An Overview of the Phytochemical Composition of Different Organs of Prunus spinosa L., Their Health Benefits and Application in Food Industry
Abstract
1. Introduction
2. Research Methodology
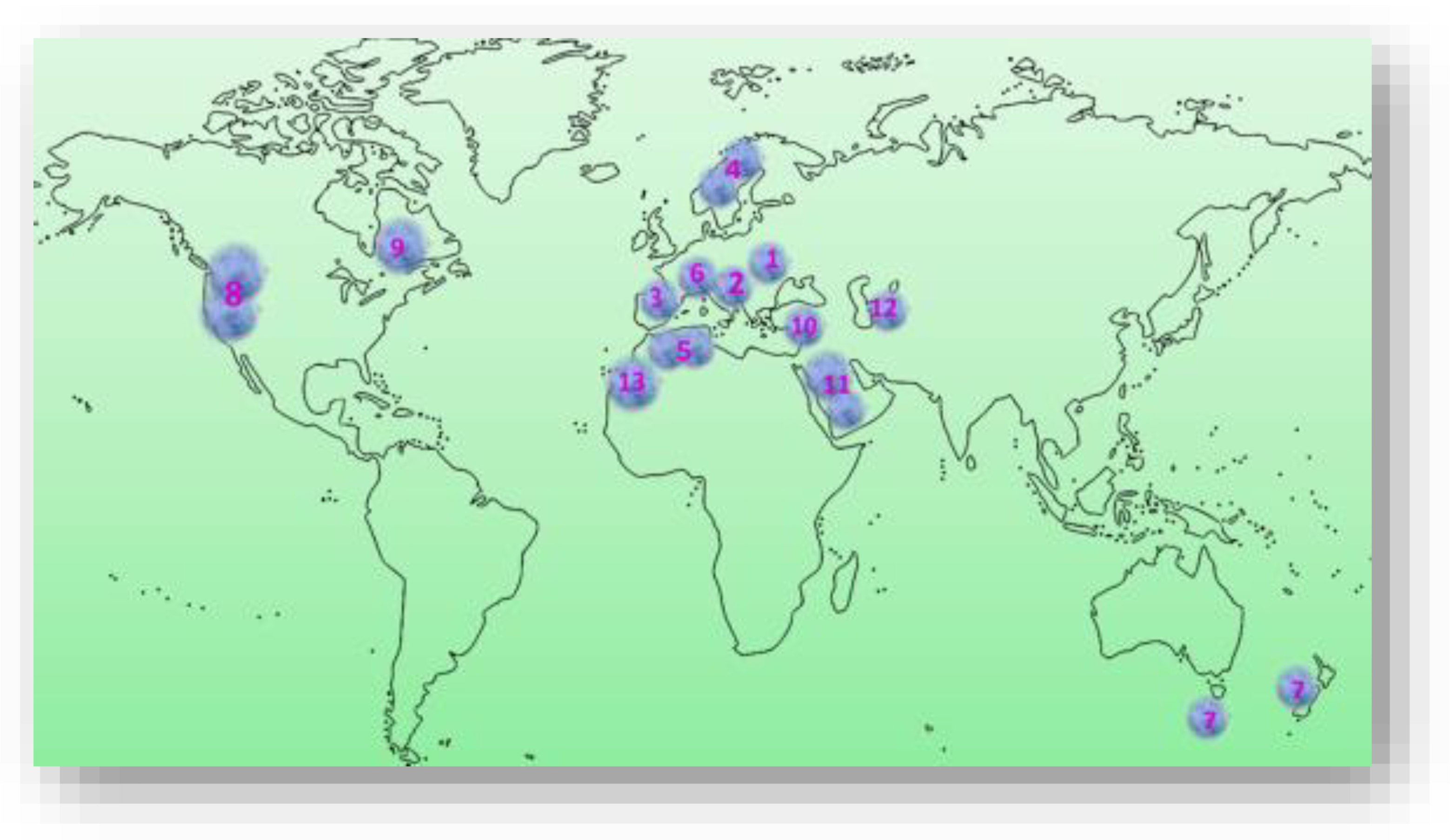
3. The Bioecology of the P. spinosa L. Shrub
Nutritional Values of the Blackthorne Fruits
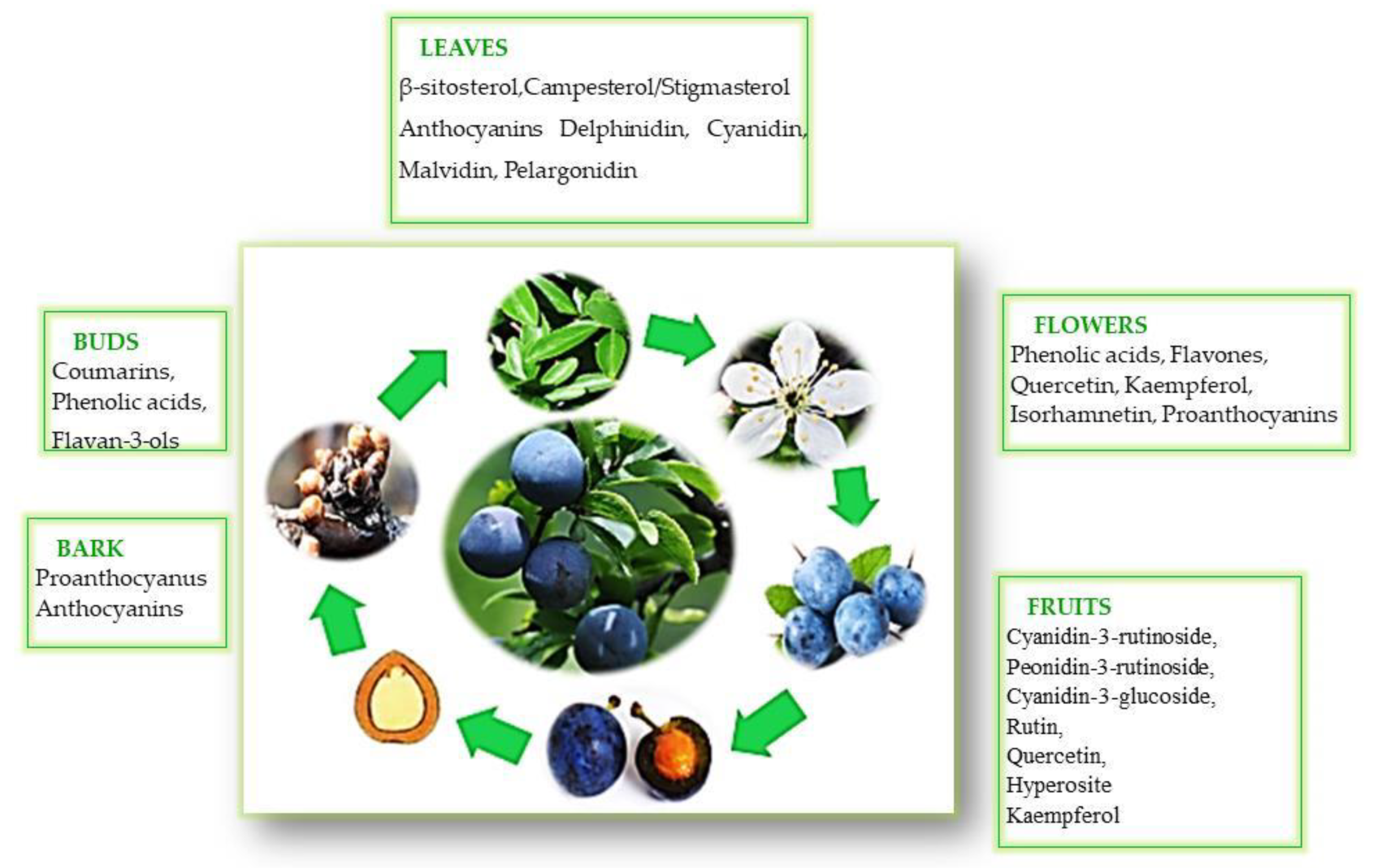
4. The Polyphenol Composition in Various Parts of the P. spinosa L. Shrub
5. The Effect of Bioactive Compounds Found in the Blackthorn Fruits in the Treatment of Various Diseases
| Bioactive Compounds | Health Effect | Main Outcomes | References |
|---|---|---|---|
| Flavonoids (Quercitin, Rutin) | Neurogenerative effect | -acetylcholinesterase inhibition -monoamine oxidase inhibition -↓ peroxyl radical capture and oxidation -neurotrophic action -maintenance of physiological functions of vital organs | [88,89] |
| Flavonoids | Cardiovascular effect | -inhibition of pro-inflammatory enzymes -anti-atherosclerotic effects -anti-atherothrombotic effects -modulation of lipid metabolism, -normalization of the LDL/HDL ratio, -improving capillary permeability -improved endothelial function, vasodilatory effects -↑ release of nitric oxide and uncoupling of endothelial nitric oxide synthesis -↓ oxidative DNA damage | [48,62,90,91,92,93,94] |
| Proantocianidine | -modulates lipid metabolism, -↑ anti-oxidant capacity of plasma, - improve vascular functions -↓ platelet activity | [48,95] | |
| Cianidin-3-rutinoside | -improve lipid ↓ mechanisms, -inhibition of lipolytic digestive enzymes -inhibition of lipid absorption processes -anti-oxidant activity on ROS, -antiglycating activity -cardioprotective activity mixed competitive inhibition of pancreatic lipase and pancreatic cholesterol esterase -inhibition of cholesterol mycelial formation linked to primary and secondary bile acid -inhibition of cholesterol mycelial absorption in the proximal jejunum. | [48,96,97] | |
| Cianidin-3-glucoside | -↑ tissue tolerance to ischemic injury -↓ risk of cardiovascular disease, hypertension, -capacity to scavenge ROS -↓ oxidative stress, enhancing inflammatory responses | [96,98] | |
| Cianidin-3-glucoside Cianidin 3-rutinoside | Diabetes and associated metabolic diseases | ↓ risk of diabetes and obesity ↓ postprandial glucose by inhibition of pancreatic α-amylase and intestinal α-glucosidase -modulates postprandial blood glucose by inhibiting carbohydrate digestive enzymes -↓ glucose transport in the small intestine. -inhibit glucose uptake in colorectal adenocarcinoma epithelial cells | [98,99] |
| Phenolic acids | Anticancer effect | -cytotoxic activity on some cancer cell lines -induction in vitro of endogenous anti-oxidant mechanisms -modulation of Nrf2 transcription factors, a regulator of cellular resistance to oxidative damage, -↓ disruption of the pro-oxidant/anti-oxidant balance with a key role in some cancers | [16,20,23,100] |
| Anthocyanins | -unchanged dietary absorption and incorporation into cells, -major contribution to establishing anti-oxidant activity, reducing cancer risk | [2,15,67,90,101,102] | |
| Fitosteroli | -anticancer activity on prostate cancer | [15] | |
| Cianidin-3-glucozide | ↓ cancer risk due to the ability to scavenge ROS | [100] |
5.1. Neurodegenerative
5.2. Dyslipidaemias and Associated Cardiovascular Diseases
5.3. Diabetes and Associated Metabolic Pathologies
5.4. Cancer Pathologies
6. Applications of P. spinosa L. in Food Industry
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bourgonje, A.R.; Feelisch, M.; Faber, K.N.; Pasch, A.; Dijkstra, G.; Van Goor, H. Oxidative Stress and Redox-Modulating Therapeutics in Inflammatory Bowel Disease. Trends Mol. Med. 2020, 26, 1034–1046. [Google Scholar] [CrossRef] [PubMed]
- Dedić, A.; Džudžević Čančar, H.; Stanojković, T.; Roje, M.; Damjanović, A.; Alispahić, A.; Jerković-Mujkić, A. HPLC Analysis of Phytosterols in Prunus spinosa L. Extracts and Their Antiproliferative Activity on Prostate Cancer Cell Lines. Kem. U Ind. 2023, 72, 323–330. [Google Scholar] [CrossRef]
- Crnić, I.; Frančić, T.; Dragičević, P.; Balta, V.; Dragović-Uzelac, V.; Đikić, D.; Landeka Jurčević, I. Blackthorn Flower Extract Impact on Glycaemic Homeostasis in Normoglycemic and Alloxan-Induced Hyperglycaemic C57BL/6 Mice. Food Technol. Biotechnol. 2021, 59, 349–359. [Google Scholar] [CrossRef] [PubMed]
- Karakas, N.; Okur, M.E.; Ozturk, I.; Ayla, S.; Karadağ, A.E.; Polat, D.Ç. Antioxidant Activity and Cytotoxic Effects of Prunus spinosa L. Fruit Extract on Various Cancer Cell Lines. MMJ 2019, 34, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Pozzo, L.; Russo, R.; Frassinetti, S.; Vizzarri, F.; Árvay, J.; Vornoli, A.; Casamassima, D.; Palazzo, M.; Della Croce, C.M.; Longo, V. Wild Italian Prunus spinosa L. Fruit Exerts In Vitro Antimicrobial Activity and Protects Against In Vitro and In Vivo Oxidative Stress. Foods 2019, 9, 5. [Google Scholar] [CrossRef] [PubMed]
- Pinacho, R.; Cavero, R.Y.; Astiasarán, I.; Ansorena, D.; Calvo, M.I. Phenolic Compounds of Blackthorn (Prunus spinosa L.) and Influence of in Vitro Digestion on Their Antioxidant Capacity. J. Funct. Foods 2015, 19, 49–62. [Google Scholar] [CrossRef]
- Barbieri, R.; Coppo, E.; Marchese, A.; Daglia, M.; Sobarzo-Sánchez, E.; Nabavi, S.F.; Nabavi, S.M. Phytochemicals for Human Disease: An Update on Plant-Derived Compounds Antibacterial Activity. Microbiol. Res. 2017, 196, 44–68. [Google Scholar] [CrossRef]
- Marchelak, A.; Kolodziejczyk-Czepas, J.; Wasielewska, P.; Nowak, P.; Olszewska, M.A. The Effects of Prunus spinosa L. Flower Extracts, Model Polyphenols and Phenolic Metabolites on Oxidative/Nitrative Modifications of Human Plasma Components with Particular Emphasis on Fibrinogen In Vitro. Antioxidants 2021, 10, 581. [Google Scholar] [CrossRef]
- Kotsou, K.; Stoikou, M.; Athanasiadis, V.; Chatzimitakos, T.; Mantiniotou, M.; Sfougaris, A.I.; Lalas, S.I. Enhancing Antioxidant Properties of Prunus spinosa Fruit Extracts via Extraction Optimization. Horticulturae 2023, 9, 942. [Google Scholar] [CrossRef]
- Najgebauer-Lejko, D.; Liszka, K.; Tabaszewska, M.; Domagała, J. Probiotic Yoghurts with Sea Buckthorn, Elderberry, and Sloe Fruit Purees. Molecules 2021, 26, 2345. [Google Scholar] [CrossRef]
- Ürkek, B.; Şengül, M.; Akgül, H.İ.; Kotan, T.E. Antioxidant Activity, Physiochemical and Sensory Characteristics of Ice Cream Incorporated with Sloe Berry (Prunus spinosa L.). Int. J. Food Eng. 2019, 15, 20180029. [Google Scholar] [CrossRef]
- Egea, I.; Sánchez-Bel, P.; Romojaro, F.; Pretel, M.T. Six Edible Wild Fruits as Potential Antioxidant Additives or Nutritional Supplements. Plant Foods Hum. Nutr. 2010, 65, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Özkan, G. Bioaccessibility of Blackthorn (Prunus spinosa) Beverage Polyphenols: Effect of Sugar Andcitric Acid Addition. Turk. J. Agric. For. 2023, 47, 1017–1024. [Google Scholar] [CrossRef]
- Backes, E.; Leichtweis, M.G.; Pereira, C.; Carocho, M.; Barreira, J.C.M.; Kamal Genena, A.; José Baraldi, I.; Filomena Barreiro, M.; Barros, L.; Ferreira, I.C.F.R. Ficus carica L. and Prunus spinosa L. Extracts as New Anthocyanin-Based Food Colorants: A Thorough Study in Confectionery Products. Food Chem. 2020, 333, 127457. [Google Scholar] [CrossRef] [PubMed]
- Sikora, E.; Bieniek, M.I.; Borczak, B. Composition and Antioxidant Properties of Fresh and Frozen Stored Blackthorn Fruits (Prunus spinosa L.). Acta Sci. Pol. Technol. Aliment. 2013, 12, 365–372. [Google Scholar]
- Popović, B.M.; Blagojević, B.; Ždero Pavlović, R.; Mićić, N.; Bijelić, S.; Bogdanović, B.; Mišan, A.; Duarte, C.M.M.; Serra, A.T. Comparison between Polyphenol Profile and Bioactive Response in Blackthorn (Prunus spinosa L.) Genotypes from North Serbia-from Raw Data to PCA Analysis. Food Chem. 2020, 302, 125373. [Google Scholar] [CrossRef]
- Suraweera, T.L.; Rupasinghe, H.P.V.; Dellaire, G.; Xu, Z. Regulation of Nrf2/ARE Pathway by Dietary Flavonoids: A Friend or Foe for Cancer Management? Antioxidants 2020, 9, 973. [Google Scholar] [CrossRef]
- González-Burgos, E.; Gómez-Serranillos, M.P. Effect of Phenolic Compounds on Human Health. Nutrients 2021, 13, 3922. [Google Scholar] [CrossRef]
- Tiboni, M.; Coppari, S.; Casettari, L.; Guescini, M.; Colomba, M.; Fraternale, D.; Gorassini, A.; Verardo, G.; Ramakrishna, S.; Guidi, L.; et al. Prunus spinosa Extract Loaded in Biomimetic Nanoparticles Evokes In Vitro Anti-Inflammatory and Wound Healing Activities. Nanomaterials 2020, 11, 36. [Google Scholar] [CrossRef]
- Condello, M.; Pellegrini, E.; Spugnini, E.P.; Baldi, A.; Amadio, B.; Vincenzi, B.; Occhionero, G.; Delfine, S.; Mastrodonato, F.; Meschini, S. Anticancer Activity of “Trigno M”, Extract of Prunus spinosa Drupes, against in Vitro 3D and in Vivo Colon Cancer Models. Biomed. Pharmacother. 2019, 118, 109281. [Google Scholar] [CrossRef]
- Sotler, R. Prooxidant Activities of Antioxidants and Their Impact on Health. Acta Clin. Croat. 2019, 58, 726–736. [Google Scholar] [CrossRef] [PubMed]
- Condello, M.; Meschini, S. Role of Natural Antioxidant Products in Colorectal Cancer Disease: A Focus on a Natural Compound Derived from Prunus spinosa, Trigno Ecotype. Cells 2021, 10, 3326. [Google Scholar] [CrossRef]
- Meschini, S.; Pellegrini, E.; Condello, M.; Occhionero, G.; Delfine, S.; Condello, G.; Mastrodonato, F. Cytotoxic and Apoptotic Activities of Prunus spinosa Trigno Ecotype Extract on Human Cancer Cells. Molecules 2017, 22, 1578. [Google Scholar] [CrossRef] [PubMed]
- Magiera, A.; Czerwińska, M.E.; Owczarek, A.; Marchelak, A.; Granica, S.; Olszewska, M.A. Polyphenols and Maillard Reaction Products in Dried Prunus spinosa Fruits: Quality Aspects and Contribution to Anti-Inflammatory and Antioxidant Activity in Human Immune Cells Ex Vivo. Molecules 2022, 27, 3302. [Google Scholar] [CrossRef] [PubMed]
- Saavedra, M.; Borges, A.; Dias, C.; Aires, A.; Bennett, R.; Rosa, E.; Simões, M. Antimicrobial Activity of Phenolics and Glucosinolate Hydrolysis Products and Their Synergy with Streptomycin against Pathogenic Bacteria. Med. Chem. 2010, 6, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Popescu, I.; Caudullo, G. Prunus cerasifera in Europe: Distribution, Habitat, Usage and Threats. In European Atlas of Forest Tree Species; Publication Office of the European Union: Luxembourg, 2016; ISBN 978-92-79-36740-3. [Google Scholar]
- Prunus spinosa, L. |Plants of the World Online|Kew Science. Available online: http://powo.science.kew.org/taxon/urn:lsid:ipni.org:names:730297-1 (accessed on 19 August 2023).
- USDA. Plants Database. Available online: https://plants.usda.gov/home (accessed on 23 November 2023).
- Fraternale, D.; Giamperi, L.; Bucchini, A.; Sestili, P.; Paolillo, M.; Ricci, D. Prunus spinosa Fresh Fruit Juice: Antioxidant Activity in Cell-Free and Cellular Systems. Nat. Prod. Commun. 2009, 4, 1934578X0900401211. [Google Scholar] [CrossRef]
- Müller, N.; Kelcey, J.G. Plants and Habitats of European Cities; Springer: New York, NY, USA, 2011; ISBN 978-0-387-89683-0. [Google Scholar]
- Komarov, V.; Shishkin, B.; Yuzepchuk, S.V.; Fedorov, A.; Kostina, K.; Kovalev, N.V.; Krishtofovich, A.N.; Linchevskii, I.A.; Poyarkova, A.I. Flora of the USSR—Volume X: Rosaceae—Rosoideae, Prunoideae; 1970; ISBN 100706510925. [Google Scholar]
- Dedić, A.; Dţudţević-Čančar, H.; Alispahić, A.; Tahirović, I.; Muratović, E. In-Vitro Antioxidant and Antimicrobial Activity of Aerial Parts of Prunus spinosa L. Growing Wild in Bosnia and Herzegovina. Int. J. Pharm. Sci. Res. 2021, 12, 3643–3653. [Google Scholar]
- Day, J.; Robertson, P.; Symes, N. The Scrub Management Handbook: Guidance on the Management of Scrub on Nature Conservation Sites; English Nature: Wetherby, UK, 2003; ISBN 978-1-85716-745-0. [Google Scholar]
- Costa, J.; Neto, C.; Aguiar, C.; Capelo, J.; Santo, J.; Honrado, C.; Pinto-Gomes, T.; Monteiro-Henriques, T.; Sequeira, M.; Lousã; et al. Vascular Plant Communities in Portugal (Continental, the Azores and Madeira). Glob. Geobot. 2012, 2, 1–180. [Google Scholar]
- Cosmulescu, S. Gruia Marius Climatic Variability in Craiova (Romania) and Its Impacts on Fruit Orchards. South-West. J. Hortic. Biol. Environ. 2016, 7, 15–26. [Google Scholar]
- Cosmulescu, S.; Baciu, A.; Gruia, M. Influence of Climatic Factors on the Phenology Spring in Southern Oltenia (Romania). J. Hortic. For. Biotechnol. 2015, 19, 147–157. [Google Scholar]
- Andronie, L.; Holonec, L.; Pop, I.; Truta, A.M.; Odagiu, A.; Sălăgean, T.; Sobolu, R.; Coroian, A.; Balta, I.; Șuba, E.E. Antioxidant Capacity of Several Romanian Forest Fruits (Rosa canina L., Prunus spinosa L., Vaccium vitis-idaea L. and Cornus mas L.). Not. Bot. Horti Agrobot. 2019, 47, 1178–1184. [Google Scholar] [CrossRef]
- Plants of the World Online|Kew Science. Available online: https://powo.science.kew.org/ (accessed on 23 November 2023).
- Franz, C.M.A.P.; Huch, M.; Abriouel, H.; Holzapfel, W.; Gálvez, A. Enterococci as Probiotics and Their Implications in Food Safety. Int. J. Food Microbiol. 2011, 151, 125–140. [Google Scholar] [CrossRef] [PubMed]
- Franz, S.; Rammelt, S.; Scharnweber, D.; Simon, J.C. Immune Responses to Implants—A Review of the Implications for the Design of Immunomodulatory Biomaterials. Biomaterials 2011, 32, 6692–6709. [Google Scholar] [CrossRef] [PubMed]
- Băbălău-Fuss, V.; Senila, L.; Becze, A.; Al-Zaben, O.; Dirja, M.; Tofana, M. Fatty Acids Composition from Rosa canina and Prunus spinosa Plant Fruit Oil. Stud. Univ. Babeș-Bolyai Chem. 2021, 66, 41–48. [Google Scholar] [CrossRef]
- Cosmulescu, S.; Baciu, A.; Cichi, M.; Gruia, M. The effect of climate changes on phenological phases in plum tree (Prunus domestica L.) in south-western romania. South West. J. Hortic. Biol. Environ. 2010, 1, 9–20. [Google Scholar]
- Ruiz-Rodríguez, B.M.; Ancos, B.d.; Sánchez-Moreno, C.; Fernández-Ruiz, V.; Sánchez-Mata, M.d.C.; Cámara, M.; Tardío, J. Wild Blackthorn (Prunus spinosa L.) and Hawthorn (Crataegus monogyna Jacq.) Fruits as Valuable Sources of Antioxidants. Fruits 2014, 69, 61–73. [Google Scholar] [CrossRef]
- De Luca, M.; Tuberoso, C.I.G.; Pons, R.; García, M.T.; Morán, M.d.C.; Ferino, G.; Vassallo, A.; Martelli, G.; Caddeo, C. Phenolic Fingerprint, Bioactivity and Nanoformulation of Prunus spinosa L. Fruit Extract for Skin Delivery. Pharmaceutics 2023, 15, 1063. [Google Scholar] [CrossRef]
- Levon, V. The Contents of Catechins and Anthocyanins in the Above-Ground Organs of Plants of Prunus spinosa L. In Agrobiodiversity for Improving of Nutrition, Health and Life Quality; Klymenko, S., Ed.; Slovak University of Agriculture: Nitra, Slovakia, 2019; pp. 265–272. ISBN 978-80-552-2108-3. [Google Scholar]
- Kuru Berk, S.; Tas, A.; Orman, E.; Gundogdu, M.; Necas, T.; Ondrasek, I.; Karatas, N.; Ercisli, S. Agro-Morphological and Biochemical Characterization of Wild Prunus spinosa L. Subsp. Dasyphylla (Schur) Domin Genotypes Naturally Grown in Western Black Sea Region of Turkey. Agronomy 2020, 10, 1748. [Google Scholar] [CrossRef]
- Colomba, M.; Benedetti, S.; Fraternale, D.; Guidarelli, A.; Coppari, S.; Freschi, V.; Crinelli, R.; Kass, G.E.N.; Gorassini, A.; Verardo, G.; et al. Nrf2-Mediated Pathway Activated by Prunus spinosa L. (Rosaceae) Fruit Extract: Bioinformatics Analyses and Experimental Validation. Nutrients 2023, 15, 2132. [Google Scholar] [CrossRef]
- Thilavech, T.; Adisakwattana, S. Cyanidin-3-Rutinoside Acts as a Natural Inhibitor of Intestinal Lipid Digestion and Absorption. BMC Complement. Altern. Med. 2019, 19, 242. [Google Scholar] [CrossRef]
- Kültür, S. Medicinal Plants Used in Kirklareli Province (Turkey). J. Ethnopharmacol. 2007, 111, 341–364. [Google Scholar] [CrossRef] [PubMed]
- Jarić, S.; Mačukanović-Jocić, M.; Djurdjević, L.; Mitrović, M.; Kostić, O.; Karadžić, B.; Pavlović, P. An Ethnobotanical Survey of Traditionally Used Plants on Suva Planina Mountain (South-Eastern Serbia). J. Ethnopharmacol. 2015, 175, 93–108. [Google Scholar] [CrossRef] [PubMed]
- Calvo, M.I.; Cavero, R.Y. Medicinal Plants Used for Cardiovascular Diseases in Navarra and Their Validation from Official Sources. J. Ethnopharmacol. 2014, 157, 268–273. [Google Scholar] [CrossRef] [PubMed]
- Barros, L.; Carvalho, A.M.; Morais, J.S.; Ferreira, I.C.F.R. Strawberry-Tree, Blackthorn and Rose Fruits: Detailed Characterisation in Nutrients and Phytochemicals with Antioxidant Properties. Food Chem. 2010, 120, 247–254. [Google Scholar] [CrossRef]
- Marakoğlu, T.; Arslan, D.; Özcan, M.; Hacıseferoğulları, H. Proximate Composition and Technological Properties of Fresh Blackthorn (Prunus spinosa L. Subsp Dasyphylla (Schur.)) Fruits. J. Food Eng. 2005, 68, 137–142. [Google Scholar] [CrossRef]
- Ozzengin, B.; Zannou, O.; Koca, I. Quality Attributes and Antioxidant Activity of Three Wild Plums from Prunus spinosa and Prunus domestica Species. Meas. Food 2023, 10, 100079. [Google Scholar] [CrossRef]
- Capek, P.; Delort, A.-M. Polysaccharides Extracted with Hot Water from Wild Prunus spinosa L. Berries. Carbohydr. Res. 2023, 529, 108852. [Google Scholar] [CrossRef] [PubMed]
- Capek, P.; Košťálová, Z. Isolation, Chemical Characterization and Antioxidant Activity of Prunus spinosa L. Fruit Phenolic Polysaccharide-Proteins. Carbohydr. Res. 2022, 515, 108547. [Google Scholar] [CrossRef]
- Babalau-Fuss, V.; Becze, A.; Roman, M.; Moldovan, A.; Cadar, O.; Tofana, M. Chemical and technological properties of blackthorn (Prunus spinosa) and rose hip (Rosa canina) fruits grown wild in cluj-napoca area. Agricultura 2020, 113–114, 1–6. [Google Scholar]
- Özcan, T.; Kahyaoğlu, G. Fatty Acid and Amino Acid Profiles in the Fruits of Prunus spinosa L. Subsp. Dasyphylla (Schur) Domin from Europe-in-Turkey. Adv. Mol. Biol. 2008, 1, 39–46. [Google Scholar]
- Özcan, T. Some Vitamin and Organic Acid Contents in the Fruits of Prunus spinosa L. Subsp. Dasyphylla (Schur) Domin from Europe-in-Turkey. IUFS J. Biol. Res. Artic. J Biol. 2008, 105, 105–114. [Google Scholar]
- Cosmulescu, S.; Calusaru, F.G. Influence of Temperature on Blackthorn (Prunus spinosa L.) Phenophases in Spring Season. J. Agric. Meteorol. 2020, 76, 53–57. [Google Scholar] [CrossRef]
- Chen, S.; Bobe, G.; Zimmerman, S.; Hammond, E.G.; Luhman, C.M.; Boylston, T.D.; Freeman, A.E.; Beitz, D.C. Physical and Sensory Properties of Dairy Products from Cows with Various Milk Fatty Acid Compositions. J. Agric. Food Chem. 2004, 52, 3422–3428. [Google Scholar] [CrossRef] [PubMed]
- Alissa, E.M.; Ferns, G.A. Functional Foods and Nutraceuticals in the Primary Prevention of Cardiovascular Diseases. J. Nutr. Metab. 2012, 2012, 569486. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, G. Harrison’s Internal Medicine, 17th Edition.—by A. S. Fauci, D.L. Kasper, D.L. Longo, E. Braunwald, S.L. Hauser, J.L. Jameson and J. Loscalzo. Intern. Med. J. 2008, 38, 932. [Google Scholar] [CrossRef]
- Özkan Karabacak, A. Effects of Different Drying Methods on Drying Characteristics, Colour and in-Vitro Bioaccessibility of Phenolics and Antioxidant Capacity of Blackthorn Pestil (Leather). Heat Mass Transf. 2019, 55, 2739–2750. [Google Scholar] [CrossRef]
- Mikulic-Petkovsek, M.; Stampar, F.; Veberic, R.; Sircelj, H. Wild Prunus Fruit Species as a Rich Source of Bioactive Compounds. J. Food Sci. 2016, 81, C1928–C1937. [Google Scholar] [CrossRef]
- Marchelak, A.; Olszewska, M.A.; Owczarek, A. Simultaneous Quantification of Thirty Polyphenols in Blackthorn Flowers and Dry Extracts Prepared Thereof: HPLC-PDA Method Development and Validation for Quality Control. J. Pharm. Biomed. Anal. 2020, 184, 113121. [Google Scholar] [CrossRef]
- Owczarek, A.; Magiera, A.; Matczak, M.; Piotrowska, D.G.; Olszewska, M.A.; Marchelak, A. Optimisation of Preparative HPLC Separation of Four Isomeric Kaempferol Diglycosides from Prunus spinosa L. by Application of the Response Surface Methodology. Phytochem. Lett. 2017, 20, 415–424. [Google Scholar] [CrossRef]
- Xu, F.; Matsuda, H.; Hata, H.; Sugawara, K.; Nakamura, S.; Yoshikawa, M. Structures of New Flavonoids and Benzofuran-Type Stilbene and Degranulation Inhibitors of Rat Basophilic Leukemia Cells from the Brazilian Herbal Medicine Cissus Sicyoides. Chem. Pharm. Bull. 2009, 57, 1089–1095. [Google Scholar] [CrossRef][Green Version]
- Ciuperca, O.T.; Tebrencu, C.E.; Ionescu, E.; Iacob, E.; Volf, I. Studies on Polyphenols Isolated from Branches of Prunus spinosa L. Species. Rev. Chim. 2019, 70, 2897–2902. [Google Scholar] [CrossRef]
- Guimarães, D.; Cavaco-Paulo, A.; Nogueira, E. Design of Liposomes as Drug Delivery System for Therapeutic Applications. Int. J. Pharm. 2021, 601, 120571. [Google Scholar] [CrossRef] [PubMed]
- Ruparelia, N.; Chai, J.T.; Fisher, E.A.; Choudhury, R.P. Inflammatory Processes in Cardiovascular Disease: A Route to Targeted Therapies. Nat. Rev. Cardiol. 2017, 14, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Roleira, F.M.F.; Tavares-da-Silva, E.J.; Varela, C.L.; Costa, S.C.; Silva, T.; Garrido, J.; Borges, F. Plant Derived and Dietary Phenolic Antioxidants: Anticancer Properties. Food Chem. 2015, 183, 235–258. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.L.; Norhaizan, M.E.; Liew, W.-P.-P.; Sulaiman Rahman, H. Antioxidant and Oxidative Stress: A Mutual Interplay in Age-Related Diseases. Front. Pharmacol. 2018, 9, 1162. [Google Scholar] [CrossRef] [PubMed]
- Kovacic, P.; Somanathan, R. Cell Signaling and Receptors with Resorcinols and Flavonoids: Redox, Reactive Oxygen Species, and Physiological Effects. J. Recept. Signal Transduct. 2011, 31, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Losada-Barreiro, S.; Bravo-Díaz, C. Free Radicals and Polyphenols: The Redox Chemistry of Neurodegenerative Diseases. Eur. J. Med. Chem. 2017, 133, 379–402. [Google Scholar] [CrossRef]
- Miguel, M.G. Anthocyanins: Antioxidant and/or Anti-Inflammatory Activities. J. Appl. Pharm. Sci. 2011, 1, 7–15. [Google Scholar]
- Balta, I.; Sevastre, B.; Mireşan, V.; Taulescu, M.; Raducu, C.; Longodor, A.L.; Marchiş, Z.; Mariş, C.S.; Coroian, A. Protective Effect of Blackthorn Fruits (Prunus spinosa) against Tartrazine Toxicity Development in Albino Wistar Rats. BMC Chem. 2019, 13, 104. [Google Scholar] [CrossRef]
- Terevinto, A.; Ramos, A.; Castroman, G.; Cabrera, M.C.; Saadoun, A. Oxidative Status, in Vitro Iron-Induced Lipid Oxidation and Superoxide Dismutase, Catalase and Glutathione Peroxidase Activities in Rhea Meat. Meat Sci. 2010, 84, 706–710. [Google Scholar] [CrossRef]
- Varga, E.; Domokos, E.; Fogarasi, E.; Steanesu, R.; Fülöp, I.; Croitoru, M.D.; Laczkó-Zöld, E. Polyphenolic compounds analysis and antioxidant activity in fruits of Prunus spinosa L. Acta Pharm. Hung. 2017, 87, 19–25. [Google Scholar] [PubMed]
- Radovanović, B.C.; Anđelković, S.M.; Radovanović, A.B.; Anđelković, M.Z. Antioxidant and Antimicrobial Activity of Polyphenol Extracts from Wild Berry Fruits Grown in Southeast Serbia. Trop. J. Pharm. Res. 2013, 12, 813–819. [Google Scholar] [CrossRef]
- Baltas, N.; Pakyildiz, S.; Can, Z.; Dincer, B.; Kolayli, S. Biochemical Properties of Partially Purified Polyphenol Oxidase and Phenolic Compounds of Prunus spinosa L. Subsp. Dasyphylla as Measured by HPLC-UV. Int. J. Food Prop. 2017, 20, 1377–1391. [Google Scholar] [CrossRef]
- Guarrera, P.M.; Forti, G.; Marignoli, S. Ethnobotanical and Ethnomedicinal Uses of Plants in the District of Acquapendente (Latium, Central Italy). J. Ethnopharmacol. 2005, 96, 429–444. [Google Scholar] [CrossRef] [PubMed]
- Alarcόn, R.; Pardo-de-Santayana, M.; Priestley, C.; Morales, R.; Heinrich, M. Medicinal and Local Food Plants in the South of Alava (Basque Country, Spain). J. Ethnopharmacol. 2015, 176, 207–224. [Google Scholar] [CrossRef]
- Gironés-Vilaplana, A.; Villaño, D.; Moreno, D.A.; García-Viguera, C. New Isotonic Drinks with Antioxidant and Biological Capacities from Berries (Maqui, Açaí and Blackthorn) and Lemon Juice. Int. J. Food Sci. Nutr. 2013, 64, 897–906. [Google Scholar] [CrossRef]
- Guimarães, R.; Barros, L.; Dueñas, M.; Carvalho, A.M.; Queiroz, M.J.R.P.; Santos-Buelga, C.; Ferreira, I.C.F.R. Characterisation of Phenolic Compounds in Wild Fruits from Northeastern Portugal. Food Chem. 2013, 141, 3721–3730. [Google Scholar] [CrossRef]
- Gandhi, G.R.; Jothi, G.; Antony, P.J.; Balakrishna, K.; Paulraj, M.G.; Ignacimuthu, S.; Stalin, A.; Al-Dhabi, N.A. Gallic Acid Attenuates High-Fat Diet Fed-Streptozotocin-Induced Insulin Resistance via Partial Agonism of PPARγ in Experimental Type 2 Diabetic Rats and Enhances Glucose Uptake through Translocation and Activation of GLUT4 in PI3K/p-Akt Signaling Pathway. Eur. J. Pharmacol. 2014, 745, 201–216. [Google Scholar] [CrossRef]
- Tzulker, R.; Glazer, I.; Bar-Ilan, I.; Holland, D.; Aviram, M.; Amir, R. Antioxidant Activity, Polyphenol Content, and Related Compounds in Different Fruit Juices and Homogenates Prepared from 29 Different Pomegranate Accessions. J. Agric. Food Chem. 2007, 55, 9559–9570. [Google Scholar] [CrossRef]
- Morita, M.; Naito, Y.; Niki, E.; Yoshikawa, T. Antioxidant Action of Fermented Grain Food Supplement: Scavenging of Peroxyl Radicals and Inhibition of Plasma Lipid Oxidation Induced by Multiple Oxidants. Food Chem. 2017, 237, 574–580. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Lankatillake, C.; Dias, D.A.; Docea, A.O.; Mahomoodally, M.F.; Lobine, D.; Chazot, P.L.; Kurt, B.; Boyunegmez Tumer, T.; Catarina Moreira, A.; et al. Impact of Natural Compounds on Neurodegenerative Disorders: From Preclinical to Pharmacotherapeutics. J. Clin. Med. 2020, 9, 1061. [Google Scholar] [CrossRef]
- Marchelak, A.; Owczarek, A.; Matczak, M.; Pawlak, A.; Kolodziejczyk-Czepas, J.; Nowak, P.; Olszewska, M.A. Bioactivity Potential of Prunus spinosa L. Flower Extracts: Phytochemical Profiling, Cellular Safety, Pro-Inflammatory Enzymes Inhibition and Protective Effects against Oxidative Stress In Vitro. Front. Pharmacol. 2017, 8, 680. [Google Scholar] [CrossRef]
- Mirmiran, P.; Hosseini-Esfahani, F.; Esfandiar, Z.; Hosseinpour-Niazi, S.; Azizi, F. Associations between Dietary Antioxidant Intakes and Cardiovascular Disease. Sci. Rep. 2022, 12, 1504. [Google Scholar] [CrossRef]
- Vermeer, M.A.; Mulder, T.P.J.; Molhuizen, H.O.F. Theaflavins from Black Tea, Especially Theaflavin-3-Gallate, Reduce the Incorporation of Cholesterol into Mixed Micelles. J. Agric. Food Chem. 2008, 56, 12031–12036. [Google Scholar] [CrossRef]
- Pandey, K.B.; Rizvi, S.I. Plant Polyphenols as Dietary Antioxidants in Human Health and Disease. Oxidative Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef]
- Hao, R.; Shan, S.; Yang, D.; Zhang, H.; Sun, Y.; Li, Z. Peonidin-3-O-Glucoside from Purple Corncob Ameliorates Nonalcoholic Fatty Liver Disease by Regulating Mitochondrial and Lysosome Functions to Reduce Oxidative Stress and Inflammation. Nutrients 2023, 15, 372. [Google Scholar] [CrossRef]
- Adisakwattana, S.; Yibchok-Anun, S.; Charoenlertkul, P.; Wongsasiripat, N. Cyanidin-3-Rutinoside Alleviates Postprandial Hyperglycemia and Its Synergism with Acarbose by Inhibition of Intestinal α-Glucosidase. J. Clin. Biochem. Nutr. 2011, 49, 36–41. [Google Scholar] [CrossRef]
- Palaniappan, K.; Holley, R.A. Use of Natural Antimicrobials to Increase Antibiotic Susceptibility of Drug Resistant Bacteria. Int. J. Food Microbiol. 2010, 140, 164–168. [Google Scholar] [CrossRef]
- Coppari, S.; Colomba, M.; Fraternale, D.; Brinkmann, V.; Romeo, M.; Rocchi, M.B.L.; Di Giacomo, B.; Mari, M.; Guidi, L.; Ramakrishna, S.; et al. Antioxidant and Anti-Inflammaging Ability of Prune (Prunus spinosa L.) Extract Result in Improved Wound Healing Efficacy. Antioxidants 2021, 10, 374. [Google Scholar] [CrossRef]
- Jayaprakasha, G.K.; Chidambara Murthy, K.N.; Pellati, F.; Patil, B.S. BetaSweet Carrot Extracts Have Antioxidant Activity and in Vitro Antiproliferative Effects against Breast Cancer Cells. J. Funct. Foods 2019, 62, 103552. [Google Scholar] [CrossRef]
- Sabatini, L.; Fraternale, D.; Di Giacomo, B.; Mari, M.; Albertini, M.C.; Gordillo, B.; Rocchi, M.B.L.; Sisti, D.; Coppari, S.; Semprucci, F.; et al. Chemical Composition, Antioxidant, Antimicrobial and Anti-Inflammatory Activity of Prunus spinosa L. Fruit Ethanol Extract. J. Funct. Foods 2020, 67, 103885. [Google Scholar] [CrossRef]
- Marčetić, M.; Samardžić, S.; Ilić, T.; Božić, D.D.; Vidović, B. Phenolic Composition, Antioxidant, Anti-Enzymatic, Antimicrobial and Prebiotic Properties of Prunus spinosa L. Fruits. Foods 2022, 11, 3289. [Google Scholar] [CrossRef] [PubMed]
- Velickovic, I.; Zizak, Z.; Rajcevic, N.; Ivanov, M.; Sokovic, M.; Marin, P.; Grujic, S. Examination of the Polyphenol Content and Bioactivities of Prunus spinosa L. Fruit Extracts. Arch. Biol. Sci. 2020, 72, 105–115. [Google Scholar] [CrossRef]
- Kumarasamy, Y.; Cox, P.J.; Jaspars, M.; Nahar, L.; Sarker, S.D. Comparative Studies on Biological Activities of Prunus padus and P. spinosa. Fitoterapia 2004, 75, 77–80. [Google Scholar] [CrossRef] [PubMed]
- Veličković, I.; Žižak, Ž.; Rajčević, N.; Ivanov, M.; Soković, M.; Marin, P.D.; Grujić, S. Prunus spinosa L. Leaf Extracts: Polyphenol Profile and Bioactivities. Not. Bot. Horti Agrobot. 2021, 49, 12137. [Google Scholar] [CrossRef]
- Seljeskog, E.; Hervig, T.; Mansoor, M.A. A Novel HPLC Method for the Measurement of Thiobarbituric Acid Reactive Substances (TBARS). A Comparison with a Commercially Available Kit. Clin. Biochem. 2006, 39, 947–954. [Google Scholar] [CrossRef] [PubMed]
- Konstantinidou, V.; Covas, M.-I.; Sola, R.; Fitó, M. Up-to Date Knowledge on the in Vivo Transcriptomic Effect of the Mediterranean Diet in Humans. Mol. Nutr. Food Res. 2013, 57, 772–783. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Liu, Y.; Xiang, J.; Wang, C.; Johnson, J.B.; Beta, T. Diverse Polyphenol Components Contribute to Antioxidant Activity and Hypoglycemic Potential of Mulberry Varieties. LWT 2023, 173, 114308. [Google Scholar] [CrossRef]
- Brandes, M.S.; Gray, N.E. NRF2 as a Therapeutic Target in Neurodegenerative Diseases. ASN Neuro 2020, 12, 175909141989978. [Google Scholar] [CrossRef]
- Cuadrado, A. Brain-Protective Mechanisms of Transcription Factor NRF2: Toward a Common Strategy for Neurodegenerative Diseases. Annu. Rev. Pharmacol. Toxicol. 2022, 62, 255–277. [Google Scholar] [CrossRef]
- Teleanu, D.M.; Niculescu, A.-G.; Lungu, I.I.; Radu, C.I.; Vladâcenco, O.; Roza, E.; Costăchescu, B.; Grumezescu, A.M.; Teleanu, R.I. An Overview of Oxidative Stress, Neuroinflammation, and Neurodegenerative Diseases. Int. J. Mol. Sci. 2022, 23, 5938. [Google Scholar] [CrossRef]
- Tossetta, G.; Marzioni, D. Targeting the NRF2/KEAP1 Pathway in Cervical and Endometrial Cancers. Eur. J. Pharmacol. 2023, 941, 175503. [Google Scholar] [CrossRef]
- Misrani, A.; Tabassum, S.; Yang, L. Mitochondrial Dysfunction and Oxidative Stress in Alzheimer’s Disease. Front. Aging Neurosci. 2021, 13, 617588. [Google Scholar] [CrossRef]
- Liu, Z.; Ren, Z.; Zhang, J.; Chuang, C.-C.; Kandaswamy, E.; Zhou, T.; Zuo, L. Role of ROS and Nutritional Antioxidants in Human Diseases. Front. Physiol. 2018, 9, 477. [Google Scholar] [CrossRef]
- Blesa, J.; Trigo-Damas, I.; Quiroga-Varela, A.; Jackson-Lewis, V.R. Oxidative Stress and Parkinson’s Disease. Front. Neuroanat. 2015, 9, 91. [Google Scholar] [CrossRef]
- Padureanu, R.; Albu, C.V.; Mititelu, R.R.; Bacanoiu, M.V.; Docea, A.O.; Calina, D.; Padureanu, V.; Olaru, G.; Sandu, R.E.; Malin, R.D.; et al. Oxidative Stress and Inflammation Interdependence in Multiple Sclerosis. J. Clin. Med. 2019, 8, 1815. [Google Scholar] [CrossRef]
- Díaz-Hung, M.L.; González Fraguela, M.E. Oxidative Stress in Neurological Diseases: Cause or Effect? Neurología 2014, 29, 451–452. [Google Scholar] [CrossRef]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative Stress, Aging, and Diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef]
- Barnham, K.J.; Masters, C.L.; Bush, A.I. Neurodegenerative Diseases and Oxidative Stress. Nat. Rev. Drug Discov. 2004, 3, 205–214. [Google Scholar] [CrossRef]
- Winiarska-Mieczan, A.; Baranowska-Wójcik, E.; Kwiecień, M.; Grela, E.R.; Szwajgier, D.; Kwiatkowska, K.; Kiczorowska, B. The Role of Dietary Antioxidants in the Pathogenesis of Neurodegenerative Diseases and Their Impact on Cerebral Oxidoreductive Balance. Nutrients 2020, 12, 435. [Google Scholar] [CrossRef]
- Chen, C.-M.; Lin, J.-K.; Liu, S.-H.; Lin-Shiau, S.-Y. Novel Regimen through Combination of Memantine and Tea Polyphenol for Neuroprotection against Brain Excitotoxicity. J. Neurosci. Res. 2008, 86, 2696–2704. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Cao, H.; Wen, J.; Xu, M. Green Tea Polyphenol (–)-Epigallocatechin-3-Gallate Enhances the Inhibitory Effect of Huperzine A on Acetylcholinesterase by Increasing the Affinity with Serum Albumin. Nutr. Neurosci. 2009, 12, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, K.; Aggarwal, B.B.; Singh, R.B.; Buttar, H.S.; Wilson, D.; De Meester, F. Food Antioxidants and Their Anti-Inflammatory Properties: A Potential Role in Cardiovascular Diseases and Cancer Prevention. Diseases 2016, 4, 28. [Google Scholar] [CrossRef]
- Jurikova, T.; Mlcek, J.; Skrovankova, S.; Sumczynski, D.; Sochor, J.; Hlavacova, I.; Snopek, L.; Orsavova, J. Fruits of Black Chokeberry Aronia Melanocarpa in the Prevention of Chronic Diseases. Molecules 2017, 22, 944. [Google Scholar] [CrossRef] [PubMed]
- Jurcau, A. The Role of Natural Antioxidants in the Prevention of Dementia—Where Do We Stand and Future Perspectives. Nutrients 2021, 13, 282. [Google Scholar] [CrossRef] [PubMed]
- Poonam, V.; Raunak, G.; Kumar, G.; Reddy, L.S.C.; Jain, R.; Sharma, S.K.; Prasad, A.K.; Parmar, V.S. Chemical Constituents of the Genus Prunus and Their Medicinal Properties. Curr. Med. Chem. 2011, 18, 3758–3824. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Rémésy, C. Bioavailability and Bioefficacy of Polyphenols in Humans. I. Review of 97 Bioavailability Studies. Am. J. Clin. Nutr. 2005, 81, 230S–242S. [Google Scholar] [CrossRef]
- Quiñones, M.; Miguel, M.; Aleixandre, A. Beneficial Effects of Polyphenols on Cardiovascular Disease. Pharmacol. Res. 2013, 68, 125–131. [Google Scholar] [CrossRef]
- Santilli, F.; D’Ardes, D.; Davì, G. Oxidative Stress in Chronic Vascular Disease: From Prediction to Prevention. Vasc. Pharmacol. 2015, 74, 23–37. [Google Scholar] [CrossRef]
- Saradhadevi, M.; Gnanadesigan, M.; Kapildev, G.; Vasanth, D. Dataset on Antitumor Properties of Silver Nanoparticles from Gloriosa superba (L.) Seed on Dalton Lymphoma Ascites (DLA) Tumor: Facile and Biocompatible Approach. Data Brief 2017, 14, 524–530. [Google Scholar] [CrossRef]
- Vita, J.A. Polyphenols and Cardiovascular Disease: Effects on Endothelial and Platelet Function. Am. J. Clin. Nutr. 2005, 81, 292S–297S. [Google Scholar] [CrossRef] [PubMed]
- Kianbakht, S.; Abasi, B.; Hashem Dabaghian, F. Improved Lipid Profile in Hyperlipidemic Patients Taking Vaccinium Arctostaphylos Fruit Hydroalcoholic Extract: A Randomized Double-Blind Placebo-Controlled Clinical Trial: Vaccinium arctostaphylos improves lipid profile in hyperlipidemia. Phytother. Res. 2014, 28, 432–436. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Ling, W.; Guo, H.; Song, F.; Ye, Q.; Zou, T.; Li, D.; Zhang, Y.; Li, G.; Xiao, Y.; et al. Anti-Inflammatory Effect of Purified Dietary Anthocyanin in Adults with Hypercholesterolemia: A Randomized Controlled Trial. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 843–849. [Google Scholar] [CrossRef] [PubMed]
- Velickovic, J.; Kostic, D.; Stojanovic, G.; Mitic, S.; Mitic, M.; Randjelovic, S.; Djordjevic, A. Phenolic Composition, Antioxidant and Antimicrobial Activity of the Extracts from Prunus spinosa L. Fruit. Hem. Ind. 2014, 68, 297–303. [Google Scholar] [CrossRef]
- Fabroni, S.; Ballistreri, G.; Amenta, M.; Romeo, F.V.; Rapisarda, P. Screening of the Anthocyanin Profile and in Vitro Pancreatic Lipase Inhibition by Anthocyanin-Containing Extracts of Fruits, Vegetables, Legumes and Cereals: Anthocyanin-Containing Extracts as Inhibitors of Pancreatic Lipase. J. Sci. Food Agric. 2016, 96, 4713–4723. [Google Scholar] [CrossRef] [PubMed]
- Gururaja, G.; Mundkinajeddu, D.; Dethe, S.; Sangli, G.; Abhilash, K.; Agarwal, A. Cholesterol Esterase Inhibitory Activity of Bioactives from Leaves of Mangifera indica L. Pharmacogn. Res. 2015, 7, 355. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Bansal, A.; Sarma, G.S.; Rawal, R.K. Chemometrics Tools Used in Analytical Chemistry: An Overview. Talanta 2014, 123, 186–199. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.K. Does the Interdependence between Oxidative Stress and Inflammation Explain the Antioxidant Paradox? Oxidative Med. Cell. Longev. 2016, 2016, 5698931. [Google Scholar] [CrossRef]
- Alzaid, F.; Cheung, H.-M.; Preedy, V.R.; Sharp, P.A. Regulation of Glucose Transporter Expression in Human Intestinal Caco-2 Cells Following Exposure to an Anthocyanin-Rich Berry Extract. PLoS ONE 2013, 8, e78932. [Google Scholar] [CrossRef]
- Murati, T.; Miletić, M.; Kolarić, J.; Lovrić, V.; Kovačević, D.B.; Putnik, P.; Jurčević, I.L.; Đikić, D.; Dragović-Uzelac, V.; Kmetič, I. Toxic Activity of Prunus spinosa L. Flower Extract in Hepatocarcinoma Cells. Arch. Ind. Hyg. Toxicol. 2019, 70, 303–309. [Google Scholar] [CrossRef]
- Sun, C.; Zhao, C.; Guven, E.C.; Paoli, P.; Simal-Gandara, J.; Ramkumar, K.M.; Wang, S.; Buleu, F.; Pah, A.; Turi, V.; et al. Dietary Polyphenols as Antidiabetic Agents: Advances and Opportunities. Food Front. 2020, 1, 18–44. [Google Scholar] [CrossRef]
- Loureiro, G.; Martel, F. The Effect of Dietary Polyphenols on Intestinal Absorption of Glucose and Fructose: Relation with Obesity and Type 2 Diabetes. Food Rev. Int. 2019, 35, 390–406. [Google Scholar] [CrossRef]
- Kang, G.G.; Francis, N.; Hill, R.; Waters, D.; Blanchard, C.; Santhakumar, A.B. Dietary Polyphenols and Gene Expression in Molecular Pathways Associated with Type 2 Diabetes Mellitus: A Review. Int. J. Mol. Sci. 2019, 21, 140. [Google Scholar] [CrossRef] [PubMed]
- Varshney, R.; Mishra, R.; Das, N.; Sircar, D.; Roy, P. A Comparative Analysis of Various Flavonoids in the Regulation of Obesity and Diabetes: An in Vitro and in Vivo Study. J. Funct. Foods 2019, 59, 194–205. [Google Scholar] [CrossRef]
- Alkhalidy, H.; Moore, W.; Wang, Y.; Luo, J.; McMillan, R.; Zhen, W.; Zhou, K.; Liu, D. The Flavonoid Kaempferol Ameliorates Streptozotocin-Induced Diabetes by Suppressing Hepatic Glucose Production. Molecules 2018, 23, 2338. [Google Scholar] [CrossRef]
- Temiz, M.A.; Okumus, E.; Yaman, T.; Keles, O.F. Mixture of Leaf and Flower Extract of Prunus spinosa L. Alleviates Hyperglycemia and Oxidative Stress in Streptozotocin-Induced Diabetic Rats. S. Afr. J. Bot. 2021, 141, 145–151. [Google Scholar] [CrossRef]
- Marzioni, D.; Mazzucchelli, R.; Fantone, S.; Tossetta, G. NRF2 Modulation in TRAMP Mice: An in Vivo Model of Prostate Cancer. Mol. Biol. Rep. 2023, 50, 873–881. [Google Scholar] [CrossRef] [PubMed]
- Ghareghomi, S.; Habibi-Rezaei, M.; Arese, M.; Saso, L.; Moosavi-Movahedi, A.A. Nrf2 Modulation in Breast Cancer. Biomedicines 2022, 10, 2668. [Google Scholar] [CrossRef]
- Dajas, F. Life or Death: Neuroprotective and Anticancer Effects of Quercetin. J. Ethnopharmacol. 2012, 143, 383–396. [Google Scholar] [CrossRef]
- Men, K.; Duan, X.; Wei, X.W.; Gou, M.L.; Huang, M.J.; Chen, L.J.; Qian, Z.Y.; Wei, Y.Q. Nanoparticle-Delivered Quercetin for Cancer Therapy. Anticancer. Agents Med. Chem. 2014, 14, 826–832. [Google Scholar] [CrossRef]
- Imran, M.; Salehi, B.; Sharifi-Rad, J.; Aslam Gondal, T.; Saeed, F.; Imran, A.; Shahbaz, M.; Tsouh Fokou, P.V.; Umair Arshad, M.; Khan, H.; et al. Kaempferol: A Key Emphasis to Its Anticancer Potential. Molecules 2019, 24, 2277. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, J.; Sharifi-Rad, M.; Salehi, B.; Iriti, M.; Roointan, A.; Mnayer, D.; Soltani-Nejad, A.; Afshari, A. In Vitro and in Vivo Assessment of Free Radical Scavenging and Antioxidant Activities of Veronica Persica Poir. Cell Mol. Biol. 2018, 64, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Ayla, S.; Gunal, M.; Sayin Sakul, A.; Biçeroğlu, Ö.; Özdemir, E.; Okur, M.; Cicek Polat, D.; Üstündağ Okur, N.; Bilgiç, B. Effects of Prunus spinosa L. Fruits on Experimental Wound Healing. Medeni. Med. J. 2017, 32. [Google Scholar] [CrossRef]
- Gegiu, G.; Branza, A.; Bucur, L.; Grigorian, M.; Tache, T.; Badea, V. Contributions to the antimicrobial and antifungal study of the aqueous extract of Prunus spinosa L. Farmacia 2015, 63, 2. [Google Scholar]
- Yahfoufi, N.; Alsadi, N.; Jambi, M.; Matar, C. The Immunomodulatory and Anti-Inflammatory Role of Polyphenols. Nutrients 2018, 10, 1618. [Google Scholar] [CrossRef] [PubMed]
- Gündüz, G.T. Antimicrobial Activity of Sloe Berry Purees on Salmonella spp. Food Control 2013, 32, 354–358. [Google Scholar] [CrossRef]
- Kavaz Yuksel, A. The Effects of Blackthorn (Prunus spinosa L.) Addition on Certain Quality Characteristics of Ice Cream: Quality Characteristics of Ice Cream. J. Food Qual. 2015, 38, 413–421. [Google Scholar] [CrossRef]
- Ulusoy, A.; Tamer, C.E. Determination of Suitability of Black Carrot (Daucus carota L. Spp. Sativus Var. Atrorubens Alef.) Juice Concentrate, Cherry Laurel (Prunus laurocerasus), Blackthorn (Prunus spinosa) and Red Raspberry (Rubus ideaus) for Kombucha Beverage Production. Food Meas. 2019, 13, 1524–1536. [Google Scholar] [CrossRef]
- Mandic, S.; Savanovic, D.; Velemir, A.; Kalaba, V.; Savanovic, J.; Jokanovic, V. Effect of Incorporating Blackthorn Fruit (Prunus spinosa L.) Extract in Natural Casing on Quality of Kranjska Sausage. Meat Technol. 2018, 59, 80–90. [Google Scholar] [CrossRef]
- Meschini, S.; Mastrodonato, F. Prunus spinosa Extracts with Anti-Tumor Activity. Patent WO2016157233A1, 1 April 2016. [Google Scholar]
- Liu, W.; Ge, Y.A. White Strawberry and Sloe Flavored Cherry Cake. Patent CN108576591A, 28 September 2018. [Google Scholar]
- Liu, D.; Song, Y.A. Fermentation Method for Making Mountain Thorn Fruit Wine. Patent CN1970719A, 30 May 2007. [Google Scholar]
- Yves, M. Liqueur Aperitive a Base vin, D’alcool, de Sucre, et de Branchages de Prunus Spinosa. Patent FR2634783A1, 2 February 1990. [Google Scholar]
- Campiche, R.; Imfeld, D.; Voegeli, R. Skin Tanning Extract. Patent EP3052202B1, 6 March 2019. [Google Scholar]
| Reference | [52] | [37] | [15] | [53] | [54] |
|---|---|---|---|---|---|
| Energy (kcal/100 g) | 383.27 ± 7.09 | 57 | 154.93 | 249 | nd |
| Moisture (%) | 60.86 ± 1.69 | 54.85 ± 2.11 | nd | 69.37 | nd |
| Carbohydrates (g/100 g dw) | 88.51 ± 2.24 | 8.64 | 31.07 ± 0.62 | nd | 15.17 ± 25.83 |
| Proteins (g/100 g dw) | 2.86 ± 0.03 | 0.75 | 2.07 ± 0.04 | 3.4 | 0.99 ± 0.25 |
| Fat (g/100 g dw) | 1.98 ± 0.32 | 1 | 2.05 ± 0.12 | 2.06 | nd |
| Ash | 6.65 ± 2.03 | nd | 0.69 ± 0.04 | 2.72 | 1.18 ± 0.56 |
| Fiber (g/100 g) | nd | 9 | 5.79 ± 0.1 | 4.6 | 0.67 ± 0.26 |
| The place/period of blackthorn harvesting | The Natural Park of Montesinho territory, in Trás-os-Montes, North-eastern Portugal/September 2008 | Area of Cluj county, Romania/September 2019 | A mountain village Łącko., in the south of Poland/ns | Konya, Turkey/September 2003 | Kastamonu (Tosya) province, Turkey/ns |
| References | [54] | [57] | [32] |
|---|---|---|---|
| Minerals elements | mg/kg | mg/kg | mg/kg |
| K | 2014.23 | 18,711.18 | 1035.82–1245.38 |
| Ca | 807.99 | 1504.41 | 19.86–34.23 |
| Mg | 188.1 | 972.21 | 8.57–11.83 |
| Na | 42.87 | 534.81 | 2.56–12.22 |
| Fe | 6.79 | 16.04 | 4.00–9.17 |
| Zn | 8.24 | nd | 0.35–1.80 |
| P | nd | 1511.37 | nd |
| Cu | 1.75 | nd | 0.93–2.45 |
| Mn | 1.44 | 4.58 | 0.80–2.38 |
| Al | nd | 26.33 | nd |
| Organs of P. spinosa | Type of Sample/ Technique | Phenols | References |
|---|---|---|---|
| Fruits | Cold solution (1% BHT [w/v], 3% formic acid [v/v] in methanol) HPLC–DAD–MS | Phenolic acids Cinnamic acid derivatives:
| [65] |
| Ethyl acetate fraction of methanol-water extract (75:25, v/v) in dried fruit UHPLC-PDA-ESI-MS | Phenolic acids Protocatechuic acid 4-O-hexoside; Protocatechuic acida; 3-O-Caffeoylquinic acid; p-Hydroxybenzoic acida; Caffeoylshikimic acid derivative; Vanilloyl malate hexoside; 3-O-p-Coumaroylquinic acid; p-Coumaric acid O-hexoside; 5-O-Caffeoylquinic acid; cis-3-O-Feruloylquinic acid; 4-O-Caffeoylquinic acid; Caffeic acid 3/4-O-hexoside; 3-O-Feruloylquinic acid; Vanillina; 4-O-Caffeoylshikimic acid; 4-O-Feruloylquinic acid; Caffeoylshikimic acid; Caffeoylshikimic acid; p-Coumaroylshikimic acid; Aromadendrin hexoside; p-Coumaroylshikimic acid; Flavonols Quercetin 3-O-β-D-galactoside; Quercetin 3-O-(6″-O-α-L-rhamnopyranosyl)-β-D-glucopyranoside; Quercetin 3-O-β-D-glucopyranoside; Quercetin 3-O-α-D-xylopyranoside; Quercetin 3-O-α-L-arabinopyranoside; Quercetin 3-O-α-L-arabinofuranoside; Quercetin 3-O-(4″-O-β-D-glucopyranosyl)-α-L-rhamnopyranoside; Quercetin 3-O-α-L-rhamnopyranoside; Quercetin malyl-pentoside; Quercetin acetyl-hexoside-rhamoside. | [24] | |
| Flowers | Defatted methanol-water extract RP-HPLC-PDA | Phenolic acids 3-O-Caffeoylquinic acid (neochlorogenic acid); 5-O-Caffeoylquinic acid (chlorogenic acid); 4-O-Caffeoylquinic acid (cryptochlorogenic acid); Caffeic acid; p-Coumaric acid; Flavanols (+)-Catechin; (–)-Epicatechin; Flavonols Kaempferol 3-O-α-L-arabinopyranoside-7-O-α-L-rhamnopyranoside; Kaempferol 3-O-β-D-xylopyranoside-7-O-α-L-rhamnopyranoside (lepidoside); Kaempferol 3,7-di-O-α-L-rhamnopyranoside (kaempferitrin); Kaempferol 3-O-α-L-arabinofuranoside-7-O-α-L-rhamnopyranoside; Kaempferol 3-O-β-D-xylopyranoside; Kaempferol 3-O-(4″-O-β-D-glucopyranosyl)-α-L-rhamnopyranoside (multiflorin B); Kaempferol 3-O-α-L-arabinofuranoside (juglanin); Kaempferol 3-O-α-L-rhamnopyranoside (afzelin); Kaempferol 7-O-α-L-rhamnopyranoside; Kaempferol 3-O-(2″-O-E-p-coumaroyl)-α-L-arabinofuranoside-7-O-α-Lrhamnopyranoside; Kaempferol 3-O-(6″-O-α-L-rhamnopyranosyl)-β-D-glucopyranoside; Kaempferol 3-O-(2″-O-E-p-coumaroyl)-α-L-arabinofuranoside. Kaempferol; Quercetin 3-O-(6″-O-α-L-rhamnopyranosyl)-β-D-glucopyranoside (rutin); Quercetin 3-O-(2″-O-β-D-glucopyranosyl)-α-L-arabinofuranoside; Quercetin 3-O-β-D-glucopyranoside (isoquercitrin); Quercetin 3-O-β-D-galactopyranoside (hyperoside); Quercetin 3-O-α-D-xylopyranoside (reinutrin); Quercetin 3-O-α-L-arabinopyranoside (guaiaverin); Quercetin 3-O-(4″-O-β-D-glucopyranosyl)-α-L-rhamnopyranoside (multinoside A); Quercetin 3-O-α-L-arabinofuranoside (avicularin); Quercetin 3-O-α-L-rhamnopyranoside (quercitrin); Quercetin; | [66] |
| Leaves | 70% (v/v) aqueous-methanolic extract UHPLC-PDA-ESI–MS | Phenolic acids
| [67] |
| Branches | Lyophilized extract HPLC/MS | Phenolic acids
Ent-(epi)-catechin-(2α→O→7,4α→8)-(epi)-catechin-3′-O-gallate; Ent-(epi)-afzelechin-(2α→O→7,4α→8)-(epi)-catechin-3′-O-gallate; Ent-(epi)-gallocatechin (2α→O→7, 4α→8)(epi)-catechin; Ent-(epi)-catechin (2α→O→7, 4 α→8)-catechin; Ent-(epi)-gallocatechin (2α→O→7, 4α→8)-(epi)-catechin; Ent-(epi)-catechin (2α→O→7, 4 α→8)-(epi)-catechin; Ent-(epi)-afzalechin (2α→O→7, 4α→8) catechin; Epigallocatechin; Ent-(epi)-afzalechin (2α→O→7, 4α→8)-(epi)-catechin; Gallocatechin; Epicatechin; Catechin; Epiafzelechin; Afzelechin; Coumarins 5-hydroxy-6-methoxy-7-O-β-D-glucosyl coumarin; 5-hydroxy-6-methoxy-7-O-β-D-rhamnosyl coumarin; Flavonols Quercetin 3-O-rutinoside; Kaempferol 3,7-O-dirhamnoside; Kaempferol 3-O-arabinoside-7-O-rhamnoside; kaempferol 3-O-arabinoside; Quercetin; Kaempferol | [6] |
| Application No. | Species/Part | Results/Mechanism | References |
|---|---|---|---|
| WO 2016/157233 A1 | P. spinosa L.-fruits, Trigno variety | The composition is used, in particular, for the treatment of human tumors but also as a dietary supplement. | [158] |
| CN108576591A | P. spinosa L.-fruits | This invention is based on the use of P. spinosa L. as a raw material to make a dessert. Its long-term consumption contributes to the elimination of cough and has a protective effect on the lungs. | [159] |
| CN1970719A | P. spinosa L. fruits | The invention reveals a technique for preparing wine from P. spinosa L. fruits by the fermentation method. | [160] |
| FR2634783A1 | P. spinosa L. branches | This invention consists of obtaining a liqueur for the appetizer. The process consists of respecting the proportions of the various constituents, wine, alcohol, sugar, and branches of P. spinosa L., during controlled fresh maceration for 48 h. | [161] |
| EP3052202B1 | P. spinosa L.—flowers extract | The invention relates to obtaining a cosmetic product in which P. spinosa L. flower extract was used as a skin tanning agent. It helps to improve melanin formation in human skin cells by topical application. | [162] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bei, M.F.; Apahidean, A.I.; Budău, R.; Rosan, C.A.; Popovici, R.; Memete, A.R.; Domocoș, D.; Vicas, S.I. An Overview of the Phytochemical Composition of Different Organs of Prunus spinosa L., Their Health Benefits and Application in Food Industry. Horticulturae 2024, 10, 29. https://doi.org/10.3390/horticulturae10010029
Bei MF, Apahidean AI, Budău R, Rosan CA, Popovici R, Memete AR, Domocoș D, Vicas SI. An Overview of the Phytochemical Composition of Different Organs of Prunus spinosa L., Their Health Benefits and Application in Food Industry. Horticulturae. 2024; 10(1):29. https://doi.org/10.3390/horticulturae10010029
Chicago/Turabian StyleBei, Mariana Florica, Alexandru Ioan Apahidean, Ruben Budău, Cristina Adriana Rosan, Raluca Popovici, Adriana Ramona Memete, Daniela Domocoș, and Simona Ioana Vicas. 2024. "An Overview of the Phytochemical Composition of Different Organs of Prunus spinosa L., Their Health Benefits and Application in Food Industry" Horticulturae 10, no. 1: 29. https://doi.org/10.3390/horticulturae10010029
APA StyleBei, M. F., Apahidean, A. I., Budău, R., Rosan, C. A., Popovici, R., Memete, A. R., Domocoș, D., & Vicas, S. I. (2024). An Overview of the Phytochemical Composition of Different Organs of Prunus spinosa L., Their Health Benefits and Application in Food Industry. Horticulturae, 10(1), 29. https://doi.org/10.3390/horticulturae10010029








