Response of Microbial Recovery Rate to Straw Return after Calcium Cyanamide Soil Disinfection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Location
2.2. Experimental Design
2.3. Cucumber Planting
2.4. Soil Sampling
2.5. Determination of Cultivable Microorganisms in Soil
2.6. Soil Physicochemical Properties
2.7. Cucumber Yield
2.8. Statistical Analysis
3. Results
3.1. Cucumber Yield
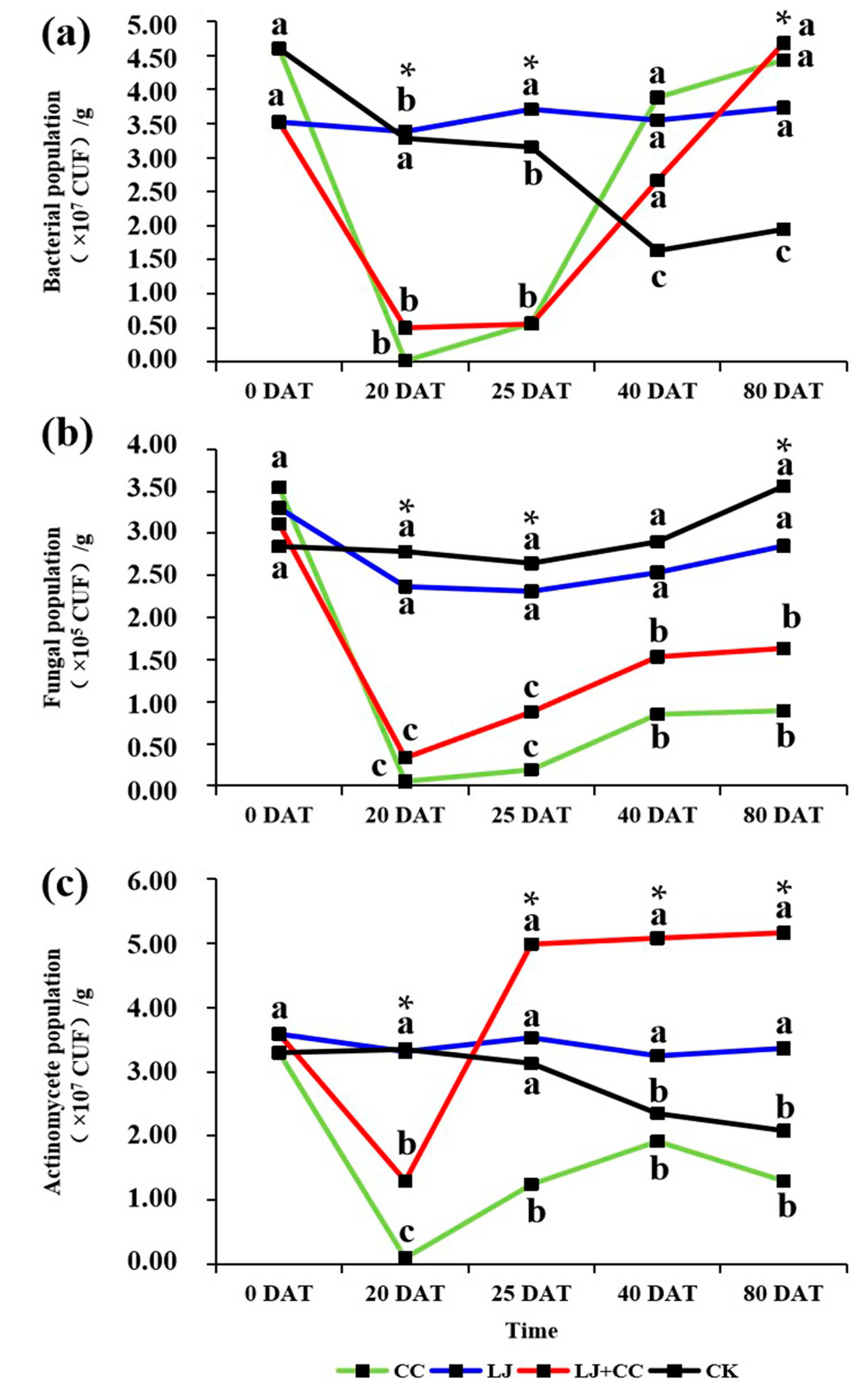
3.2. Culturable Microorganism Analysis
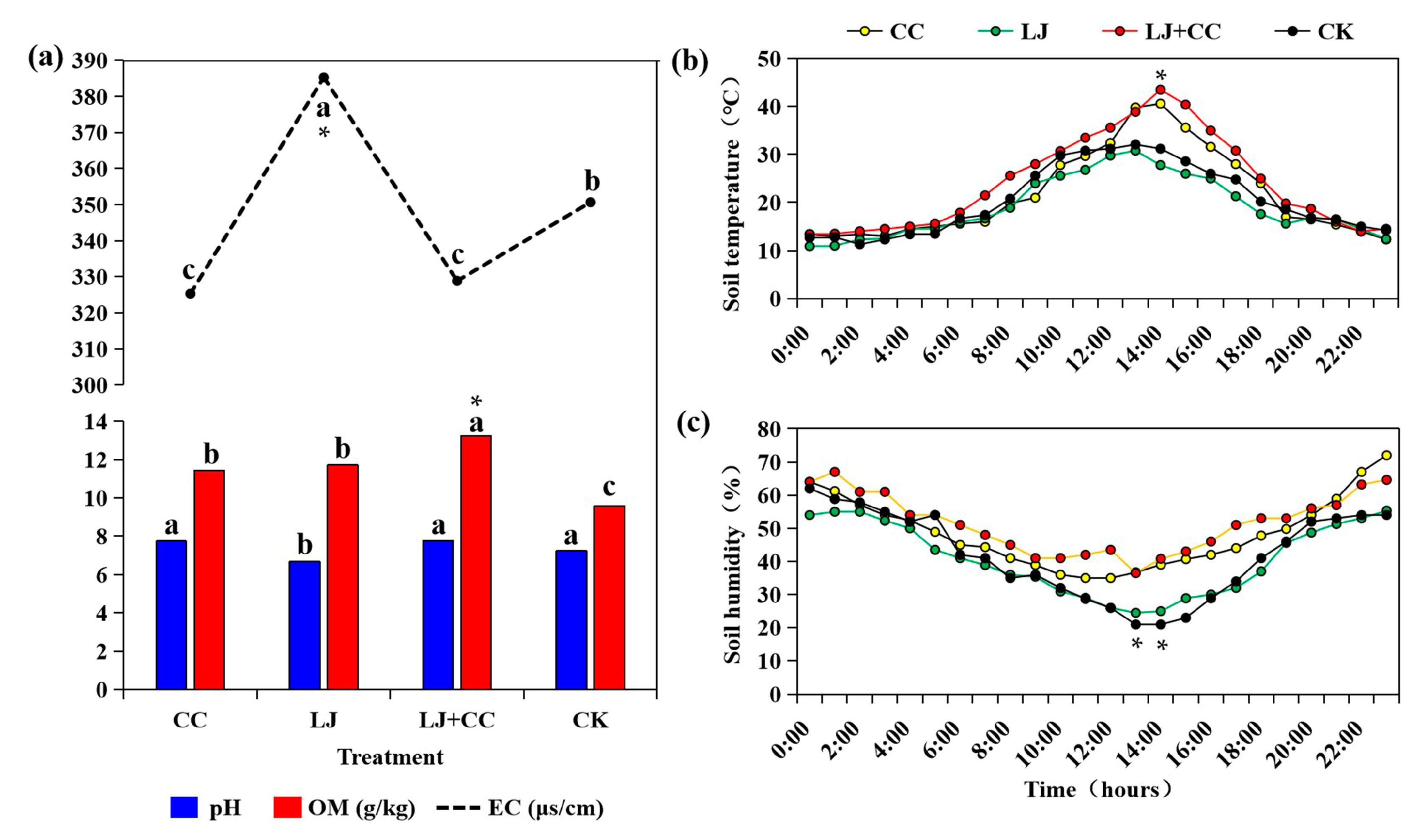
3.3. Soil Physicochemical Analysis
4. Discussion
4.1. Vegetable Straw Added to Soil Promotes the Microbial Recovery Rate after Soil Disinfection
4.2. CaCN2 Promotes the Decomposition Efficiency of Nutrients in Vegetable Straw
4.3. Analysis of the Interaction between Soil Microorganisms and Physicochemical Properties after Straw Return
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, H.; Cao, Y.; Wang, X.; Ge, X.; Li, B.; Jin, C. Evaluation on the production of food crop straw in China from 2006 to 2014. BioEnergy Res. 2017, 10, 949–957. [Google Scholar] [CrossRef]
- Li, H.; Dai, M.W.; Dai, S.L.; Dong, X.J. Current status and environment impact of direct straw return in china’s cropland—A review. Ecotoxicol. Environ. Saf. 2018, 159, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Ros, M.; de Souza Oliveira Filho, J.; Murcia, M.D.P.; Bustamante, M.A.; Moral, R.; Coll, M.D.; Santisima-Trinidad, A.B.L.; Pascual, J.A. Mesophilic anaerobic digestion of pig slurry and fruit and vegetable waste: Dissection of the microbial community structure. J. Clean. Prod. 2017, 156, 757–765. [Google Scholar] [CrossRef]
- Cui, H.Y.; Zhang, S.B.; Zhao, M.Y.; Zhao, Y.; Wei, Z.M. Parallel faction analysis combined with two-dimensional correlation spectroscopy reveal the characteristics of mercury-composting-derived dissolved organic matter interactions. J. Hazard. Mater. 2020, 384, 121395. [Google Scholar] [CrossRef] [PubMed]
- Bouallagui, H.; Lahdheb, H.; Ben Romdan, E.; Rachdi, B.; Hamdi, M. Improvement of fruit and vegetable waste anaerobic digestion performance and stability with co-substrates addition. J. Environ. Manag. 2009, 90, 1844–1849. [Google Scholar] [CrossRef] [PubMed]
- Awasthi, M.K.; Sarsaiya, S.; Wainaina, S.; Rajendran, K.; Kumar, S.; Quan, W.; Duan, Y.; Awasthi, S.K.; Chen, H.; Pandey, A.; et al. A critical review of organic manure biorefinery models toward sustainable circular bioeconomy: Technological challenges, advancements, innovations, and future perspectives. Renew. Sustain. Energy Rev. 2019, 111, 115–131. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, Y.; Chen, Y.; Lu, Q.; Li, M.; Wang, X.; Wei, Y.; Xie, X.; Wei, Z. A regulating method for reducing nitrogen loss based on enriched ammonia-oxidizing bacteria during composting. Bioresour. Technol. 2016, 221, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Sun, B.; Lu, F.; Zhang, G.; Wang, X.; Ouyang, Z. Straw incorporation strategy on cereal crop yield in China. Crop Sci. 2015, 55, 1773–1781. [Google Scholar] [CrossRef]
- Liu, L.; Cheng, M.; Yang, L.; Gu, X.Y.; Jin, J.Y.; Fu, M.J. Regulation of straw decomposition and its effect on soil function by the amount of returned straw in a cool zone rice crop system. Sci. Rep. 2023, 13, 15673. [Google Scholar] [CrossRef]
- Sun, R.; Zhang, X.X.; Guo, X.; Wang, D.; Chu, H. Bacterial diversity in soils subjected to long-term chemical fertilization can be more stably maintained with the addition of livestock manure than wheat straw. Soil Biol. Biochem. 2015, 88, 9–18. [Google Scholar] [CrossRef]
- Wang, W.; Li, L.; Wu, K.; Zhang, K.; Jie, J.; Yang, Y. Preparation of Ni-Mo-S catalysts by hydrothermal method and their hydrodeoxygenation properties. Appl. Catal. A Gen. 2015, 495, 8–16. [Google Scholar] [CrossRef]
- Wei, X.X.; Li, Y.S.; Fan, X.G.; He, C.X.; Yan, Y.; Sun, M.T. Techno-economic feasibility of in situ vegetable residue return in the Chinese solar greenhouse. Agronomy 2021, 11, 1828. [Google Scholar] [CrossRef]
- Jiang, W.; Yan, T.; Chen, B. Impact of media channels and social interactions on the adoption of straw return by Chinese farmers. Sci. Total. Environ. 2021, 756, 144078. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.W.; Chen, L.D.; Shi, Y.X.; Chai, A.L.; Fan, T.F.; Li, L.; Li, B.J. The calcium cyanamide and polyethylene blocks the secondary transmission and infection of vegetable leaf diseases. Front. Plant Sci. 2022, 13, 1027584. [Google Scholar] [CrossRef]
- Shi, K.; Wang, L.; Zhou, Y.H.; Yu, Y.L.; Yu, J.Q. Effects of calcium cyanamide on soil microbial communities and fusarium oxysporum f. sp. cucumberinum. Chemosphere 2009, 75, 872–877. [Google Scholar] [CrossRef]
- Chen, L.D.; Xie, X.W.; Kang, H.J.; Liu, R.C.; Shi, Y.X.; Li, L.; Xie, J.M.; Li, B.J.; Chai, A.L. Efficiency of calcium cyanamide on the control of tomato soil-borne disease and their impacts on the soil microbial community. Appl. Soil Ecol. 2022, 176, 104522. [Google Scholar] [CrossRef]
- Leytur, M.; Duarte, A.V.; Sala, A.; Giardina, E.B.; Benedetto, A.D. Pot growing Media Amendment with Calcium Cyanamide and Weed Control Relationships. Inter. J. Plant Soil Sci. 2018, 23, 42425. [Google Scholar] [CrossRef]
- Agamalian, H. Evaluation of calcium cyanamide for preplant weed control in horticultural crops. Proc. West. Soc. Weed Sci. 1990, 43, 90–95. [Google Scholar]
- Liu, Y.X.; Shi, J.X.; Feng, Y.G.; Yang, X.M.; Li, X.; Shen, Q.R. Tobacco bacterial wilt can be biologically controlled by the application of antagonistic strains in combination with organic fertilizer. Biol. Fert. Soils 2013, 49, 447–464. [Google Scholar] [CrossRef]
- Shen, Z.Z.; Zhong, S.T.; Wang, Y.G.; Wang, B.B.; Mei, X.L.; Li, R.; Ruan, Y.Z.; Shen, Q.R. Induced soil microbial suppression of banana Fusarium wilt disease using compost and biofertilizers to improve yield and quality. Eur. J. Soil Biol. 2013, 57, 1–8. [Google Scholar] [CrossRef]
- Shen, Z.Z.; Ruan, Y.Z.; Wang, B.B.; Zhong, S.T.; Su, L.X.; Li, R.; Shen, Q.R. Effect of biofertilizer for suppressing Fusarium wilt disease of banana as well as enhancing microbial and chemical properties of soil under greenhouse trial. Appl. Soil Ecol. 2015, 93, 111–119. [Google Scholar] [CrossRef]
- Zhang, T.T.; Huang, J.; Deng, S.B.; Yu, G. Influence of pesticides contamination on the emission of PCDD/PCDF to the land from open burning of corn straws. Environ. Pollut. 2011, 159, 1744–1748. [Google Scholar] [CrossRef] [PubMed]
- Lou, Y.L.; Liang, W.J.; Xu, M.G.; He, X.H.; Wang, Y.; Zhao, K. Straw coverage alleviates seasonal variability of the topsoil microbial biomass and activity. Catena 2011, 86, 117–120. [Google Scholar] [CrossRef]
- Ding, X.L.; Han, X.; Zhang, X. Long-term impacts of manure, straw, and fertilizer on amino sugars in a silty clay loam soil under temperate conditions. Biol. Fert. Soils 2013, 49, 949–954. [Google Scholar] [CrossRef]
- Vineela, C.; Wani, S.P.; Srinivasarao, C.; Padmaja, B.; Vittal, K.P.R. Microbial properties of soils as affected by cropping and nutrient management practices in several long-term manorial experiments in the semi-arid tropics of India. Appl. Soil Ecol. 2008, 40, 165–173. [Google Scholar] [CrossRef]
- Singh, V.K.; Singh, A.K.; Kumar, A. Disease management of tomato through PGPB: Current trends and future perspective. 3 Biotech 2017, 7, 255. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; He, Z.C.; Yang, Y.H.; Jia, S.Q.; Yu, M.; Chen, X.J.; Shen, A.L. Linking soil microbial community dynamics to straw-carbon distribution in soil organic carbon. Sci. Rep. 2020, 10, 5526. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Wu, M.; Petropoulos, E.; Zhang, J.W.; Nie. J.; Liao, Y.L.; Li, Z.P.; Lin, X.G.; Feng, Y.Z. Responses of paddy soil bacterial community assembly to different long-term fertilizations in southeast China. Sci. Total Environ. 2019, 656, 625–633. [Google Scholar] [CrossRef]
- El-Tarabily, K.A.; Sivasithamparam, K. Non-streptomycete actinomycetes as biocontrol agents of soilborne fungal plant pathogens and as plant growth promoters. Soil Biol. Biochem. 2006, 38, 1505–1520. [Google Scholar] [CrossRef]
- Zhao, J.; Ni, T.; Xun, W.; Huang, X.; Huang, Q.; Ran, W.; Shen, B.; Zhang, R.; Shen, Q. Influence of straw incorporation with and without straw decomposer on soil bacterial community structure and function in a rice-wheat cropping system. Appl. Microbiol. Biotechnol. 2017, 101, 4761–4773. [Google Scholar] [CrossRef]
- Chen, L.D.; Shi, Y.X.; Li, L.; Chai, A.L.; Guo, N.; Fan, T.F.; Xie, X.W.; Li, B.J. Effect of calcium cyanamide and straw returning cooperative treatment on the control of lettuce soil-borne diseases and soil quality. Acta Phytopathol. Sin. 2022, 52, 621–629. [Google Scholar]
- Guo, J.H.; Liu, X.J.; Zhang, Y.; Shen, J.L.; Han, W.X.; Zhang, W.F.; Christie, P.; Goulding, K.W.T.; Vitousek, P.M.; Zhang, F.S. Significant acidification in major Chinese croplands. Science 2010, 327, 1008–1010. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Liang, F.; Yu, A.; Li, B.; Yang, L. Evaluation of stability and maturity during forced-aeration composting of chicken manure and sawdust at different C/N ratios. Chemosphere 2010, 78, 614–619. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhou, X.; Jiang, A.Q.; Fan, J.Z.; Lan, T.; Zhang, J.B.; Cai, Z.C. Distinct impacts of reductive soil disinfestation and chemical soil disinfestation on soil fungal communities and memberships. Appl. Microbiol. Biotechnol. 2018, 102, 7623–7634. [Google Scholar] [CrossRef] [PubMed]
- Turmel, M.S.; Speratti, A.; Baudron, F.; Verhulst, N.; Govaets, B. Crop residue management and soil health: A systems analysis. Agric. Syst. 2014, 134, 6–16. [Google Scholar] [CrossRef]
- Su, Y.; Lv, J.L.; Yu, M.; Ma, Z.H.; Xi, H.; Kou, C.L. Long-term decomposed straw return positively affects the soil microbial community. J. Appl. Microbiol. 2019, 128, 138–150. [Google Scholar] [CrossRef] [PubMed]
- Dolan, M.S.; Clapp, C.E.; Allmaras, R.R.; Baker, J.M.; Molina, J.A.E. Soil or ganic carbon and nitrogen in a Minnesota soil as related to tillage, residue and nitrogen management. Soil Tillage Res. 2006, 89, 221–231. [Google Scholar] [CrossRef]
- Shan, Y.; Cai, Z.; Han, Y.; Johnson, S.E.; Buresh, R.J. Organic acid accumulation under fooded soil conditions in relation to the incorporation of wheat and rice straws with different C: N ratios. Soil Sci. Plant Nutr. 2008, 54, 46–56. [Google Scholar] [CrossRef]
- Wilhelm, W.W.; Doran, J.W.; Power, J.F. Corn and soybean yield response to crop residue management under no-tillage production systems. Agron. J. 1986, 78, 184–189. [Google Scholar] [CrossRef]
- Cheng, M.; Xie, W.Y.; Yang, Z.X.; Zhou, H.P. Effects of long-term straw return on corn yield, soil nutrient contents and en zyme activities in dryland of the Loess Plateau, China. Chin. J. Eco-Agric. 2019, 27, 1528–1536. [Google Scholar]
- Sheng, X.F.; He, L.Y. Solubilization of potassium-bearing minerals by a wild-type strain of Bacillus edaphicus and its mutants and increased potassium uptake by wheat. Can. J. Microbiol. 2006, 52, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Zhang, X.; Liu, J.; Gao, L.H. Effects of summer cover crop and residue management on cucumber growth in intensive Chinese production systems: Soil nutrients, microbial properties and nematodes. Plant Soil 2011, 339, 299–315. [Google Scholar] [CrossRef]
- Bainard, L.D.; Lu, P.; Ma, B.; Liu, J.H. Bio-fertilizer and rotten straw amendments alter the rhizosphere bacterial community and increase oat productivity in a saline–alkaline environment. Sci. Rep. 2020, 10, 19896. [Google Scholar]
- Turmuktini, T.; Kantikowati, E.; Natalie, B.; Setiawati, M.; Yuwariah, Y.Y.; Joy, B.; Simarmata, T. Restoring the health of paddy soil by using straw compost and biofertilizers to increase fertilizer efciency and rice production with SOBARI (system of organic based aerobic rice intensifcation) technology. Asian J. Agric. Rural. Dev. 2012, 2, 519–526. [Google Scholar]
- Bletsos, F.A. Grafting and calcium cyanamide as alternatives to methyl bromide for greenhouse eggplant production. Sci. Hortic. 2006, 107, 325–331. [Google Scholar] [CrossRef]
- Simujide, H.; Aorigele, C.; Wang, C.J.; Zhang, T.H.; Manda, B. Evaluation of calcium cyanamide addition during co-composting of manure and maize straw in a forced-aeration static-pile system. J. Environ. Health Sci. Eng. 2016, 14, 18. [Google Scholar] [CrossRef]
- Schimel, J.P.; Schaeffer, S.M. Microbial control over carbon cycling in soil. Front. Microbiol. 2012, 3, 348. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, Z.; Wang, X.; Sun, Q.; Dong, H.; Wang, G.; Chen, X.; Yin, C.; Han, Z.; Mao, Z. Effects of biochar on the growth of apple seedlings, soil enzyme activities and fungal communities in replant disease soil. Sci. Hortic. 2019, 256, 108641. [Google Scholar] [CrossRef]
- Mitchell, R.J.; Hester, A.J.; Campbell, C.D.; Chapman, S.J.; Cameron, C.M.; Hewison, R.L.; Potts, J.M. Explaining the variation in the soil microbial community: Do vegetation composition and soil chemistry explain the same or different parts of the microbial variation? Plant Soil 2012, 351, 355–362. [Google Scholar] [CrossRef]
- Qiu, M.H.; Li, S.Q.; Zhou, X.; Cui, X.S.; Vivanco, J.M.; Zhang, N.; Shen, Q.R.; Zhang, R.F. De-coupling of root-microbiome associations followed by antagonist inoculation improves rhizosphere soil suppressiveness. Biol. Fertil. Soils 2014, 50, 217–224. [Google Scholar] [CrossRef]
- Glab, T.; Kulig, B. Effect of mulch and tillage system on soil porosity under wheat (Triticum aestivum). Soil Tillage Res. 2008, 99, 169–178. [Google Scholar] [CrossRef]
- Lu, P.; Lin, Y.H.; Yang, Z.Q.; Xu, Y.P.; Tan, F.; Jia, X.D.; Wang, M.; Xu, D.R.; Wang, X.Z. Effects of application of corn straw on soil microbial community structure during the maize growing season. J. Basic Microbiol. 2015, 55, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Eichorst, S.A.; Kuske, C.R. Identification of cellulose-responsive bacterial and fungal communities in geographically and edaphically different soils by using stable isotope probing. Appl. Environ. Microb. 2012, 78, 2316–2327. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Mondejar, R.; Zuhlke, D.; Becher, D.; Riedel, K.; Baldrian, P. Cellulose and hemicellulose decomposition by forest soil bacteria proceeds by the action of structurally variable enzymatic systems. Sci. Rep. 2016, 6, 25279. [Google Scholar] [CrossRef] [PubMed]
- Brabcova, V.; Stursova, M.; Baldrian, P. Nutrient content affects the turnover of fungal biomass in forest topsoil and the composition of associated microbial communities. Soil Biol. Biochem. 2018, 118, 187–198. [Google Scholar] [CrossRef]
- Su, J.Q.; Wei, B.; Ouyang, W.Y.; Huang, F.Y.; Zhao, Y.; Xu, H.J.; Zhu, Y.G. Antibiotic resistome and its association with bacterial communities during sewage sludge composting. Environ. Sci. Technol. 2015, 49, 7356–7363. [Google Scholar] [CrossRef]
- Wei, H.; Wang, L.; Hassan, M.; Xie, B. Succession of the functional microbial communities and the metabolic functions in maize straw composting process. Bioresour. Technol. 2018, 256, 333–341. [Google Scholar] [CrossRef]
- Prietzel, J.; Spielvogel, S. Rapid Degradation of Soil Organic Matter Sampled from Anoxic Surface Horizons of Fens after Contact with Oxygen-Implications for Sampling and Sample Pretreatment. In Proceedings of the Joint Annual Meeting, ASA-CSSA-SSSA, Pittsburgh, PA, USA, 1–5 November 2008. [Google Scholar]
- Peng, J.; Wegner, C.E.; Bei, Q.; Liu, P.; Liesack, W. Metatranscriptomics reveals a differential temperature effect on the structural and functional organization of the anaerobic food web in rice field soil. Microbiome 2018, 6, 169. [Google Scholar] [CrossRef]
- Dai, Z.M.; Zang, H.D.; Chen, J.; Fu, Y.Y.; Wang, X.H.; Liu, H.T.; Shen, C.C.; Wang, J.J.; Kuzyakov, Y.; Becker, J.N.; et al. Metagenomic insights into soil microbial communities involved in carbon cycling along an elevation climosequences. Environ. Microbiol. 2021, 23, 4631–4645. [Google Scholar] [CrossRef]
- Babujia, L.C.; Hungria, M.; Franchini, J.C.; Brookes, P.C. Microbial biomass and activity at various soil depths in a Brazilian oxisol after two decades of no-tillage and conventional tillage. Soil Biol. Biochem. 2010, 42, 2174–2181. [Google Scholar] [CrossRef]
- Souza, R.C.; Hungria, M.; Cantão, M.E.; Vasconcelos, A.T.R.; Nogueira, M.A.; Vicente, V.A. Metagenomic analysis reveals microbial functional redundancies and specificities in a soil under different tillage and crop-management regimes. Appl. Soil Ecol. 2015, 86, 106–112. [Google Scholar] [CrossRef]
| Samples | pH | EC (μS/cm) | OM (g/kg) | TN (g/kg) | NH4-N (mg/kg) | NO3-N (mg/kg) | TP (mg/g) | AP (mg/kg) | TK (mg/g) | AK (mg/kg) | TC (g/kg) | Water Content/% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Soil | 7.23 | 350.67 | 9.58 | 0.74 | 19.48 | 35.92 | 0.64 | 21.83 | 5.07 | 148.61 | - | 65.20 |
| Pepper straw | - | - | - | 13.42 | - | - | 1.80 | - | 20.22 | - | 284.90 | 68.92 |
| Treatments | Plant Height (cm) | Leaf Area (cm2) | Single Fruit Weight (kg) | Stimulation Effect (%) |
|---|---|---|---|---|
| CC | 27 ± 0.2 b | 245 ± 1.42 b | 0.26 ± 0.01 b | 4 |
| LJ | 27 ± 1.33 b | 239 ± 9.2 b | 0.25 ± 0.05 b | 0 |
| LJ+CC | 31 ± 1.68 a | 272 ± 13.88 a | 0.3 ± 0.08 a | 20 |
| CK | 25 ± 2 c | 215 ± 16.61 c | 0.25 ± 0.07 b | - |
| Significance | * | * | * |
| Treatment | TN (g/kg) | NH4-N (mg/kg) | NO3-N (mg/kg) | TP (mg/g) | AP (mg/kg) | TK (mg/g) | AK (mg/kg) |
|---|---|---|---|---|---|---|---|
| CC | 0.91 ± 0.07 b | 18.59 ± 0.06 b | 39.07 ± 0.58 a * | 0.60 ± 0.05 b | 25.74 ± 0.23 c | 4.93 ± 0.12 b | 165.83 ± 1.41 b |
| LJ | 0.89 ± 0.04 b | 23.34 ± 0.83 a * | 38.83 ± 0.90 a * | 0.78 ± 0.02 a * | 33.66 ± 0.38 a * | 5.90 ± 0.46 a * | 179.17 ± 6.07 a * |
| LJ+CC | 1.01 ± 0.05 a * | 23.27 ± 0.37 a * | 33.42 ± 0.37 b | 0.62 ± 0.05 b | 28.71 ± 2.25 b | 5.29 ± 0.10 b | 161.94 ± 7.61 b |
| CK | 0.74 ± 0.03 c | 19.48 ± 1.25 b | 35.92 ± 2.39 b | 0.64 ± 0.14 b | 21.83 ± 0.37 c | 5.07 ± 0.78 b | 148.61 ± 9.93 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, X.; Chen, L.; Shi, Y.; Chai, A.; Fan, T.; Li, B.; Li, L. Response of Microbial Recovery Rate to Straw Return after Calcium Cyanamide Soil Disinfection. Horticulturae 2024, 10, 2. https://doi.org/10.3390/horticulturae10010002
Xie X, Chen L, Shi Y, Chai A, Fan T, Li B, Li L. Response of Microbial Recovery Rate to Straw Return after Calcium Cyanamide Soil Disinfection. Horticulturae. 2024; 10(1):2. https://doi.org/10.3390/horticulturae10010002
Chicago/Turabian StyleXie, Xuewen, Lida Chen, Yanxia Shi, Ali Chai, Tengfei Fan, Baoju Li, and Lei Li. 2024. "Response of Microbial Recovery Rate to Straw Return after Calcium Cyanamide Soil Disinfection" Horticulturae 10, no. 1: 2. https://doi.org/10.3390/horticulturae10010002






