Fine-Tuning Grape Phytochemistry: Examining the Distinct Influence of Oak Ash and Potassium Carbonate Pre-Treatments on Essential Components
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Grape Sampling
2.3. Preparation for the Drying Process
2.4. Analytical Methods for Phenolic Compounds
2.5. Analytical Methods for the Anthocyanin Content
2.6. Analytical Methods for Phenolic Acids
2.7. Analytical Methods for Flavonoids
2.8. Analytical Methods for the Phytoalexins
2.9. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Keskin, N.; Kunter, B.; Çelik, H.; Kaya, O.; Keskin, S. ANOM approach for the statistical evaluation of organic acid contents of clones of the grape variety ‘Kalecik Karası’. Mitteilungen Klosterneubg. 2021, 71, 126–138. [Google Scholar]
- Ates, F.; Kaya, O.; Keskin, N.; Turan, M. Bi ogenic amines in raisins of one vintage year: In fluence of two chemical pre-treatments (dipping in oak ash solution or potassium carbonate solution). Mitteilungen Klosterneubg. 2022, 72, 51–59. [Google Scholar]
- Keskin, N.; Kunter, B.; Celik, H.; Kaya, O.; Keskin, S. Evaluation of Clonal Variability of Berry Phenolics in Vitis vinifera L. Cv. Kalecik Karası. Erwerbs-Obstbau 2022, 64 (Suppl. S1), 65–72. [Google Scholar] [CrossRef]
- Kalkan, N.N.; Karadoğan, B.; Kadioğlu, Z.; Esmek, İ.; Albayrak, S.; Kaya, O. Response of Karaerik Grape Cultivar (Vitis vinifera L.) to Two Training Systems and Three Trunk Heights. Erwerbs-Obstbau 2022, 64 (Suppl. S1), 119–127. [Google Scholar] [CrossRef]
- World Health Organization. Fruit and Vegetable Promotion Initiative. 2022. Available online: https://www.who.int/dietphysicalactivity/publications/trs916/intro/en/ (accessed on 20 May 2022).
- Kaliora, A.C.; Kountouri, A.M.; Karathanos, V.T.; Andrikopoulos, N.K. Vitamin C content in grapes, fresh and dried, and in products made from them. J. Food Compos. Anal. 2016, 46, 30–37. [Google Scholar]
- Karakus, S.; Ates, F.; Keskin, N.; Turan, M.; Kaya, O. Comparison of contents of sugars, organic acids and free amino acids in raisins obtained from Gök Üzüm (Vitis vinifera L.). Mitteilungen Klosterneubg. 2023, 73, 98–113. [Google Scholar]
- Karakus, S.; Kaya, O.; Hajizadeh, H.S.; Gutiérrez-Gamboa, G.; Ates, F.; Turan, M.; Araya-Alman, M. Characterization of volatile compounds of Gök Üzüm raisins produced from grapes pre-treated with different dipping solutions. Chem. Biol. Technol. Agric. 2023, 10, 55. [Google Scholar] [CrossRef]
- Nile, S.H.; Park, S.W. Edible Oil. Inhibitory effects of anthocyanins and fruit extracts from Vitis coignetiae on melanin formation in B16F10 cells. Int. J. Mol. Sci. 2014, 15, 21930–21947. [Google Scholar]
- González-Gallego, J.; García-Mediavilla, M.V.; Sánchez-Campos, S.; Tuñón, M.J. Fruit polyphenols, immunity and inflammation. Br. J. Nutr. 2010, 104 (Suppl. S3), S15–S27. [Google Scholar] [CrossRef]
- Tsuda, T. Dietary anthocyanin-rich plants: Biochemical basis and recent progress in health benefits studies. Mol. Nutr. Food Res. 2012, 56, 159–170. [Google Scholar] [CrossRef]
- Nunes, T.; Almeida, L.; Rocha, J.F.; Laranjinha, J.; Barbosa, R.M. Inhibition of human in vitro osteoclastogenesis by anthocyanin-rich açai extract. Inflammopharmacology 2017, 25, 261–268. [Google Scholar]
- Chukwumah, Y.; Walker, L.; Verghese, M. Peanut skin color: A biomarker for total polyphenolic content and antioxidative capacities of peanut cultivars. Int. J. Mol. Sci. 2009, 10, 4941–4952. [Google Scholar] [CrossRef] [PubMed]
- Keskin, N.; Kaya, O.; Ates, F.; Turan, M.; Gutiérrez-Gamboa, G. Drying grapes after the application of different dipping solutions: Effects on hormones, minerals, vitamins, and antioxidant enzymes in Gök Üzüm (Vitis vinifera L.) raisins. Plants 2022, 11, 529. [Google Scholar] [CrossRef]
- USDA. Statistical Data. 2022. Available online: https://apps.fas.usda.gov/psdonline/circulars/Raisins.pdf (accessed on 20 October 2023).
- Kaya, O.; Ates, F.; Kara, Z.; Turan, M.; Gutiérrez-Gamboa, G. Study of Primary and Secondary Metabolites of Stenospermocarpic, Parthenocarpic and Seeded Raisin Varieties. Horticulturae 2022, 8, 1030. [Google Scholar] [CrossRef]
- Vendramin, V.; Viel, A.; Vincenzi, S. Caftaric Acid Isolation from Unripe Grape: A “Green” Alternative for Hydroxycinnamic Acids Recovery. Molecules 2021, 26, 1148. [Google Scholar] [CrossRef] [PubMed]
- Yousef, G.G.; Brown, A.F.; Funakoshi, Y.; Mbeunkui, F.; Grace, M.H.; Ballington, J.R.; Loraine, A.; Lila, M.A. Efficient quantification of the health-relevant anthocyanin and phenolic acid profiles in commercial cultivars and breeding selections of blueberries (Vaccinium spp.). J. Agric. Food Chem. 2013, 61, 4806–4815. [Google Scholar] [CrossRef]
- Pantelić, M.M.; Zagorac, D.Č.D.; Ćirić, I.Ž.; Pergal, M.V.; Relić, D.J.; Todić, S.R.; Natić, M.M. Phenolic profiles, antioxidant activity and minerals in leaves of different grapevine varieties grown in Serbia. J. Food Compos. Anal. 2017, 62, 76–83. [Google Scholar] [CrossRef]
- Brossa, R.; Casals, I.; Pintó-Marijuan, M.; Fleck, I. Leaf flavonoid content in Quercus ilex L. resprouts and its seasonal variation. Trees 2009, 23, 401–408. [Google Scholar] [CrossRef]
- Jeandet, P.; Breuil, A.C.; Adrian, M.; Weston, L.A.; Debord, S.; Meunier, P.; Maume, G.; Bessis, R. HPLC analysis of grapevine phytoalexins coupling photodiode array detection and fluorometry. Sci. Hortic. 1997, 183, 115–126. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013; Available online: http://www.R-project.org/ (accessed on 8 August 2022).
- Wickham, H.; Wickham, H. Data Analysis; Springer International Publishing: Cham, Switzerland, 2016; pp. 189–201. [Google Scholar]
- Kolde, R. Pheatmap: Pretty Heatmaps_.R Package Version 1.0.12. 2019. Available online: https://rdrr.io/cran/pheatmap/ (accessed on 25 February 2022).
- Ünal, M.S.; Güler, E.; Yaman, M. Changes in Antioxidant and Color Properties of Raisins According to Variety and Drying Method. Horticulturae 2023, 9, 771. [Google Scholar] [CrossRef]
- Teixeira, A.; Eiras-Dias, J.; Castellarin, S.D.; Gerós, H. Berry phenolics of grapevine under challenging environments. Int. J. Mol. Sci. 2013, 14, 18711–18739. [Google Scholar] [CrossRef]
- Badhani, B.; Sharma, N.; Kakkar, R. Gallic acid: A versatile antioxidant with promising therapeutic and industrial applications. RSC Adv. 2015, 5, 27540–27557. [Google Scholar] [CrossRef]
- Williamson, G.; Carughi, A. Polyphenol content and health benefits of raisins. Nutr. Res. 2010, 30, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Olmo-Cunillera, A.; Escobar-Avello, D.; Pérez, A.J.; Marhuenda-Muñoz, M.; Lamuela-Raventós, R.M.; Vallverdú-Queralt, A. Is eating raisins healthy? Nutrients 2019, 12, 54. [Google Scholar] [CrossRef] [PubMed]
- Thiruchelvi, R.; Jayashree, P.; Nayik, G.A.; Gull, A.; Ahad, T.; Thakur, M.; Shah, T.R.; Paray, M.A.; Haq, R.U. Raisin. In Antioxidants in Vegetables and Nuts-Properties and Health Benefits; Springer: Berlin/Heidelberg, Germany, 2020; pp. 523–537. [Google Scholar]
- Sabel, A.; Bredefeld, S.; Schlander, M.; Claus, H. Wine phenolic compounds: Antimicrobial properties against yeasts, lactic acid and acetic acid bacteria. Beverages 2017, 3, 29. [Google Scholar] [CrossRef]
- Campos, F.; Peixoto, A.F.; Fernandes, P.A.; Coimbra, M.A.; Mateus, N.; de Freitas, V.; Fernandes, I.; Fernandes, A. The antidiabetic effect of grape pomace polysaccharide-polyphenol complexes. Nutrients 2021, 13, 4495. [Google Scholar] [CrossRef] [PubMed]
- Squadrito, M.; Corona, O.; Ansaldi, G.; Di Stefano, R. Evolution of anthocyanin profile from grape to wine. Oeno One 2010, 44, 167–177. [Google Scholar] [CrossRef]
- Karadeniz, F.; Durst, R.W.; Wrolstad, R.E. Polyphenolic composition of raisins. J. Agric. Food Chem. 2000, 48, 5343–5350. [Google Scholar] [CrossRef]
- Kalkan, N.N.; Bozkurt, A.; Gecim, T.; Karadogan, B.; Bahar, E.; Kaya, O. Influence of pruning severity in the table grape variety ‘Karaerik’ (Vitis vinifera L.). Mitteilungen Klosterneubg. 2022, 72, 137–145. [Google Scholar]
- Kelebek, H.; Jourdes, M.; Selli, S.; Teissedre, P.L. Comparative evaluation of the phenolic content and antioxidant capacity of sun-dried raisins. J. Sci. Food Agric. 2013, 93, 2963–2972. [Google Scholar] [CrossRef]

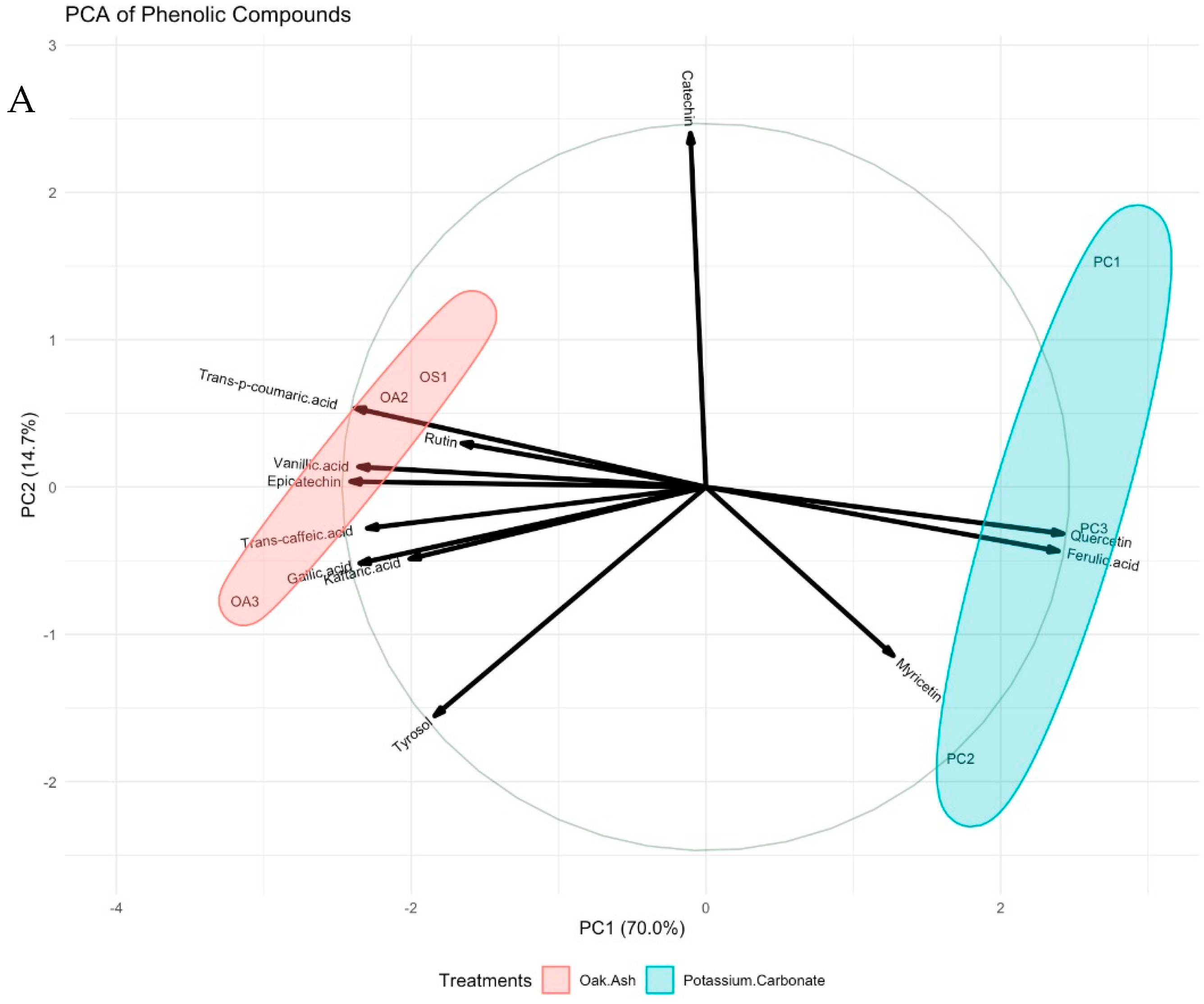
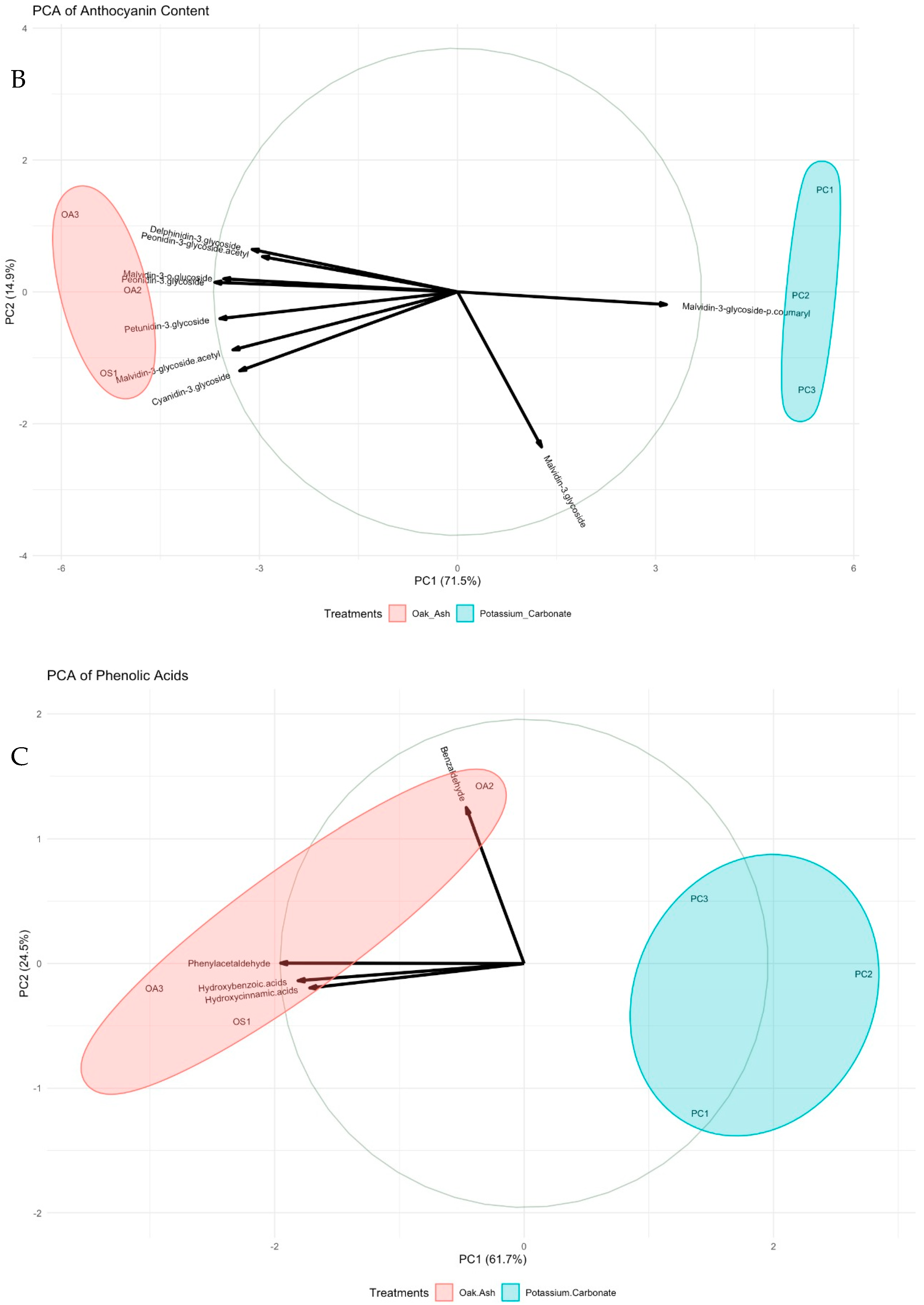
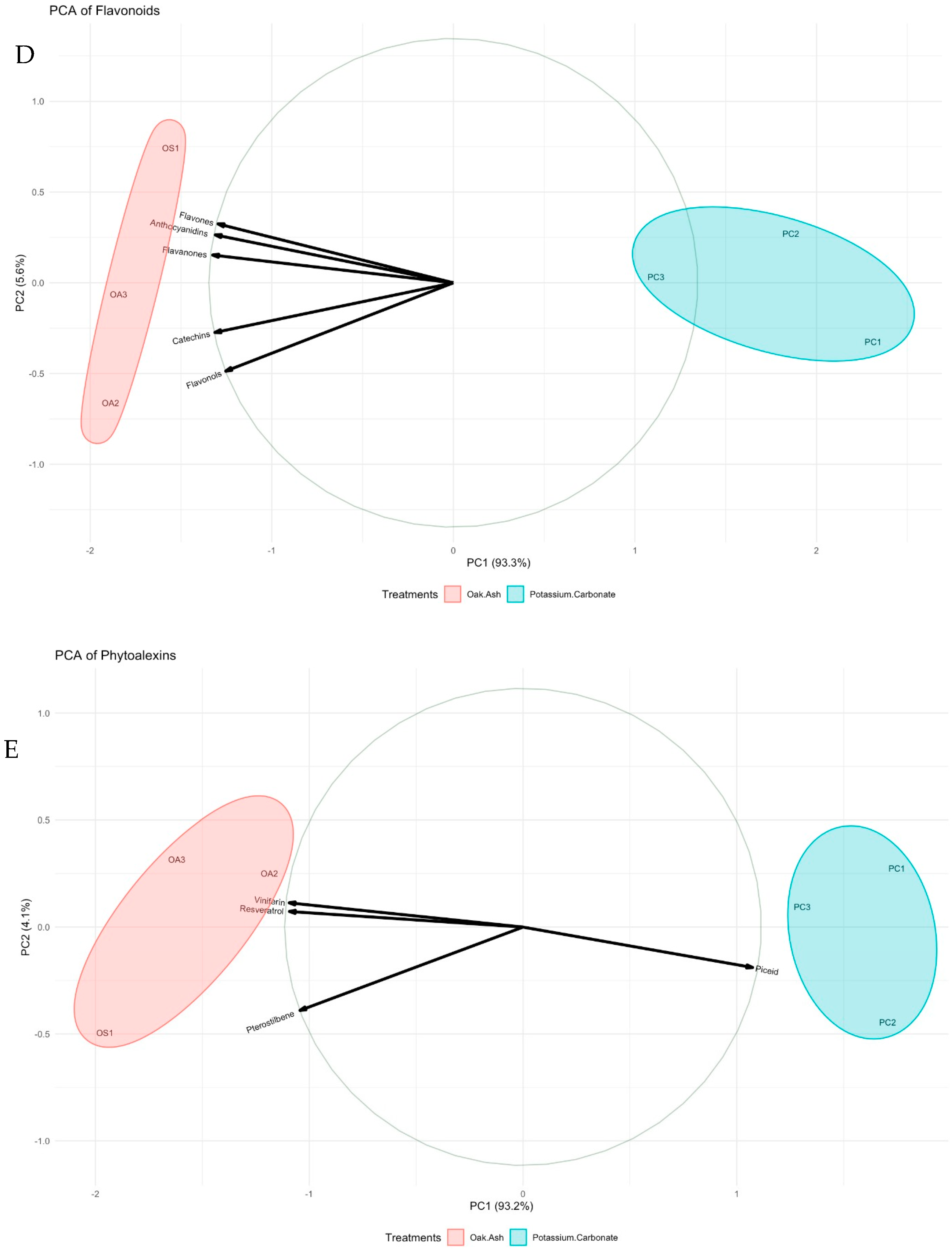
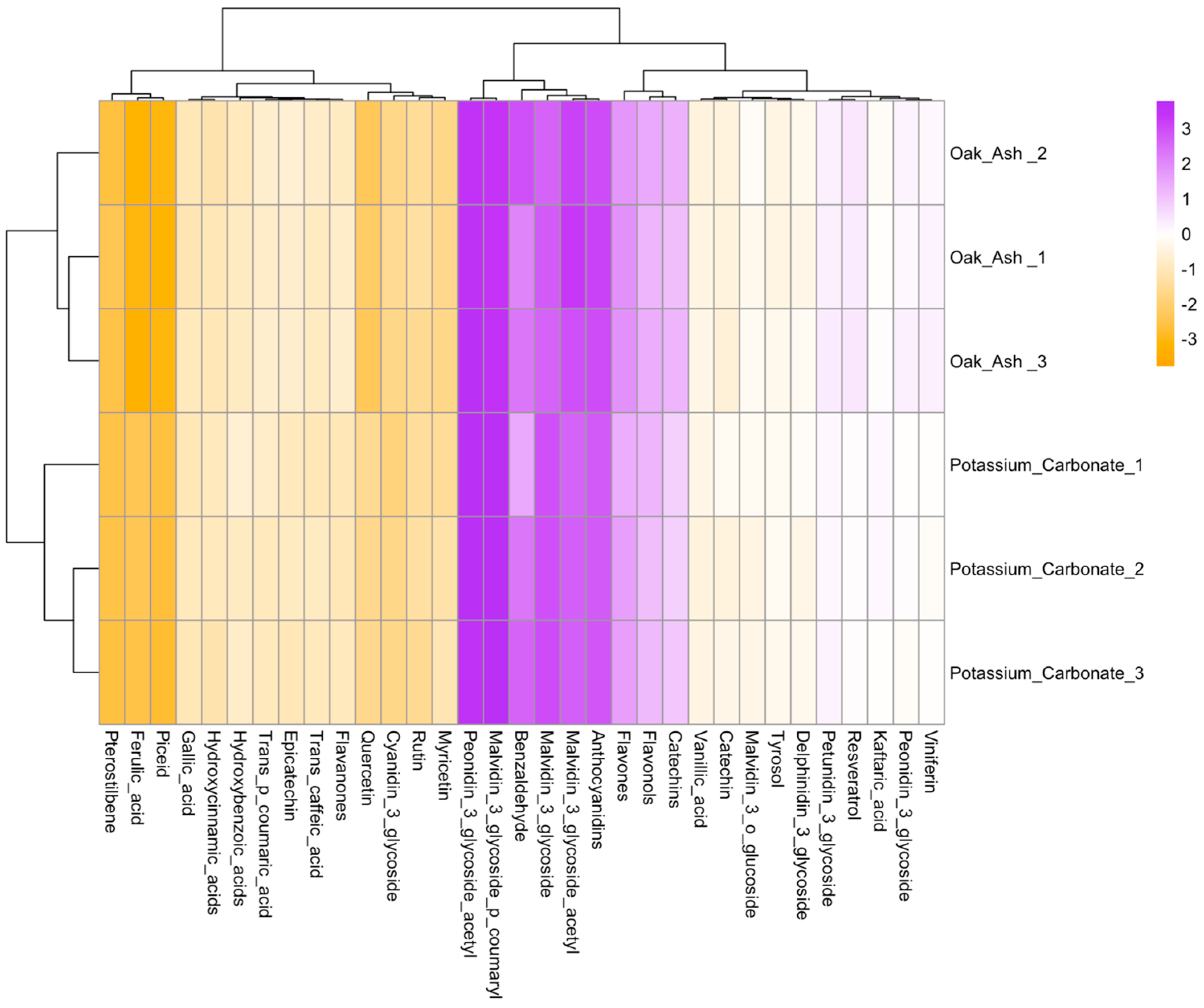
| Oak Ash | K2CO3 | p-Value | Significance Level | |
|---|---|---|---|---|
| Flavonoids (mg/kg) | ||||
| Anthocyanidins | 215.0 ± 5.621 b | 154.0 ± 5.132 a | 0.001 | ** |
| Flavonols | 68.0 ± 2.14 b | 50.5 ± 2.72 a | 0.010 | * |
| Flavones | 92.6 ± 3.31 b | 66.0 ± 3.71 a | 0.007 | ** |
| Flavanones | 15.1 ± 2.02 b | 11.0 ± 0.35 a | 0.001 | ** |
| Catechins | 61.6 ± 2.12 b | 39.8 ± 2.89 a | 0.005 | ** |
| Phytoalexins (mg/kg) | ||||
| Resveratrol | 33.8 ± 0.12 b | 23.2 ± 0.40 a | 0.020 | *** |
| Pterostilbene | 4.6 ± 0.3 b | 3.8 ± 0.12 a | 0.016 | * |
| Piceid | 3.0 ± 0.1 | 3.4 ± 0.1 | 0.003 | ** |
| Viniferin | 29.2 ± 0.22 b | 21.4 ± 0.61 a | 0.001 | ** |
| Oak Ash | K2CO3 | p-Values | Significance Level | |
|---|---|---|---|---|
| Anthocyanin content | ||||
| Malvidin-3-O-glycoside | 165.0 ± 5.332 | 171.0 ± 5.834 | 0.514 | ns |
| Delphinidin-3-O-glycoside | 21.5 ± 0.15 b | 19.1 ± 0.62 a | 0.047 | * |
| Cyanidin-3-O-glycoside | 8.3 ± 0.9 b | 6.5 ± 0.3 a | 0.022 | * |
| Petunidin-3-O-glycoside | 32.3 ± 0.74 b | 24.8 ± 0.94 a | 0.004 | ** |
| Peonidin-3-O-glycoside | 29.9 ± 0.52 b | 21.9 ± 0.71 a | 0.001 | ** |
| Malvidin-3-O-glucoside | 22.7 ± 0.21 b | 17.9 ± 0.57 a | 0.003 | ** |
| Malvidin-3-O-glycoside acetyl | 226.0 ± 13.134 b | 143.0 ± 11.722 a | 0.010 | * |
| Peonidin-3-O-glycoside acetyl | 312.0 ± 11.732 | 273.0± 11.234 | 0.081 | ns |
| Malvidin-3-O-glycoside-p-coumaryl | 273.0 ± 5.543 a | 299.0 ± 5.577 b | 0.030 | * |
| Phenolic acids | ||||
| Hydroxycinnamic acids | 12.5 ± 0.31 b | 11.0 ± 0.21 a | 0.036 | * |
| Hydroxybenzoic acids | 14.0 ± 0.12 | 13.2 ± 0.23 | 0.131 | ns |
| Benzaldehyde | 148.0 ± 12.733 | 103.0 ± 26.134 | 0.291 | ns |
| Phenylacetaldehyde | 115.7 ± 8.943 b | 82.3 ± 8.42 a | 0.048 | * |
| Oak Ash | K2CO3 | p-Values | Significance Level | |
|---|---|---|---|---|
| Phenolic Compounds (µg/L) | ||||
| Tyrosol | 20.2 ± 0.32 | 19.1 ± 0.51 | 0.210 | ns |
| Gallic acid | 12.9 ± 0.13 b | 11.0 ± 0.32 a | 0.018 | * |
| Vanillic acid | 19.5 ± 0.41 b | 16.5 ± 0.33 a | 0.004 | ** |
| Trans-caffeic acid | 14.6 ± 0.25 b | 11.7 ± 0.41 a | 0.008 | ** |
| Trans-p-coumaric acid | 15.4 ± 0.17 b | 12.1 ± 0.24 a | 0.001 | *** |
| Ferulic acid | 2.9 ± 0.1 a | 4.2 ± 0.1 b | 0.001 | *** |
| Kaftaric acid | 25.4 ± 0.19 | 23.5 ± 0.65 | 0.093 | ns |
| Catechin | 18.2 ± 0.22 | 17.8 ± 0.52 | 0.666 | ns |
| (-)-Epicatechin | 16.1 ± 0.24 b | 11.7 ± 0.39 a | 0.001 | ** |
| Quercetin | 5.4 ± 0.1 a | 7.3 ± 0.1 b | 0.001 | *** |
| Rutin | 9.6 ± 0.3 | 8.4 ± 0.4 | 0.101 | ns |
| Myricetin | 8.2 ± 0.3 | 9.2 ± 0.5 | 0.292 | ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaya, O.; Delavar, H.; Ates, F.; Yilmaz, T.; Sahin, M.; Keskin, N. Fine-Tuning Grape Phytochemistry: Examining the Distinct Influence of Oak Ash and Potassium Carbonate Pre-Treatments on Essential Components. Horticulturae 2024, 10, 95. https://doi.org/10.3390/horticulturae10010095
Kaya O, Delavar H, Ates F, Yilmaz T, Sahin M, Keskin N. Fine-Tuning Grape Phytochemistry: Examining the Distinct Influence of Oak Ash and Potassium Carbonate Pre-Treatments on Essential Components. Horticulturae. 2024; 10(1):95. https://doi.org/10.3390/horticulturae10010095
Chicago/Turabian StyleKaya, Ozkan, Hava Delavar, Fadime Ates, Turhan Yilmaz, Muge Sahin, and Nurhan Keskin. 2024. "Fine-Tuning Grape Phytochemistry: Examining the Distinct Influence of Oak Ash and Potassium Carbonate Pre-Treatments on Essential Components" Horticulturae 10, no. 1: 95. https://doi.org/10.3390/horticulturae10010095
APA StyleKaya, O., Delavar, H., Ates, F., Yilmaz, T., Sahin, M., & Keskin, N. (2024). Fine-Tuning Grape Phytochemistry: Examining the Distinct Influence of Oak Ash and Potassium Carbonate Pre-Treatments on Essential Components. Horticulturae, 10(1), 95. https://doi.org/10.3390/horticulturae10010095









