Melatonin Promotes Accumulation of Resveratrol and Its Derivatives through Upregulation of PAL, 4CL, C4H, and STS in Grape Seeds
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Experimental Treatments
2.2. Determination of Total Soluble Solids (TSS), Titratable Acid, and Total Anthocyanins
2.3. Extraction and Determination of Total Proanthocyanidins, Flavonoids, and Phenols
2.4. Melatonin Extraction and Determination
2.5. Widely Targeted Metabolomics Analysis
2.6. RNA-Seq and Quantitative RT–PCR (qRT-PCR)
2.7. Resveratrol Extraction and Content Determination
2.8. Statistical Analysis
3. Results
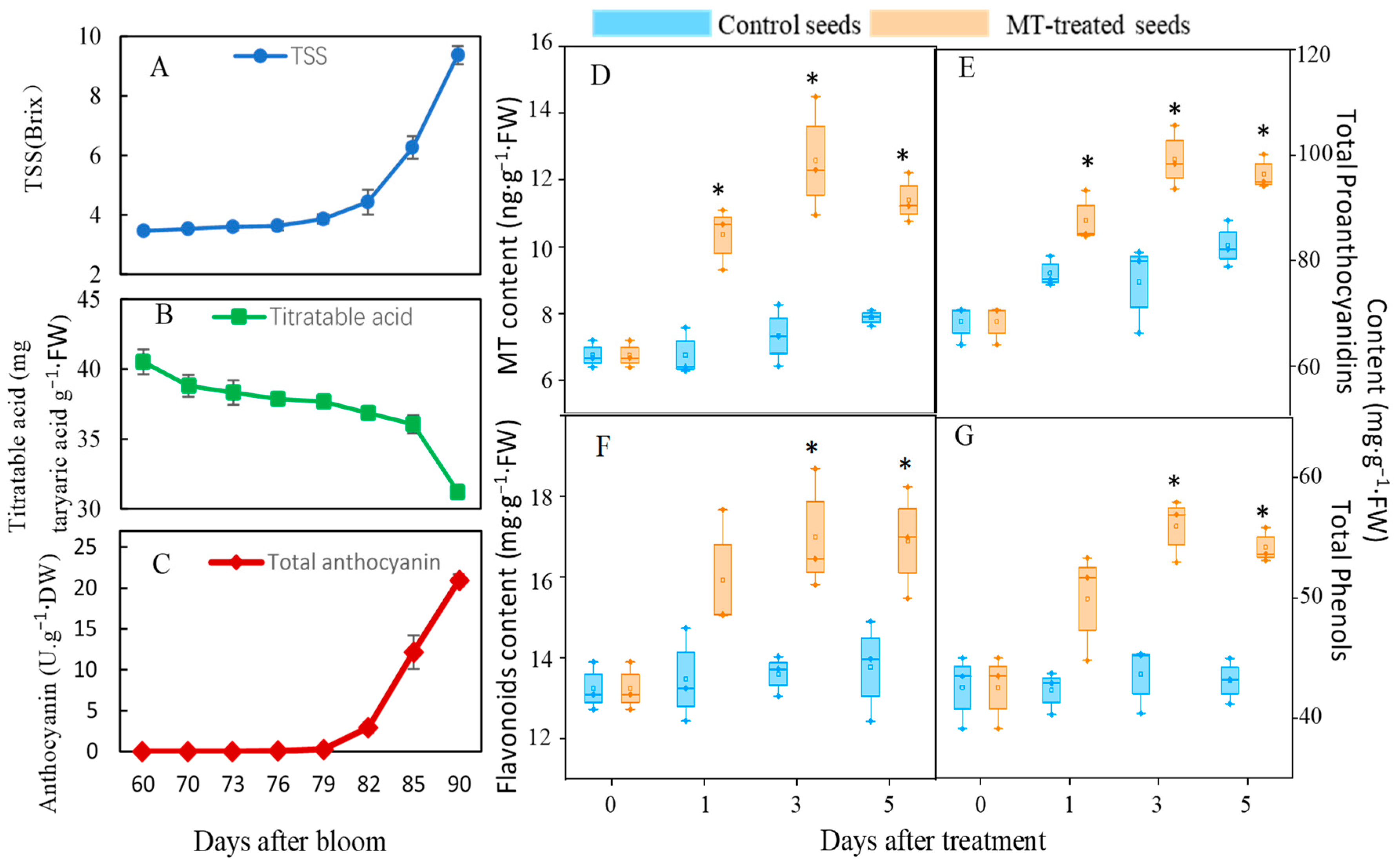
3.1. Melatonin Treatment of Preveraison Grape Berries Promotes Phenolic Accumulation in Seeds
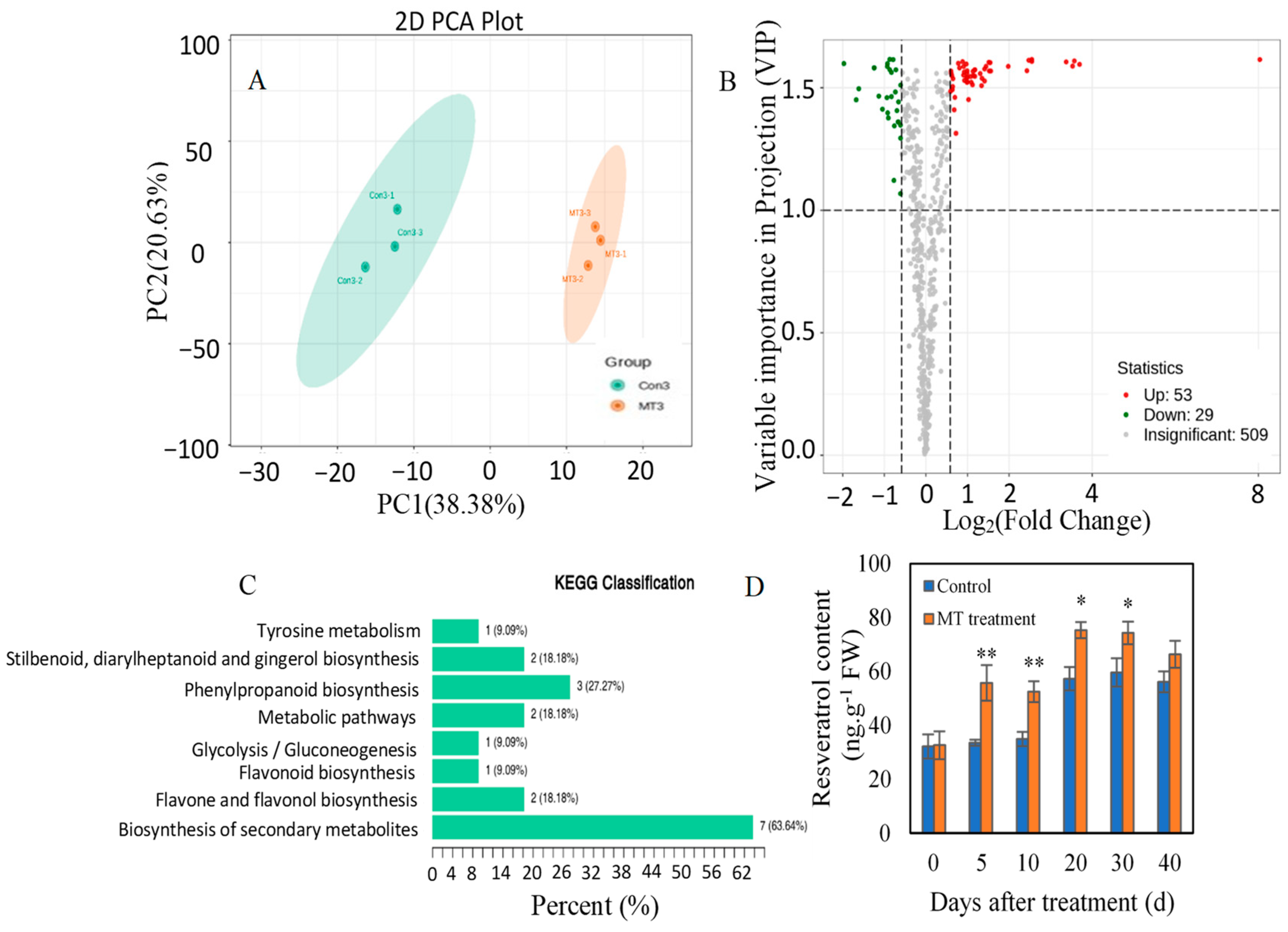
3.2. Identification of Differentially Accumulated Metabolites (DAMs) in Response to Melatonin
3.3. The Changes in the Transcriptome Profile of Grape Seeds in Response to Melatonin
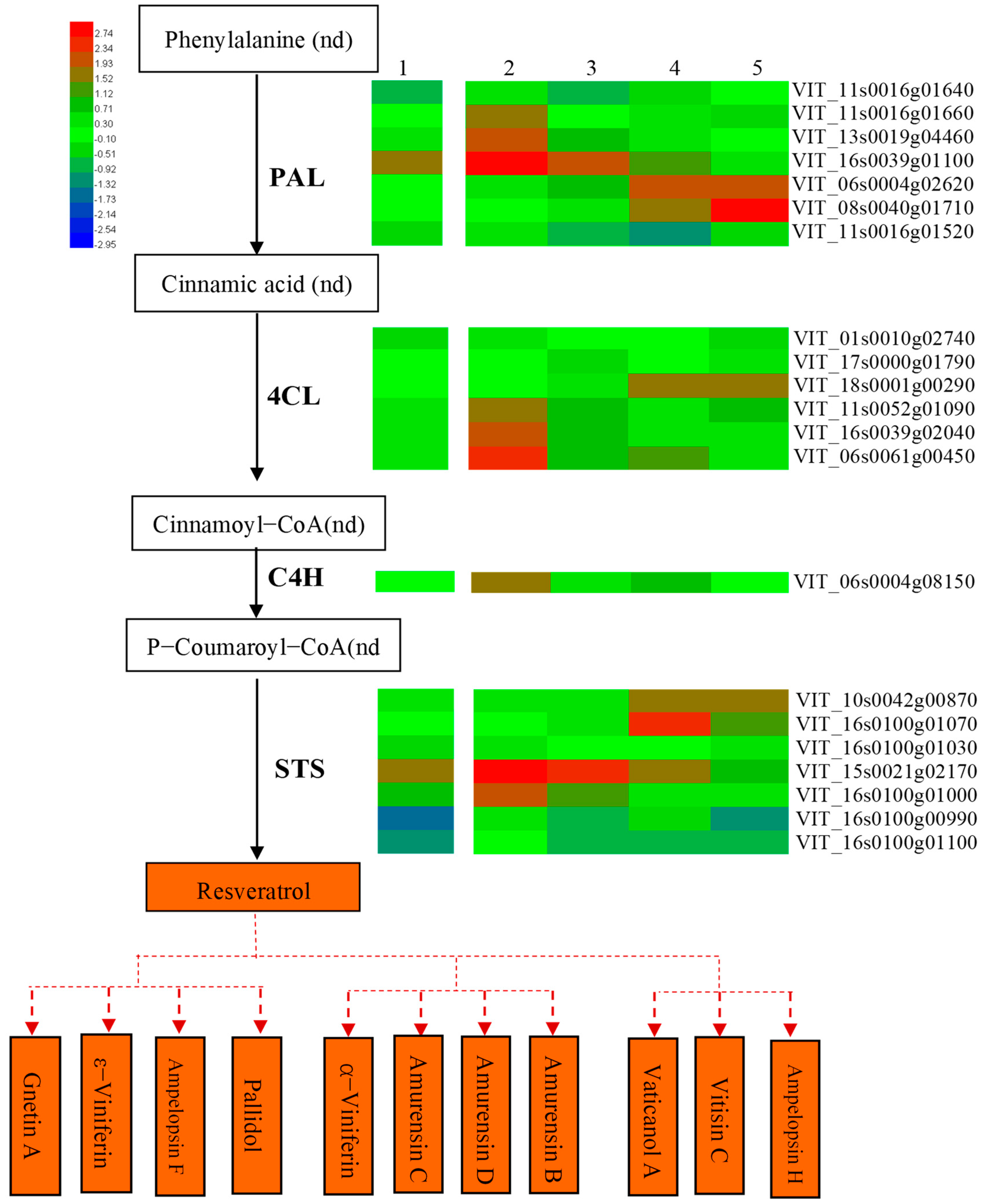
3.4. Association Analysis of DAMs and DEGs Related to Resveratrol Metabolism
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Guerrero, R.F.; García-Parrilla, M.C.; Puertas, B.; Cantos-Villar, E. Wine, Resveratrol and Health: A Review. Nat. Prod. Commun. 2009, 4, 635–658. [Google Scholar] [CrossRef] [PubMed]
- Weiskirchen, S.; Weiskirchen, R. Resveratrol: How Much Wine Do You Have to Drink to Stay Healthy? Adv. Nutr. 2016, 7, 706–718. [Google Scholar] [CrossRef] [PubMed]
- Iveta, P.; Ingus, P.; Pawe, G. Identification and determination of stilbenes by Q-TOF in grape skins, seeds, juice and stems. J. Food Compos. Anal. 2018, 74, 44–52. [Google Scholar] [CrossRef]
- Estrela, J.M.; Ortega, A.; Mena, S.; Rodriguez, M.L.; Asensi, M. Pterostilbene: Biomedical applications. Crit. Rev. Clin. Lab. Sci. 2013, 50, 65–78. [Google Scholar] [CrossRef] [PubMed]
- Kalantari, H.; Das, D.K. Physiological effects of resveratrol. Biofactors 2010, 36, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Liu, C.; Xiang, W.; Chen, H.; Qin, X.; Huang, X. Advances in study of oxyresveratrol. Int. J. Pharmacol. 2014, 10, 44–54. [Google Scholar] [CrossRef]
- Pangeni, R.; Sahni, J.K.; Ali, J.; Sharma, S.; Baboota, S. Resveratrol: Review on therapeutic potential and recent advances in drug delivery. Expert Opin. Drug Deliv. 2014, 11, 1285–1298. [Google Scholar] [CrossRef]
- Takaoka, M. Resveratrol, a new phenolic compound, from Veratrum grandiflorum. Nippon Kagaku Kaishi 1939, 60, 1090–1100. [Google Scholar] [CrossRef]
- Nonomura, S.; Kanagawa, H.; Makimoto, A. Chemical constituents of polygonaceous plants. I. Studies on the components of ko-j o-kon. (Polygonum cuspidatum Sieb. et Zucc.). J. Pharm. Soc. Jpn. 1963, 83, 988–990. [Google Scholar] [CrossRef]
- Langcake, P.; Pryce, R.J. The production of resveratrol by Vitis vinifera and other members of the Vitaceae as a response to infection or injury. Physiol. Plant Pathol. 1976, 9, 77–86. [Google Scholar] [CrossRef]
- Li, X.; Wu, B.; Wang, L.; Li, S. Extractable amounts of trans-resveratrol in seed and berry skin in Vitis evaluated at the germplasm level. J. Agric. Food Chem. 2006, 54, 8804–8811. [Google Scholar] [CrossRef]
- Vannozzi, A.; Dry, I.B.; Fasoli, M.; Zenoni, S.; Lucchin, M. Genome-wide analysis of the grapevine stilbene synthase multigenic family: Genomic organization and expression profiles upon biotic and abiotic stresses. BMC Plant Biol. 2012, 12, 130. [Google Scholar] [CrossRef] [PubMed]
- Holl, J.; Vannozzi, A.; Czemmel, S.; D”Onofrio, C.; Walker, A.R.; Rausch, T.; Lucchin, M.; Boss, P.K.; Dry, I.B.; Bogs, J. The R2R3-MYB transcription factors MYB14 and MYB15 regulate stilbene biosynthesis in Vitis vinifera. Plant Cell 2013, 25, 4135–4149. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Hou, Y.; Wang, L.; Xin, H.; Wang, N.; Li, S. Myb14, a direct activator of STS, is associated with resveratrol content variation in berry skin in two grape cultivars. Plant Cell Rep. 2014, 33, 1629–1640. [Google Scholar] [CrossRef] [PubMed]
- Darren; Chern; Jan; Wong; Rudolf; Schlechter; Alessandro; Vannozzi; Janine; Höll. A systems-oriented analysis of the grapevine R2R3-MYB transcription factor family uncovers new insights into the regulationof stilbene accumulation. DNA Res. 2016, 23, 454–466. [Google Scholar] [CrossRef]
- Galano, A.; Tan, D.X.; Reiter, R.J. Melatonin as a natural ally against oxidative stress: A physicochemical examination. J. Pineal Res. 2011, 51, 1–16. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin: A New Plant Hormone and/or a Plant Master Regulator? Trends Plant Sci. 2018, 24, 38–48. [Google Scholar] [CrossRef]
- Qianqian, S.; Na, Z.; Jinfang, W.; Haijun, Z.; Dianbo, L.; Jin, S.; Ren, L.; Sarah, W.; Bing, Z.; Shuxin, R. Melatonin promotes ripening and improves quality of tomato fruit during postharvest life. J. Exp. Bot. 2015, 66, 657–668. [Google Scholar] [CrossRef]
- Lili, X.; Qianyu, Y.; Feng’e, B.; Hong, S.; Heng, Z.; Yuxin, Y. Melatonin Enhances Phenolics Accumulation Partially via Ethylene Signaling and Resulted in High Antioxidant Capacity in Grape Berries. Front. Plant Sci. 2017, 8, 1426. [Google Scholar] [CrossRef]
- Hu, W.; Yang, H.; Tie, W.; Yan, Y.; Ding, Z.; Liu, Y.; Wu, C.; Wang, J.; Reiter, R.J.; Tan, D.X. Natural Variation in Banana Varieties Highlights the Role of Melatonin in Postharvest Ripening and Quality. J. Agric. Food Chem. 2017, 65, 9987–9994. [Google Scholar] [CrossRef]
- Zeng, W.; Mostafa, S.; Lu, Z.; Jin, B. Melatonin-mediated abiotic stress tolerance in plants. Front. Plant Sci. 2022, 13, 847175. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Xu, L.; Gao, S.; Lyu, X.; Yao, Y. Melatonin alters the secondary metabolite profile of grape berry skin by promoting VvMYB14-mediated ethylene biosynthesis. Hortic. Res. 2021, 8, 43. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Ma, W.; Guan, X.; Wang, F.; Fan, Z.; Gao, S.; Yao, Y. VvMYB14 participates in melatonin-induced proanthocyanidin biosynthesis by upregulating expression of VvMYBPA1 and VvMYBPA2 in grape seeds. Hortic. Res. 2023, 10, 214–226. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Ma, W.; Lyu, X.; Cao, X.; Yao, Y. Melatonin may increase disease resistance and flavonoid biosynthesis through effects on DNA methylation and gene expression in grape berries. BMC Plant Biol. 2020, 20, 231. [Google Scholar] [CrossRef] [PubMed]
- Dewanto, S.; Wu, X.; Adom, K.; Liu, H. Thermal processing enhances the nutritional values of tomatoes by increasing the total antioxidant activity. J. Agric. Food Chem. 2002, 50, 3010–3014. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Ricardo-da-Silva, M.; Spranger, I. Critical factors of vanillin assay for catechins and proanthocyanidins. J. Agric. Food Chem. 1998, 46, 4267–4274. [Google Scholar] [CrossRef]
- Guo, H.; Guo, H.; Zhang, L.; Tang, Z.; Yu, X.; Wu, J.; Zeng, F. Metabolome and Transcriptome Association Analysis Reveals Dynamic Regulation of Purine Metabolism and Flavonoid Synthesis in Transdifferentiation during Somatic Embryogenesis in Cotton. Int. J. Mol. Sci. 2019, 20, 2070. [Google Scholar] [CrossRef]
- Jiang, J.; Xi, H.; Dai, Z.; Lecourieux, F.; Yuan, L.; Liu, X.; Patra, B.; Wei, Y.; Li, S.; Wang, L. VvWRKY8 represses stilbene synthase genes through direct interaction with VvMYB14 to control resveratrol biosynthesis in grapevine. J. Exp. Bot. 2019, 70, 715–729. [Google Scholar] [CrossRef]
- Xu, L.; Yue, Q.; Xiang, G.; Bian, F.e.; Yao, Y. Melatonin promotes ripening of grape berry via increasing the levels of ABA, H2O2, and particularly ethylene. Hortic. Res. 2018, 5, 41. [Google Scholar] [CrossRef]
- Li, C.; Chen, L.; Fan, Q.; He, P.; Wang, C.; Huang, H.; Huang, R.; Tang, J.; Tadda, S.A.; Qiu, D. Weighted Gene Co-Expression Network Analysis to Explore Hub Genes of Resveratrol Biosynthesis in Exocarp and Mesocarp of ‘Summer Black’ Grape. Plants 2023, 12, 578. [Google Scholar] [CrossRef]
- Huang, J.; Gu, M.; Lai, Z.; Fan, B.; Shi, K.; Zhou, Y.H.; Yu, J.Q.; Chen, Z. Functional analysis of the Arabidopsis PAL gene family in plant growth, development, and response to environmental stress. Plant Physiol. 2010, 153, 1526–1538. [Google Scholar] [CrossRef] [PubMed]
- Fraser, C.M.; Chapple, C. The Phenylpropanoid Pathway in Arabidopsis. Arab. Book 2011, 9, PMC3268504. [Google Scholar] [CrossRef] [PubMed]
- Salas-Navarrete, C.; Hernández-Chávez, G.; Flores, N.; Martínezet, L.M.; Martinez, A. Increasing pinosylvin production in Escherichia coli by reducing the expression level of the gene fabI -encoded enoyl-acyl carrier protein reductase. Electron. J. Biotechnol. 2018, 33, 11–16. [Google Scholar] [CrossRef]
- Wang, D.; Jiang, C.Y.; Liu, W.D.; Wang, Y.J. The WRKY53 transcription factor enhances stilbene synthesis and disease resistance by interacting with MYB14 and MYB15 in Chinese wild grape. J. Exp. Bot. 2020, 71, 3211–3226. [Google Scholar] [CrossRef]
- Keylor, M.H.; Matsuura, B.S.; Stephenson, C.R. Chemistry and biology of resveratrol-derived natural products. Chem. Rev. 2015, 115, 8976–9027. [Google Scholar] [CrossRef]

| Compounds | Con3 | MT3 |
|---|---|---|
| pinosylvin | 9.00 ± 0.00 | 2356.28 ± 505.41 * |
| α-Viniferin | 188,847.90 ± 54,182.28 | 2,449,201.31 ± 426,335.38 ** |
| Vaticanol A | 5830.19 ± 1146.64 | 69,281.18 ± 5266.76 ** |
| Amurensin C | 188,718.79 ± 53,271.80 | 2,179,123.95 ± 616,648.37 * |
| Amurensin D | 190,930.54 ± 27,811.23 | 1,980,980.38 ± 408,186.50 * |
| Ampelopsin H | 50,520.08 ± 5251.23 | 295,471.18 ± 37,293.55 ** |
| Vitisin C | 42,901.96 ± 1562.33 | 250,819.85 ± 6649.66 ** |
| Neohopeaphenol A | 5899.16 ± 293.83 | 32,810.76 ± 3416.07 ** |
| Amurensin B | 109,458.38 ± 22,593.39 | 589,960.05 ± 182,509.44 * |
| epsilon-Viniferin | 9817.16 ± 1935.81 | 28,930.87 ± 2644.88 ** |
| Ampelopsin F | 32,124.87 ± 2397.95 | 93,821.43 ± 9448.45 ** |
| Gnetin A | 28,488.77 ± 1737.31 | 81,414.05 ± 7458.81 ** |
| Pallidol | 26,675.34 ± 6608.20 | 70,994.99 ± 12,082.73 * |
| ε-Viniferin | 8985.18 ± 2752.81 | 22,726.13 ± 1918.08 * |
| Resveratrol | 92,312.43 ± 2404.19 | 200,857.58 ± 46,631.23 * |
| Leachianol B | 29,326.08 ± 3670.84 | 59,727.30 ± 16,682.69 * |
| gnetupendin A | 670,566.02 ± 10,249.34 | 388,370.45 ± 4479.28 ** |
| 2,3,5,4′-Tetrahydroxystilbene-2-O-glucoside | 812,403.24 ± 14,592.55 | 457,287.42 ± 58,580.91 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, S.; Wei, D.; Pan, D.; Wang, F.; Kang, H.; Yao, Y. Melatonin Promotes Accumulation of Resveratrol and Its Derivatives through Upregulation of PAL, 4CL, C4H, and STS in Grape Seeds. Horticulturae 2024, 10, 65. https://doi.org/10.3390/horticulturae10010065
Gao S, Wei D, Pan D, Wang F, Kang H, Yao Y. Melatonin Promotes Accumulation of Resveratrol and Its Derivatives through Upregulation of PAL, 4CL, C4H, and STS in Grape Seeds. Horticulturae. 2024; 10(1):65. https://doi.org/10.3390/horticulturae10010065
Chicago/Turabian StyleGao, Shiwei, Dezheng Wei, Dandan Pan, Fei Wang, Hui Kang, and Yuxin Yao. 2024. "Melatonin Promotes Accumulation of Resveratrol and Its Derivatives through Upregulation of PAL, 4CL, C4H, and STS in Grape Seeds" Horticulturae 10, no. 1: 65. https://doi.org/10.3390/horticulturae10010065
APA StyleGao, S., Wei, D., Pan, D., Wang, F., Kang, H., & Yao, Y. (2024). Melatonin Promotes Accumulation of Resveratrol and Its Derivatives through Upregulation of PAL, 4CL, C4H, and STS in Grape Seeds. Horticulturae, 10(1), 65. https://doi.org/10.3390/horticulturae10010065






