Abstract
In recent years, researchers have turned their attention to the co-cultivation of microalgae and plants as a means to enhance the growth of hydroponically cultivated plants while concurrently producing microalgal biomass. However, the techniques used require precise calibration based on plant growth responses and their interactions with the environment and cultivation conditions. This study initially focused on examining the impact of hydroponic nutrient concentrations on the growth of the microalga Chlorella sp. AARL G049. The findings revealed that hydroponic nutrient solutions with electrical conductivities (EC) of 450 µS/cm and 900 µS/cm elicited a positive response in microalgae growth, resulting in high-quality biomass characterized by an elevated lipid content and favorable properties for renewable biodiesel. The biomass also exhibited high levels of polyunsaturated fatty acids (PUFAs), indicating excellent nutritional indices. The microalgae culture and microalgae-free culture, along with inoculation-free lettuce (Lactuca sativa L. var. longifolia) and lettuce that was inoculated with plant growth actinobacteria, specifically the actinomycete Streptomyces thermocarboxydus S3, were subsequently integrated into a hydroponic deep-water culture system. The results indicated that several growth parameters of lettuce cultivated in treatments incorporating microalgae experienced a reduction of approximately 50% compared to treatments without microalgae, and lowering EC levels in the nutrient solution from 900 µS/cm to 450 µS/cm resulted in a similar approximately 50% reduction in lettuce growth. Nevertheless, the adverse impacts of microalgae and nutrient stress were alleviated through the inoculation with actinomycetes. Even though the co-cultivation system leads to reduced lettuce growth, the system enables the production of high-value microalgal biomass with exceptional biodiesel fuel properties, including superior oxidative stability (>13 h), a commendable cetane number (>62), and a high heating value (>40 MJ/kg). This biomass, with its potential as a renewable biodiesel feedstock, has the capacity to augment the overall profitability of the process. Hence, the co-cultivation of microalgae and actinomycete-inoculated lettuce appears to be a viable approach not only for hydroponic lettuce cultivation but also for the generation of microalgal biomass with potential applications in renewable energy.
1. Introduction
Recently, global population expansion, coupled with increasing urbanization and evolving climatic conditions, has intensified the demands on conventional agricultural practices for ensuring both food security and sustainable energy resources [1]. Conventional agricultural systems face formidable challenges, including limited arable land, diminishing water resources, and greenhouse gas emissions [2,3]. Hydroponic systems are now acknowledged as a highly efficient approach to crop production, demonstrating 20–25% higher yields than soil-based systems, with productivity between two and five times higher [2]. Additionally, hydroponics requires relatively less space compared to traditional soil-based farming practices because the water in which the plants are situated is already filled with nutrients. This eliminates the need for the plant roots to spread out extensively to obtain these nutrients [3]. However, the extent of the reduction in farming area will be influenced by factors such as the specific crops grown and the local context of agricultural practices. Despite the soilless cultivation systems yielding premium-quality toxin-free fruits and vegetables, necessitating fewer insecticides and pesticides, their widespread adoption at the field scale is hindered by the need for substantial capital investment and high operational costs [4]. Additionally, the effluent discharged from traditional hydroponic units contains significant levels of nitrates and phosphorous, posing the risk of eutrophication upon release into aquatic ecosystems [5]. In response to these critical challenges, the innovative integration of microalgae cultivation with traditional agriculture, exemplified by the co-cultivation of microalgae and crops in hydroponic systems [6,7], emerges as a promising solution to effectively address the multifaceted issues within the food, agriculture, and energy nexus.
Microalgae, specifically eukaryotic, unicellular algae, exhibit the capacity to assimilate nutrients from diverse sources, including wastewater, atmospheric carbon dioxide (CO2), and industrial flue gas, leveraging sunlight through the photosynthesis process to generate biomass [8]. This algal biomass is notably rich in lipids primarily employed in biodiesel production, proteins utilized as dietary supplements, and carbohydrates serving as feedstock for bioethanol and biohydrogen production [9]. In addition to these applications, microalgae are seamlessly integrated into modern agriculture due to their potential to produce biostimulants. These include endogenous phytohormones, volatile compounds, acetoin, and 2,3-butanediol, thereby promoting plant growth, fortifying plant resistance to abiotic stress, and facilitating efficient nutrient uptake [6]. Particularly noteworthy is the widespread commercialization and development of Chlorella spp., a highly versatile microalgae species, in various biotechnological applications [6,7]. Microalgae are frequently cultivated in hydroponic systems, especially those involving nutrition. The presence of microalgae can influence water quality parameters including dissolved oxygen and pH levels. Moreover, instances may arise where microalgae compete with primary plants for nutrient uptake [10]. Nonetheless, microalgae, in certain cases, contribute to nutrient buffering, alleviating the impact of sanitizing chemicals such as bleach treatments. Therefore, it is imperative to regulate and maintain low levels of microalgae in hydroponic systems [3]. Another notable advantage of microalgae lies in their capacity to produce oxygen through photosynthesis in the root zone of plants, preventing anaerobic conditions and mitigating sulfide damage in plants susceptible to sulfate reduction. Furthermore, the respiration and exudation from plant roots serve as carbon inputs that enhance the biomass of the microalgae [11].
Several investigations have been conducted to enhance plant growth through the symbiotic association achieved by co-cultivating microalgae with plants. In a study by Zhang et al. [12], a research group explored potential hydroponic systems for cultivating various tomato varieties in conjunction with Chlorella infusionum. The results of their investigation revealed heightened microalgal biomass productivity and improved plant productivity in the hydroponic system utilizing C. infusionum alongside tomato plants, surpassing the outcomes observed in monoculture scenarios. Additionally, simultaneous cultivation of Chlorella vulgaris in a hydroponic medium with tomato plants demonstrated synergistic and positive effects on autotroph growth, as indicated by studies conducted by Supraja et al. [6] and Barone et al. [11]. Further research by Huo et al. [3] and Uyar and Mismil [7] documented increased total nitrogen and phosphorus removal, along with enhanced algal and leafy vegetable yields, in the co-cultivation mode involving microalga C. vulgaris and vegetables (mint, purple kohlrabi, and lettuce). The increase in the yield of leafy vegetables in co-cultivation was up to 50% compared to cultivation without microalgae. While microalgae play a beneficial role in supporting plant growth within co-cultivation systems, the simultaneous existence of microalgae and plants within a unified system often results in the convergence of specific operational conditions for both autotrophs. Subsequently, this coexistence triggers undesired competition, resulting in a reduction in the efficiency of these systems in terms of both yield and nutrient removal [6,10]. This circumstance commonly creates a limited margin for the success of co-cultivation systems, implying that techniques require precise calibration based on plant growth responses and their interactions with the environment and cultivation conditions.
Despite the widespread acknowledgment of the positive impacts of microalgae on plants highlighted by various researchers [3,6,7,10,11,12], there remains a paucity of scientific evidence supporting their effects on plants in hydroponic systems. This lack of substantiation is particularly conspicuous when juxtaposed with other microbial plant biostimulants, warranting a thorough examination of their relative efficiency. Beyond the application of microalgae in hydroponic systems, the inoculation of plants with plant growth-promoting bacteria (PGPB) through microbial inoculation techniques has exhibited the capacity to enhance plant growth in hydroponic cultivation [13,14]. In a recent investigation, Kitwetch et al. [5] observed improved lettuce growth in a hydroponic system following inoculation with the plant probiotic actinomycete Streptomyces thermocarboxydus S3. This strategy involves introducing root-colonizing bacteria into plants, which are renowned for their ability to produce plant growth phytohormones such as indole-3-acetic acid, auxin, cytokinin, gibberellin, and abscisic acid. Actinomycetes, a subset of bacteria recognized for promoting plant growth, have garnered increasing recognition as effective plant biostimulants, contributing to the enhancement of crop yields through environmentally sustainable agricultural practices [15]. Actinomycetes have demonstrated their capacity to augment the growth of diverse plants under both standard and challenging conditions, employing various mechanisms, including the synthesis of phytohormones and siderophores (iron-chelating molecules), along with phosphate solubilization [16]. The application of beneficial microorganisms presents a promising approach to the cultivation of nutritious and high-quality food, reducing reliance on excessive fertilizers, and enhancing the productivity of arugula in hydroponic farming. However, research on the co-cultivation of microalgae and actinomycete-inoculated plants in hydroponic systems is currently lacking. Regrettably, there is an absence of studies that have investigated the feasibility of utilizing microalgal biomass from co-cultivation systems for the production of biodiesel with desired fuel properties, with a specific focus on alterations in the fatty acid composition of microalgal lipids which significantly influence biodiesel properties. Furthermore, the utilization of microalgal biodiesel aligns with the implementation of recent fuel usage regulations, contributing to the overarching goal of reducing carbon emissions [9].
Therefore, this study aimed to systematically investigate the interplay between microalgae and lettuce growth within a hydroponic deep-water culture system, with consideration given to the influence of actinomycete inoculation. This research involved a comprehensive evaluation of microalgae growth, focusing on biomass production and lipid accumulation, and an in-depth analysis of the diverse growth parameters associated with lettuce. Additionally, the investigation delved into the prospective application of microalgal biomass as a biodiesel feedstock, involving the detailed scrutiny of fatty acid compositions, the estimation of nutritional indices, and a thorough assessment of biodiesel fuel properties. To the best of the author’s knowledge, this is the first study involving an investigation of the co-cultivation of microalgae and actinomycete-inoculated plants within hydroponic systems. These inquiries are crucial in unveiling the symbiotic capabilities of microalgae and plants, contributing to the concurrent development of a sustainable agricultural economy and rendering the economically viable field-scale integration of algal cultivation with renewable energy production.
2. Materials and Methods
2.1. Microalgae
The microalga Chlorella sp. AARL G049 was sourced from the Algal and Cyanobacterial Research Laboratory, Department of Biology, Faculty of Science, Chiang Mai University, Thailand. The initial cultivation of the microalga involved using 1 L of Jaworski’s medium (JM) with the following composition: 0.02 g/L Ca(NO3)2·4H2O, 0.0124 g/L KH2PO4, 0.05 g/L MgSO4·7H2O, 0.0159 g/L NaHCO3, 0.00225 g/L EDTAFeNa, 0.00225 g/L EDTANa2, 0.00248 g/L H3BO3, 0.00139 g/L MnCl2·4H2O, 0.001 g/L (NH4)6Mo7O24·4H2O, 0.004 g/L cyanocobalamin, 0.04 mg/L thiamine HCl, 0.04 mg/L biotin, 0.08 g/L NaNO3, and 0.036 g/L Na2HPO4·12H2O. The initial pH of the medium was adjusted to 7.0 [17]. The microalgae were cultivated initially in a 400 mL total volume of JM medium, which was aerated using air for a duration of seven days. During this cultivation phase, the microalgae were exposed to a white light-emitting diode (LED) with an intensity of 2400 lux, following a light-to-dark cycle consisting of 24 h of light followed by 0 h of darkness. A controlled temperature of 30 ± 2 °C was maintained throughout the cultivation period. Afterward, the microalgal cells were harvested through centrifugation at 6000 rpm for 15 min. The collected cells were subsequently re-suspended in 40 mL of fresh medium, serving as the initial culture for further experimental procedures.
2.2. Lettuce
Lettuce seeds (Lactuca sativa L. var. longifolia) were obtained from the Vegetable Seed Production and Organic Farming Learning Center at Maejo University in Thailand. To ensure sterility, the lettuce seeds underwent a sterilization process: they were first immersed in 70% v/v ethanol for 1 min, followed by a 12 min soak in a 1.2% v/v NaClO solution. Subsequently, the seeds were rinsed three times for 1 min each using sterilized deionized water. After surface sterilization, the lettuce seeds were inoculated with a spore suspension of the actinomycete Streptomyces thermocarboxydus S3 at a concentration of 108 spores/mL. This actinomycete was isolated from spores of Funneliformis mosseae, which was originally isolated from the soil of an Aquilaria crassna plantation in Rayong Province, Thailand [16]. The inoculated seeds were placed on a shaker at room temperature (120 rpm) for 3 h before being sown in a growth tray for subsequent experimental procedures. Uninoculated lettuce seeds were included as a control experiment.
2.3. Hydroponic Nutrient Solution
A commercially available hydroponic liquid fertilizer was procured from Kitsuwan Farm in Nonthaburi, Thailand. The fertilizer consisted of two separate solutions: Solution A, comprising 115 g/L of Ca(NO3)2, 2 g/L of 7% Fe-DTPA, and 4 g/L of 13.2% Fe-EDTA and Solution B, composed of 60 g/L of KNO3, 50 g/L of MgSO4, 26.5 g/L of KH2PO4, 5 g/L of Dissolvine® ABC EDTA (Nouryon B.V., Amsterdam, The Netherlands), and 1 g/L of 13% Mn-EDTA. The blending of these solutions followed the manufacturer’s instructions. The resulting nutrient solution, referred to as AB, was prepared at full strength, exhibiting an electrical conductivity (EC) of 1800 µS/cm.
2.4. Cultivation of Microalgae in Hydroponic Nutrient Solution
The growth performance of Chlorella sp. AARL G049 was investigated in various hydroponic nutrient solutions with electrical conductivities (ECs) ranging from 225 to 1800 µS/cm. The aim was to compare its growth in these solutions with its growth in the traditional JM medium (EC of 320 µS/cm). The microalgae, initially at an optical density (OD665) of 0.16 ± 0.01 (2.5 × 106 cells/mL), were cultured in a total volume of 200 mL of medium. The microalgae were subjected to continuous white light at an intensity of 2400 lux, following a light-to-dark cycle where light was provided for 24 h followed by a dark phase of 0 h. The culture was agitated at 130 rpm for a duration of 12 days.
2.5. Co-Cultivation of Microalgae and Actinomycete-Inoculated Lettuce in a Hydroponic Deep-Water Culture System
The lettuce seeds, whether inoculated with the actinomycete or left uninoculated, were meticulously planted in a growth tray containing a mixture of perlite and vermiculite in a 3:1 ratio. These trays were then placed in a controlled laboratory environment with specific conditions. The experimental setup included a 16 h photoperiod to ensure ample light exposure, with photosynthetically active radiation reaching an intensity of 2400 lux. The laboratory temperature was consistently maintained at 30 °C. To support robust growth, the lettuce plants received regular daily watering using distilled water. After seven days of seedling growth, when the plants had reached an approximate length of 5 cm and had developed three to four true leaves, they were delicately transplanted into a laboratory hydroponic deep water culture system. The transplants were subjected to two conditions: one with the addition of microalgal cells (OD665 of 0.2) and the other without. The plants were then cultivated in the controlled laboratory setting, maintaining the previously mentioned conditions for a period of 32 days. The experiments were designed with optimized EC values and included various treatments:
Treatment I: Actinomycete-inoculated lettuce seeds + microalgae cells
Treatment II: Actinomycete-inoculated lettuce seeds
Treatment III: Uninoculated lettuce seeds + microalgae cells
Treatment IV: Uninoculated lettuce seeds
Every four days, the ECs of all treatments were regularly checked and modified to maintain the specified value for each treatment throughout the experiment. This adjustment was accomplished by adding the required amount of stock solution. Additionally, the pH level was assessed at the same four-day intervals.
2.6. Analytical Methods
2.6.1. Determination of Microalgal Growth
The evaluation of microalgae growth was contingent upon the parameter termed “biomass concentration” (g/L). To determine the biomass concentration based on dry weight, we employed a gravimetric methodology utilizing 10 mL aliquots. Microalgal biomass was filtrated using pre-weighed glass fiber filters of grade GF/C and then dried at a controlled temperature of 60 °C until achieving a state of consistent weight. Subsequently, the specific growth rate (µ, 1/day) was calculated using the following equation (Equation (1)):
where Xt and X0 are the biomass concentration (g/L) at a given sampling time (tt) and on the preceding sampling (t0) days.
µ = [Ln (Xt/X0)]/(tt − t0)
2.6.2. Determination of Lettuce Growth
Multiple parameters were evaluated, including the leaf count, shoot and root length, and the fresh and dry weights of both the shoot and root. The drying process involved placing the samples in an oven at 60 °C until a consistent weight was attained.
2.6.3. Determination of Microalgal Lipid Content
The method for lipid extraction from microalgal biomass was conducted following the procedure outlined by Pekkoh et al. [9] with some minor adjustments. Specifically, 100 mg of dried microalgae biomass was subjected to extraction using 10 mL of CH2Cl2/MeOH (2:1 v/v) at room temperature, followed by sonication for 30 min utilizing a 40 kHz sonicator. This extraction process was repeated twice for thoroughness. Once the lipids were successfully extracted, they were then centrifuged to produce a clear supernatant, and the solvent was subsequently removed by applying a stream of nitrogen gas. Following the removal of the solvent, the lipids were allowed to air-dry completely before being weighed. The determination of lipid content was carried out through a calculation that considered the ratio of lipids to dried microalgal biomass.
2.6.4. FAME Characterizations
The transformation of the obtained lipid into biodiesel, specifically converting it into fatty acid methyl esters (FAMEs), was achieved through an acid-catalyzed transesterification process. To initiate this transesterification reaction, an exact quantity of the extracted lipid sample (10 mg) was mixed with toluene (0.5 mL), methanol (1.5 mL), and concentrated hydrochloric acid (HCl) (50 μL, 35%). The mixture underwent thorough blending and was subsequently subjected to controlled heating at a temperature of 98 °C for a duration of 1 h and 30 min. After the reaction was completed, the mixture was allowed to cool, and an aliquot of hexane (1 mL) was introduced. Through vigorous agitation, the mixture was homogenized, resulting in the separation of distinct layers. Specifically, the upper layer, referred to as the FAME layer, containing the fatty acid methyl esters (FAMEs), was carefully isolated and collected for further detailed analysis.
To examine the composition of the FAMEs, we utilized a 7890B Gas Chromatograph equipped with a cross-linked capillary HP-5 column (30 m in length, 0.32 mm in inner diameter, and a 0.25 μm film thickness) along with a flame ionization detector (Agilent Technologies, Santa Clara, CA, USA). The gas chromatograph was operated under precise conditions, with an inlet temperature set at 230 °C. Initially, the oven temperature was established at 45 °C for a period of 2 min. Subsequently, it underwent a gradual increase to 100 °C, maintained for 4 min, followed by an increase to 200 °C, maintained for 8 min, a further increase to 250 °C, maintained for 6 min, and a detector temperature held at 230 °C. By comparing the retention times of the fatty acids to a reference set of 33 pure FAME standards (Figure S1), we were able to identify the specific fatty acids present in the sample using OpenLab CDS Software version C.01.09 (Agilent Technologies, Santa Clara, CA, USA). The peak area of each sample was treated as a triangle and quantified by multiplying the peak height (h) from the baseline to the half-width (w1/2) at the central point, using the formula A = h × w1/2. To determine the proportion of individual FAMEs present in the biodiesel samples, the following equation (Equation (2)) was employed:
FAMEs of individual fatty acids = (area of a peak/total area of all the peaks) × 100
2.6.5. Estimation of Biodiesel Properties
Fatty acid profiling was conducted to examine the essential characteristics of microalgal biodiesel. Biodiesel properties were estimated using the model equations outlined by Pekkoh et al. [18]. This comprehensive analysis involved determining various fuel properties, including the saponification value (SV, mg KOH/g), iodine value (IV, g I2/100 g), cetane number (CN), degree of unsaturation (DU, %wt), long-chain saturated factor (LCSF, %wt), cold filter plugging point (CFPP, °C), pour point (PP, °C), high heating value (HHV, MJ/kg), cloud point (CP, °C), kinematic viscosity (υ, ln KV at 40 °C in mm2/s), density (ρ, g/cm3), oxidative stability (OS; h), allylic position equivalents (APE), and bis-allylic position equivalents (BAPE).
2.7. Statistical Analysis
The experiments were conducted three times independently. To determine significant distinctions among the treatments, one-way analysis of variance (ANOVA) coupled with Duncan’s multiple range test (DMRT) was employed. In cases where the p-value was below 0.05, denoting statistical significance, the least significant difference was employed to differentiate the means. This analysis was executed using SPSS Statistics 17.0.
3. Results and Discussion
3.1. Growing Microalgae in a Hydroponic Fertilizer-Based Culture Medium
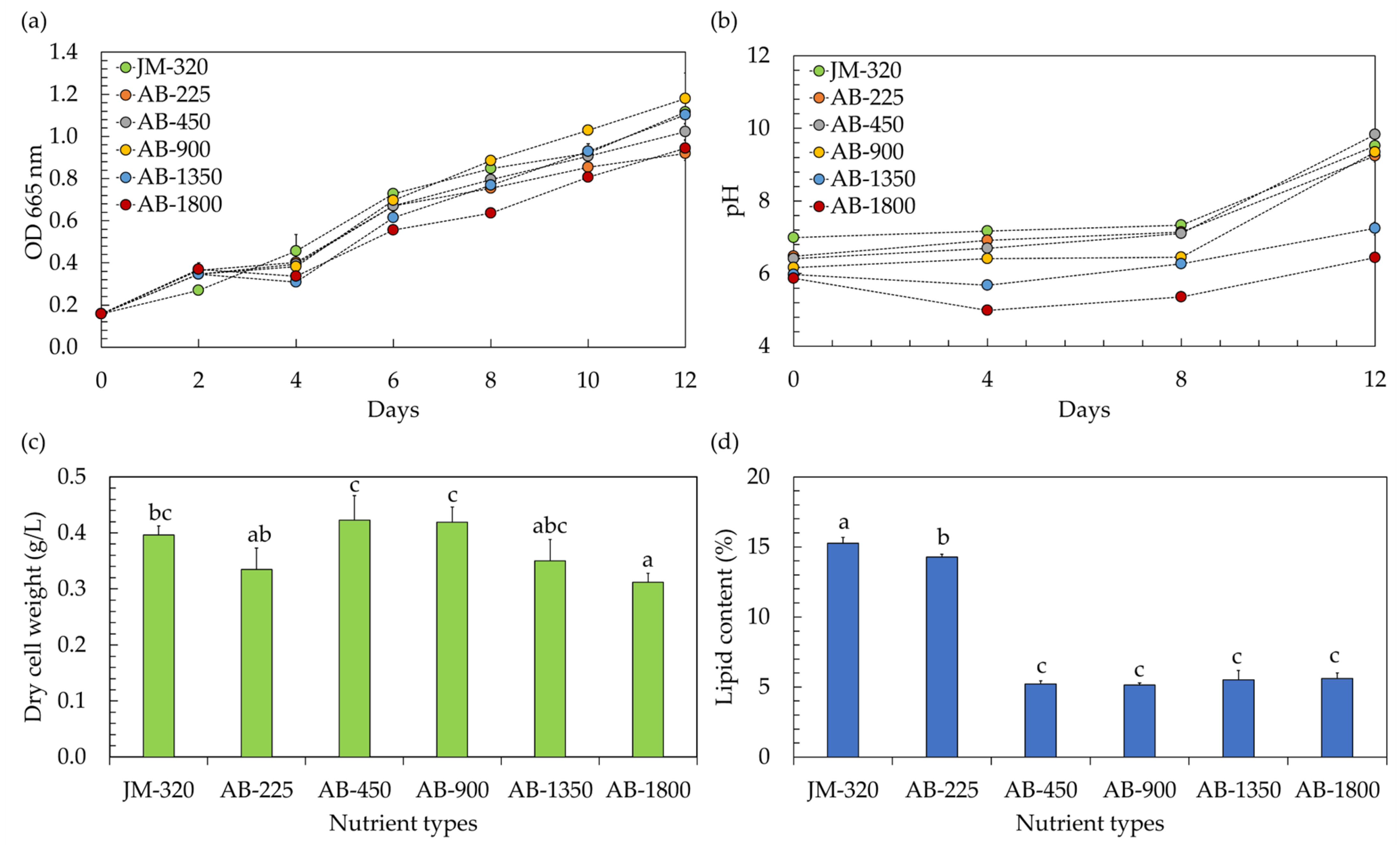
In the context of utilizing microalgae within a hydroponic system, the precise nutrient concentration becomes a matter of paramount importance. Traditionally, when cultivating vegetables hydroponically, a hydroponic nutrient solution is employed with an electrical conductivity (EC) typically surpassing 450 µS/cm, while the exact EC level varies based on the specific type of vegetables being grown [5]. However, any variations in nutrient levels, whether they are insufficient or excessively elevated, have the potential to inhibit the growth of microalgae [19]. In this study, the effects of hydroponic nutrient solutions at different EC levels on the growth of microalga Chlorella sp. AARL G049 were therefore investigated. The study included a comparison of EC levels ranging from 225 to 1800 µS/cm (EC-225, EC-450, EC-900, EC-1800). These varied EC conditions corresponded to nitrate (NO3–N) levels within the range of 4.3–43.4 mg/L. The findings, illustrated in Figure 1, were contrasted with those obtained using the conventional JM medium, characterized by an EC of 320 µS/cm and a NO3–N level of 5.2 mg/L (JM-320). Referring to the growth curve, as depicted in Figure 1a, it is evident that the microalga Chlorella sp. AARL G049 exhibited robust growth over a 12 day cultivation period in a hydroponic nutrient solution-based culture medium. As the cultivation progressed, it was also observed that the culture pH increased (Figure 1b). Elevated pH levels in the medium signify the proliferation of microalgae. This growth is attributed to the cells’ absorption of carbon dioxide (CO2) and bicarbonate through the membrane, resulting in the release of hydroxide ions (OH−) into the culture medium [9]. Additionally, the uptake of nitrates by the microalgae contributes to the increase in pH [9,19]. The specific growth rates (µ) ranged from 0.112 to 0.138 day−1. Notably, the µ values at EC-450 (0.138 day−1) and EC-900 (0.137 day−1) were comparable to those of JM-320 (0.133 day−1), while the other ECs exhibited lower µ values in the range of 0.112–0.122 day−1, implying that hydroponic nutrient solutions with EC-450 and EC-900 levels have demonstrated their suitability for cultivating Chlorella sp. AARL G049 in a hydroponic system. Towards the end of the cultivation period, it was observed that cultivating Chlorella sp. AARL G049 with hydroponic nutrient solutions at EC-450 and EC-900 levels resulted in significantly higher biomass concentrations, measured in terms of dry cell weight, ranging from 0.419 to 0.423 g/L (Figure 1c). These values were slightly higher than those achieved with JM-320 (0.395 g/L) and other ECs (0.312–0.350 g/L). The lower biomass production of Chlorella sp. AARL G049 under EC-1350 and EC-1800 conditions may result from substrate inhibition at elevated EC levels. Specifically, nitrogen exerts a pivotal influence on microalgae growth. An elevation in inorganic nitrogen levels results in enhanced microalgae growth and biomass production. However, if the nitrogen concentration becomes excessive, it can induce substrate inhibition, negatively impacting microalgae growth and metabolic activity [19]. The hydroponic nutrient solution having an EC-225 value might not offer the ideal combination of necessary nutrients essential for the growth of Chlorella sp. AARL G049, resulting in a decrease in biomass production.
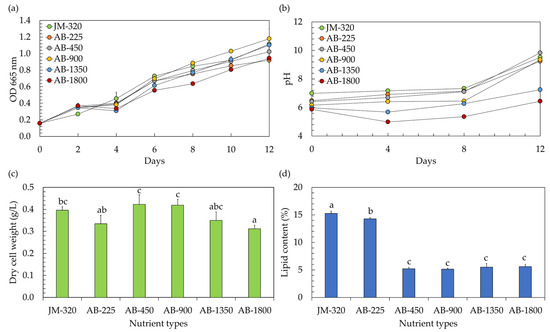
Figure 1.
Optical density at 665 nm (a) and culture pH (b) during cultivation, along with biomass production (c) and lipid yield (d) after cultivation of microalgae in a hydroponic fertilizer-based culture medium with varying electrical conductivity (EC) levels of 225–1800 µS/cm (EC-225, EC-450, EC-900, EC-1350, and EC-1800). The traditional JM medium, serving as a control, exhibited a detected EC level of 320 µS/cm (JM-320). Different lowercase letters (a, b, or c) indicate significant differences among the treatments (p < 0.05).
The EC of the growth medium can influence the availability and mobility of essential nutrients for microalgal growth [19]. Changes in EC may affect the solubility and accessibility of nutrients such as nitrogen and phosphorus, which are crucial for lipid synthesis [19,20]. Considering the lipid accumulation of Chlorella sp. AARL G049 (Figure 1d), the results revealed a noticeable variation in lipid content depending on the EC level of the nutrient solution. At an EC of 225 µS/cm, Chlorella sp. AARL G049 exhibited the highest lipid content at 14.28%. As the EC level increased, the lipid content progressively decreased, with values of 5.21%, 5.15%, 5.52%, and 5.61% recorded for EC levels of 450, 900, 1350, and 1800 µS/cm, respectively. In comparison, the lipid content obtained with the JM-320 was slightly higher at 15.26%. These findings suggest that the use of the hydroponic nutrient solution at an EC of 225 µS/cm resulted in a higher lipid content compared to the other EC levels tested and was only slightly lower than the lipid content obtained with the traditional JM medium. The observed decrease in lipid content with increasing EC levels may be attributed to various factors, including changes in nutrient availability, osmotic stress, and alterations in metabolic processes [19,20]. Nutrient availability plays a crucial role in influencing the growth and proliferation of microalgae, with far-reaching consequences for their lipid and fatty acid (FA) composition [21]. When microalgae face a deficiency in nitrogen, they reduce the production of numerous proteins engaged in various cellular functions. This reduction in protein synthesis during nitrogen limitation serves to conserve energy and enables the microalgae to allocate more resources toward the synthesis of lipids [22]. This suggests that a lower nitrogen concentration at lower EC levels may result in increased lipid contents in microalgae. However, it is important to note that there exists a tradeoff between biomass and lipid content, as illustrated in Figure 1c,d.
Within the context of biofuel production, the generation of feedstock for biodiesel purposes must not solely prioritize high lipid contents but should also ensure an appropriate distribution of fatty acids. This is because the relative composition of various fatty acids significantly influences the overall attributes of the fuel. Furthermore, earlier studies have indicated that biofuel production benefits from the incorporation of microbial lipids enriched with elevated levels of C16–C18 fatty acids [9]. Hence, the assessment of biodiesel quality relies heavily on the examination of fatty acid profiles within microalgal biomass. Considering that medium nutrient supplementation has been widely recognized as a significant influencer of the fatty acid profiles of microalgae [23], an investigation was conducted into the fatty acid profiling of Chlorella lipids (Figures S2–S7). The findings are presented as percentages of fatty acid methyl esters (FAMEs) and can be found in Table 1. The lipid of Chlorella sp. AARL G049 contains a range of fatty acid chain lengths from C6 to C24. The most prevalent fatty acids are those with 16 and 18 carbon atoms, constituting 98.44% for JM-320, 98.43% for EC-225, 94.70% for EC-450, 94.14% for EC-900, 89.98% for EC-1350, and 88.42% for EC-1800. This suggests that an increase in EC may restrict the synthesis of C16–18 fatty acids, possibly because high nitrogen levels might lead to increased protein synthesis or alter the carbon-to-nitrogen ratio, diverting metabolic pathways away from fatty acid synthesis [24]. However, biodiesel feedstocks with a combined C16–C18 content exceeding 90% are typically considered suitable for biodiesel production [19].

Table 1.
Fatty acid profiling of lipid-derived biodiesel from microalga Chlorella sp. AARL G049 cultured in traditional JM medium (JM-320) and hydroponic nutrient solution with varied electrical conductivity (EC) levels (225–1800 µS/cm).
In the FA composition of Chlorella sp. AARL G049, an increase in EC levels was found to elevate the concentrations of pentadecanoic acid (C15:0), palmitoleic acid (C16:1), stearic acid (C18:0), and linoleic acid (C18:2n6c) while limiting the accumulation of palmitic acid (C16:0), oleic acid (C18:1n9c), and elaidic acid (C18:1n9t). Moreover, a noteworthy finding was that Chlorella sp. AARL G049 exhibited minimal quantities of C18:3 fatty acid (<0.10%) (Table 1). This indicates the appropriateness of the acquired lipids for biodiesel production. Typically, the existence of substantial concentrations of C18:3 (>12%) in the microalgae fatty acid composition has been associated with compromised oxidative stability in biodiesel. This is due to the unsaturated bond in C18:3 being positioned closer to the terminal methyl group, rendering it more susceptible to oxidation [18]. Changes in the fatty acid (FA) composition of microalgal lipids can be ascribed to variations in the transcription of genes linked to fatty acid synthesis. These alterations are often responsive to fluctuating EC levels, particularly in the context of nutrient availability [21].
Moreover, a decrease in EC was associated with higher proportions of saturated fatty acids (SFAs) and monounsaturated fatty acids (MUFAs) compared to polyunsaturated fatty acids (PUFAs) (Table 1). Biodiesel compositions characterized by elevated ratios of SFAs and MUFAs contribute to improved energy production, oxidation stability, and combustion characteristics. In contrast, a substantial presence of unsaturated bonds in fatty acids leads to reduced oxidation stability, energy yield, and combustion properties, despite promoting favorable cold-flow qualities [25]. The variations in the proportions of SFAs, MUFAs, and PUFAs among the treatments reflect their distinctive attributes. These apparently conflicting outcomes may arise from gene regulation involved in fatty acid synthesis. There might be a correlation between the expression of four genes related to fatty acid biosynthesis—specifically the β-ketoacyl ACP synthase I (KAS I) gene, stearoyl-ACP desaturase (SAD) gene, omega-6 fatty acid desaturase (ω-6 FAD) gene, and omega-3 fatty acid desaturase (ω-3 FAD) gene—and changes in the fatty acid composition [26].
More notably, lipids from Chlorella sp. AARL G049 cultured in nutrient hydroponic fertilizer exhibited PUFA contents ranging from 8.66% to 29.28%, whereas JM-320 yielded only 6.07% PUFAs (Table 1). The overall quantities of omega-3 PUFAs, reaching a maximum of 0.3%, were less than the total levels of omega-6 PUFAs, which could go up to 29.28%. Generally, fatty acids abundant in PUFAs, especially omega-3 PUFAs, confer health benefits by reducing the risk of cardiovascular disease, atherosclerosis, cancer, and inflammatory conditions. Omega-6 PUFAs also offer benefits to humans and mammals when consumed in very small amounts, as they act as precursors for omega-3 PUFAs [27]. For promoting a healthy lifestyle, lipids with PUFA to SFA ratios ≥ 0.4 are considered favorable for potential applications in pharmaceuticals, cosmetics, and dietary supplements [28]. The ratio of PUFAs/SFAs serves as a widely employed indicator for appraising the nutritional quality of dietary products. It is a standard measure utilized to evaluate the influence of diet on cardiovascular health (CVH), where elevated PUFA/SFA levels are associated with a more favorable impact [29]. In this study, the PUFA/SFA ratio derived from Chlorella sp. AARL G049 cultivated in nutrient hydroponic fertilizer surpassed that of JM-320 (0.15). More specifically, the ratios were 0.21 for EC-225, 0.61 for EC-450, 0.70 for EC-900, 0.41 for EC-1350, and 0.40 for EC-1800 (Table 1), highlighting their nutritional quality. However, in addition to assessing lipid production, it is crucial to further broaden the analysis to include various essential hydrophobic components, such as isoprenoids. This category includes compounds such as carotenoids, phytol, squalene, and others, all of which necessitate a further comprehensive investigation for a thorough understanding of the biochemical context of nutritional qualities.
Based on the above findings, it should be concluded that a hydroponic nutrient solution with EC levels of 450 µS/cm and 900 µS/cm could be considered suitable for cultivating the microalga Chlorella sp. AARL G049 in a hydroponic system, owing to the high observed biomass production. Furthermore, the results of fatty acid profiling at these EC levels suggest that lipids are not only appropriate as biodiesel feedstock but also show promise for incorporation into dietary supplements, highlighting their elevated nutritional qualities.
3.2. Growing Microalgae and Actinomycete-Inoculated Lettuce in a Hydroponic Deep-Water Culture System
In this study, a hydroponic solution with electrical conductivity (EC) levels of 450 µS/cm and 900 µS/cm was utilized in a hydroponic deep-water culture system for the co-cultivation of microalgae and lettuce. The study compared the growth of lettuce seeds that were inoculated and uninoculated with the actinomycete Streptomyces thermocarboxydus S3 under conditions both with and without the addition of microalgae in the co-cultivation system (Figure 2). The decision to incorporate S. thermocarboxydus S3 in the inoculation of lettuce seeds was based on earlier findings. A previous study revealed the effectiveness of this actinomycete in mitigating the adverse effects of low nutrients and drought stress on rice crops. Additionally, mung beans (Vigna radiata) subjected to inoculation with this strain exhibited notable enhancements in parameters such as fresh weight, root length, and total length of the plants [16]. The growth parameters, including the shoot and root fresh weight, shoot and root dry weight, leaf number, and shoot and root length, were measured following 39 days of lettuce cultivation, and the results are presented in Table 2 and Figure 3a. Results revealed that the shoot and root fresh weight, shoot and root dry weight, leaf number, and shoot and root length of lettuce cultivated at an EC level of 900 µS/cm were greater than those observed at an EC level of 450 µS/cm. In microalgae-containing treatments, there was a decrease of approximately 50% in the shoot and root fresh weight (3.49–4.87 g for EC-450 and 9.38–9.39 g for EC-900), shoot and root dry weight (0.37–0.42 g for EC-450 and 0.66–0.72 g for EC-900), and leaf number (9.33 leaves for EC-450 and 12 leaves for EC-900) in uninoculated lettuce compared to treatments without microalgae (shoot and root fresh weight of 8.95–9.41 g for EC-450 and 14.92–17.80 g for EC-900, shoot and root dry weight of 0.89–1.06 g for EC-450 and 1.45–1.70 g for EC-900, and leaf number of 11.33–12.00 leaves for EC-450 and 13.50–14.50 leaves for EC-900) (Table 2). This difference could be attributed to nutrient availability in the co-cultivation system. Even though nutrient concentrations, measured in terms of EC values, were consistently maintained every 4 days, the nutrients were shared between microalgae and lettuce in the co-cultivation groups, leading to competition in nutrient uptake between those organisms. In contrast, in the microalgae-free treatments, all nutrients were exclusively allocated for the growth of lettuce, resulting in high growth parameters. Typically, the inclusion of microalgae in co-cultivation has a positive impact on plant growth by facilitating the release of secondary metabolites and other allelochemicals, including phytohormones, volatile compounds, acetoin, and 2,3 butanediol that promote plant development [6]. Nevertheless, the reduced growth rate observed in lettuce in microalgae-containing treatments in this study could be linked to the possibility that the microalgal presence might not be substantial enough to establish a favorable interaction with the lettuce.

Figure 2.
Growth progress of actinomycete-inoculated and uninoculated lettuce over 39 days in a hydroponic deep-water culture system with electrical conductivity (EC) set at 450 µS/cm (EC-450) and 900 µS/cm (EC-900). Microalgae were introduced into the co-cultivation system on day 7. The treatment involving microalgae is denoted as “M”, and actinomycete-inoculated lettuce is denoted by “A”.

Table 2.
Growth parameters of lettuce inoculated and uninoculated with actinomycete Streptomyces thermocarboxydus S3 cultivated in a hydroponic system at electrical conductivity levels of 450 µs/cm (EC-450) and 900 µs/cm (EC-900) with and without microalgae addition.

Figure 3.
Growth assessment of actinomycete-inoculated and uninoculated lettuce (a) and light microscopic examination of lettuce roots (b) on day 39 of cultivation in a hydroponic deep-water culture system with electrical conductivity (EC) set at 450 µS/cm (EC-450) and 900 µS/cm (EC-900). The treatment involving microalgae is denoted as “M”, and actinomycete-inoculated lettuce is denoted by “A”.
Remarkably, the inoculation with the actinomycete improved the shoot and root fresh weight, shoot and root dry weight, and leaf number of lettuce in microalgae-containing treatments (Table 2). This enhancement could be attributed to the ability of the actinomycete S. thermocarboxydus S3 employed in this study to colonize lettuce roots [5] and produce plant growth agents, such as phytohormones (including indole-3-acetic acid and siderophore) and organic acids (including citric, oxalic, gluconic, malic, succinic, acetic, and lactic acids), as documented in prior research [16], potentially contributing to the improved growth of the lettuce. However, the values in microalgae-containing treatments were still lower than those of microalgae-free treatments. The observed effect is linked to the inadequate quantities of plant growth agents produced by the actinomycete in the co-cultivation system. Conversely, the shoot length and root length of lettuce in treatments containing microalgae surpassed those in microalgae-free treatments (Table 2). Furthermore, both microalgae-containing and microalgae-free treatments showed improved shoot length and root length of lettuce due to the inoculation with the actinomycete (Table 2). The rise in shoot length and root length in treatments with microalgae could be linked to the phenomenon of cell swelling, facilitating the swift absorption of nutrients [6]. This suggests that in response to competition for nutrient uptake, lettuce may enhance its ability to capture light by enlarging the size of its light-harvesting complex for photosynthesis [5], leading to the improvement in shoot length. In addition, the increase in root length could be attributed to the colonization of microalgae both inside and on the surface of the roots (Figure 3b). This colonization could contribute to a reduced root respiration rate and lower nutrient uptake. As a result, lettuce may respond by developing longer roots to enhance the absorption and transport of nutrients as well as the root respiration rate [30].
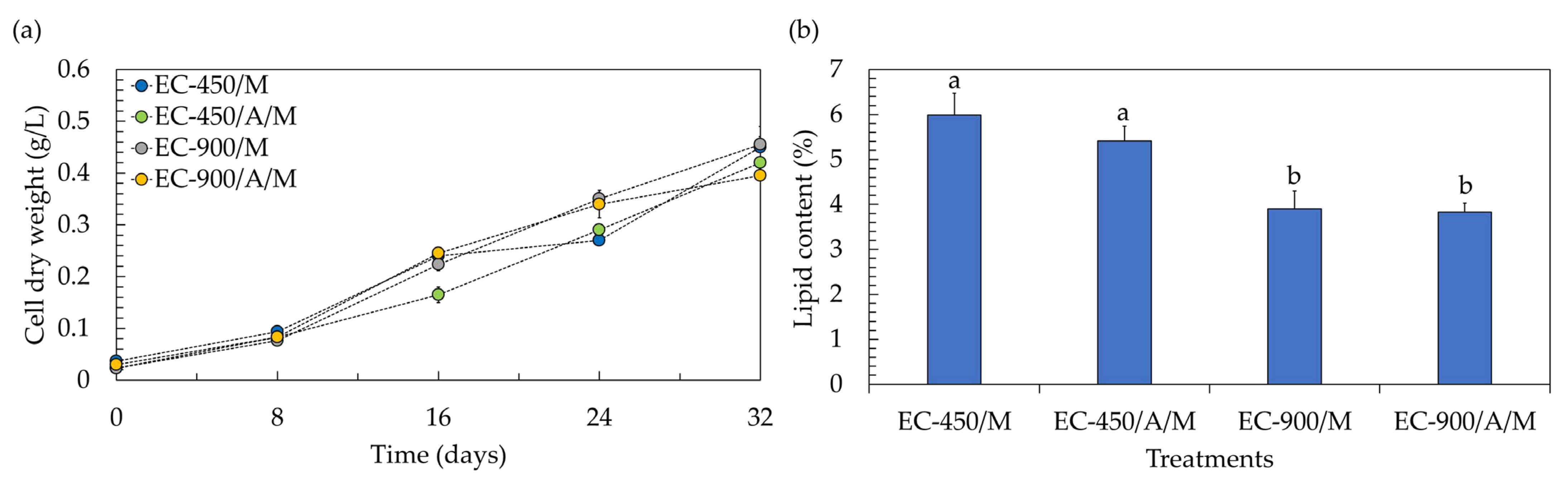
One crucial attribute of lettuce is its fresh weight, given its status as a naturally marketable vegetable. Achieving the highest fresh weight is advantageous, as it allows for the use of only one plant to constitute the bundle (marketable unit). Profitability is negatively impacted when increasing the number of plants to form a marketable unit; for instance, the use of two plants per pack can result in a 50% reduction in profitability [31]. According to the results, the fresh weight of lettuce in treatments containing microalgae decreased by approximately two-fold, being lower than that in microalgae-free treatments. This suggests that the co-cultivation system led to a reduction of about 50% in the marketable profitability of lettuce. However, within a co-cultivation system, both lettuce and microalgal biomass gain market value, potentially enhancing the overall profitability of the process. Examining the biomass production of the microalga Chlorella sp. AARL G049 in the context of co-cultivation reveals that it thrives across all treatments (Figure 4a), showcasing specific growth rates (µ) within the range of 0.050 to 0.054 day−1. The biomass concentrations at the end of co-cultivation were as follows: 0.450 g/L (µ = 0.054 day−1) for treatments with uninoculated seeds, no microalgae, and EC of 450 µS/cm; 0.420 g/L (µ = 0.052 day−1) for treatments with inoculated seeds, microalgae, and EC of 450 µS/cm; 0.455 g/L (µ = 0.054 day−1) for treatments with uninoculated seeds, no microalgae, and EC of 900 µS/cm; and 0.395 g/L (µ = 0.050 day−1) for treatments with inoculated seeds, microalgae, and EC of 900 µS/cm. The diminished concentration of microalgal biomass in treatments with inoculated seeds could be attributed to the adverse impact of the actinomycete during their synergistic interaction. Typically, actinomycetes not only produce phytohormones but also release other compounds, including inhibitory chemicals. These substances may constrain microalgal biomass production and potentially hinder gene expression and/or physiological activities [32], leading to lower biomass concentrations. Furthermore, the reduced biomass concentrations in treatments with the elevated EC level of 900 µS/cm could be attributed to substrate inhibition, likely associated with the need to adjust and maintain the EC value every 4 days during the lettuce growth. While a recommended EC level of 900 µS/cm was mentioned for microalgae growth in Section 3.1, it is important to note that in this experiment, no additional nutrients were introduced during cultivation. Therefore, substrate inhibition may not have occurred under these conditions. To enhance the biomass concentration, it is recommended to explore semi-continuous culturing for microalgae in co-cultivation systems, as this approach could help mitigate substrate inhibition issues.
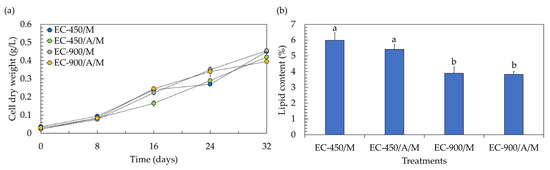
Figure 4.
Biomass production (a) and lipid yield (b) of microalgae after co-cultivation with lettuce in a hydroponic deep-water culture system with electrical conductivity (EC) set at 450 µS/cm (EC-450) and 900 µS/cm (EC-900). The treatment involving microalgae is denoted as “M”, and actinomycete-inoculated lettuce is denoted by “A”. Different lowercase letters (a or b) indicate significant differences among the treatments (p < 0.05).
Considering lipid accumulation, the lipid contents were in the range of 3.83–5.99% (Figure 4b). It was noted that the introduction of the actinomycete resulted in a slight limitation of lipid accumulation in microalgae cells, causing a decrease of approximately 2–10% compared to uninoculated treatments. Additionally, at the elevated EC level of 900 µS/cm, the lipid accumulation was lower than that observed at an EC level of 450 µS/cm. As mentioned earlier, the actinomycete might produce inhibitory compounds such as antibiotics and volatile organic compounds, exerting a negative impact on microalgal lipid production [32]. The findings also suggest that the phytohormones generated by the actinomycete may not be sufficient to alter the carbon metabolism towards microalgal lipid biosynthesis [33]. Furthermore, the nutrient availability resulting from maintaining the EC value every 4 days during lettuce growth might not induce microalgal lipid synthesis. High lipid accumulation in microalgae cells typically occurs under conditions of nutrient limitation, starvation, and/or depletion [34]. Despite the low lipid accumulation in microalgae cells, microalgal lipids in both inoculated and uninoculated treatments at the elevated EC level of 900 µS/cm comprised more than 93% of the C16–C18 fatty acids (Table 3 and Figures S8–S11). This suggests that the lipids produced in these treatments could potentially be utilized as biodiesel feedstock. In contrast, under the EC of 450 µS/cm, microalgae accumulated only 88–89% of the C16–C18 fatty acids (Table 3), which does not meet the standard requirements for biodiesel production [19]. Nevertheless, the limited accumulation of C18:3 fatty acid (<0.16%) in all treatments (Table 3) could enhance the feasibility of utilizing microalgal lipids for biodiesel production [18].

Table 3.
Fatty acid profiling of lipid-derived biodiesel from microalga Chlorella sp. AARL G049 cultured in a hydroponic deep-water culture system with actinomycete-inoculated and uninoculated lettuce under electrical conductivity (EC) levels of 450 and 900 µS/cm.
According to Table 3, it was also found that when the EC level was maintained at 900 µS/cm, there was a decrease in pentadecanoic acid (C15:0), palmitoleic acid (C16:1), elaidic acid (C18:1n9c), and linoleic acid (C18:2n6c), while palmitic acid (C16:0), stearic acid (C18:0), and oleic acid (C18:1n9c) increased. The total quantities of saturated fatty acids (SFAs) also increased with rising EC levels, whereas unsaturated fatty acids (UFAs), including both monounsaturated fatty acids (MUFAs) and polyunsaturated fatty acids (PUFAs), decreased. Moreover, the introduction of the actinomycete has the potential to enhance the accumulation of C18:1n9c and overall MUFAs, indicating that actinomycetes may exert both positive and negative effects on microalgae. A similar increase in the levels of C18:1n9c and MUFAs was observed in the co-cultivation of microalgae with the actinomycete Streptomyces leeuwenhoekii WA12 [32]. However, it is well-known that elevated levels of SFAs are associated with a higher cetane number, reduced NOx emissions, a shorter ignition delay time, and enhanced oxidative stability [15]. The reduced levels of PUFAs in both inoculated and uninoculated treatments at an EC value of 900 µS/cm also indicate their viability as biodiesel feedstock (Table 3). This is because the presence of PUFAs necessitates the use of an oxidative stabilizer for safe application. The vulnerability of PUFAs to autoxidation can lead to biodiesel degradation and potential issues in the fuel system [9]. Although there are higher levels of PUFAs (8.56–8.68%) and a higher PUFA/SFA ratio (0.13–0.14) in both inoculated and uninoculated treatments at an EC value of 450 µS/cm compared to an EC value of 900 µS/cm (PUFAs of 4.07–5.77% and PUFA/SFA ratio of 0.06–0.08) (Table 3), indicating their potential use as nutrient supplements, these PUFA/SFA ratios are still below the minimum requirement for potential applications in pharmaceuticals, cosmetics, and dietary supplements. These results suggest that microalgal lipids from co-cultivation systems might only be good for making biodiesel. Therefore, microalgal lipids obtained from both inoculated and uninoculated treatments at the EC value of 900 µS/cm can serve as suitable biodiesel feedstock. This is attributed to their high concentration of long-chain fatty acids spanning from 16 to 18 carbon atoms and a substantial ratio of SFAs, indicating positive attributes for biodiesel properties.
In the context of biodiesel production, it is essential to investigate how the fatty acid composition affects the fuel characteristics of biodiesel. The fuel attributes of the generated biodiesel samples exposed to both inoculated and uninoculated treatments are outlined in Table 4. These attributes were determined empirically based on the FAMEs composition detailed in Table 3. The results were evaluated in comparison to biodiesel standards, including TH 2020 (utilized in Thailand) [9], EN 14214 (utilized in Europe) [35], and ASTM D6751 (utilized in the United States) [36]. Examination of the biodiesel composition indicated that the introduction of the actinomycete did not negatively affect the fuel quality of the microalgal biodiesel. Nonetheless, there were differences in fuel quality across the tested EC levels. Importantly, the quality of the produced biodiesel adhered to the specified parameters of TH 2020, EN 14214, and ASTM D6751 (Table 4). In this study, model equations were used to estimate indicators defining biodiesel quality and properties, aiming to alleviate the need for laborious physiochemical measurements and to expedite the selection of efficient processes. However, it is worth noting that these models exhibit a maximum error of approximately 13% when compared to experimental data [37,38]. Despite this limitation, a model establishing a connection between the fatty acid profile and the resulting biodiesel proves valuable for quickly assessing the potential viability of a new biodiesel feedstock [39]. Such models are currently widely used for the rapid estimation of microalgal biodiesel [18,19] and plant-derived biodiesel [40].

Table 4.
Estimated fuel properties of lipid-derived biodiesel from microalga Chlorella sp. AARL G049 cultured in a hydroponic deep-water culture system with actinomycete-inoculated and uninoculated lettuce under electrical conductivity (EC) levels of 450 and 900 µS/cm.
According to Table 4, the saponification value (SV) for Chlorella sp. AARL G049 biodiesel ranged from 209.98 to 212.65 mg KOH per gram of oil. This measurement signifies the quantity of KOH required to saponify 1 g of oil and is expressed in milligrams. These SVs were comparable to those observed in biodiesel derived from different microalgae species such as Chlorella spp., Scenedesmus spp., and Chlamydomonas spp., as reported by Sivaramakrishnan and Incharoensakdi [33]. The iodine value (IV) of Chlorella biodiesel was determined to be 29.13–42.91 g I2/100 g oil (Table 4), aligning with the specifications outlined in biodiesel standards (EN 14214 and TH 2020), which prescribe a value below 120 g I2/100 g oil. The IV typically serves as an indicator of the overall unsaturation of biodiesel, offering insights into its oxidative stability. Contrary to expectations, the oxidative stability of the fuel in this instance was notably lower. A diminished IV pointed to a heightened level of oxidative stability [9], underscoring the oxidation-resistant characteristics of Chlorella biodiesel. The cetane number (CN) of biodiesel fell within the range of 62.64–65.51 (Table 4), indicating enhanced ignition quality. This aligns closely with the biodiesel quality standards set in Thailand (>51), Europe (>51), and the United States (>47). Wu et al. [41] suggested that blending traditional diesel with biodiesel possessing a higher cetane number contributes to a more smoothly operating engine with improved cold-starting capabilities. The degree of unsaturation (DU) in biodiesel varied between 46.51% and 54.61% (Table 4), closely resembling the DU (45–70%) observed in microalgae such as Chlorella spp., Scenedesmus spp., and Chlamydomonas spp., as documented by Sivaramakrishnan and Incharoensakdi [33]. As demonstrated in the findings of Serrano et al. [42], biodiesel exhibiting a lower DU value displays greater resistance to oxidation during prolonged storage. The higher heating value (HHV) of biodiesel pertains to the quantity of fuel in a specific amount that produces heat even after complete combustion. The assessed HHV for Chlorella biodiesel ranged from 40.09 to 40.36 MJ/kg (Table 4), a value closely approaching the one documented for petroleum-derived diesel (46 MJ/kg) as reported by Pekkoh et al. [18]. The long-chain saturated factor (LCSF) and the cold filter plugging point (CFPP) values for Chlorella biodiesel fell within the ranges of 13.84–14.57% and 26.93–38.02 °C (Table 4), respectively. These findings suggest that the fuel may not meet quality standards in cold climates but is well-suited for use in tropical regions. The temperature at which the gasoline in a vehicle starts to appear cloudy is known as the cloud point (CP). At this temperature, wax crystals begin to form in the fuel, potentially leading to blockages in the car’s fuel filters and fuel lines. While the international standards for biodiesel lack a specified minimum temperature requirement, Pekkoh et al. [9] concluded that a narrower cloud point (CP) range is preferable. The CP range of 12.36–16.67 °C (Table 4) identified in Chlorella biodiesel enhances its suitability for usage in tropical regions.
As indicated in Table 4, Chlorella biodiesel exhibited the same kinematic viscosity (υ) of 5.10. This aligns with the international standards for biodiesel, with values falling within the range of 1.9–6.0 for ASTM D6751, 3.5–5.0 for EN 14214, and 8 for TH 2020. Viscosity, measuring the resistance of a fluid to flow, plays a crucial role in controlling the spray characteristics of the fuel. Improved viscosity contributes to an overall enhancement in fuel quality, as viscosity and density impact fuel spray qualities. In compression ignition (CI) engines, higher viscosity results in reduced fuel spray, as noted by Sharma et al. [21]. This can lead to incomplete combustion, causing carbon deposition on the injector and valve sheet, posing potential challenges for the vehicle engine. Moreover, density (ρ) influences the spray characteristics of fuel in engines, thereby directly impacting combustion behavior. The research results indicate that the density of Chlorella biodiesel, at 0.85 g/cm3 (Table 4), falls within the standard range for biodiesel (0.85–0.90 g/cm3). Oxidation stability (OS) is a significant challenge contributing to the overall undesirable properties of biodiesel. An increase in the quantity of UFAs correlates with a decrease in OS [9]. The examination revealed that the oxidation stability of Chlorella biodiesel ranged from 13.32 h to 28.57 h (Table 4), surpassing the established standard for biodiesel. This suggests that Chlorella biodiesel exhibits robust oxidation stability. The OS of biodiesel is influenced by both the type and quantity of alkyl esters it contains, which can be assessed through indices such as the allylic position equivalent (APE) and bis-allylic position equivalent (BAPE). The APE values for Chlorella biodiesel ranged from 38.64 to 50.04, while the BAPE values varied from 3.79 to 7.58 (Table 4). In a similar vein, various vegetable oil feedstocks exhibit APE values ranging from 17 to 189 and BAPE values ranging from 4 to 85 (Verma et al., 2016). The quality of biodiesel is often associated with high oxidation stability and a low BAPE level, emphasizing the importance of producing biodiesel from oils with the lowest BAPE concentrations [18].
Considering the mentions of biodiesel in TH 2020, EN 14214, and ASTM D6751, it is reasonable to affirm that the fuel characteristics of Chlorella lipids meet these specifications. Notably, microalgal lipids obtained from both inoculated and uninoculated treatments at an EC value of 900 µS/cm can serve as viable biodiesel feedstock. This is attributed to their elevated concentration of long-chain fatty acids ranging from 16 to 18 carbon atoms and a substantial ratio of saturated fatty acids (SFAs), signifying favorable attributes for biodiesel properties. This indicates that the co-cultivation of microalgae and actinomycete-inoculated lettuce holds promise, not only for hydroponic lettuce cultivation but also for generating microalgal biomass with potential applications in renewable energy. Despite the observed lower yields of lipids, it is suggested that future studies should focus on enhancing lipid production by developing various strategies and methods within a co-cultivated hydroponic system. This may include selecting high-lipid producing microalgae strains, cultivating microalgae using phytohormones, and enhancing microalgae growth coupled with lipid accumulation through the use of chemicals and/or environmental factors. Additionally, co-producing other high-value compounds through a zero-waste biorefinery approach could further contribute to making this approach more practical and effective.
4. Conclusions
The findings presented in this study mark the initial documentation of the co-cultivation of microalgae and lettuce that has been inoculated with the actinomycete Streptomyces thermocarboxydus S3 or left uninoculated in a hydroponic deep-water culture system. It has been demonstrated that the microalga Chlorella sp. AARL G049 can thrive in a co-cultivation system, but it hinders lettuce growth by approximately 50% compared to treatments without microalgae. However, the introduction of actinomycete inoculation proves effective in alleviating the adverse impact of microalgae and nutrient limitations, resulting in the enhancement of lettuce growth. Despite the co-cultivation treatments with microalgae yielding lower lettuce yields, the system facilitates the production of high-value microalgal biomass with superior biodiesel fuel properties. This biomass, with its potential as a valuable biodiesel feedstock, could enhance the overall profitability of the process. Therefore, the co-cultivation of microalgae and actinomycete-inoculated lettuce in hydroponic systems could greatly contribute to the sustainable development of the food–agriculture–energy nexus.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/horticulturae10010070/s1, Figure S1: GC chromatography of pure fatty acid methyl ester (FAME) standards, Figure S2: GC chromatography of lipid-derived biodiesel from microalga Chlorella sp. AARL G049 cultured in traditional JM medium (JM-320), Figure S3: GC chromatography of lipid-derived biodiesel from microalga Chlorella sp. AARL G049 cultured in hydroponic nutrient solution with an electrical conductivity (EC) level at 225 µS/cm, Figure S4: GC chromatography of lipid-derived biodiesel from microalga Chlorella sp. AARL G049 cultured in hydroponic nutrient solution with an electrical conductivity (EC) level at 450 µS/cm, Figure S5: GC chromatography of lipid-derived biodiesel from microalga Chlorella sp. AARL G049 cultured in hydroponic nutrient solution with an electrical conductivity (EC) level at 900 µS/cm, Figure S6: GC chromatography of lipid-derived biodiesel from microalga Chlorella sp. AARL G049 cultured in hydroponic nutrient solution with an electrical conductivity (EC) level at 1350 µS/cm, Figure S7: GC chromatography of lipid-derived biodiesel from microalga Chlorella sp. AARL G049 cultured in hydroponic nutrient solution with an electrical conductivity (EC) level at 1800 µS/cm, Figure S8: GC chromatography of lipid-derived biodiesel from microalga Chlorella sp. AARL G049 cultured in a hydroponic deep-water culture system with actinomycete-inoculated lettuce under EC levels of 450 µS/cm, Figure S9: GC chromatography of lipid-derived biodiesel from microalga Chlorella sp. AARL G049 cultured in a hydroponic deep-water culture system with actinomycete-inoculated lettuce under EC levels of 900 µS/cm, Figure S10: GC chromatography of lipid-derived biodiesel from microalga Chlorella sp. AARL G049 cultured in a hydroponic deep-water culture system with actinomycete-uninoculated lettuce under EC levels of 450 µS/cm, Figure S11: GC chromatography of lipid-derived biodiesel from microalga Chlorella sp. AARL G049 cultured in a hydroponic deep-water culture system with actinomycete-uninoculated lettuce under EC levels of 900 µS/cm.
Author Contributions
Conceptualization, S.S. (Sirasit Srinuanpan); methodology, W.P.-a., S.S. (Sritip Sensupa), J.P. and S.S. (Sirasit Srinuanpan); software, S.S. (Sritip Sensupa) and S.S. (Sirasit Srinuanpan); validation, W.P.-a., J.P. and S.S. (Sirasit Srinuanpan); formal analysis, S.S. (Sritip Sensupa); investigation, S.S. (Sritip Sensupa), A.W., N.S., B.K. and S.S. (Sirasit Srinuanpan); resources, W.P.-a., J.P., Y.C. and S.S. (Sirasit Srinuanpan); data curation, S.S. (Sritip Sensupa) and S.S. (Sirasit Srinuanpan); writing—original draft preparation, S.S. (Sirasit Srinuanpan); writing—review and editing, W.P.-a., S.S. (Sritip Sensupa), A.W., N.S., B.K., J.P., P.S., S.L., Y.C. and S.S. (Sirasit Srinuanpan); visualization, S.S. (Sirasit Srinuanpan); supervision, W.P.-a., J.P., Y.C. and S.S. (Sirasit Srinuanpan); project administration, S.S. (Sritip Sensupa); funding acquisition, W.P.-a. and S.S. (Sirasit Srinuanpan). All authors have read and agreed to the published version of the manuscript.
Funding
This research project was supported by Fundamental Fund 2023, Chiang Mai University under Grant No. FF66/036. The project is also funded by the National Research Council of Thailand (NRCT) and Chiang Mai University under Grant No. N42A660462.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Acknowledgments
The authors would like to thank Kitsuwan Farm, Notaburi, Thailand, for providing their hydroponic nutrient solution.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Hemathilake, D.M.K.S.; Gunathilake, D.M.C.C. Agricultural productivity and food supply to meet increased demands. In Future Foods; Academic Press: Cambridge, MA, USA, 2022; pp. 539–553. [Google Scholar] [CrossRef]
- Gashgari, R.; Alharbi, K.; Mughrbil, K.; Jan, A.; Glolam, A. Comparison between growing plants in hydroponic system and soil based system. In Proceedings of the 4th World Congress on Mechanical, Chemical, and Material Engineering, ICMIE, Madrid, Spain, 16–18 August 2018; pp. 1–7. [Google Scholar] [CrossRef]
- Huo, S.; Liu, J.; Addy, M.; Chen, P.; Necas, D.; Cheng, P.; Li, K.; Chai, H.; Liu, Y.; Ruan, R. The influence of microalgae on vegetable production and nutrient removal in greenhouse hydroponics. J. Clean. Prod. 2019, 243, 118563. [Google Scholar] [CrossRef]
- Ronga, D.; Biazzi, E.; Parati, K.; Carminati, D.; Carminati, E.; Tava, A. Microalgal biostimulants and biofertilisers in crop productions. Agronomy 2019, 9, 192. [Google Scholar] [CrossRef]
- Kitwetch, B.; Rangseekaew, P.; Chromkaew, Y.; Pathom-Aree, W.; Srinuanpan, S. Employing a plant probiotic actinomycete for growth promotion of lettuce (Lactuca sativa L. var. longifolia) cultivated in a hydroponic system under nutrient limitation. Plants 2023, 12, 3793. [Google Scholar] [CrossRef] [PubMed]
- Supraja, K.V.; Behera, B.; Balasubramanian, P. Performance evaluation of hydroponic system for co-cultivation of microalgae and tomato plant. J. Clean. Prod. 2020, 272, 122823. [Google Scholar] [CrossRef]
- Özer Uyar, G.E.; Mısmıl, N. Symbiotic association of microalgae and plants in a deep water culture system. PeerJ 2022, 10, e14536. [Google Scholar] [CrossRef]
- Klinthong, W.; Yang, Y.H.; Huang, C.H.; Tan, C.S. A review: Microalgae and their applications in CO2 capture and renewable energy. Aerosol. Air Qual. Res. 2015, 15, 712–742. [Google Scholar] [CrossRef]
- Pekkoh, J.; Duangjan, K.; Phinyo, K.; Kaewkod, T.; Ruangrit, K.; Thurakit, T.; Pumas, C.; Pathom-aree, W.; Cheirsilp, B.; Gu, W.; et al. Turning waste CO2 into value-added biorefinery co-products using cyanobacterium Leptolyngbya sp. KC45 as a highly efficient living photocatalyst. Chem. Eng. J. 2023, 460, 141765. [Google Scholar] [CrossRef]
- Adi, E.B.M.; Priadi, D.; Deswina, P.; Agustini, N.W.S. September. The growth of pak choy (Brassica rapa L.) on the microalgae (Spirulina platensis) biomass-based nutrient solution. IOP Conf. Ser. Earth Environ. Sci. 2023, 1230, 012205. [Google Scholar] [CrossRef]
- Barone, V.; Puglisi, I.; Fragalà, F.; Piero, A.R.L.; Giuffrida, F.; Baglieri, A. Novel bioprocess for the cultivation of microalgae in hydroponic growing system of tomato plants. J. Appl. Phycol. 2019, 31, 465–470. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, X.; Zhou, Q. Co-cultivation of Chlorella spp. and tomato in a hydroponic system. Biomass Bioenergy 2017, 97, 132–138. [Google Scholar] [CrossRef]
- Ayuso-Calles, M.; García-Estévez, I.; Jiménez-Gómez, A.; Flores-Félix, J.D.; Escribano-Bailón, M.T.; Rivas, R. Rhizobium laguerreae improves productivity and phenolic compound content of lettuce (Lactuca sativa L.) under saline stress conditions. Foods 2020, 9, 1166. [Google Scholar] [CrossRef] [PubMed]
- Sebring, R.L.; Duiker, S.W.; Berghage, R.D.; Regan, J.M.; Lambert, J.D.; Bryant, R.B. Gluconacetobacter diazotrophicus inoculation of two lettuce cultivars affects leaf and root growth under hydroponic conditions. Appl. Sci. 2022, 12, 1585. [Google Scholar] [CrossRef]
- Boukhatem, Z.F.; Merabet, C.; Tsaki, H. Plant growth promoting actinobacteria, the most promising candidates as bioinoculants? Front. Agron. 2022, 4, 849911. [Google Scholar] [CrossRef]
- Lasudee, K.; Tokuyama, S.; Lumyong, S.; Pathom-Aree, W. Actinobacteria associated with arbuscular mycorrhizal Funneliformis mosseae spores, taxonomic characterization and their beneficial traits to plants: Evidence obtained from mung bean (Vigna radiata) and thai jasmine rice (Oryza sativa). Front. Microbiol. 2018, 9, 1247. [Google Scholar] [CrossRef] [PubMed]
- Jaworski, G.H.M.; Talling, J.F.; Heaney, S.I. The influence of carbon dioxide-depletion on growth and sinking rate of two planktonic diatoms in culture. Br. Phycol. J. 1981, 16, 395–410. [Google Scholar] [CrossRef]
- Pekkoh, J.; Lomakool, S.; Chankham, J.; Duangjan, K.; Thurakit, T.; Phinyo, K.; Ruangrit, K.; Tragoolpua, Y.; Pumas, C.; Pathom-aree, W.; et al. Maximizing biomass productivity of cyanobacterium Nostoc sp. through high-throughput bioprocess optimization and application in multiproduct biorefinery towards a holistic zero waste. Biomass Conv. Biorefin. 2022, 1–21. [Google Scholar] [CrossRef]
- Srinuanpan, S.; Cheirsilp, B.; Kitcha, W.; Prasertsan, P. Strategies to improve methane content in biogas by cultivation of oleaginous microalgae and the evaluation of fuel properties of the microalgal lipids. Renew. Energy 2017, 113, 1229–1241. [Google Scholar] [CrossRef]
- Lizzul, A.M.; Hellier, P.; Purton, S.; Baganz, F.; Ladommatos, N.; Campos, L. Combined remediation and lipid production using Chlorella sorokiniana grown on wastewater and exhaust gases. Bioresour. Technol. 2014, 151, 12–18. [Google Scholar] [CrossRef]
- Sharma, K.K.; Schuhmann, H.; Schenk, P.M. High lipid induction in microalgae for biodiesel production. Energies 2012, 5, 1532–1553. [Google Scholar] [CrossRef]
- Singh, R.P.; Yadav, P.; Kumar, I.; Solanki, M.K.; Roychowdhury, R.; Kumar, A.; Gupta, R.K. Advancement of abiotic stresses for microalgal lipid production and its bioprospecting into sustainable biofuels. Sustainability 2023, 15, 13678. [Google Scholar] [CrossRef]
- Fernandes, T.; Fernandes, I.; Andrade, C.A.P.; Cordeiro, N. Changes in fatty acid biosynthesis in marine microalgae as a response to medium nutrient availability. Algal Res. 2016, 18, 314–320. [Google Scholar] [CrossRef]
- Yaakob, M.A.; Mohamed, R.M.S.R.; Al-Gheethi, A.; Ravishankar, G.A.; Ambati, R.R. Influence of nitrogen and phosphorus on microalgal growth, biomass, lipid, and fatty acid production: An overview. Cells 2021, 10, 393. [Google Scholar] [CrossRef] [PubMed]
- Saranya, D.; Shanthakumar, S. Insights into the influence of CO2 supplement on phycoremediation and lipid accumulation potential of microalgae: An exploration for biodiesel production. Environ. Technol. Innov. 2021, 23, 101596. [Google Scholar] [CrossRef]
- Jusoh, M.; Loh, S.H.; Chuah, T.S.; Aziz, A.; San Cha, T. Indole-3-acetic acid (IAA) induced changes in oil content, fatty acid profiles and expression of four fatty acid biosynthetic genes in Chlorella vulgaris at early stationary growth phase. Phytochemistry 2015, 111, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Mariamenatu, A.H.; Abdu, E.M. Overconsumption of omega-6 polyunsaturated fatty acids (PUFAs) versus deficiency of omega-3 PUFAs in modern-day diets: The disturbing factor for their “balanced antagonistic metabolic functions” in the human body. J. Lipids 2021, 2021, 8848161. [Google Scholar] [CrossRef] [PubMed]
- Habeebullah, S.F.K.; Alagarsamy, S.; Al-Haddad, S.; Al-Yamani, F. Composition, in vitro antioxidant and angiotensin-converting enzyme inhibitory effects of lipids isolated from fifteen species of seaweeds. Food Chem. Adv. 2023, 3, 100352. [Google Scholar] [CrossRef]
- Chen, J.; Liu, H. Nutritional indices for assessing fatty acids: A mini-review. Int. J. Mol. Sci. 2020, 21, 5695. [Google Scholar] [CrossRef]
- Bharti, A.; Prasanna, R.; Kumar, G.; Kumar, A.; Nain, L. Co-cultivation of cyanobacteria for raising nursery of chrysanthemum using a hydroponic system. J. Appl. Phycol. 2019, 31, 3625–3635. [Google Scholar] [CrossRef]
- Oliveira, T.J.S.S.; Oliveira, C.E.S.; Jalal, A.; Gato, I.M.B.; Rauf, K.; Moreira, V.A.; Lima, B.H.; Vitória, L.S.; Giolo, V.M.; Teixeira Filho, M.C.M. Inoculation reduces nitrate accumulation and increases growth and nutrient accumulation in hydroponic arugula. Sci. Hortic. 2023, 320, 112213. [Google Scholar] [CrossRef]
- Kumsiri, B.; Pekkoh, J.; Pathom-aree, W.; Lumyong, S.; Phinyo, K.; Pumas, C.; Srinuanpan, S. Enhanced production of microalgal biomass and lipid as an environmentally friendly biodiesel feedstock through actinomycete co-culture in biogas digestate. Bioresour. Technol. 2021, 337, 125446. [Google Scholar] [CrossRef]
- Sivaramakrishnan, R.; Incharoensakdi, A. Plant hormone induced enrichment of Chlorella sp. omega-3 fatty acids. Biotechnol. Biofuels 2020, 13, 7. [Google Scholar] [CrossRef] [PubMed]
- Mulgund, A. Increasing lipid accumulation in microalgae through environmental manipulation, metabolic and genetic engineering: A review in the energy NEXUS framework. Energy Nexus 2022, 5, 100054. [Google Scholar] [CrossRef]
- CEN. Liquid Petroleum Products—Fatty Acid Methyl Esters (FAME) for Use in Diesel Engines and Heating Applications—Requirements and Test Methods; CEN Publications: Brussels, Belgium, 2012. [Google Scholar]
- ASTM. Standard specification for biodiesel fuel (B100) blend stock for distillate fuels. In Annual Book of ASTM Standards; ASTM International: West Conshohocken, PA, USA, 2012. [Google Scholar]
- Saxena, P.; Jawale, S.; Joshipura, M.H. A review on prediction of properties of biodiesel and blends of biodiesel. Proc. Eng. 2013, 51, 395–402. [Google Scholar] [CrossRef]
- Giakoumis, E.G.; Sarakatsanis, C.K. Estimation of biodiesel cetane number, density, kinematic viscosity and heating values from its fatty acid weight composition. Fuel 2018, 222, 574–585. [Google Scholar] [CrossRef]
- Talebi, A.F.; Tabatabaei, M.; Chisti, Y. BiodieselAnalyzer: A user-friendly software for predicting the properties of prospective biodiesel. Biofuel Res. J. 2014, 2, 55–57. [Google Scholar] [CrossRef]
- Klajn, F.F.; Gurgacz, F.; Lenz, A.M.; Iacono, G.E.P.; de Souza, S.N.M.; Ferruzzi, Y. Comparison of the emissions and performance of ethanol-added diesel–biodiesel blends in a compression ignition engine with those of pure diesel. Environ. Technol. 2018, 41, 511–520. [Google Scholar] [CrossRef]
- Wu, G.; Ge, J.C.; Choi, N.J. A comprehensive review of the application characteristics of biodiesel blends in diesel engines. Appl. Sci. 2020, 10, 8015. [Google Scholar] [CrossRef]
- Serrano, M.; Martínez, M.; Aracil, J. Long term storage stability of biodiesel: Influence of feedstock, commercial additives and purification step. Fuel Process. Technol. 2013, 116, 135–141. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).