kinetic Study
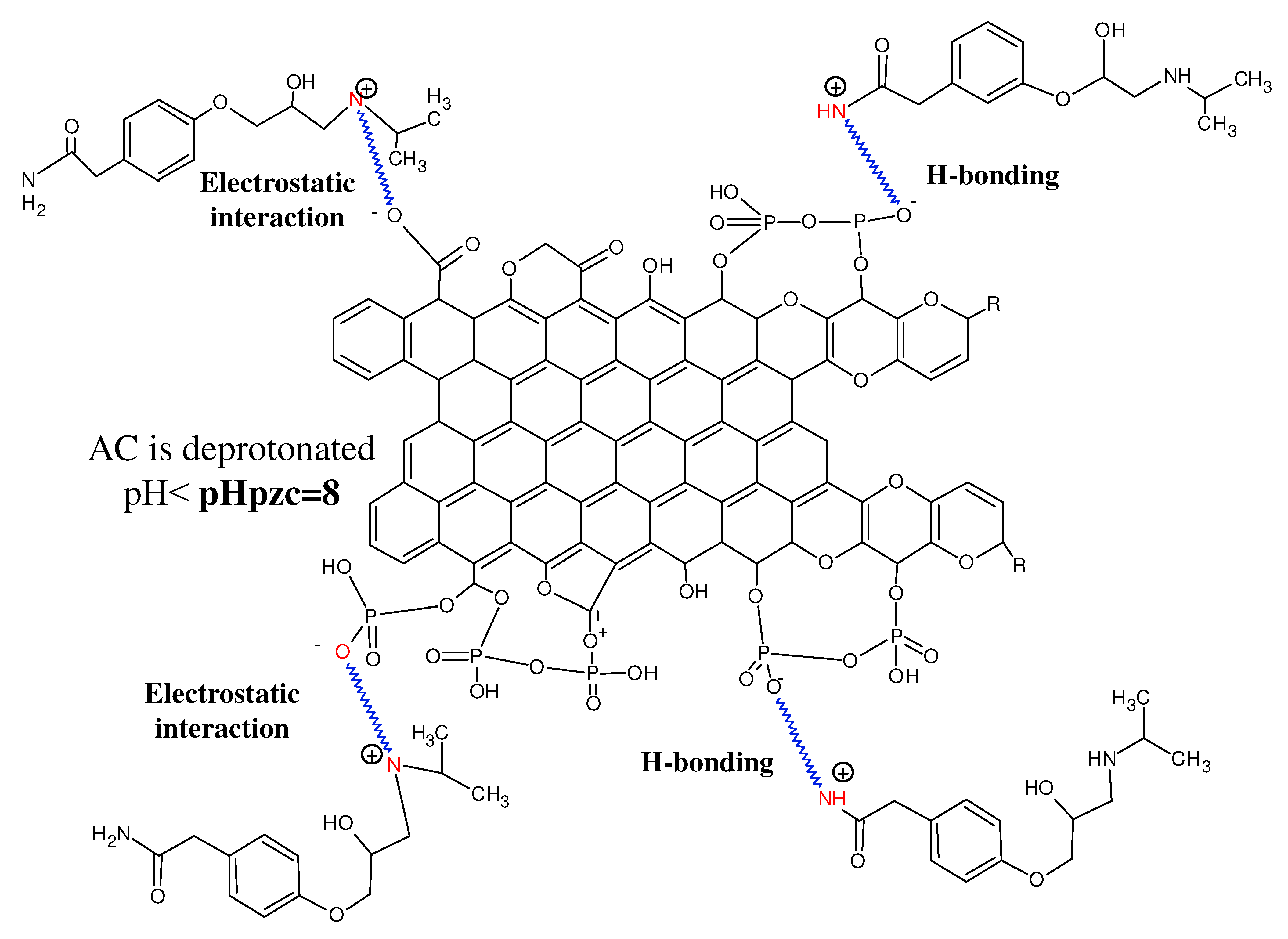
3.2.3. Mechanism of Atenolol Adsorption
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Azwar, E.; Mahari, W.A.W.; Chuah, J.H.; Vo, D.-V.N.; Ma, N.L.; Lam, W.H.; Lam, S.S. Transformation of biomass into carbon nanofiber for supercapacitor application—A review. Int. J. Hydrogen Energy 2018, 43, 20811–20821. [Google Scholar] [CrossRef]
- Moulefera, I.; Trabelsi, M.; Mamun, A.; Sabantina, L. Electrospun Carbon Nanofibers from Biomass and Biomass Blends—Current Trends. Polymers 2021, 13, 1071. [Google Scholar] [CrossRef]
- Nor, N.M.; Lau, L.C.; Lee, K.T.; Mohamed, A.R. Synthesis of activated carbon from lignocellulosic biomass and its applications in air pollution control—A review. J. Environ. Chem. Eng. 2013, 1, 658–666. [Google Scholar] [CrossRef]
- Vargas, A.M.; Cazetta, A.L.; Garcia, C.A.; Moraes, J.C.; Nogami, E.M.; Lenzi, E.; Costa, W.F.; Almeida, V.C. Preparation and characterization of activated carbon from a new raw lignocellulosic material: Flamboyant (Delonix regia) pods. J. Environ. Manag. 2011, 92, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Z.-Y.; Yang, Q.; Li, X.-M.; Luo, K.; Liu, Y.; Zeng, G.-M. Preparation of peanut hull-based activated carbon by microwave-induced phosphoric acid activation and its application in Remazol Brilliant Blue R adsorption. Ind. Crops Prod. 2012, 37, 178–185. [Google Scholar] [CrossRef]
- Liou, T.-H. Development of mesoporous structure and high adsorption capacity of biomass-based activated carbon by phosphoric acid and zinc chloride activation. Chem. Eng. J. 2010, 158, 129–142. [Google Scholar] [CrossRef]
- Yahya, M.A.; Al-Qodah, Z.; Zanariah Ngah, C.W. Agricultural bio-waste materials as potential sustainable precursors used for activated carbon production: A review. Renew. Sustain. Energy Rev. 2015, 46, 218–235. [Google Scholar] [CrossRef]
- Allied Market Research. Activated Carbon Market by Product Type (Powdered, Granular), Application (Liquid, Gaseous) and End Use (Water Treatment, Food & Beverage Processing, Air purification)—Global Opportunity Analysis and Industry Forecast, 2014–202. Allied Mark. Res. 2022. Available online: https://www.alliedmarketresearch.com/activated-carbon-market (accessed on 4 August 2022).
- Acharya, J.; Sahu, J.N.; Mohanty, C.; Meikap, B. Removal of lead(II) from wastewater by activated carbon developed from Tamarind wood by zinc chloride activation. Chem. Eng. J. 2009, 149, 249–262. [Google Scholar] [CrossRef]
- Tsai, W.-T.; Lai, C.-W.; Su, T.-Y. Adsorption of bisphenol-A from aqueous solution onto minerals and carbon adsorbents. J. Hazard. Mater. 2006, 134, 169–175. [Google Scholar] [CrossRef]
- García-Mateos, F.; Ruiz-Rosas, R.; Marqués, M.; Cotoruelo, L.; Rodríguez-Mirasol, J.; Cordero, T. Removal of paracetamol on biomass-derived activated carbon: Modeling the fixed bed breakthrough curves using batch adsorption experiments. Chem. Eng. J. 2015, 279, 18–30. [Google Scholar] [CrossRef]
- Kalkan, Ç.; Yapsakli, K.; Mertoglu, B.; Tufan, D.; Saatci, A. Evaluation of Biological Activated Carbon (BAC) process in wastewater treatment secondary effluent for reclamation purposes. Desalination 2011, 265, 266–273. [Google Scholar] [CrossRef]
- Guo, J.; Xu, W.S.; Chen, Y.L.; Lua, A.C. Adsorption of NH3 onto activated carbon prepared from palm shells impregnated with H2SO4. J. Colloid Interface Sci. 2004, 281, 285–290. [Google Scholar] [CrossRef]
- Sun, Y.; Yang, G.; Zhang, J.; Wang, Y.; Yao, M. Activated Carbon Preparation from Lignin by H3PO4 Activation and Its Application to Gas Separation. Chem. Eng. Technol. 2011, 35, 309–316. [Google Scholar] [CrossRef]
- Dolores, L.-C.; Juan, P.M.-L.; Falco, C.; Titirici, M.-M.; Diego, C.-A. Porous Biomass-Derived Carbons: Activated Carbons. In Sustainable Carbon Materials from Hydrothermal Processes; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2013; pp. 75–100. [Google Scholar] [CrossRef]
- Calvo-Muñoz, E.M.; García-Mateos, F.J.; Rosas, J.; Rodríguez-Mirasol, J.; Cordero, T. Biomass Waste Carbon Materials as adsorbents for CO2 Capture under Post-Combustion Conditions. Front. Mater. 2016, 3, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Ozdemir, S.; Ozdemir, S.; Yetilmezsoy, K. Poultry abattoir sludge as bio-nutrient source for walnut plantation in low-fertility soil. Environ. Prog. Sustain. Energy 2019, 38, e13066. [Google Scholar] [CrossRef]
- García-Mateos, F.J.; Moulefera, I.; Rosas, J.M.; Benyoucef, A.; Rodríguez-Mirasol, J.; Cordero, T. Alcohol Dehydrogenation on Kraft Lignin-Derived Chars with Surface Basicity. Catalysts 2017, 7, 308. [Google Scholar] [CrossRef] [Green Version]
- Matos, I.; Bernardo, M.; Fonseca, I. Porous carbon: A versatile material for catalysis. Catal. Today 2017, 285, 194–203. [Google Scholar] [CrossRef]
- Zhang, S.; Yin, H.; Wang, J.; Zhu, S.; Xiong, Y. Catalytic cracking of biomass tar using Ni nanoparticles embedded carbon nanofiber/porous carbon catalysts. Energy 2020, 216, 119285. [Google Scholar] [CrossRef]
- Szymański, G.; Rychlicki, G. Catalytic conversion of propan-2-ol on carbon catalysts. Carbon 1993, 31, 247–257. [Google Scholar] [CrossRef]
- Arampatzidou, A.C.; Deliyanni, E.A. Comparison of activation media and pyrolysis temperature for activated carbons development by pyrolysis of potato peels for effective adsorption of endocrine disruptor bisphenol-A. J. Colloid Interface Sci. 2016, 466, 101–112. [Google Scholar] [CrossRef]
- Moulefera, I.; García-Mateos, F.J.; Benyoucef, A.; Rosas, J.M.; Rodríguez-Mirasol, J.; Cordero, T. Effect of Co-solution of Carbon Precursor and Activating Agent on the Textural Properties of Highly Porous Activated Carbon Obtained by Chemical Activation of Lignin with H3PO4. Front. Mater. 2020, 7, 1–14. [Google Scholar] [CrossRef]
- Xu, J.; Chen, L.; Qu, H.; Jiao, Y.; Xie, J.; Xing, G. Preparation and characterization of activated carbon from reedy grass leaves by chemical activation with H3PO4. Appl. Surf. Sci. 2014, 320, 674–680. [Google Scholar] [CrossRef]
- Kan, Y.; Yue, Q.; Li, D.; Wu, Y.; Gao, B. Preparation and characterization of activated carbons from waste tea by H 3 PO 4 activation in different atmospheres for oxytetracycline removal. J. Taiwan Inst. Chem. Eng. 2017, 71, 494–500. [Google Scholar] [CrossRef]
- Romero, A.; Lillo-Rodenas, M.A.; de Lecea, C.S.-M.; Linares-Solano, A. Hydrothermal and conventional H3PO4 activation of two natural bio-fibers. Carbon 2012, 50, 3158–3169. [Google Scholar] [CrossRef]
- Bouchenafa-Saïb, N.; Mekarzia, A.; Bouzid, B.; Mohammedi, O.; Khelifa, A.; Benrachedi, K.; Belhaneche-Bensemra, N. Removal of malathion from polluted water by adsorption onto chemically activated carbons produced from coffee grounds. Desalin. Water Treat. 2013, 52, 4920–4927. [Google Scholar] [CrossRef]
- Marsh, H.; Rodríguez-Reinoso, F. Preface. In Activated Carbon; Marsh, H., Rodríguez-Reinoso, F., Eds.; Elsevier Science Ltd.: Oxford, UK, 2006. [Google Scholar] [CrossRef]
- Rodríguez-Reinoso, F.; Molina-Sabio, M. Textural and chemical characterization of microporous carbons. Adv. Colloid Interface Sci. 1998, 76–77, 271–294. [Google Scholar] [CrossRef]
- Nielsen, L.; Biggs, M.J.; Skinner, W.; Bandosz, T.J. The effects of activated carbon surface features on the reactive adsorption of carbamazepine and sulfamethoxazole. Carbon 2014, 80, 419–432. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.P.; Sun, Y.; Woo, M.W.; Zhang, L.; Xu, K.Z. Preparation of steam activated carbon from black liquor by flue gas precipitation and its performance in hydrogen sulfide removal: Experimental and simulation works. J. Taiwan Inst. Chem. Eng. 2016, 59, 395–404. [Google Scholar] [CrossRef]
- Vernersson, T.; Bonelli, P.R.; Cerrella, E.G.; Cukierman, A.L. Arundo donax cane as a precursor for activated carbons preparation by phosphoric acid activation. Bioresour. Technol. 2002, 83, 95–104. [Google Scholar] [CrossRef]
- Jagtoyen, M.; Groppo, J.; Derbyshire, F. Activated carbons from bituminous coals by reaction with H3PO4: The influence of coal cleaning. Fuel Process. Technol. 1993, 34, 85–96. [Google Scholar] [CrossRef]
- Nabais, J.V.; Laginhas, C.; Carrott, M.R.; Carrott, P.; Amorós, J.C.; Gisbert, A.N. Surface and porous characterisation of activated carbons made from a novel biomass precursor, the esparto grass. Appl. Surf. Sci. 2013, 265, 919–924. [Google Scholar] [CrossRef]
- Fierro, V.; Torné-Fernández, V.; Celzard, A.; Montané, D. Influence of the demineralisation on the chemical activation of Kraft lignin with orthophosphoric acid. J. Hazard. Mater. 2007, 149, 126–133. [Google Scholar] [CrossRef]
- Tancredi, N.; Cordero, T.; Mirasol, J.R.; Rodríguez, J.J. Activated carbons from Uruguayan eucalyptus wood. Fuel 1996, 75, 1701–1706. [Google Scholar] [CrossRef]
- Hernández, V. Lignocellulosic Precursors Used in the Synthesis of Activated Carbon-Characterization Techniques and Applications in the Wastewater Treatment; InTech: Houston, TX, USA, 2012. [Google Scholar] [CrossRef] [Green Version]
- González-García, P. Activated carbon from lignocellulosics precursors: A review of the synthesis methods, characterization techniques and applications, Renew. Sustain. Energy Rev. 2018, 82, 1393–1414. [Google Scholar] [CrossRef]
- Lin, Z.; Wang, D.; Zhang, H.; Li, L.; Huang, Z.; Shen, J.; Lin, Y. Extraction and determination of malachite green from aquatic products based on molecularly imprinted polymers. Sep. Sci. Technol. 2016, 51, 1684–1689. [Google Scholar] [CrossRef]
- Das, A.K.; Saha, S.; Pal, A.; Maji, S.K. Surfactant-modified alumina: An efficient adsorbent for malachite green removal from water environment. J. Environ. Sci. Health Part A 2009, 44, 896–905. [Google Scholar] [CrossRef]
- Farooqi, Z.H.; Sultana, H.; Begum, R.; Usman, M.; Ajmal, M.; Nisar, J.; Irfan, A.; Azam, M. Catalytic degradation of malachite green using a crosslinked colloidal polymeric system loaded with silver nanoparticles. Int. J. Environ. Anal. Chem. 2020. [Google Scholar] [CrossRef]
- Zaccariello, G.; Moretti, E.; Storaro, L.; Riello, P.; Canton, P.; Gombac, V.; Montini, T.; Rodríguez-Castellón, E.; Benedetti, A. TiO2–mesoporous silica nanocomposites: Cooperative effect in the photocatalytic degradation of dyes and drugs. RSC Adv. 2014, 4, 37826–37837. [Google Scholar] [CrossRef]
- Al Qarni, H.; Collier, P.; O’Keeffe, J.; Akunna, J. Investigating the removal of some pharmaceutical compounds in hospital wastewater treatment plants operating in Saudi Arabia. Environ. Sci. Pollut. Res. 2016, 23, 13003–13014. [Google Scholar] [CrossRef] [Green Version]
- Rezaei, R.; Aghapour, A.A.; Khorsandi, H. Investigating the biological degradation of the drug β-blocker atenolol from wastewater using the SBR. Biogeochemistry 2022, 33, 267–281. [Google Scholar] [CrossRef]
- Marques, S.C.; Mestre, A.S.; Machuqueiro, M.; Gotvajn, A.; Marinšek, M.; Carvalho, A.P. Apple tree branches derived activated carbons for the removal of β-blocker atenolol. Chem. Eng. J. 2018, 345, 669–678. [Google Scholar] [CrossRef]
- Haro, N.K.; Del Vecchio, P.; Marcilio, N.R.; Feris, L. Removal of atenolol by adsorption—Study of kinetics and equilibrium. J. Clean. Prod. 2017, 154, 214–219. [Google Scholar] [CrossRef]
- Ahmad, A.A.; Din, A.T.M.; Yahaya, N.K.E.; Karim, J.; Ahmad, M.A. Atenolol sequestration using activated carbon derived from gasified Glyricidia sepium. Arab. J. Chem. 2020, 13, 7544–7557. [Google Scholar] [CrossRef]
- Madani, N.; Bouchenafa-Saib, N.; Mohammedi, O.; Varela-Gandía, F.; Cazorla-Amorós, D.; Hamada, B.; Cherifi, O. Removal of heavy metal ions by adsorption onto activated carbon prepared from Stipa tenacissima leaves. Desalin. Water Treat. 2017, 64, 179–188. [Google Scholar] [CrossRef]
- Miroslav, F.; Radka, K.; Oksana, G.; Zuzana, S.; Aleš, K.; Antonín, N.; Martin, K.; Roman, G. Sorption of atenolol, sulfamethoxazole and carbamazepine onto soil aggregates from the illuvial horizon of the Haplic Luvisol on loess. Soil Water Res. 2018, 13, 177–183. [Google Scholar] [CrossRef] [Green Version]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of Gases in Multimolecular Layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Dubinin, M.M.; Zaverina, E.D.; Timofeyev, D.P. Sorption and structure of active carbons. I. Adsorption of organic vapors. Zhurnal Fiz. Khimii 1947, 21, 1352–1362. [Google Scholar]
- Sayğılı, H.; Güzel, F.; Önal, Y. Conversion of grape industrial processing waste to activated carbon sorbent and its performance in cationic and anionic dyes adsorption. J. Clean. Prod. 2015, 93, 84–93. [Google Scholar] [CrossRef]
- Demiral, I.; Şamdan, C.A. Preparation and Characterisation of Activated Carbon from Pumpkin Seed Shell Using H3PO4. Anadolu Univ. J. Sci. Technol. Appl. Sci. Eng. 2016, 17, 125–138. [Google Scholar] [CrossRef] [Green Version]
- Tadda, M.A.; Ahsan, A.; Shitu, A.; ElSergany, M.; Arunkumar, T.; Jose, B.; Daud, N.N. A review on activated carbon: Process, application and prospects. J. Adv. Civ. Eng. Pract. Res. 2016, 2, 7–13. [Google Scholar]
- Das, D.; Samal, D.P.; Bc, M. Preparation of Activated Carbon from Green Coconut Shell and its Characterization. J. Biosens. Bioelectron. 2015, 6, 100248. [Google Scholar] [CrossRef]
- Durán-Valle, C.J. Techniques Employed in the Physicochemical Characterization of Activated Carbons. In Lignocellulosic Precursors Used in the Synthesis of Activated Carbon-Characterization Techniques and Applications in the Wastewater Treatment; Montoya, V.H., Petriciolet, A.B., Eds.; IntechOpen: London, UK, 2012; pp. 38–58. Available online: https://books.google.dz/books?id=DnmszQEACAAJ (accessed on 4 August 2022).
- Yakout, S.; El-Deen, G.S. Characterization of activated carbon prepared by phosphoric acid activation of olive stones. Arab. J. Chem. 2016, 9, S1155–S1162. [Google Scholar] [CrossRef] [Green Version]
- Angin, D. Production and characterization of activated carbon from sour cherry stones by zinc chloride. Fuel 2014, 115, 804–811. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Jagtoyen, M.; Thwaites, M.; Stencel, J.; McEnaney, B.; Derbyshire, F. Adsorbent carbon synthesis from coals by phosphoric acid activation. Carbon 1992, 30, 1089–1096. [Google Scholar] [CrossRef]
- Kriaa, A.; Hamdi, N.; Srasra, E. Removal of Cu (II) from water pollutant with Tunisian activated lignin prepared by phosphoric acid activation. Desalination 2010, 250, 179–187. [Google Scholar] [CrossRef]
- Girgis, B.S.; Yunis, S.S.; Soliman, A.M. Characteristics of activated carbon from peanut hulls in relation to conditions of preparation. Mater. Lett. 2002, 57, 164–172. [Google Scholar] [CrossRef]
- Jagtoyen, M.; Derbyshire, F. Activated carbons from yellow poplar and white oak by H3PO4 activation. Carbon 1998, 36, 1085–1097. [Google Scholar] [CrossRef]
- Prahas, D.; Kartika, Y.; Indraswati, N.; Ismadji, S. Activated carbon from jackfruit peel waste by H3PO4 chemical activation: Pore structure and surface chemistry characterization. Chem. Eng. J. 2008, 140, 32–42. [Google Scholar] [CrossRef]
- Jun, T.Y.; Latip, N.H.A.; Abdullah, A.M.; Latif, P.A. Effect of Activation Temperature and Heating Duration on Physical Characteristics of Activated Carbon Prepared from Agriculture Waste. Environ. Asia 2010, 3, 143–148. [Google Scholar]
- Donald, J.; Ohtsuka, Y.; Xu, C. Effects of activation agents and intrinsic minerals on pore development in activated carbons derived from a Canadian peat. Mater. Lett. 2011, 65, 744–747. [Google Scholar] [CrossRef]
- Giles, C.H.; Smith, D.; Huitson, A. A general treatment and classification of the solute adsorption isotherm. I. Theoretical. J. Colloid Interface Sci. 1974, 47, 755–765. [Google Scholar] [CrossRef]
- Qu, W.; Yuan, T.; Yin, G.; Xu, S.; Zhang, Q.; Su, H. Effect of properties of activated carbon on malachite green adsorption. Fuel 2019, 249, 45–53. [Google Scholar] [CrossRef]
- Duan, C.; Wang, J.; Liu, Q.; Zhou, Y.; Zhou, Y. Efficient removal of Salbutamol and Atenolol by an electronegative silanized β-cyclodextrin adsorbent. Sep. Purif. Technol. 2021, 282, 120013. [Google Scholar] [CrossRef]
- García-Rosero, H.; Romero-Cano, L.A.; Aguilar-Aguilar, A.; Bailón-García, E.; Carvalho, A.P.; Pérez-Cadenas, A.F.; Carrasco-Marín, F. Adsorption and thermal degradation of Atenolol using carbon materials: Towards an advanced and sustainable drinking water treatment. J. Water Process. Eng. 2022, 49, 102978. [Google Scholar] [CrossRef]

| Proximate Analysis | Weight (%) |
|---|---|
| Ash | 1.19 |
| Fixed carbon | 24.50 |
| Volatile matter | 62.81 |
| Moisture | 11.50 |
| Samples | N | C | H | O * | O/C × 102 | H/C × 102 |
|---|---|---|---|---|---|---|
| STLs | 1.10 | 47.70 | 6.40 | 44.80 | 93.92 | 13.42 |
| R1-400 | 2.50 | 76.50 | 2.40 | 18.60 | 24.31 | 3.14 |
| R1-450 | 0.50 | 81.60 | 2.10 | 15.80 | 19.36 | 2.57 |
| R1-500 | 0.50 | 82.60 | 1.90 | 15.00 | 18.16 | 2.30 |
| R1-600 | 0.40 | 91.00 | 1.60 | 7.00 | 7.69 | 1.76 |
| R2-400 | 2.30 | 76.20 | 2.00 | 19.50 | 25.59 | 2.62 |
| R2-450 | 0.40 | 78.40 | 1.70 | 19.50 | 24.87 | 2.17 |
| R2-500 | 0.40 | 79.10 | 1.40 | 19.10 | 24.15 | 1.77 |
| R2-600 | 0.30 | 81.60 | 1.40 | 16.70 | 20.47 | 1.72 |
| R3-400 | 1.50 | 70.30 | 1.80 | 26.40 | 37.55 | 2.56 |
| R3-450 | 0.20 | 72.00 | 1.50 | 26.30 | 36.53 | 2.08 |
| R3-500 | 0.20 | 75.20 | 1.30 | 23.30 | 30.98 | 1.73 |
| R3-600 | 0.20 | 78.30 | 1.30 | 20.20 | 25.80 | 1.66 |
| Samples | SBET (m2/g) | Vtotal (cm3/g) | Vµp (cm3/g) | Vmeso (cm3/g) | Vµp/Vtot | Dp (nm) |
|---|---|---|---|---|---|---|
| R1-400 | 1204 | 0.61 | 0.53 | 0.07 | 86.88 | 2.03 |
| R1-450 | 1371 | 0.69 | 0.54 | 0.10 | 78.26 | 2.01 |
| R1-500 | 1478 | 0.78 | 0.57 | 0.13 | 73.08 | 2.11 |
| R1-600 | 1340 | 0.69 | 0.52 | 0.11 | 75.36 | 2.06 |
| R2-400 | 1258 | 0.71 | 0.49 | 0.16 | 69.01 | 2.26 |
| R2-450 | 1503 | 0.86 | 0.59 | 0.21 | 68.60 | 2.29 |
| R2-500 | 1387 | 1.17 | 0.53 | 0.53 | 45.29 | 3.37 |
| R2-600 | 1340 | 1.07 | 0.50 | 0.43 | 46.73 | 3.19 |
| R3-400 | 1286 | 0.88 | 0.50 | 0.11 | 56.81 | 2.73 |
| R3-450 | 1317 | 1.05 | 0.47 | 0.45 | 44.76 | 3.18 |
| R3-500 | 1100 | 1.27 | 0.43 | 0.59 | 33.86 | 4.62 |
| R3-600 | 838 | 1.18 | 0.33 | 0.33 | 27.97 | 5.63 |
| ACs | R1-500 | R2-500 | R3-500 | CAC |
|---|---|---|---|---|
| Langmuir isotherm parameters | ||||
| qL (mg/g) | 98.65 | 169.69 | 115.69 | 126.52 |
| KL (L/mg) | 0.08 | 0.04 | 0.05 | 0.06 |
| R2 | 0.99 | 0.95 | 0.99 | 0.99 |
| Freundlich isotherm parameters | ||||
| KF (m2/g) | 14.24 | 13.66 | 11.09 | 12.54 |
| 1/n | 0.44 | 0.56 | 0.51 | 0.62 |
| R2 | 0.98 | 0.91 | 0.97 | 0.98 |
| Temkin isotherm parameters | ||||
| K1 (J/mol) | 121.06 | 93.45 | 101.34 | 47.98 |
| K2 (L/mg) | 0.93 | 0.75 | 0.55 | 0.23 |
| R2 | 0.81 | 0.81 | 0.83 | 0.87 |
| Acs | First-Order Model | Experiment | Second-Order Model | ||||
|---|---|---|---|---|---|---|---|
| qm (mg/g) | K·102 (L/mg) | R2 | qexp (mg/g) | qm (mg/g) | K·102 (L/mg) | R2 | |
| R1-500 | 18.77 | 1.63 | 0.68 | 70.92 | 69.01 | 6.63 | 0.99 |
| R2-500 | 36.51 | 2.12 | 0.94 | 71.27 | 69.75 | 2.16 | 0.99 |
| R3-500 | 41.61 | 1.78 | 0.84 | 75.53 | 71.53 | 2.07 | 0.99 |
| CAC | 19.74 | 0.20 | 0.68 | 74.06 | 70.45 | 7.62 | 0.99 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Madani, N.; Moulefera, I.; Boumad, S.; Cazorla-Amorós, D.; Gandía, F.J.V.; Cherifi, O.; Bouchenafa-Saib, N. Activated Carbon from Stipa tenacissima for the Adsorption of Atenolol. C 2022, 8, 66. https://doi.org/10.3390/c8040066
Madani N, Moulefera I, Boumad S, Cazorla-Amorós D, Gandía FJV, Cherifi O, Bouchenafa-Saib N. Activated Carbon from Stipa tenacissima for the Adsorption of Atenolol. C. 2022; 8(4):66. https://doi.org/10.3390/c8040066
Chicago/Turabian StyleMadani, Nesrine, Imane Moulefera, Souad Boumad, Diego Cazorla-Amorós, Francisco José Varela Gandía, Ouiza Cherifi, and Naima Bouchenafa-Saib. 2022. "Activated Carbon from Stipa tenacissima for the Adsorption of Atenolol" C 8, no. 4: 66. https://doi.org/10.3390/c8040066
APA StyleMadani, N., Moulefera, I., Boumad, S., Cazorla-Amorós, D., Gandía, F. J. V., Cherifi, O., & Bouchenafa-Saib, N. (2022). Activated Carbon from Stipa tenacissima for the Adsorption of Atenolol. C, 8(4), 66. https://doi.org/10.3390/c8040066










