Release of Bioactive Molecules from Graphene Oxide-Alginate Hybrid Hydrogels: Effect of Crosslinking Method
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Ionically Crosslinked Hybrid Hydrogels
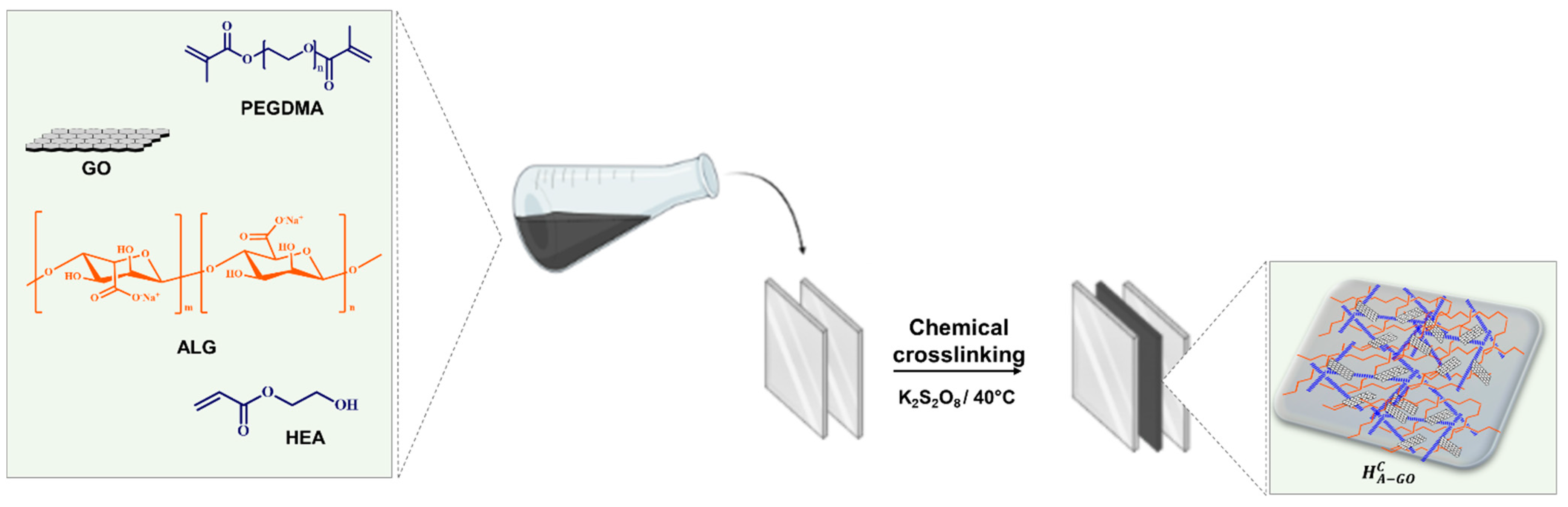
2.2. Synthesis of Chemically Crosslinked Hybrid Hydrogels
2.3. Instrumentation
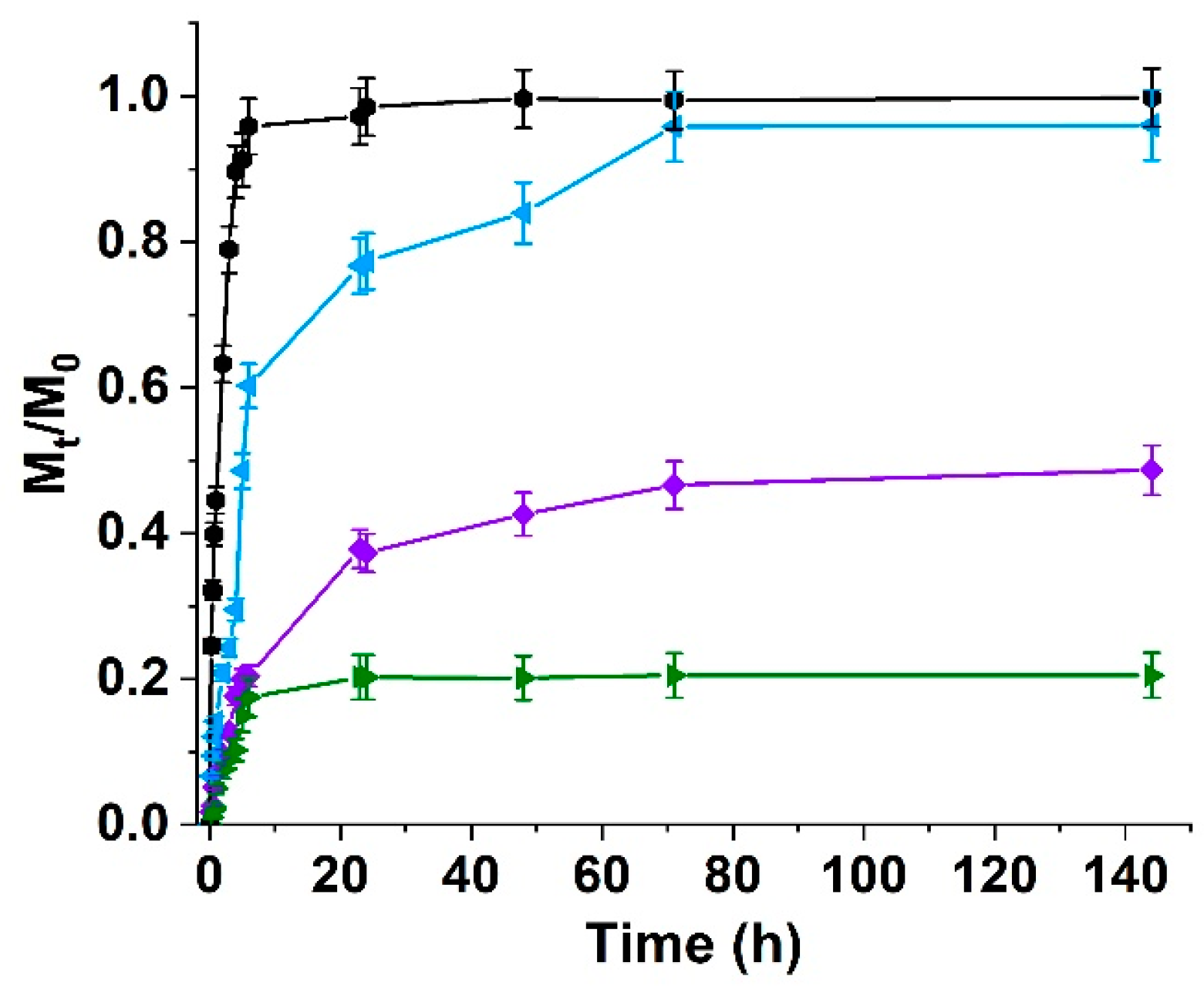
2.4. Determination of Weight Variation in Water Media
2.5. Determination of In Vitro Release Profiles
2.6. Statistical Analyses
3. Results and Discussion
3.1. Synthesis of Physically Crosslinked Hydrogels and Curcumin Loading
3.2. Synthesis of Chemically Crosslinked Hydrogels and Curcumin Loading
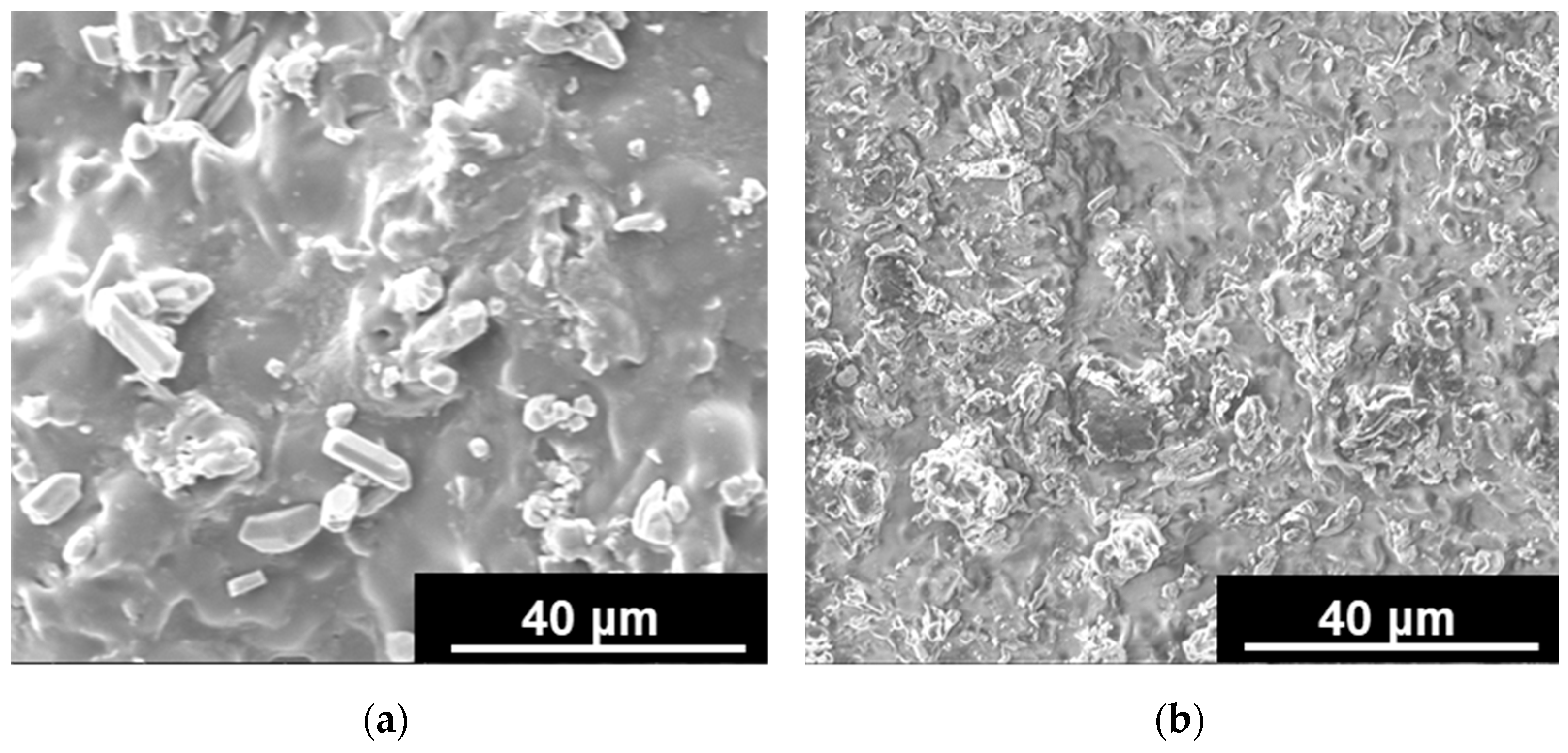
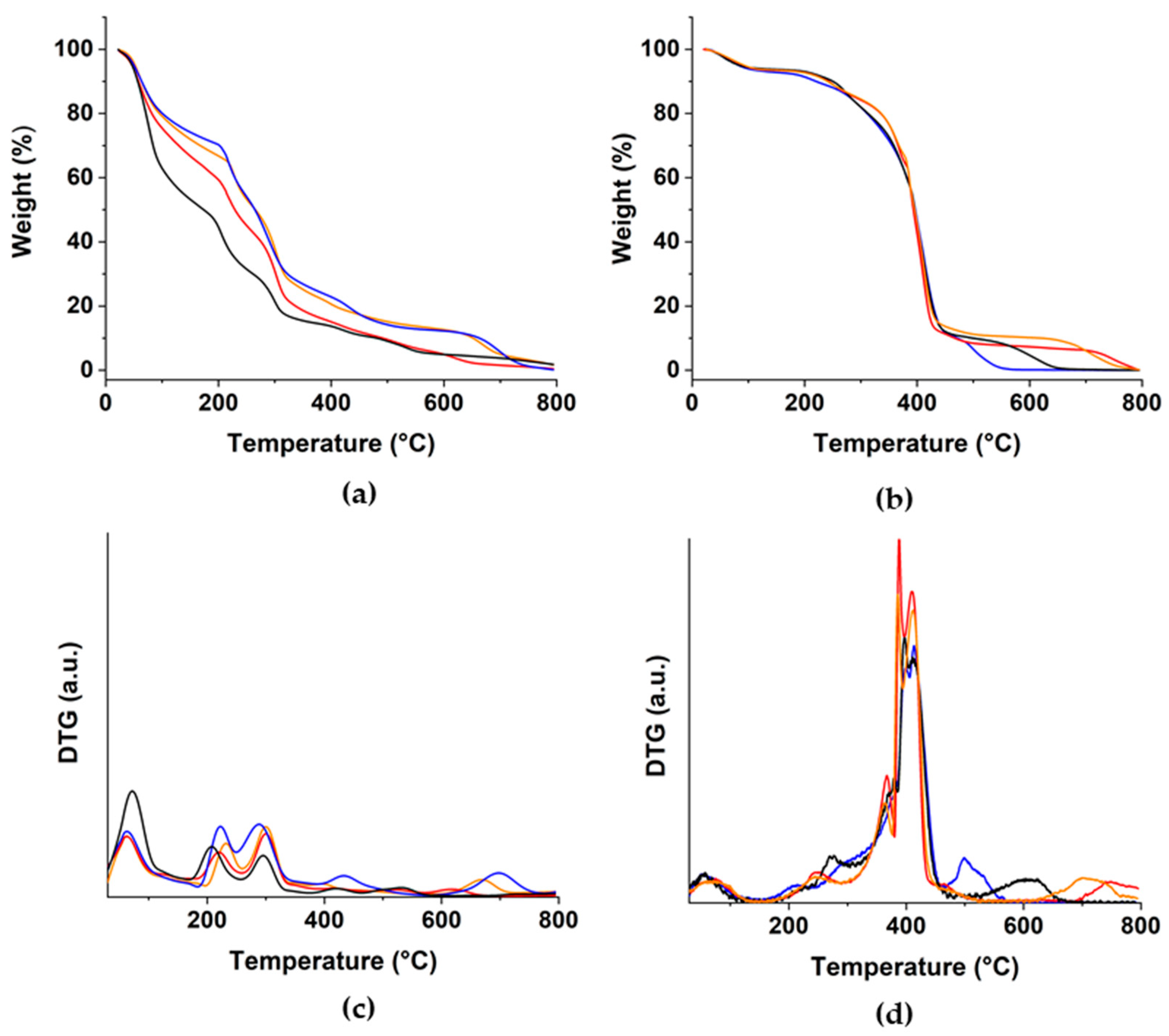
3.3. Characterization of Hydrogel Systems
3.4. Curcumin Delivery Behavior of Hydrogel Systems
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, B.Q.; Dragomir, M.P.; Yang, C.; Li, Q.Q.; Horst, D.; Calin, G.A. Targeting non-coding RNAs to overcome cancer therapy resistance. Signal Transduct. Target. Ther. 2022, 7, 121. [Google Scholar] [CrossRef] [PubMed]
- Lei, W.; Yang, C.; Wu, Y.; Ru, G.Q.; He, X.L.; Tong, X.M.; Wang, S.B. Nanocarriers surface engineered with cell membranes for cancer targeted chemotherapy. J. Nanobiotechnol. 2022, 20, 45. [Google Scholar] [CrossRef] [PubMed]
- Makharza, S.A.; Cirillo, G.; Vittorio, O.; Valli, E.; Voli, F.; Farfalla, A.; Curcio, M.; Lemma, F.; Nicoletta, F.P.; El-Gendy, A.A.; et al. Magnetic Graphene Oxide Nanocarrier for Targeted Delivery of Cisplatin: A Perspective for Glioblastoma Treatment. Pharmaceuticals 2019, 12, 76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oh, J.S.; Lee, E.J. Photodynamic Graphene Oxide Combined Alginate Hydrogel for Controlled Drug Release. Macromol. Res. 2021, 29, 383–390. [Google Scholar] [CrossRef]
- Curcio, M.; Brindisi, M.; Cirillo, G.; Frattaruolo, L.; Leggio, A.; Rago, V.; Nicoletta, F.P.; Cappello, A.R.; Iemma, F. Smart Lipid-Polysaccharide Nanoparticles for Targeted Delivery of Doxorubicin to Breast Cancer Cells. Int. J. Mol. Sci. 2022, 23, 2386. [Google Scholar] [CrossRef]
- Islam, M.S.; Renner, F.; Azizighannad, S.; Mitra, S. Direct incorporation of nano graphene oxide (nGO) into hydrophobic drug crystals for enhanced aqueous dissolution. Colloids Surf. B Biointerfaces 2020, 189, 110827. [Google Scholar] [CrossRef] [PubMed]
- Priyadarsini, S.; Mohanty, S.; Mukherjee, S.; Basu, S.; Mishra, M. Graphene and graphene oxide as nanomaterials for medicine and biology application. J. Nanostruct. Chem. 2018, 8, 123–137. [Google Scholar] [CrossRef] [Green Version]
- Curcio, M.; Farfalla, A.; Saletta, F.; Valli, E.; Pantuso, E.; Nicoletta, F.P.; Iemma, F.; Vittorio, O.; Cirillo, G. Functionalized Carbon Nanostructures Versus Drug Resistance: Promising Scenarios in Cancer Treatment. Molecules 2020, 25, 2102. [Google Scholar] [CrossRef] [PubMed]
- di Luca, M.; Vittorio, O.; Cirillo, G.; Curcio, M.; Czuban, M.; Voli, F.; Farfalla, A.; Hampel, S.; Nicoletta, F.P.; Iemma, F. Electro-responsive graphene oxide hydrogels for skin bandages: The outcome of gelatin and trypsin immobilization. Int. J. Pharm. 2018, 546, 50–60. [Google Scholar] [CrossRef]
- Ligorio, C.; O'Brien, M.; Hodson, N.W.; Mironov, A.; Iliut, M.; Miller, A.F.; Vijayaraghavan, A.; Hoyland, J.A.; Saiani, A. TGF-P3-loaded graphene oxide—Self-assembling peptide hybrid hydrogels as functional 3D scaffolds for the regeneration of the nucleus pulposus. Acta Biomater. 2021, 127, 116–130. [Google Scholar] [CrossRef]
- Chung, S.; Revia, R.A.; Zhang, M.Q. Graphene Quantum Dots and Their Applications in Bioimaging, Biosensing, and Therapy. Adv. Mater. 2021, 33, 1904362. [Google Scholar] [CrossRef] [PubMed]
- Radic, M.B.M.; Filipovic, V.V.; Vukomanovic, M.; Runic, J.N.; Tomic, S.L. Degradable 2-Hydroxyethyl Methacrylate/Gelatin/Alginate Hydrogels Infused by Nanocolloidal Graphene Oxide as Promising Drug Delivery and Scaffolding Biomaterials. Gels 2022, 8, 22. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zeng, X.; Ma, C.; Yi, H.; Ali, Z.; Mou, X.B.; Li, S.; Deng, Y.; He, N.Y. Injectable hydrogels for cartilage and bone tissue engineering. Bone Res. 2017, 5, 17014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, X.; Wu, H.; Guo, B.L.; Dong, R.N.; Qiu, Y.S.; Ma, P.X. Antibacterial anti-oxidant electroactive injectable hydrogel as self-healing wound dressing with hemostasis and adhesiveness for cutaneous wound healing. Biomaterials 2017, 122, 34–47. [Google Scholar] [CrossRef] [PubMed]
- Fan, F.; Saha, S.; Hanjaya-Putra, D. Biomimetic Hydrogels to Promote Wound Healing. Front. Bioeng. Biotechnol. 2021, 9, 718377. [Google Scholar] [CrossRef]
- Liang, Y.P.; He, J.H.; Guo, B.L. Functional Hydrogels as Wound Dressing to Enhance Wound Healing. ACS Nano 2021, 15, 12687–12722. [Google Scholar] [CrossRef]
- Qu, J.; Zhao, X.; Liang, Y.P.; Zhang, T.L.; Ma, P.X.; Guo, B.L. Antibacterial adhesive injectable hydrogels with rapid self-healing, extensibility and compressibility as wound dressing for joints skin wound healing. Biomaterials 2018, 183, 185–199. [Google Scholar] [CrossRef]
- Stan, D.; Tanase, C.; Avram, M.; Apetrei, R.; Mincu, N.B.; Mateescu, A.L.; Stan, D. Wound healing applications of creams and “smart” hydrogels. Exp. Dermatol. 2021, 30, 1218–1232. [Google Scholar] [CrossRef]
- Liu, H.; Wang, C.Y.; Li, C.; Qin, Y.G.; Wang, Z.H.; Yang, F.; Li, Z.H.; Wang, J.C. A functional chitosan-based hydrogel as a wound dressing and drug delivery system in the treatment of wound healing. RSC Adv. 2018, 8, 7533–7549. [Google Scholar] [CrossRef] [Green Version]
- di Luca, M.; Curcio, M.; Valli, E.; Cirillo, G.; Voli, F.; Butini, M.E.; Farfalla, A.; Pantuso, E.; Leggio, A.; Nicoletta, F.P.; et al. Combining antioxidant hydrogels with self-assembled microparticles for multifunctional wound dressings. J. Mater. Chem. B 2019, 7, 4361–4370. [Google Scholar] [CrossRef]
- Li, J.Y.; Mooney, D.J. Designing hydrogels for controlled drug delivery. Nat. Rev. Mater. 2016, 1, 16071. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.K.; Wang, Z.J.; Xiao, Y.; Zhang, S.M.; Wang, J.L. Advances in crosslinking strategies of biomedical hydrogels. Biomater. Sci. 2019, 7, 843–855. [Google Scholar] [CrossRef]
- Zhao, X.; Liang, Y.P.; Huang, Y.; He, J.H.; Han, Y.; Guo, B.L. Physical Double-Network Hydrogel Adhesives with Rapid Shape Adaptability, Fast Self-Healing, Antioxidant and NIR/pH Stimulus-Responsiveness for Multidrug-Resistant Bacterial Infection and Removable Wound Dressing. Adv. Funct. Mater. 2020, 30, 1910748. [Google Scholar] [CrossRef]
- Li, M.; Liang, Y.P.; He, J.H.; Zhang, H.L.; Guo, B.L. Two-Pronged Strategy of Biomechanically Active and Biochemically Multifunctional Hydrogel Wound Dressing To Accelerate Wound Closure and Wound Healing. Chem. Mater. 2020, 32, 9937–9953. [Google Scholar] [CrossRef]
- Zheng, W.; Wang, L.; Jiao, H.; Wu, Z.; Zhao, Q.; Lin, T.; Ma, H.; Zhang, Z.; Xu, X.; Cao, J.; et al. A cost-effective, fast cooling, and efficient anti-inflammatory multilayered topological hydrogel patch for burn wound first aid. Chem. Eng. J. 2022; in press. [Google Scholar] [CrossRef]
- Zhao, Q.; Liu, J.Y.; Wu, Z.X.; Xu, X.Y.; Ma, H.D.; Hou, J.; Xu, Q.L.; Yang, R.P.; Zhang, K.Y.; Zhang, M.M.; et al. Robust PEDOT:PSS-based hydrogel for highly efficient interfacial solar water purification. Chem. Eng. J. 2022, 442, 136284. [Google Scholar] [CrossRef]
- Aparicio-Collado, J.L.; Garcia-San-Martin, N.; Molina-Mateo, J.; Cabanilles, C.T.; Quiles, V.D.; Serrano-Aroca, A.; Serra, R.S.I. Electroactive calcium-alginate/polycaprolactone/reduced graphene oxide nanohybrid hydrogels for skeletal muscle tissue engineering. Colloidc Surf. B Biointerfaces 2022, 214, 112455. [Google Scholar] [CrossRef]
- Garcia-Couce, J.; Vernhes, M.; Bada, N.; Aguero, L.; Valdes, O.; Alvarez-Barreto, J.; Fuentes, G.; Almirall, A.; Cruz, L.J. Synthesis and Evaluation of AlgNa-g-Poly(QCL-co-HEMA) Hydrogels as Platform for Chondrocyte Proliferation and Controlled Release of Betamethasone. Int. J. Mol. Sci. 2021, 22, 5730. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, I.; Kumar, S.S.S.S.; Gupta, K.; Chakraborty, S. In-vitro release study through novel graphene oxide aided alginate based pH-sensitive drug carrier for gastrointestinal tract. Mater. Today Commun. 2021, 26, 101737. [Google Scholar] [CrossRef]
- Geng, Z.J.; Ji, Y.X.; Yu, S.; Liu, Q.F.; Zhou, Z.B.; Guo, C.P.; Lu, D.H.; Pei, D.T. Preparation and characterization of a dual cross-linking injectable hydrogel based on sodium alginate and chitosan quaternary ammonium salt. Carbohydr. Res. 2021, 507, 108389. [Google Scholar] [CrossRef]
- Wang, L.N.; Zhang, H.J.; Liu, X.Q.; Liu, Y.; Zhu, X.; Liu, X.H.; You, X.Y. A Physically Cross-Linked Sodium Alginate-Gelatin Hydrogel with High Mechanical Strength. ACS Appl. Polym. Mater. 2021, 3, 3197–3205. [Google Scholar] [CrossRef]
- Sanchez-Fernandez, J.A.; Presbitero-Espinosa, G.; Pena-Paras, L.; Pizana, E.I.R.; Galvan, K.P.V.; Vopalensky, M.; Kumpova, I.; Elizalde-Herrera, L.E. Characterization of Sodium Alginate Hydrogels Reinforced with Nanoparticles of Hydroxyapatite for Biomedical Applications. Polymers 2021, 13, 2927. [Google Scholar] [CrossRef] [PubMed]
- Theodorakis, N.; Saravanou, S.F.; Kouli, N.P.; Iatridi, Z.; Tsitsilianis, C. pH/Thermo-Responsive Grafted Alginate-Based SiO2 Hybrid Nanocarrier/Hydrogel Drug Delivery Systems. Polymers 2021, 13, 1228. [Google Scholar] [CrossRef]
- Shahriari-Khalaji, M.; Hong, S.Y.; Hu, G.Q.; Ji, Y.; Hong, F.F. Bacterial Nanocellulose-Enhanced Alginate Double-Network Hydrogels Cross-Linked with Six Metal Cations for Antibacterial Wound Dressing. Polymers 2020, 12, 2683. [Google Scholar] [CrossRef]
- Wang, Q.Q.; Liu, Y.; Zhang, C.J.; Zhang, C.; Zhu, P. Alginate/gelatin blended hydrogel fibers cross-linked by Ca2+ and oxidized starch: Preparation and properties. Mat. Sci. Eng. C 2019, 99, 1469–1476. [Google Scholar] [CrossRef] [PubMed]
- Raja, C.A.; Balakumar, S.; Anandkumar, B.; George, R.P.; Mudali, U.K. Formation of bioactive nano hybrid thin films on anodized titanium via electrophoretic deposition intended for biomedical applications. Mater. Today Commun. 2020, 25, 101666. [Google Scholar] [CrossRef]
- Devi, G.V.Y.; Prabhu, A.; Anil, S.; Venkatesan, J. Preparation and characterization of dexamethasone loaded sodium alginate-graphene oxide microspheres for bone tissue engineering. J. Drug Deliv. Sci. Technol. 2021, 64, 102624. [Google Scholar] [CrossRef]
- Abasalizadeh, F.; Moghaddam, S.V.; Alizadeh, E.; Akbari, E.; Kashani, E.; Fazljou, S.M.B.; Torbati, M.; Akbarzadeh, A. Alginate-based hydrogels as drug delivery vehicles in cancer treatment and their applications in wound dressing and 3D bioprinting. J. Biol. Eng. 2020, 14, 8. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.U.A.; Abd Razak, S.I.; Haider, S.; Mannan, H.A.; Hussain, J.; Hasan, A. Sodium alginate-f-GO composite hydrogels for tissue regeneration and antitumor applications. Int. J. Biol. Macromol. 2022, 208, 475–485. [Google Scholar] [CrossRef] [PubMed]
- Yun, Y.J.; Wu, H.W.; Gao, J.; Dai, W.; Deng, L.H.; Lv, O.; Kong, Y. Facile synthesis of Ca2+-crosslinked sodium alginate/graphene oxide hybrids as electro- and pH-responsive drug carrier. Mat. Sci. Eng. C 2020, 108, 110380. [Google Scholar] [CrossRef] [PubMed]
- Lentz, L.; Mayer, D.A.; Dogenski, M.; Ferreira, S.R.S. Hybrid aerogels of sodium alginate/graphene oxide as efficient adsorbents for wastewater treatment. Mater. Chem. Phys. 2022, 283, 125981. [Google Scholar] [CrossRef]
- Jin, Y.; Zhang, W.K.; Zhang, Y.H.; Yang, Y.Q.; Fang, Z.W.; Song, J.L.; Qian, Y.; Yuan, W.E. Multifunctional biomimetic hydrogel based on graphene nanoparticles and sodium alginate for peripheral nerve injury therapy. Biomater. Adv. 2022, 135, 212727. [Google Scholar] [CrossRef]
- Khapre, M.A.; Pandey, S.; Jugade, R.M. Glutaraldehyde-cross-linked chitosan-alginate composite for organic dyes removal from aqueous solutions. Int. J. Biol. Macromol. 2021, 190, 862–875. [Google Scholar] [CrossRef]
- Fan, L.H.; Ge, H.Y.; Zou, S.Q.; Xiao, Y.; Wen, H.H.; Li, Y.; Feng, H.; Nie, M. Sodium alginate conjugated graphene oxide as a new carrier for drug delivery system. Int. J. Biol. Macromol. 2016, 93, 582–590. [Google Scholar] [CrossRef] [PubMed]
- Serrano-Aroca, A.; Ferrandis-Montesinos, M.; Wang, R.B. Antiviral Properties of Alginate-Based Biomaterials: Promising Antiviral Agents against SARS-CoV-2. ACS Appl. Bio Mater. 2021, 4, 5897–5907. [Google Scholar] [CrossRef]
- Cirillo, G.; Pantuso, E.; Curcio, M.; Vittorio, O.; Leggio, A.; Iemma, F.; De Filpo, G.; Nicoletta, F.P. Alginate Bioconjugate and Graphene Oxide in Multifunctional Hydrogels for Versatile Biomedical Applications. Molecules 2021, 26, 1355. [Google Scholar] [CrossRef] [PubMed]
- Madeo, L.F.; Sarogni, P.; Cirillo, G.; Vittorio, O.; Voliani, V.; Curcio, M.; Shai-Hee, T.; Buchner, B.; Mertig, M.; Hampel, S. Curcumin and Graphene Oxide Incorporated into Alginate Hydrogels as Versatile Devices for the Local Treatment of Squamous Cell Carcinoma. Materials 2022, 15, 1648. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Burgess, D.J. In vitro dissolution testing strategies for nanoparticulate drug delivery systems: Recent developments and challenges. Drug Deliv. Transl. Res. 2013, 3, 409–415. [Google Scholar] [CrossRef] [Green Version]
- Zaborniak, I.; Chmielarz, P. Comestible curcumin: From kitchen to polymer chemistry as a photocatalyst in metal-free ATRP of (meth)acrylates. J. Ind. Eng. Chem. 2022, 105, 481–490. [Google Scholar] [CrossRef]
- Zheng, H.C.; Yang, J.S.; Han, S.Y. The synthesis and characteristics of sodium alginate/graphene oxide composite films crosslinked with multivalent cations. J. Appl. Polym. Sci. 2016, 133, 43616. [Google Scholar] [CrossRef]
- Yong, Q.W.; Liang, C.Z. Synthesis of an Aqueous Self-Matting Acrylic Resin with Low Gloss and High Transparency via Controlling Surface Morphology. Polymers 2019, 11, 322. [Google Scholar] [CrossRef] [Green Version]
- Wallmersperger, T.; Ballhause, D.; Kroplin, B. On the modeling of polyelectrolyte gels. Macromol. Symp. 2007, 254, 306–313. [Google Scholar] [CrossRef]
- Cirillo, G.; Curcio, M.; Spizzirri, U.G.; Vittorio, O.; Tucci, P.; Picci, N.; Iemma, F.; Hampel, S.; Nicoletta, F.P. Carbon nanotubes hybrid hydrogels for electrically tunable release of Curcumin. Eur. Polym. J. 2017, 90, 1–12. [Google Scholar] [CrossRef]
- Talebian, S.; Mehrali, M.; Raad, R.; Safaei, F.; Xi, J.T.; Liu, Z.F.; Foroughi, J. Electrically Conducting Hydrogel Graphene Nanocomposite Biofibers for Biomedical Applications. Front. Chem. 2020, 8, 88. [Google Scholar] [CrossRef] [Green Version]
- Saikia, A.K.; Aggarwal, S.; Mandal, U.K. Electrically induced swelling and methylene blue release behaviour of poly (N-isopropylacrylamide-co-acrylamido-2-methylpropyl sulphonic acid) hydrogels. Colloid Polym. Sci. 2015, 293, 3533–3544. [Google Scholar] [CrossRef]
- Wanjale, M.V.; Jaikumar, V.S.; Sivakumar, K.C.; Paul, R.A.; James, J.; Kumar, G.S.V. Supramolecular Hydrogel Based Post-Surgical Implant System for Hydrophobic Drug Delivery Against Glioma Recurrence. Int. J. Nanomed. 2022, 17, 2203–2224. [Google Scholar] [CrossRef] [PubMed]
- Kalepu, S.; Nekkanti, V. Insoluble drug delivery strategies: Review of recent advances and business prospects. Acta Pharm. Sin. B 2015, 5, 442–453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reis, A.V.; Guilherme, M.R.; Rubira, A.F.; Muniz, E.C. Mathematical model for the prediction of the overall profile of in vitro solute release from polymer networks. J. Colloid Interface Sci. 2007, 310, 128–135. [Google Scholar] [CrossRef]
- Siepmann, J.; Gopferich, A. Mathematical modeling of bioerodible, polymeric drug delivery systems. Adv. Drug Deliv. Rev. 2001, 48, 229–247. [Google Scholar] [CrossRef] [PubMed]
- Malekjani, N.; Jafari, S.M. Modeling the release of food bioactive ingredients from carriers/nanocarriers by the empirical, semiempirical, and mechanistic models. Compr. Rev. Food Sci. Food Saf. 2021, 20, 3–47. [Google Scholar] [CrossRef]
- Unagolla, J.M.; Jayasuriya, A.C. Drug transport mechanisms and in vitro release kinetics of vancomycin encapsulated chitosan-alginate polyelectrolyte microparticles as a controlled drug delivery system. Eur. J. Pharm. Sci. 2018, 114, 199–209. [Google Scholar] [CrossRef]
- Li, X.S.; Li, Q.; Zhao, C. Zero-Order Controlled Release of Water-Soluble Drugs Using a Marker Pen Platform. ACS Omega 2021, 6, 13774–13778. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Kao, W.J. Drug release kinetics and transport mechanisms of non-degradable and degradable polymeric delivery systems. Expert Opin. Drug Deliv. 2010, 7, 429–444. [Google Scholar] [CrossRef] [PubMed]
- Sulttan, S.; Rohani, S. Controlled Drug Release of Smart Magnetic Self-Assembled Micelle, Kinetics and Transport Mechanisms. J. Pharm. Sci. 2022, 111, 2378–2388. [Google Scholar] [CrossRef] [PubMed]


| Hydrogel | pH | Voltage (V) | WV (%) | (%) |
|---|---|---|---|---|
| 5.5 | 0 | 221 | ||
| 12 | 224 | 1.4 | ||
| 24 | 227 | 2.7 | ||
| 48 | 230 | 4.1 | ||
| 7.4 | 0 | 401 | ||
| 12 | 411 | 2.5 | ||
| 24 | 406 | 1.2 | ||
| 48 | 409 | 2.0 | ||
| 5.5 | 0 | 258 | ||
| 12 | 296 | 14.7 | ||
| 24 | 384 | 48.8 | ||
| 48 | 348 | 34.9 | ||
| 7.4 | 0 | 517 | ||
| 12 | 651 | 25.9 | ||
| 24 | 691 | 33.7 | ||
| 48 | 673 | 30.2 | ||
| 5.5 | 0 | 251 | ||
| 12 | 208 | −17.1 | ||
| 24 | 187 | −25.4 | ||
| 48 | 164 | −34.6 | ||
| 7.4 | 0 | 554 | ||
| 12 | 202 | −63.5 | ||
| 24 | 152 | −72.5 | ||
| 48 | 124 | −77.6 | ||
| 5.5 | 0 | 157 | ||
| 12 | 147 | −6.3 | ||
| 24 | 138 | −12.2 | ||
| 48 | 114 | −27.4 | ||
| 7.4 | 0 | 436 | ||
| 12 | 309 | −29.1 | ||
| 24 | 252 | −42.2 | ||
| 48 | 197 | −54.8 |
| Hydrogel | CUR Loading (wt%) | Reversible First Order | Reversible Second Order | ||||
|---|---|---|---|---|---|---|---|
| R2 | Fmax | α | R2 | Fmax | α | ||
| 2.5 | 0.8074 | - | - | 0.9105 | 0.60 | 1.51 | |
| 5.0 | 0.7954 | - | - | 0.9471 | 0.99 | 99 | |
| 7.5 | 0.6877 | - | - | 0.9322 | 0.99 | 99 | |
| 2.5 | 0.7688 | - | - | 0.9386 | 0.39 | 0.64 | |
| 5.0 | 0.6992 | - | - | 0.9112 | 0.80 | 4.01 | |
| 7.5 | 0.7598 | - | - | 0.9054 | 0.92 | 11.5 | |
| 2.5 | 0.9322 | 0.99 | 99 | 0.8877 | - | - | |
| 4.5 * | 0.9547 | 0.99 | 99 | 0.9258 | - | - | |
| 5.0 | 0.9471 | 0.99 | 99 | 0.8954 | - | - | |
| 7.5 | 0.9954 | 0.98 | 53.35 | 0.9411 | - | - | |
| 2.5 | 0.9054 | 0.92 | 11.50 | 0.8598 | - | - | |
| 4.5 * | 0.9479 | 0.94 | 99 | 0.9025 | - | - | |
| 5.0 | 0.9112 | 0.80 | 4.01 | 0.8992 | - | - | |
| 7.5 | 0.9833 | 0.45 | 0.8 | 0.9584 | - | - | |
| Hydrogel | Zero Order | First Order | Ritger-Peppas | Peppas-Sahlin | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R2 | k1 (10−3) | R2 | k2 | R2 | kP | n | R2 | m | (10−2) | |||
| 0.692 | 9.36 | 0.794 | 0.52 | 0.996 | 0.29 | 0.19 | 0.998 | 0.21 | 0.31 | 1.7 | 18 | |
| 0.690 | 6.02 | 0.826 | 0.38 | 0.993 | 0.19 | 0.19 | 0.997 | 0.24 | 0.20 | 2.0 | 10 | |
| 0.565 | 1.07 | 0.995 | 0.17 | 0.950 | 0.31 | 0.28 | 0.967 | 0.49 | 0.26 | 1.57 | 17 | |
| 0.734 | 0.50 | 0.985 | 0.11 | 0.924 | 0.11 | 0.34 | 0.988 | 0.54 | 0.08 | 0.34 | 23 | |
| Hydrogel | Voltage | Zero Order | First Order | Ritger-Peppas | Peppas-Sahlin | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R2 | k1 (10−2) | R2 | k2 | R2 | kP | n | R2 | m | (10−2) | ||||
| 12 | 0.555 | 1.08 | 0.993 | 0.18 | 0.926 | 0.32 | 0.27 | 0.967 | 0.48 | 0.27 | 1.67 | 16 | |
| 24 | 0.567 | 1.08 | 0.991 | 0.17 | 0.931 | 0.31 | 0.28 | 0.961 | 0.49 | 0.26 | 1.54 | 17 | |
| 48 | 0.549 | 1.09 | 0.987 | 0.19 | 0.905 | 0.34 | 0.26 | 0.973 | 0.46 | 0.29 | 2.03 | 14 | |
| 12 | 0.635 | 0.97 | 0.969 | 0.14 | 0.909 | 0.24 | 0.31 | 0.963 | 0.51 | 0.20 | 0.97 | 21 | |
| 24 | 0.518 | 0.23 | 0.986 | 0.23 | 0.901 | 0.07 | 0.25 | 0.921 | 0.45 | 0.07 | 0.49 | 14 | |
| 48 | 0.405 | 1.11 | 0.984 | 0.62 | 0.914 | 0.15 | 0.57 | 0.915 | 0.32 | 0.62 | 8.83 | 7 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Madeo, L.F.; Curcio, M.; Iemma, F.; Nicoletta, F.P.; Hampel, S.; Cirillo, G. Release of Bioactive Molecules from Graphene Oxide-Alginate Hybrid Hydrogels: Effect of Crosslinking Method. C 2023, 9, 8. https://doi.org/10.3390/c9010008
Madeo LF, Curcio M, Iemma F, Nicoletta FP, Hampel S, Cirillo G. Release of Bioactive Molecules from Graphene Oxide-Alginate Hybrid Hydrogels: Effect of Crosslinking Method. C. 2023; 9(1):8. https://doi.org/10.3390/c9010008
Chicago/Turabian StyleMadeo, Lorenzo Francesco, Manuela Curcio, Francesca Iemma, Fiore Pasquale Nicoletta, Silke Hampel, and Giuseppe Cirillo. 2023. "Release of Bioactive Molecules from Graphene Oxide-Alginate Hybrid Hydrogels: Effect of Crosslinking Method" C 9, no. 1: 8. https://doi.org/10.3390/c9010008
APA StyleMadeo, L. F., Curcio, M., Iemma, F., Nicoletta, F. P., Hampel, S., & Cirillo, G. (2023). Release of Bioactive Molecules from Graphene Oxide-Alginate Hybrid Hydrogels: Effect of Crosslinking Method. C, 9(1), 8. https://doi.org/10.3390/c9010008







