Cinnamaldehyde Acts as a Fungistat by Disrupting the Integrity of Fusarium oxysporum Fox-1 Cell Membranes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Fungus Strains
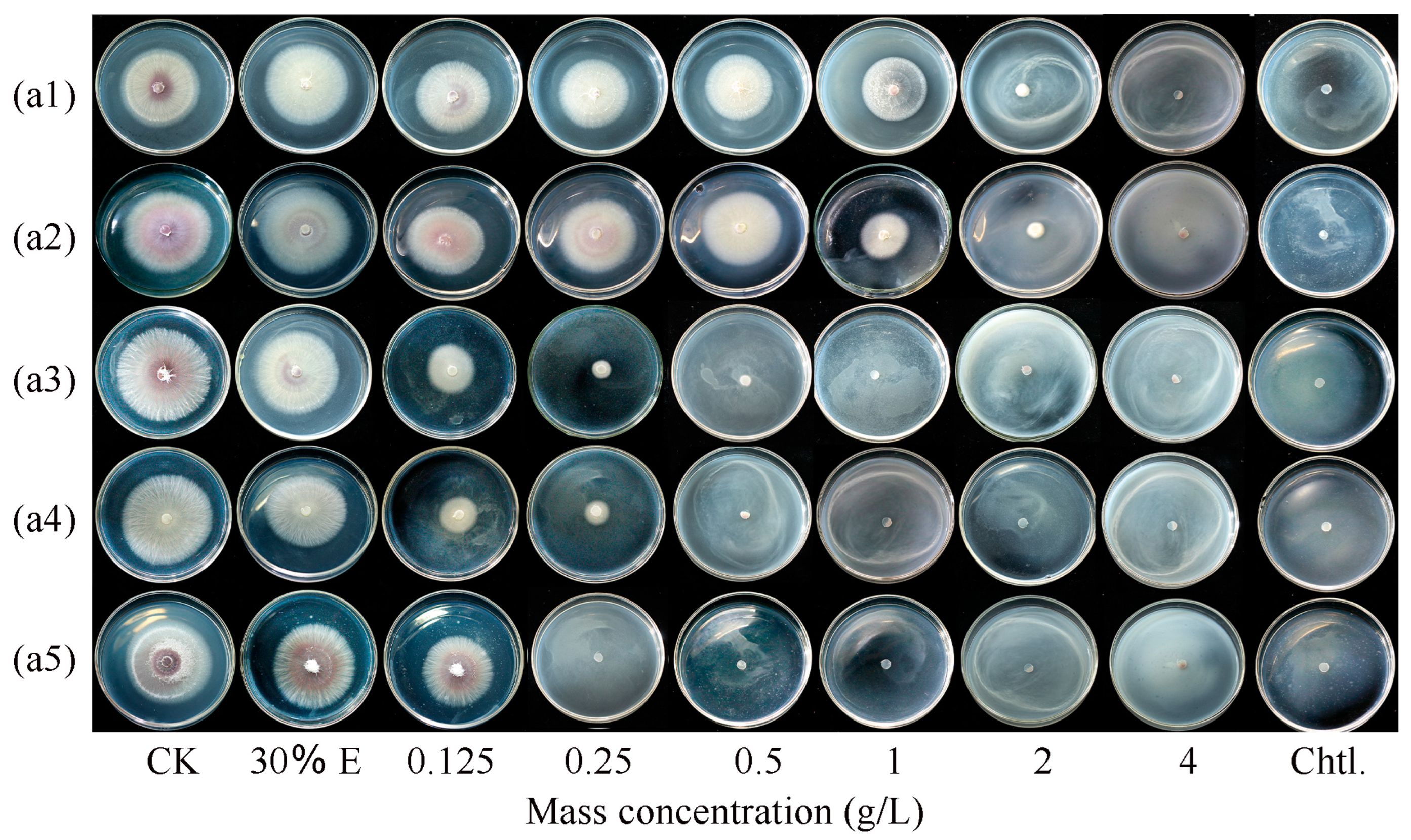
2.2. Antifungal Activity Assay of Ginger EO and Selected Volatile Compounds
2.3. Influence of Ginger EO and Selected Volatile Compounds on the Germination of Spores
2.4. Determination of Cell Membrane Integrity in CA-Treated F. oxysporum FOX-1 Cells
2.4.1. Observation of F. oxysporum FOX-1 Morphology Using Scanning Electron Microscopy
2.4.2. Propidium Iodide Staining Analysis
2.4.3. Cellular Leakage Assay
2.4.4. Determination of Ergosterol Content in Cell Membranes
2.5. Determination of the Effect of CA on the Pathogenicity of F. oxysporum FOX-1
2.6. Statistical Analyses
3. Results
3.1. Ginger EO and Selected Volatile Compounds Inhibited the Growth of F. oxysporum FOX-1
3.2. Ginger EO and Selected Volatile Compounds Affected Spore Germination in F. oxysporum FOX-1
3.3. CA Altered F. oxysporum FOX-1 Morphology
3.4. CA-Induced Cell Membrane Injury of F. oxysporum FOX-1
3.5. CA-Induced Cellular Leakage of F. oxysporum FOX-1
3.6. Changes in Malondialdehyde and Ergosterol Content
3.7. Changes in the Infectivity of F. oxysporum FOX-1 after Treatment with Different Mass Concentrations of CA
4. Discussion
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhou, J.; Liu, X.; Sun, C.; Li, G.; Yang, P.; Jia, Q.; Cai, X.; Zhu, Y.; Yin, J.; Liu, Y. Silica Nanoparticles Enhance the Disease Resistance of Ginger to Rhizome Rot during Postharvest Storage. Nanomaterials 2022, 12, 1418. [Google Scholar] [CrossRef] [PubMed]
- Park, C.J.; Kim, H.S.; Lee, D.W.; Kim, J.; Choi, Y.H. Identification of antifungal constituents of essential oils extracted from Boesenbergia pulcherrima against Fusarium wilt (Fusarium oxysporum). Appl. Biol. Chem. 2020, 63, 34. [Google Scholar] [CrossRef]
- Peng, H.; Hu, H.; Xi, K.; Zhu, X.; Zhou, J.; Yin, J.; Guo, F.; Liu, Y.; Zhu, Y. Silicon Nanoparticles Enhance Ginger Rhizomes Tolerance to Postharvest Deterioration and Resistance to Fusarium solani. Front. Plant Sci. 2022, 13, 816143. [Google Scholar] [CrossRef] [PubMed]
- Şesan, T.E.; Enache, E.; Iacomi, B.M.; Oprea, M.; Oancea, F.; Iacomi, C. In vitro antifungal activity of some plant extracts against Fusarium oxysporum in blackcurrant (Ribes nigrum L.). Acta Sci. Pol. Hortorum Cultus 2017, 16, 167–176. [Google Scholar] [CrossRef]
- Wang, Q.F.; Wang, X.Y.; Li, H.S.; Yang, X.Y.; Zhang, R.M.; Gong, B.; Shi, Q.H. Effects of linalool on Botrytis cinerea growth and control of tomato gray mold. J. Appl. Ecol. 2023, 34, 213–220. (In Chinese) [Google Scholar] [CrossRef]
- El Khetabi, A.; Ezrari, S.; El Ghadraoui, L.; Tahiri, A.; Ait Haddou, L.; Belabess, Z.; Lahlali, R. In vitro and in vivo antifungal activities of nine commercial essential oils against brown rot in apples. Horticulturae 2021, 7, 545. [Google Scholar] [CrossRef]
- Ju, J.; Xie, Y.; Yu, H.; Guo, Y.; Cheng, Y.; Qian, H.; Yao, W. Synergistic interactions of plant essential oils with antimicrobial agents: A new antimicrobial therapy. Crit. Rev. Food Sci. Nutr. 2022, 62, 1740–1751. (In Chinese) [Google Scholar] [CrossRef] [PubMed]
- Reddy, D.N. Essential oils extracted from medicinal plants and their applications. In Natural Bio-Active Compounds: Volume 1: Production and Applications; Springer: Berlin/Heidelberg, Germany, 2019; pp. 237–283. [Google Scholar] [CrossRef]
- Cheny, R.; Luol, M.; Liuz, Y. Comparison and analysis of chemical constituents of volatile oil from ginger from different habitats. Jiangxi J. Tradit. Chin. Med. 2022, 53, 67–70. [Google Scholar]
- Abdullahi, A.; Khairulmazmi, A.; Yasmeen, S.; Ismail, I.S.; Norhayu, A.; Sulaiman, M.R.; Ahmed, O.H.; Ismail, M.R. Phytochemical profiling and antimicrobial activity of ginger (Zingiber officinale) essential oils against important phytopathogens. Arab. J. Chem. 2020, 13, 8012–8025. [Google Scholar] [CrossRef]
- Ma, Q.; Zhang, Y.; Wang, Z.G. Antimicrobial activity of the essential oils from five plants. Plant Prot. 2020, 46, 161–167. (In Chinese) [Google Scholar] [CrossRef]
- Liao, S.; Yang, G.; Huang, S.; Li, B.; Li, A.; Kan, J. Chemical composition of Zanthoxylum schinifolium Siebold & Zucc. essential oil and evaluation of its antifungal activity and potential modes of action on Malassezia Restricta. Ind. Crops Prod. 2022, 180, 114698. [Google Scholar] [CrossRef]
- Badr, M.M.; Badawy, M.E.; Taktak, N.E. Preparation, characterization, and antimicrobial activity of cinnamon essential oil and cinnamaldehyde nanoemulsions. J. Essent. Oil Res. 2022, 34, 544–558. [Google Scholar] [CrossRef]
- Liu, Y.; Wisniewski, M.; Kennedy, J.F.; Jiang, Y.; Tang, J.; Liu, J. Chitosan and oligochitosan enhance ginger (Zingiber officinale Roscoe) resistance to rhizome rot caused by Fusarium oxysporum in storage. Carbohydr. Polym. 2016, 151, 474–479. [Google Scholar] [CrossRef]
- Ma, X.Y.; Yu, C. Bacteriostatic Ability and Field Control Effect of Eight Fungicides against Fusarium oxysporum. J. Eest China For. Sci. 2023, 52, 69–74. (In Chinese) [Google Scholar] [CrossRef]
- Weng, T.; Wang, Y.; Long, C. Study on Mechanism of Geraniol against Geotrichum citri-aurantii of Citrus. Food Sci. 2023, 44, 14–21. (In Chinese). Available online: https://kns.cnki.net/kcms/detail/11.2206.TS.20220425.1801.064.html (accessed on 12 October 2023).
- Huang, J.; Liu, S.; Liu, R.; Yi, Y.; Li, C.; Xiao, Z.; Tu, J.; Xiao, J. Mechanisms of Litsea cubeba essential oil in the control of Colletotrichum scovillei in pepper (Capsicum annuum L.): Cell membrane/wall perspective. Physiol. Mol. Plant Pathol. 2023, 127, 102103. [Google Scholar] [CrossRef]
- Wang, B.; Li, P.; Yang, J.; Yong, X.; Yin, M.; Chen, Y.; Wang, Q. Inhibition efficacy of Tetradium glabrifolium fruit essential oil against Phytophthora capsici and potential mechanism. Ind. Crops Prod. 2022, 176, 114310. [Google Scholar] [CrossRef]
- Bao, Z.; Fan, M.; Hannachi, K.; Li, T.; Zhao, J.; Li, Y.; Qian, H.; Wang, L. Antifungal activity of star anise extract against Penicillium roqueforti and Aspergillus niger for bread shelf life. Food Res. Int. 2023, 172, 113225. [Google Scholar] [CrossRef]
- Kong, W.; Huo, H.; Gu, Y.; Cao, Y.; Wang, J.; Liang, J.; Niu, S. Antifungal activity of camphor against four phytopathogens of Fusarium. S. Afr. J. Bot. 2022, 148, 437–445. [Google Scholar] [CrossRef]
- Matheron, M.E.; Porchas, M. Impact of azoxystrobin, dimethomorph, fluazinam, fosetyl-Al, and metalaxyl on growth, sporulation and zoospore cyst germination of Three Phytophthora spp. Plant Dis. 2000, 84, 454–458. [Google Scholar] [CrossRef]
- Xing, F.; Hua, H.; Selvaraj, J.N.; Zhao, Y.; Zhou, L.; Liu, X.; Liu, Y. Growth inhibition and morphological alterations of Fusarium verticillioides by cinnamon oil and cinnamaldehyde. Food Control 2014, 46, 343–350. [Google Scholar] [CrossRef]
- Wang, L.; Jin, J.; Liu, X.; Wang, Y.; Liu, Y.; Zhao, Y.; Xing, F. Effect of cinnamaldehyde on morphological alterations of Aspergillus ochraceus and expression of key genes involved in ochratoxin A biosynthesis. Toxins 2018, 10, 340. [Google Scholar] [CrossRef]
- Xu, L.; Tao, N.; Yang, W.; Jing, G. Cinnamaldehyde damaged the cell membrane of Alternaria alternata and induced the degradation of mycotoxins in vivo. Ind. Crops Prod. 2018, 112, 427–433. [Google Scholar] [CrossRef]
- Xi, K.Y.; Xiong, S.J.; Li, G.; Guo, C.Q.; Zhou, J.; Ma, J.W.; Yin, J.L.; Liu, Y.Q.; Zhu, Y.X. Antifungal Activity of Ginger Rhizome Extract against Fusarium solani. Horticulturae 2022, 8, 983. [Google Scholar] [CrossRef]
- Jing, C.; Gou, J.; Han, X.; Wu, Q.; Zhang, C. In vitro and in vivo activities of eugenol against tobacco black shank caused by Phytophthora nicotianae. Pestic. Biochem. Physiol. 2017, 142, 148–154. [Google Scholar] [CrossRef]
- Wei, J.; Bi, Y.; Xue, H.; Wang, Y.; Zong, Y.; Prusky, D. Antifungal activity of cinnamaldehyde against Fusarium sambucinum involves inhibition of ergosterol biosynthesis. J. Appl. Microbiol. 2020, 129, 256–265. [Google Scholar] [CrossRef]
- Niu, A.; Wu, H.; Ma, F.; Tan, S.; Wang, G.; Qiu, W. The antifungal activity of cinnamaldehyde in vapor phase against Aspergillus niger isolated from spoiled paddy. LWT 2022, 159, 113181. [Google Scholar] [CrossRef]
- Sun, Q.; Li, J.; Sun, Y.; Chen, Q.; Zhang, L.; Le, T. The antifungal effects of cinnamaldehyde against Aspergillus niger and its application in bread preservation. Food Chem. 2020, 317, 126405. [Google Scholar] [CrossRef]
- Perina, F.J.; de Andrade, C.C.L.; Moreira, S.I.; Nery, E.M.; Ogoshi, C.; Alves, E. Cinnamomun zeylanicum oil and trans-cinnamaldehyde against Alternaria brown spot in tangerine: Direct effects and induced resistance. Phytoparasitica 2019, 47, 575–589. [Google Scholar] [CrossRef]
- Yousef, N.; Niloufar, M.; Elena, P. Antipathogenic effects of emulsion and nanoemulsion of cinnamon essential oil against Rhizopus rot and grey mold on strawberry fruits. Foods Raw Mater. 2019, 7, 210–216. [Google Scholar] [CrossRef]
- Gow, N.A.; Latge, J.P.; Munro, C.A. The fungal cell wall: Structure, biosynthesis, and function. Microbiol. Spectr. 2017, 5, 1–25. [Google Scholar] [CrossRef]
- Ishii, H. Target sites of tubulin-binding fungicides. In Target Sites of Fungicide Action; CRC Press: Boca Raton, FL, USA, 2018; pp. 43–52. [Google Scholar]




| Chemical | Regression Equation | Correlation Coefficient | EC50 (g/L) | MIC (g/L) |
|---|---|---|---|---|
| Ginger essential oil | y = 25.929x + 9.395 | 0.876 | 1.102 | 2 |
| Linalool | y = 26.483x + 10.254 | 0.832 | 1.183 | 2 |
| Eugenol | y = 18.26x + 47.879 | 0.373 | 0.235 | 0.5 |
| Citral | y = 12.178x + 18.943 | 0.899 | 0.141 | 0.5 |
| Cinnamaldehyde | y = 0.002x + 0.016 | 0.971 | 0.242 | 0.25 |
| Spore Germination Rate (%) | |||
|---|---|---|---|
| Chemical | Essential Oil Concentrations (g/L) | ||
| CK | 1/2 MIC | MIC | |
| Ginger essential oil | 89.27 ± 1.85 a | 68.45 ± 1.51 a | 58.88 ± 1.70 a |
| Linalool | 92.67 ± 1.58 a | 17.89 ± 1.76 b | 5.99 ± 0.21 b |
| Eugenol | 88.57 ± 1.81 a | 7.40 ± 0.69 d | 3.86 ± 0.12 b,c |
| Citral | 88.65 ± 1.48 a | 12.40 ± 1.87 c | 4.47 ± 0.10 b,c |
| Cinnamaldehyde | 91.85 ± 1.35 a | 13.50 ± 1.05 b,c | 3.48 ± 0.19 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, L.-R.; Hu, H.-J.; Wang, J.; Zhu, Y.-X.; Zhu, X.-D.; Ma, J.-W.; Liu, Y.-Q. Cinnamaldehyde Acts as a Fungistat by Disrupting the Integrity of Fusarium oxysporum Fox-1 Cell Membranes. Horticulturae 2024, 10, 48. https://doi.org/10.3390/horticulturae10010048
Zhou L-R, Hu H-J, Wang J, Zhu Y-X, Zhu X-D, Ma J-W, Liu Y-Q. Cinnamaldehyde Acts as a Fungistat by Disrupting the Integrity of Fusarium oxysporum Fox-1 Cell Membranes. Horticulturae. 2024; 10(1):48. https://doi.org/10.3390/horticulturae10010048
Chicago/Turabian StyleZhou, Li-Rong, Hai-Jun Hu, Jie Wang, Yong-Xing Zhu, Xue-Dong Zhu, Jia-Wei Ma, and Yi-Qing Liu. 2024. "Cinnamaldehyde Acts as a Fungistat by Disrupting the Integrity of Fusarium oxysporum Fox-1 Cell Membranes" Horticulturae 10, no. 1: 48. https://doi.org/10.3390/horticulturae10010048






