A Genome-Wide Identification and Expression Analysis of the Xyloglucan Endotransglucosylase/Hydrolase Gene Family in Melon (Cucumis melo L.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Genome-Wide Characterization of Melon XTH Genes
2.2. Physicochemical Property Analysis of CmXTH Proteins
2.3. Phylogenetic Tree of CmXTH Proteins
2.4. Chromosomal Localization of CmXTH Genes
2.5. Gene Structure, Protein Structural Domain, and Conserved Motif Analysis of CmXTHs
2.6. Cis-Acting Element Analysis of CmXTH Genes
2.7. Synteny and Duplation Analysis of CmXTH Genes
2.8. Plant Material and Abiotic Stresses
3. Results
3.1. Identification of XTH Family Genes in Melon
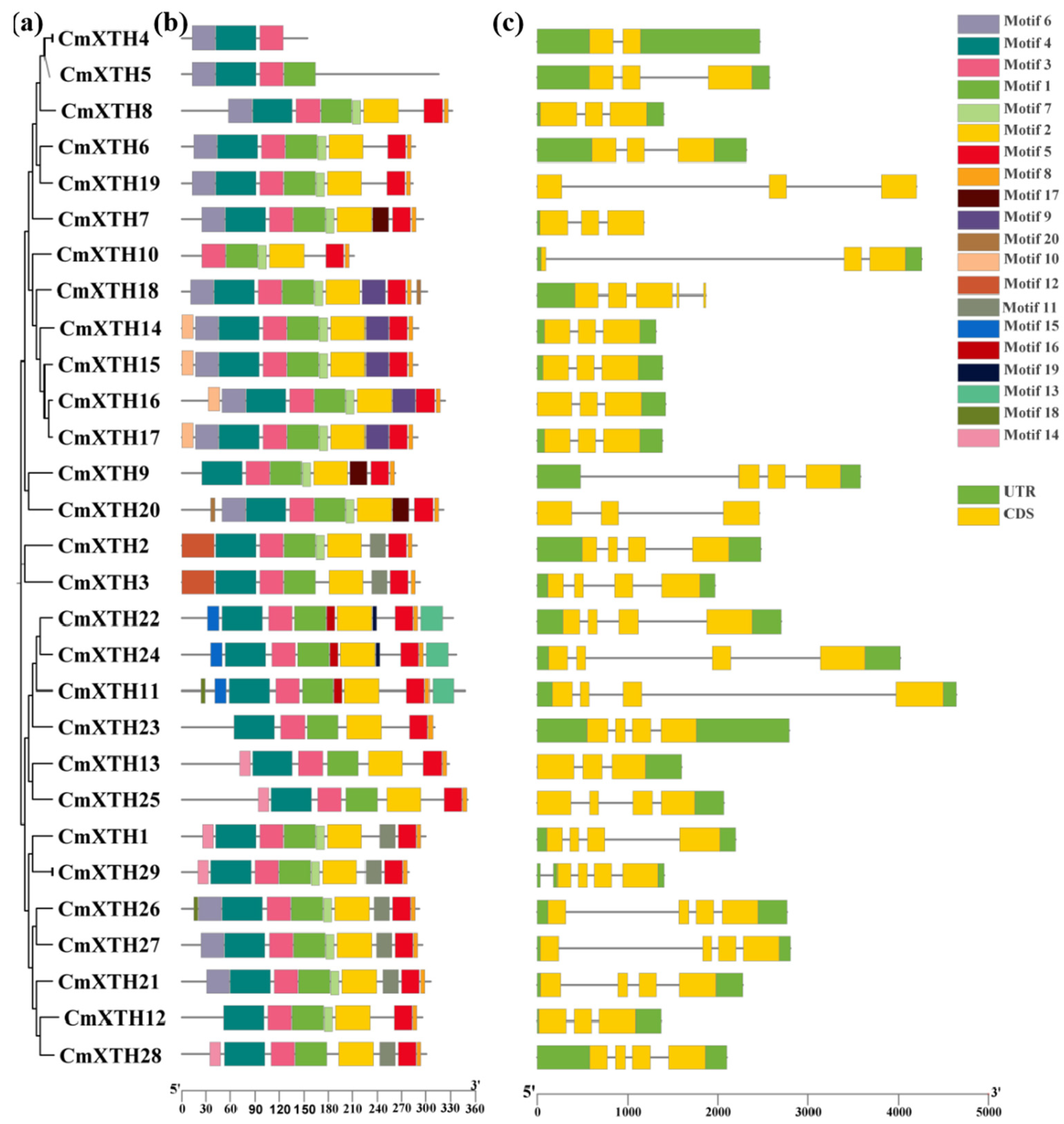
3.2. Phylogenetic Tree Analysis of Melon XTH Family Genes
3.3. Chromosomal Localization of Melon XTH Family Genes
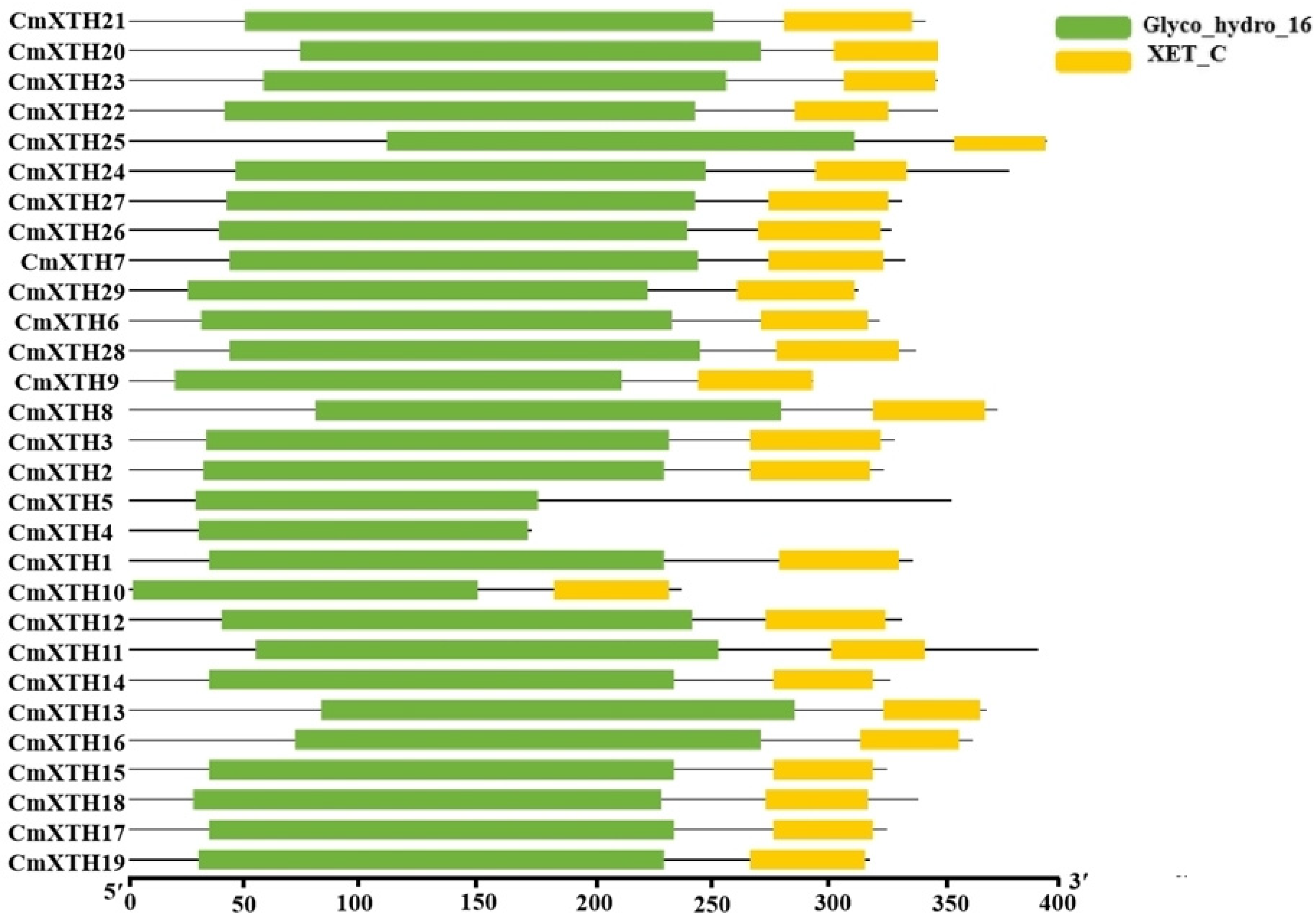
3.4. Gene Structure, Protein Structural Domain, and Conserved Motif Analysis of XTH Family in Melon
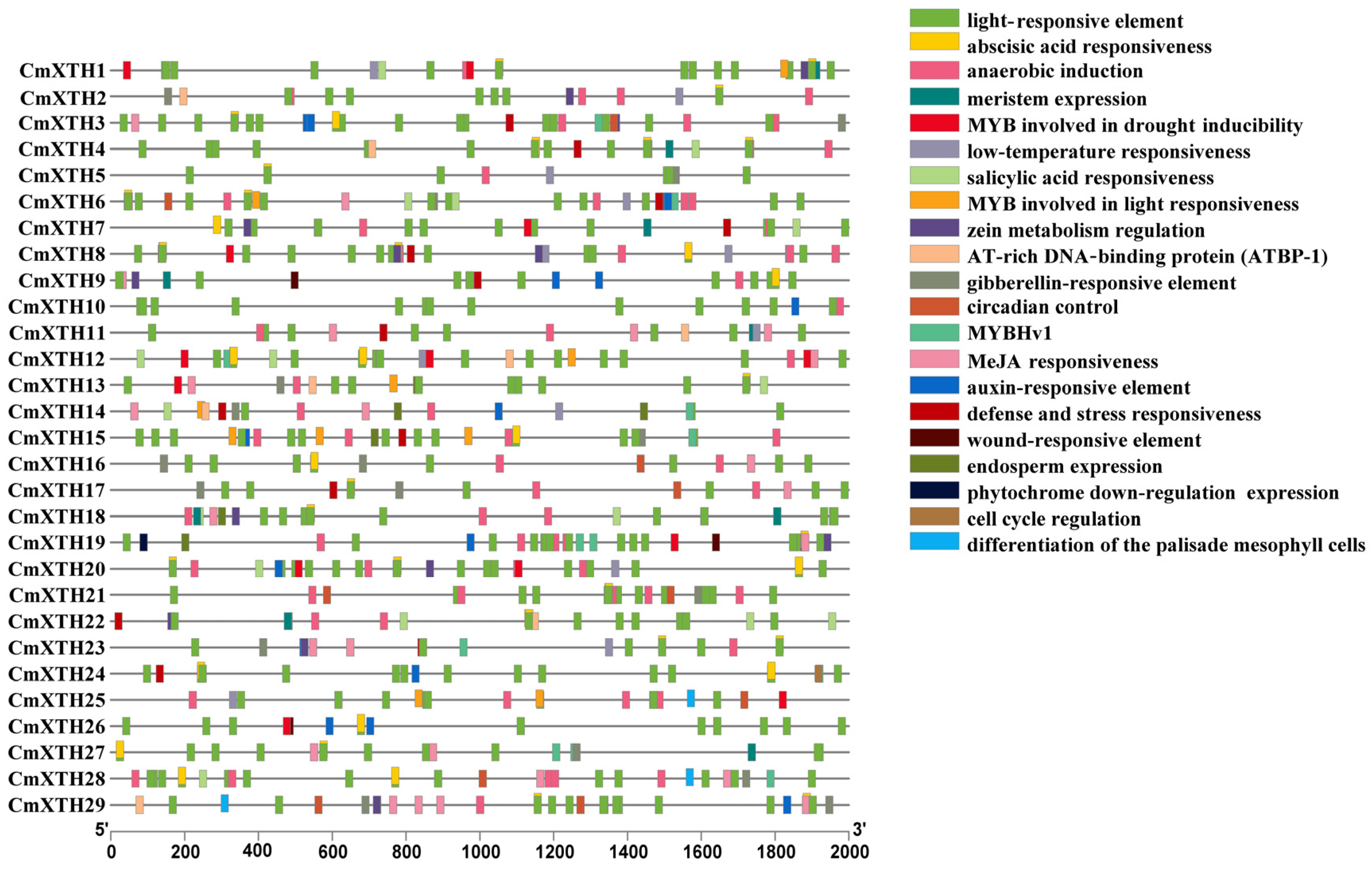
3.5. Cis-Acting Element Analysis of Melon XTH Family Genes
3.6. Synteny Analysis of Melon XTH Family Genes
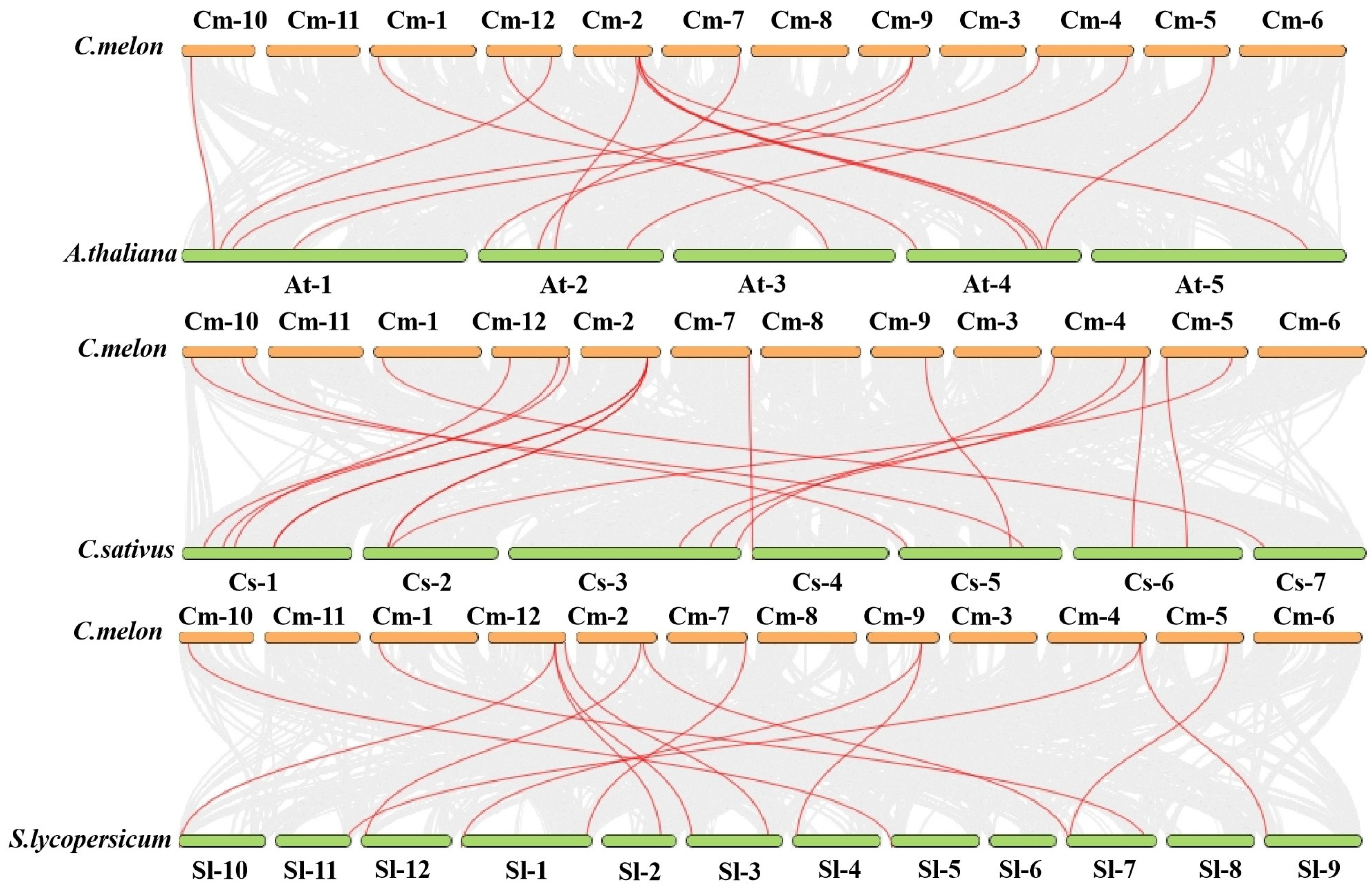
3.7. Collinearity Analysis of Melon XTH Genes with Three Other Species
3.8. CmXTH Gene Expression in Response to Multiple Stresses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rui, Y.; Dinneny, J.R. A wall with integrity: Surveillance and maintenance of the plant cell wall under stress. New Phytol. 2020, 225, 1428–1439. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.B.; Cosgrove, D.J. Xyloglucan and its interactions with other components of the growing cell wall. Plant Cell Physiol. 2015, 56, 180–194. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.B.; Cosgrove, D.J. A revised architecture of primary cell walls based on biomechanical changes induced by substrate-specific endoglucanases. Plant Physiol. 2012, 158, 1933–1943. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.E.; Ban, Q.; Li, H.; Hou, Y.; Jin, M.; Han, S.; Rao, J. DkXTH8, a novel xyloglucan endotransglucosylase/hydrolase in persimmon, alters cell wall structure and promotes leaf senescence and fruit postharvest softening. Sci. Rep. 2016, 6, 39155. [Google Scholar] [CrossRef]
- Viborg, A.H.; Terrapon, N.; Lombard, V.; Michel, G.; Czjzek, M.; Henrissat, B.; Brumer, H. A subfamily roadmap of the evolutionarily diverse glycoside hydrolase family 16 (GH16). J. Biol. Chem. 2019, 294, 15973–15986. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Xu, Z.C.; Ding, A.M.; Kong, Y.Z. Genome-wide identification and expression profiling analysis of the Xyloglucan Endotransglucosylase/hydrolase gene family in tobacco (Nicotiana tabacum L.). Genes 2018, 9, 273. [Google Scholar] [CrossRef]
- Li, M.; Xie, F.; He, Q.; Li, J.; Liu, J.L.; Sun, B.; Luo, Y.Y.; Zhang, J.L.; Liu, J.L.; Sun, B.; et al. Expression analysis of XTH in stem swelling of stem mustard and selection of reference genes. Genes 2020, 11, 113. [Google Scholar] [CrossRef]
- Eklöf, J.M.; Brumer, H. The XTH gene family: An update on enzyme structure, function, and phylogeny in xyloglucan remodeling. Plant Physiol. 2010, 153, 456–466. [Google Scholar] [CrossRef]
- Baumann, M.J.; Eklöf, J.M.; Michel, G.; Kallas, A.M.; Teeri, T.T.; Czjzek, M.; Brumer, H. Structural evidence for the evolution of xyloglucanase activity from xyloglucan endo-transglycosylases: Biological implications for cell wall metabolism. Plant Cell 2007, 19, 1947–1963. [Google Scholar] [CrossRef]
- Yokoyama, R.; Nishitani, K. A comprehensive expression analysis of all members of a gene family encoding cell-wall enzymes allowed us to predict cis- regulatory regions involved in cell-wall construction in specific organs of arabidopsis. Plant Cell Physiol. 2001, 42, 1025–1033. [Google Scholar] [CrossRef]
- Yokoyama, R.; Rose, J.K.C.; Nishitani, K. A surprising diversity and abundance of xyloglucan endotransglucosylase/hydrolases in rice. Classification and expression analysis. Plant Physiol. 2004, 134, 1088–1099. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.H.; Zhang, X.M.; Yao, W.J.; Gao, Y.; Zhao, K.; Guo, Q.; Zhou, B.R.; Jiang, T.B. Genome-wide identification and expression analysis of the xyloglucan endotransglucosylase/hydrolase gene family in poplar. BMC Genom. 2021, 22, 804. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, D.C.; Zhang, H.Y.; Gao, H.B.; Guo, X.L.; Wang, D.M.; Zhang, X.Q.; Zhang, A.M. The alpha- and beta-expansin and xyloglucan endotransglucosylase/hydrolase gene families of wheat: Molecular cloning, gene expression, and EST data mining. Genomics 2007, 90, 516–529. [Google Scholar] [CrossRef]
- Miedes, E.; Lorences, E.P. Xyloglucan endotransglucosylase/hydrolases (XTHs) during tomato fruit growth and ripening. Plant Physiol. 2009, 166, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, R.G.; Johnston, S.L.; Yauk, Y.K.; Sharma, N.N.; Schroder, R. Analysis of xyloglucan endotransglucosylase/hydrolase (XTH) gene families in kiwifruit and apple. Postharvest Biol. Technol. 2009, 51, 149–157. [Google Scholar] [CrossRef]
- Maris, A.; Suslov, D.; Fry, S.C.; Verbelen, J.P.; Vissenberg, K. Enzymic characterization of two recombinant xyloglucan endotransglucosylase/hydrolase (XTH) proteins of Arabidopsis and their effect on root growth and cell wall extension. J. Exp. Bot. 2009, 60, 3959–3972. [Google Scholar] [CrossRef]
- Osato, Y.; Yokoyama, R.; Nishitani, K. A principal role for AtXTH18 in Arabidopsis thaliana root growth: A functional analysis using RNAi plants. J. Plant Res. 2006, 119, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Nishikubo, N.; Takahashi, J.; Roos, A.A.; Derba-Maceluch, M.; Piens, K.; Brumer, H.; Teeri, T.T.; Stålbrand, H.; Mellerowicz, E.J. Xyloglucan endo- transglycosylase-mediated xyloglucan rearrangements in developing wood of hybrid aspen. Plant Physiol. 2011, 155, 399–413. [Google Scholar] [CrossRef]
- Li, Q.Y.; Li, H.Y.; Yin, C.Y.; Wang, X.T.; Jiang, Q.; Zhang, R.; Ge, F.F.; Chen, Y.D.; Yang, L. Genome-wide identification and characterization of xyloglucan endotransglycosylase/hydrolase in ananas comosus during development. Genes 2019, 10, 537. [Google Scholar] [CrossRef]
- Du, H.; Hu, X.; Yang, W.; Hu, W.; Yan, W.; Li, Y.; He, W.; Cao, M.; Zhang, X.; Luo, B. ZmXTH, a xyloglucan endotransglucosylase/hydrolase gene of maize, conferred aluminum tolerance in Arabidopsis. Plant Physiol. 2021, 266, 153520. [Google Scholar] [CrossRef]
- Cho, S.K.; Kim, J.E.; Park, J.A.; Eom, T.J.; Kim, W.T. Constitutive expression of abiotic stress-inducible hot pepper CaXTH3, which encodes a xyloglucan endotransglucosylase/hydrolase homolog, improves drought and salt tolerance in transgenic Arabidopsis plants. FEBS Lett. 2006, 580, 3136–3144. [Google Scholar] [CrossRef] [PubMed]
- Harada, T.; Torii, Y.; Morita, S.; Onodera, R.; Hara, Y.; Yokoyama, R.; Nishitani, K.; Satoh, S. Cloning, characterization, and expression of xyloglucan endotransglucosylase/hydrolase and expansin genes associated with petal growth and development during carnation flower opening. J. Exp. Bot. 2011, 62, 815–823. [Google Scholar] [CrossRef]
- Han, Y.S.; Sa, G.; Sun, J.; Shen, Z.D.; Zhao, R.; Ding, M.Q.; Deng, S.R.; Lu, Y.J.; Zhang, Y.H.; Shen, X.; et al. Overexpression of Populus euphratica xyloglucan endotransglucosylase/hydrolase gene confers enhanced cadmium tolerance by the restriction of root cadmium uptake in transgenic tobacco. Environ. Exp. Bot. 2014, 100, 74–83. [Google Scholar] [CrossRef]
- Zhu, X.F.; Shi, Y.Z.; Lei, G.J.; Fry, S.C.; Zhang, B.C.; Zhou, Y.H.; Braam, J.; Jiang, T.; Xu, X.Y.; Mao, C.Z.; et al. XTH31, Encoding an in vitro XEH/XET-active enzyme, regulates aluminum sensitivity by modulating in vivo XET action, cell wall xyloglucan content, and aluminum binding capacity in arabidopsis. aluminum binding capacity in arabidopsis. Plant Cell 2012, 24, 4731–4747. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.Y.; Seo, Y.S.; Kim, S.J.; Kim, W.T.; Shin, J.S. Constitutive expression of CaXTH3, a hot pepper xyloglucan endotransglucosylase/hydrolase, enhanced tolerance to salt and drought stresses in tomato plants (Solanum lycopersicum cv. Dotaerang). Plant Cell Rep. 2011, 30, 867–877. [Google Scholar] [CrossRef]
- Han, Y.; Han, S.K.; Ban, Q.Y.; He, Y.H.; Jin, M.J.; Rao, J.P. Overexpression of persimmon DkXTH1 enhanced tolerance to abiotic stress and delayed fruit softening in transgenic plants. Plant Cell Rep. 2017, 36, 583–596. [Google Scholar] [CrossRef] [PubMed]
- Malinowski, R.; Fry, S.C.; Zuzga, S.; Wiśniewska, A.; Godlewski, M.; Noyszewski, A. Developmental expression of the cucumber Cs-XTH1 and Cs-XTH3 genes, encoding xyloglucan endotransglucosylase/hydrolases, can be influenced by mechanical stimuli. Acta Physiol. Plant. 2018, 40, 130–140. [Google Scholar] [CrossRef]
- Takahashi, D.; Johnson, K.; Hao, P.F.; Tuong, T.; Erban, A.; Sampathkumar, A.; Bacic, A.; Livingston, D.P.; Kopka, J.; Kuroha, T.; et al. Cell wall modification by the xyloglucan endotransglucosylase/hydrolase XTH19 influences freezing tolerance after cold and sub-zero acclimation. Plant Cell Environ. 2021, 44, 915–930. [Google Scholar] [CrossRef]
- Chikh-Rouhou, H.; Abdedayem, W.; Solmaz, I.; Sari, N.; Garcés-Claver, N. Melon (Cucumis melo L.): Genomics and breeding. In Smart Plant Breeding for Vegetable Crops in Post-Genomics Era; Springer Nature: Singapore, 2023; pp. 25–52. [Google Scholar]
- DellaPenna, D.; Pogson, B.J. Vitamin synthesis in plants: Tocopherols and Carotenoids. Annu. Rev. Plant Biol. 2006, 57, 711–738. [Google Scholar] [CrossRef]
- Nguyen, P.D.T.; Tran, T.; Thieu, H.H.; Lao, T.; Le, T.; Nguyen, N. Hybridization Between the canary Melon and a Vietnamese Non-sweet Melon Cultivar. Aiming to Improve the Growth Performance and Fruit Quality in Melon (Cucumis melo L.). Mol. Biotechnol. 2024, 66, 1673–1683. [Google Scholar] [CrossRef]
- Cabello, M.J.; Castellanos, M.T.; Romojaro, F.; Martínez-Madrid, C.; Ribas, F. Yield and quality of melon grown under different irrigation and nitrogen rates. Agric. Water Manag. 2009, 96, 866–874. [Google Scholar] [CrossRef]
- Akrami, M.; Arzani, A. Physiological alterations due to field salinity stress in melon (Cucumis melo L.). Acta Physiol. Plant. 2018, 40, 91. [Google Scholar] [CrossRef]
- Chevilly, S.; Dolz-Edo, L.; Martínez-Sánchez, G.; Morcillo, L.; Vilagrosa, A.; López-Nicolás, J.M.; Blanca, J.; Yenush, L.; Mulet, J.M. Distinctive traits for drought and salt stress tolerance in melon (Cucumis melo L.). Front. Plant Sci. 2021, 12, 777060. [Google Scholar] [CrossRef]
- Akrami, M.; Arzani, A.; Majnoun, Z. Leaf ion content, yield and fruit quality of field-grown melon under saline conditions. Exp. Agric. 2019, 55, 707–722. [Google Scholar] [CrossRef]
- Yu, J.; Wu, S.; Sun, H.; Wang, X.; Tang, X.; Guo, S.; Zhang, Z.; Huang, S.; Xu, Y.; Weng, Y.; et al. CuGenDBv2: An updated database for cucurbit genomics. Nucleic Acids Res. 2022, 51, D1457–D1464. [Google Scholar] [CrossRef]
- Mistry, J.; Chuguransky, S.; Williams, L.; Qureshi, M.; Salazar, G.A.; Sonnhammer, E.L.L.; Tosatto, S.C.E.; Lisanna, P.; Shriya, R.; Richardson, L.J. Pfam: The protein families database in 2021. Nucleic Acids Res. 2021, 49, D412–D419. [Google Scholar] [CrossRef]
- Chen, C.; Wu, Y.; Li, J.; Wang, X.; Zeng, Z.; Xu, J.; Liu, Y.; Feng, J.; Chen, H.; He, Y. TBtools-II: A “one for all, all for one” bioinformatics platform for biological big-data mining. Mol. Plant 2023, 16, 1733–1742. [Google Scholar] [CrossRef]
- Lu, S.N.; Wang, J.Y.; Chitsaz, F.; Derbyshire, M.K.; Geer, R.C.; Gonzales, N.R.; Gwadz, M.; Hurwitz, D.I.; Marchler, G.H.; Song, J.S. CDD/SPARCLE: The conserved domain database in 2020. Nucleic Acids Res. 2020, 48, D265–D268. [Google Scholar] [CrossRef]
- Wilkins, M.R.; Gasteiger, E.; Bairoch, A.; Sanchez, J.C.; Williams, K.L.; Appel, R.D.; Hochstrasser, D.F. Protein identification and analysis tools in the ExPASy server. Methods Mol. Biol. 1999, 112, 531–552. [Google Scholar]
- Larkin, M.A.; Blackshields, G.; Brown, N.P.; Chenna, R.; McGettigan, P.A.; McWilliam, H.; Valentin, F.; Wallace, I.M.; Wilm, A.; Lopez, R. Clustal W and Clustal X version 2.0. Bioinformatics 2007, 23, 2947–2948. [Google Scholar] [CrossRef]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar] [PubMed]
- Zhang, Z.; Li, J.; Zhao, X.Q.; Wang, J.; Wong, G.K.S.; Yu, J. KaKs_calculator: Calculating Ka and Ks through model selection and model averaging. Genom. Proteom. Bioinform. 2006, 4, 259–263. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Zheng, L.; Wu, H.H.; Qanmber, G.; Ali, F.; Wang, L.L.; Liu, Z.D.; Yu, Q.; Wang, Q.; Xu, A.X.; Yang, Z.R. Genome-wide study of the GATL gene family in Gossypium hirsutum L. reveals that GhGATL genes act on pectin synthesis to regulate plant growth and fiber elongation. Genes 2020, 11, 64. [Google Scholar] [CrossRef]
- Tuskan, G.A.; DiFazio, S.; Jansson, S.; Bohlmann, J.; Grigoriev, I.; Hellsten, U.; Putnam, N.; Ralph, S.; Rombauts, S.; Salamov, A.; et al. The genome of black cottonwood, Populus trichocarpa (Torr. & gray). Science 2006, 313, 1596–1604. [Google Scholar]
- Flagel, L.E.; Wendel, J.F. Gene duplication and evolutionary novelty in plants. New Phytol. 2009, 183, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Li, J.H.; Pan, Y.; Zhang, Y.; Ni, L.; Wang, Y.L. Genome-wide identification and expression analysis of the UGlcAE gene family in tomato. Int. J. Mol. Sci. 2018, 19, 1583. [Google Scholar] [CrossRef]
- Shan, T.; Rong, W.; Xu, H.; Du, L.; Liu, X.; Zhang, Z. The wheat R2R3-MYB transcription factor TaRIM1 participates in resistance response against the pathogen Rhizoctonia cerealis infection through regulating defense genes. Sci. Rep. 2016, 6, 28777. [Google Scholar] [CrossRef]
- Ma, R.; Liu, B.; Geng, X.; Ding, X.; Yan, N.; Sun, X.; Wang, W.; Sun, X.; Zheng, C. Biological function and stress response mechanism of MYB transcription factor family genes. J. Plant Growth Regul. 2023, 42, 83–95. [Google Scholar] [CrossRef]
- Wani, S.H.; Kumar, V.; Shriram, V.; Sah, S.K. Phytohormones and their metabolic engineering for abiotic stress tolerance in crop plants. Crop J. 2016, 4, 162–176. [Google Scholar] [CrossRef]
- Liu, Y.B.; Lu, S.M.; Zhang, J.F.; Liu, S.; Lu, Y.T. A xyloglucanendotransglucosylase/hydrolase involves in the growth of primaryroot and alters the deposition of cellulose in Arabidopsis. Planta 2007, 226, 1547–1560. [Google Scholar] [CrossRef]
- Jan, A. Characterization of a xyloglucan endotransglucosylase gene that is up-regulated by gibberellin in rice. Plant Physiol. 2004, 136, 3670–3681. [Google Scholar] [CrossRef]
- Catala, C.; Rose, J.K.C.; York, W.S.; Albersheim, P.; Darvill, A.G.; Bennett, A.B. Characterization of a Tomato Xyloglucan Endotransglycosylase Gene That Is Down-Regulated by Auxin in Etiolated Hypocotyls. Plant Physiol. 2001, 127, 1180–1192. [Google Scholar] [CrossRef]
- Becnel, J.; Natarajan, M.; Kipp, A.; Braam, J. Developmental expression patterns of Arabidopsis XTH genes reported by transgenes and Genevestigator. Plant Mol. Biol. 2006, 61, 451–467. [Google Scholar] [CrossRef]
- Ahuja, I.; de Vos, R.C.H.; Bones, A.M.; Hall, R.D. Plant molecular stress responses face climate change. Trends Plant Sci. 2010, 15, 664–674. [Google Scholar] [CrossRef]
- Cosgrove, D.J. Growth of the plant cell wall. Nat. Rev. Mol. Cell Biol. 2005, 6, 850–861. [Google Scholar] [CrossRef]
- Dhar, S.; Kim, J.; Yoon, E.K.; Jang, S.; Ko, K.; Lim, J. SHORT-ROOT controls cell elongation in the etiolated arabidopsis hypocotyl. Mol. Cells 2002, 45, 243–256. [Google Scholar] [CrossRef]
- Takeda, T.; Furuta, Y.; Awano, T.; Mizuno, K.; Mitsuishi, Y.; Hayashi, T. Suppression and acceleration of cell elongation by integration of xyloglucans in pea stem segments. Proc. Natl. Acad. Sci. USA 2002, 99, 9055–9060. [Google Scholar] [CrossRef]
- Soga, K.; Wakabayashi, K.; Kamisaka, S.; Hoson, T. Effects of hypergravity on expression of XTH genes in azuki bean epicotyls. Physiol. Plant. 2007, 131, 332–340. [Google Scholar] [CrossRef]
- Bi, H.; Liu, Z.; Liu, S.; Qiao, W.; Zhang, K.; Zhao, M.; Wang, D. Genome-wide analysis of wheat xyloglucan endotransglucosylase/hydrolase (XTH) gene family revealed TaXTH17 involved in abiotic stress responses. BMC Plant Biol. 2024, 24, 640. [Google Scholar] [CrossRef]
- Clauw, P.; Coppens, F.; De Beuf, K.; Dhondt, S.; Van Daele, T.; Maleux, K.; Veronique, S.; Lieven, C.; Nathalie, G.; Inzé, D. Leaf responses to mild drought stress in natural variants of Arabidopsis. Plant Physiol. 2015, 167, 800–816. [Google Scholar] [CrossRef]
- Yan, J.; Huang, Y.; He, H.; Han, T.; Di, P.; Sechet, J.; Fang, L.; Liang, Y.; Scheller, H.V.; Mortimer, J.C.; et al. Xyloglucan endotransglucosylase-hydrolase30 negatively affects salt tolerance in Arabidopsis. J. Exp. Bot. 2019, 70, 5495–5506. [Google Scholar] [CrossRef]




| Gene | Gene Locus ID | Location | CDS (bp) | Peptide (aa) | Instability Index | Molecular Weight (Da) | PI |
|---|---|---|---|---|---|---|---|
| CmXTH1 | MELO3C018785 | chr01: 2797273–2799471 | 903 bp | 300 aa | 45.05 | 33,588.34 | 5.26 |
| CmXTH2 | MELO3C024704 | chr02: 21323928–21326399 | 870 bp | 289 aa | 34.95 | 32,917.35 | 9.53 |
| CmXTH3 | MELO3C024706 | chr02: 21333890–21335856 | 882 bp | 293 aa | 45.2 | 34,131.82 | 9.51 |
| CmXTH4 | MELO3C017482 | chr02: 22499632–22502092 | 465 bp | 154 aa | 22.88 | 17,581.87 | 4.55 |
| CmXTH5 | MELO3C017481 | chr02: 22503328–22505897 | 948 bp | 315 aa | 35.85 | 35,381.2 | 7.01 |
| CmXTH6 | MELO3C017480 | chr02: 22534180–22536499 | 864 bp | 287 aa | 30.69 | 32,324.17 | 6.08 |
| CmXTH7 | MELO3C017479 | chr02: 22546478–22547656 | 894 bp | 297 aa | 34.24 | 33,828.34 | 8.77 |
| CmXTH8 | MELO3C017478 | chr02: 22549665–22551067 | 999 bp | 332 aa | 46.86 | 37,429.94 | 5.55 |
| CmXTH9 | MELO3C017476 | chr02: 22553781–22557361 | 789 bp | 262 aa | 39.77 | 30,083.25 | 6.95 |
| CmXTH10 | MELO3C011706 | chr03: 9439998–9444256 | 639 bp | 212 aa | 43.38 | 24,762.61 | 7.80 |
| CmXTH11 | MELO3C003441 | chr04: 1148942–1153580 | 1047 bp | 348 aa | 48.95 | 40,244.39 | 8.72 |
| CmXTH12 | MELO3C026755 | chr04: 24982870–24984241 | 891 bp | 296 aa | 43.07 | 34,184.68 | 8.45 |
| CmXTH13 | MELO3C009367 | chr04: 31375327–31376928 | 987 bp | 328 aa | 54.7 | 38,015.38 | 9.46 |
| CmXTH14 | MELO3C014469 | chr05: 2181987–2183301 | 876 bp | 291 aa | 46.63 | 32,561.62 | 9.16 |
| CmXTH15 | MELO3C014468 | chr05: 2185616–2187003 | 873 bp | 290 aa | 47.39 | 32,447.42 | 8.75 |
| CmXTH16 | MELO3C014467 | chr05: 2192378–2193764 | 873 bp | 290 aa | 47.03 | 32,473.39 | 8.92 |
| CmXTH17 | MELO3C014466 | chr05: 2192362–2193783 | 972 bp | 323 aa | 46.58 | 36,197.89 | 9.26 |
| CmXTH18 | MELO3C014465 | chr05: 2199276–2201139 | 909 bp | 302 aa | 43.56 | 34,044.34 | 8.66 |
| CmXTH19 | MELO3C004087 | chr05: 23648856–23653057 | 855 bp | 284 aa | 38.41 | 32,001.04 | 8.7 |
| CmXTH20 | MELO3C004089 | chr05: 23693098–23695553 | 966 bp | 321 aa | 34.75 | 37,539.19 | 6.04 |
| CmXTH21 | MELO3C018033 | chr07: 26383224–26385502 | 918 bp | 305 aa | 37.8 | 35,560.35 | 8.66 |
| CmXTH22 | MELO3C005245 | chr09: 17959997–17962698 | 1002 bp | 333 aa | 40.86 | 38,027.88 | 6.5 |
| CmXTH23 | MELO3C012004 | chr10: 3321938–3324728 | 933 bp | 310 aa | 43.85 | 3051.95 | 6.39 |
| CmXTH24 | MELO3C018292 | chr10: 19947383–19951402 | 1014 bp | 337 aa | 48.32 | 38,689.82 | 6.89 |
| CmXTH25 | MELO3C004941 | chr12: 5989364–5991429 | 1056 bp | 351 aa | 44.8 | 40,418.45 | 8.2 |
| CmXTH26 | MELO3C021685 | chr12: 15335053–15337819 | 879 bp | 292 aa | 43.5 | 33,181.48 | 6.59 |
| CmXTH27 | MELO3C021686 | chr12: 15354703–15357507 | 891 bp | 296 aa | 40.01 | 33,782.4 | 6.22 |
| CmXTH28 | MELO3C002480 | chr12: 22365305–22367404 | 906 bp | 301 aa | 35.16 | 35,230.49 | 5.05 |
| CmXTH29 | MELO3C001951 | chr12: 25813539–25814945 | 840 bp | 279 aa | 39 | 31,972.97 | 5.34 |
| Motif | Length | Motif Consensus | Functional Annotation |
|---|---|---|---|
| 1 | 39 | REQQFHLWFDPTKDFHTYSILWNPQSIVFLVDNIPIRVF | GH16 |
| 2 | 42 | PMRLYSSJWNADDWATRGGLVKTDWTKAPFTASYRBFNABGC | GH16 |
| 3 | 30 | HDEIDFEFLGNLSGDPYTLHTNVYSQGKGB | GH16 |
| 4 | 50 | NGRLLTLSLDKDSGSGFQSKNZYLFGKFDMQIKLVPGNSAGTVTAFYLSS | GH16 |
| 5 | 34 | GPASSGLDAAQRNRLRWVQKKYMIYBYCTDTKRF | XET |
| 6 | 29 | MASIMTASAGNFLQDVDITWGDGRAKILB | Unknown |
| 7 | 11 | KNWENKGVPFP | Unknown |
| 8 | 8 | QGLPPECK | Unknown |
| 9 | 15 | MHSPHKGLTIMLLQC | Unknown |
| 10 | 15 | ASSGSSSCGSKFSST | Unknown |
| 11 | 41 | MAKFRSFFIAFLVCVIVYNHIQVEAKMSKNMVJNWGNSQSK | Unknown |
| 12 | 27 | PDEAERLRKFDPVTFGKGRRHRGKIRH | Unknown |
| 13 | 12 | PQCAPKSPNNWW | Unknown |
| 14 | 14 | FDEGYRPLWGPDHL | Unknown |
| 15 | 8 | MGGDYPSK | Unknown |
| 16 | 7 | WCCQGSA | Unknown |
| 17 | 6 | CFCNFV | Unknown |
| 18 | 6 | VDPIEQ | Unknown |
| 19 | 10 | CGPQGQRWWD | Unknown |
| Sequence1 | Sequence2 | Ka | Ks | Ka/Ks |
|---|---|---|---|---|
| CmXTH6 | CmXTH20 | 0.310543605 | 2.090064483 | 0.148580873 |
| CmXTH13 | CmXTH25 | 0.258461427 | 3.495944739 | 0.073931783 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, S.; Kang, Y.; Lin, Y.; Zheng, X.; Wu, Y.; Yang, Z. A Genome-Wide Identification and Expression Analysis of the Xyloglucan Endotransglucosylase/Hydrolase Gene Family in Melon (Cucumis melo L.). Horticulturae 2024, 10, 1017. https://doi.org/10.3390/horticulturae10101017
Zhao S, Kang Y, Lin Y, Zheng X, Wu Y, Yang Z. A Genome-Wide Identification and Expression Analysis of the Xyloglucan Endotransglucosylase/Hydrolase Gene Family in Melon (Cucumis melo L.). Horticulturae. 2024; 10(10):1017. https://doi.org/10.3390/horticulturae10101017
Chicago/Turabian StyleZhao, Shiwen, Yushi Kang, Yuqin Lin, Xue Zheng, Yongjun Wu, and Zhenchao Yang. 2024. "A Genome-Wide Identification and Expression Analysis of the Xyloglucan Endotransglucosylase/Hydrolase Gene Family in Melon (Cucumis melo L.)" Horticulturae 10, no. 10: 1017. https://doi.org/10.3390/horticulturae10101017
APA StyleZhao, S., Kang, Y., Lin, Y., Zheng, X., Wu, Y., & Yang, Z. (2024). A Genome-Wide Identification and Expression Analysis of the Xyloglucan Endotransglucosylase/Hydrolase Gene Family in Melon (Cucumis melo L.). Horticulturae, 10(10), 1017. https://doi.org/10.3390/horticulturae10101017





