From Weeds to Feeds: Exploring the Potential of Wild Plants in Horticulture from a Centuries-Long Journey to an AI-Driven Future
Abstract
:1. Introduction
2. Materials and Methods
2.1. Research Design and Approach
2.2. Gathering Ethnobotanical Knowledge in the Field
2.3. Building a Global Database of Wild Food Plants
2.4. Conceptual Analysis
2.5. Specific Models
2.6. Proposal for a Specialized Model Combining Multimodal Data
3. Results and Discussion
3.1. Literature Background—Concept of Wild Plants and Their Potential Use as a Novel Crop
3.1.1. Steps in the Concept of Wild Plants
3.1.2. Horticultural Perspective of the “Wild Plant”
- Post-cultivated wild plants, which merely extend a previously domesticated line but have been left uncultivated thereafter.
- Sub-spontaneous wild plants that originate in uncultivated soil from seeds of cultivated plants.
- Spontaneous wild plants that represent a natural element in the local flora. These spontaneous wild plants can, at least theoretically, have a triple origin:
- They may derive from sub-spontaneous wild plants that have found favorable conditions in the natural environment for a return to a wild state. We will refer to them as colonial wild plants.
- They may descend from ancestors that have never passed through the cultivated stage. We will refer to them as autochthonous, or indigenous, wild plants.
- Hybrid mixed wild plants. They may result from the hybridization of indigenous wild plants with either of the forms, and these are referred to as hybrid wild plants.
3.1.3. “Weeds” Versus “Invasive Species”
3.1.4. Weeds as Novel Crops
3.1.5. Indigenous Wild Plants Versus Weeds as Potential Novel Crops
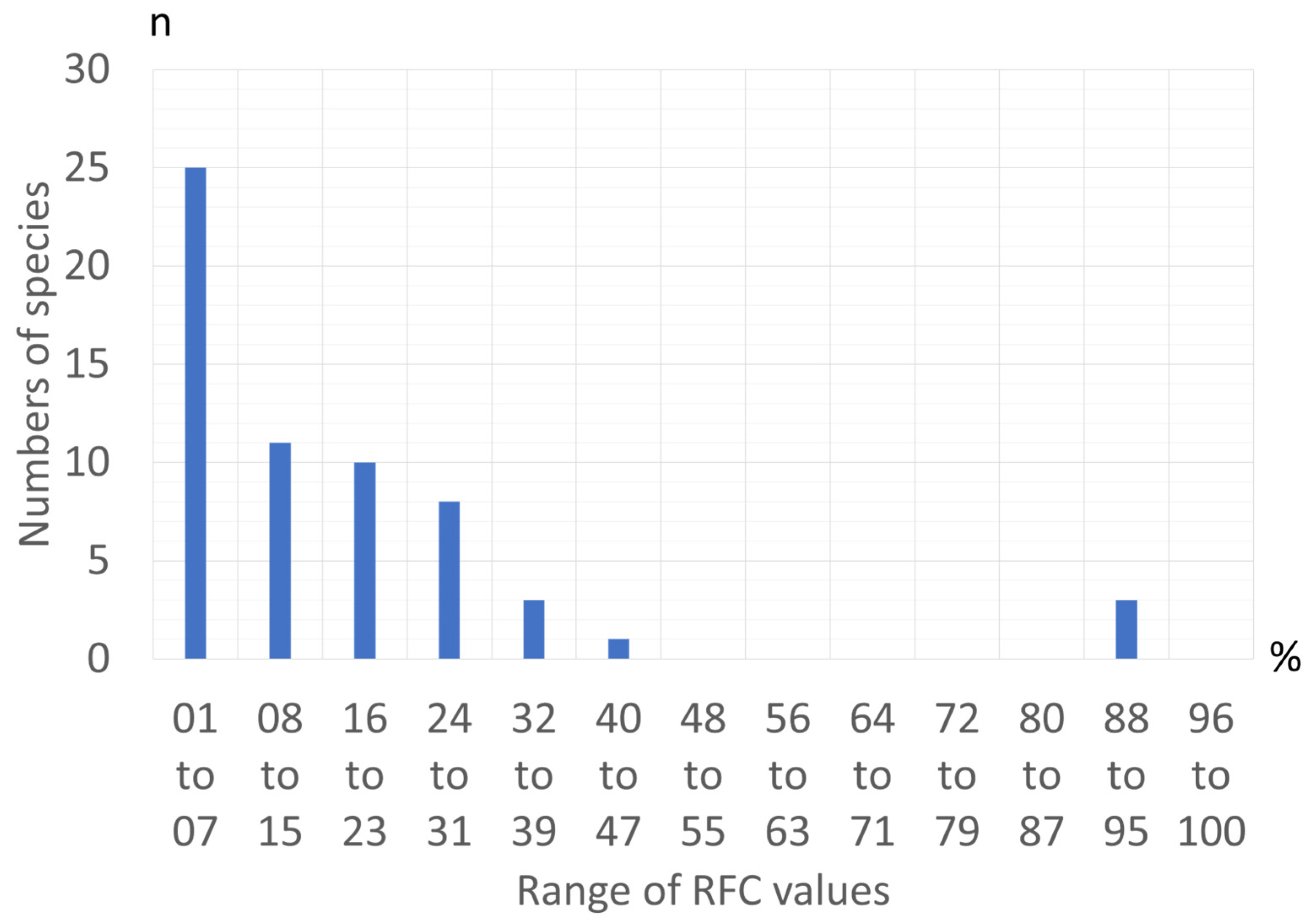
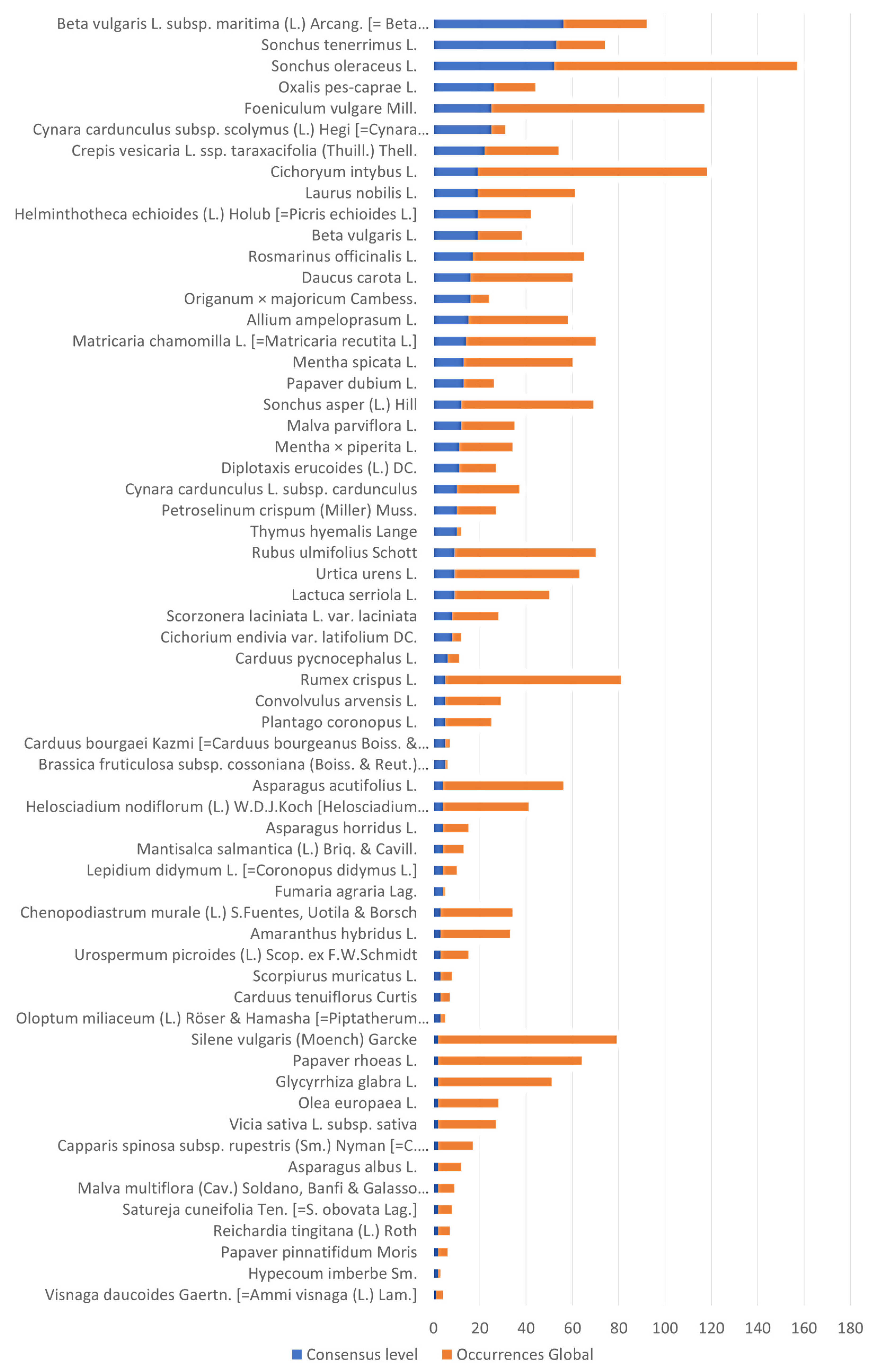
3.2. Occurrences, Extension of Use, and Local Consensus for Selecting Wild Food Plant Species as Potential Crops
The Local Consensus Model for the Huerta de Murcia (Spain)
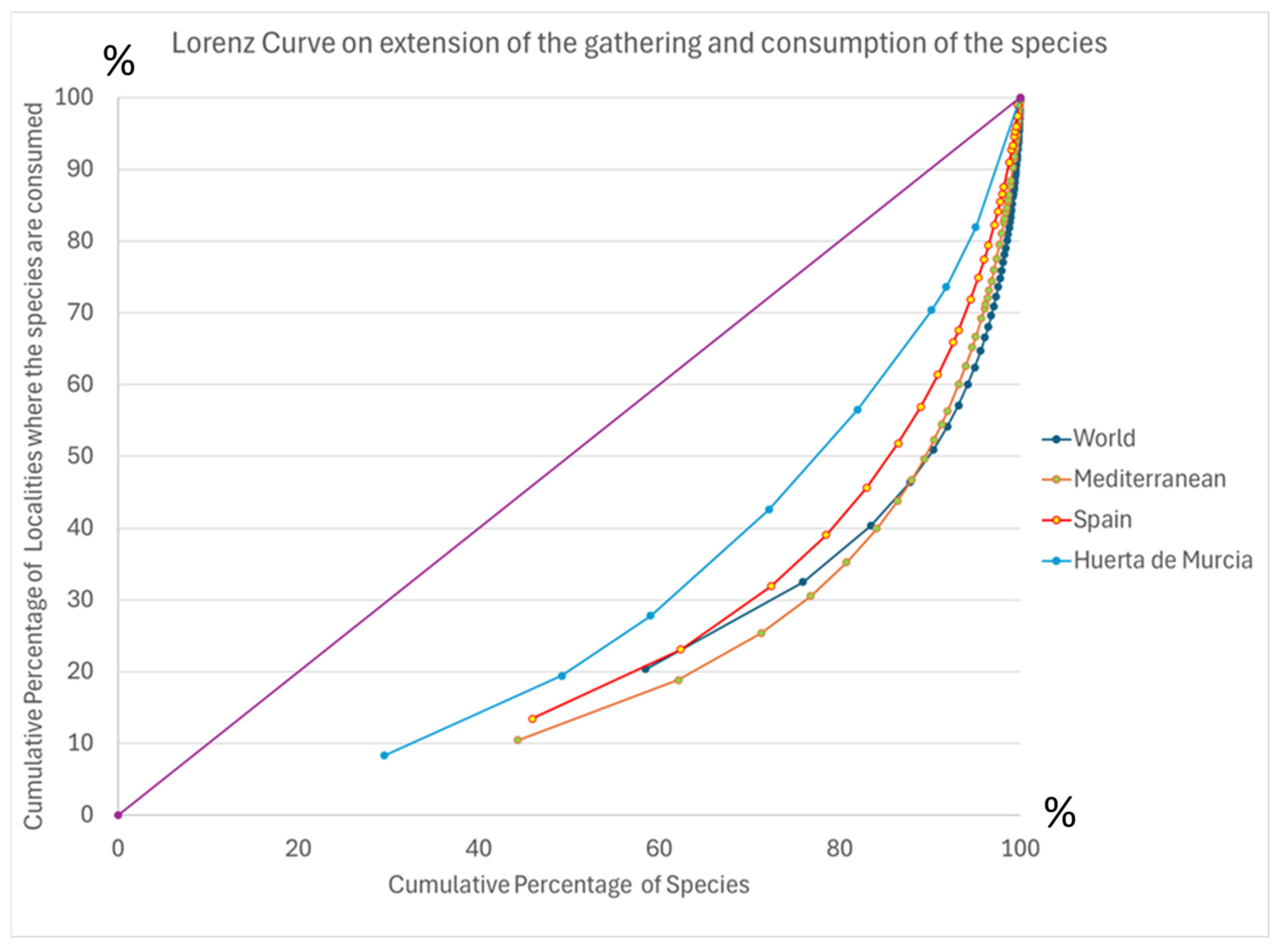
3.3. Analysis at Different Scales: A Comparison of Murcia and World Data
3.3.1. The Global Occurrence Patterns of Potential Crops
3.3.2. Integrating Diverse Sources of Evidence
3.4. Developing a General Model to Integrate Multimodal Data
3.4.1. Preliminary Considerations
3.4.2. Tools and Techniques Available
- Machine Learning Algorithms [57]: Supervised learning is useful for predicting outcomes based on labeled training data. Common algorithms include decision trees, random forests, support vector machines, and neural networks. Unsupervised learning helps in clustering and finding patterns in data without pre-existing labels. Algorithms like k-means clustering and hierarchical clustering can be used to group plants with similar traits.
- Natural Language Processing (NLP) [58]: Text mining and data extraction: Tools like NLTK [59] (it’s an open-source project developed and maintained by a community of contributors), spaCy [60] (it is an open-source project developed and maintained by a community of contributors), and BERT [61] (Google AI. While Google AI, Alphabet Inc., Mountain View, CA, USA) can extract relevant information about plant characteristics, nutritional value, health benefits, and cultivation requirements from the scientific literature, databases, and online resources. Sentiment analysis and entity recognition tools can be used to understand and categorize qualitative data from various sources.
- Geographic Information Systems (GISs): Spatial analysis, tools like ArcGIS (Esri, Redlands, CA, USA) and QGIS (it is developed by a global community of volunteers and is not affiliated with any specific company or country) can help analyze the geographic distribution of wild plants and their environmental conditions, providing insights into optimal growing regions. Remote sensing, satellites, and drones can gather data on land use, soil types, and climate conditions to support decision-making.
- Genomic and Bioinformatics Tools: Gene sequencing data analysis [62]: Tools like BLAST [63] (NCBI, Bethesda, MD, USA), ClustalW (its development and maintenance have been primarily carried out at the University of Cambridge in the United Kingdom), and Bioconductor (initially developed at the Fred Hutchinson Cancer Research Center in Seattle, WA, USA, it has since become a collaborative effort) can analyze genetic information to identify plants with desirable traits for breeding and cultivation. Genome-wide association studies (GWAS) (is not associated with a specific company or country) [64] are used to find correlations between genetic variants and traits of interest in plants.
- Big Data Platforms: Data storage and processing in platforms like Hadoop (the Apache Software Foundation, which oversees Hadoop’s and Spark’s development and governance, is a global collaboration of individuals and organizations), Spark, and Google Cloud (Google LLC, Mountain View, CA, USA) to manage and process large datasets efficiently. Data integration [65] with tools like KNIME (it’s a collaborative project involving researchers and developers from various institutions worldwide. The KNIME AG company, based in Switzerland, provides commercial support and additional features for enterprise users) and Alteryx (Alteryx, Inc. Irvine, CA, USA) can integrate data from multiple sources, including genomic, phenotypic, and environmental data.
- Decision Support Systems (DSSs) [66]: multi-criteria decision analysis (MCDA): Tools like DEXi (Dexi.io San Francisco, CA, USA) and PROMETHEE (it is not associated with a specific company or country) can evaluate multiple criteria to rank and select candidate species based on their suitability for cultivation. Expert systems: AI-driven systems that use rule-based approaches to simulate expert-level decision-making in selecting crop candidates.
- AI and Machine Learning Platforms: TensorFlow (Google Brain, a research division of Google AI, which is part of Alphabet Inc., the parent company of Google, Mountain View, CA, USA) [67] and PyTorch (Facebook AI Research (FAIR), Menlo Park, CA, USA) [68]: widely used frameworks for developing and deploying machine learning models. AutoML Tools [69,70]: tools like Google AutoML (Google Cloud, Alphabet Inc., Mountain View, CA, USA) and H2O.ai (H2O.ai, Mountain View, California, Estados Unidos) [71] automate the process of model selection, hyperparameter tuning, and feature engineering.
- Data Visualization Tools [72]: Visualization libraries: libraries like Matplotlib (it is not associated with a specific company or country) [73], Seaborn (it is an open-source project developed and maintained by a community of contributors) [74], and Plotly (Plotly, Inc., Montreal, QC, Canada) [75] in Python (it was created by Guido van Rossum in the late 1980s and released to the public in 1991) can create insightful visualizations to interpret complex data. Interactive dashboards: tools like Tableau (Salesforce, Seattle, WA, USA) and Power BI (Microsoft Power Platform, Microsoft Corporation, Redmond, WA, USA) [76] can create interactive dashboards to help stakeholders understand the data and results.
- Collaborative Platforms and Research Collaboration Tools [77]: Platforms like GitHub (GitHub, San Francisco, CA, USA. It was acquired by Microsoft in 2018) [78] and Jupyter Notebooks (Jupyter Notebooks don’t have a specific physical location) [79] facilitate collaboration among researchers by enabling code sharing and collaborative data analysis.
3.4.3. Critical Points
- Multimodal Data Integration: The model must build a coherent data system integrating ecological (environmental conditions where the species thrive, such as soil type, climate, and geographic distribution), genetic (genomic information to understand the genetic diversity, adaptation mechanisms, and potential for breeding), agronomic (growth rates, yield potential, resistance to pests and diseases, and watering requirements) [80], socioeconomic (local and global market demand, cultural importance, and economic viability), ethnobotanical (historical and traditional uses of the species, including medicinal, nutritional, and cultural significance), nutritional, toxicological, and pharmacological data (active substances present, their relative abundance and their physiological and pharmacological properties in humans and livestock) [81].
- AI Techniques: Machine learning supervised and unsupervised learning algorithms to identify patterns and make predictions based on integrated data. Deep learning, neural networks for complex data analysis, such as image recognition of plant species or genomic data interpretation [82]. Natural language processing (NLP) [83] to analyze textual data from the scientific literature, reports, and ethnobotanical records. Geospatial analysis, GIS, and remote sensing data to map species distribution and environmental conditions.
- Model Development: Feature selection, identifying the most relevant variables from the multimodal data that influence the potential of species as crops. Model training using historical data to train AI models to predict the suitability and potential productivity of plant species. Cross-validation and testing with independent datasets to ensure model accuracy and reliability.
3.4.4. Key Steps
- Data collection and preprocessing: Gather data from various sources, including ecological databases, genetic repositories, agronomic trials, and socioeconomic surveys. Clean and preprocess the data to manage missing values, normalize scales, and encode categorical variables.
- Feature Engineering: derive new features that capture interactions between diverse types of data, such as genotype–environment interactions or socio-economic factors influencing plant use. Use domain knowledge to guide feature selection and ensure meaningful variables are included.
- Model Selection: Start with basic machine learning models (e.g., decision trees [84], random forests [85]) to establish baselines. Progress to more complex models (e.g., neural networks [86], ensemble methods [87]) for improved accuracy and robustness. Implement geospatial models to incorporate environmental data effectively.
- Training and Validation: Split the data into training and validation sets. Train the model on the training set, using cross-validation techniques to fine-tune hyperparameters. Validate the model on the validation set, iterating to improve performance.
- Deployment, Monitoring, and Updating: Deployment involves implementing the AI model in a practical and accessible format, such as a software application or online platform so that stakeholders—such as researchers, practitioners, or decision-makers—can easily interact with and utilize the model. The goal is to make the AI tool user-friendly, ensuring that it meets the needs of its intended audience and integrates smoothly into their workflows. Monitoring involves evaluating its accuracy, efficiency, and overall effectiveness as it processes new data. Continuous monitoring helps to identify any issues or discrepancies that may arise and ensures that the model remains reliable and relevant. Updating involves regularly refining the model based on performance evaluations and new information to maintain its relevance and effectiveness.
- Case Studies and Pilot Projects: Conduct case studies or pilot projects to validate the model using real-world scenarios and gather feedback from agronomists, farmers, and other stakeholders to refine the model and its application.
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Smith, L.C.; El Obeid, A.E.; Jensen, H.H. The geography and causes of food insecurity in developing countries. Agric. Econ. 2000, 22, 199–215. [Google Scholar] [CrossRef]
- Minnis, P.E. Famine Foods of the North American Desert Borderlands in Historical Context. In Ethnobotany a Reader; Minnis, P.E., Ed.; University of Oklahoma Press: Norman, OK, USA, 2000; pp. 214–239. [Google Scholar]
- Begossi, A. Food Taboos—A Scientific Reason? In Plants for Food and Medicine; Prendergast, H.D.V., Etkin, N., Harris, D., Houghton, P., Eds.; Royal Botanic Gardens: Kew, UK, 1998; pp. 41–46. [Google Scholar]
- Winstead, D.J.; Jacobson, M.G. Food resilience in a dark catastrophe: A new way of looking at tropical wild edible plants. Ambio 2022, 51, 1949–1962. [Google Scholar] [CrossRef] [PubMed]
- Handa, S.S. The Integration of food and medicine in India. In Plants for Food and Medicine; Prendergast, H.D.V., Etkin, N., Harris, D., Houghton, P., Eds.; Royal Botanic Gardens: Kew, UK, 1998; pp. 57–68. [Google Scholar]
- Sinha, R.; Sinha, S. Ethnobiology; Surhaby Publications: Jaipur, India, 2001; pp. 1–335. [Google Scholar]
- Ding, X.Y.; Zhang, Y.; Wang, L.; Zhuang, H.F.; Chen, W.Y.; Wang, Y.H. Collection calendar: The diversity and local knowledge of wild edible plants used by Chenthang Sherpa people to treat seasonal food shortages in Tibet, China. J. Ethnobiol. Ethnomed. 2021, 17, 40. [Google Scholar] [CrossRef]
- Gomes, L.C.A.; Medeiros, P.M.; Prata, A. Patterns of use of wild food plants by Brazilian local communities: Systematic review and meta-analysis. J. Ethnobiol. Ethnomed. 2023, 19, 47. [Google Scholar] [CrossRef] [PubMed]
- Guarrera, P.M.; Savo, V. Wild food plants used in traditional vegetable mixtures in Italy. J. Ethnopharmacol. 2016, 185, 202–234. [Google Scholar] [CrossRef]
- Motti, R. Wild Plants Used as Herbs and Spices in Italy: An Ethnobotanical Review. Plants 2021, 10, 563. [Google Scholar] [CrossRef]
- Fongnzossie, E.F.; Nyangono, C.F.B.; Biwole, A.B.; Ebai, P.N.B.; Ndifongwa, N.B.; Motove, J.; Dibong, S.D. Wild edible plants and mushrooms of the Bamenda Highlands in Cameroon: Ethnobotanical assessment and potentials for enhancing food security. J. Ethnobiol. Ethnomed. 2020, 16, 12. [Google Scholar] [CrossRef]
- Zohary, M. Geobotanical Foundations of the Middle East; Gustav Fisher Verlag: Stuttgart, Germany, 1973; Volumes 1 and 2. [Google Scholar]
- Ertuğ, F. An ethnobotanical study in Central Anatolia (Turkey). Econ. Bot. 2000, 54, 155–182. Available online: https://www.jstor.org/stable/4256288 (accessed on 14 September 2024).
- Pyke, G.H.; Starr, C.K. Optimal foraging theory. In Encyclopedia of Social Insects; Starr, C.K., Ed.; Springer International Publishing: Berlin/Heidelberg, Germany, 2021; pp. 677–685. [Google Scholar] [CrossRef]
- O’Connell, J.F.; Hawkes, K. Alyawara plant use and optimal foraging theory. In Hunter-Gatherer Foraging Strategies: Ethnographic and Archaeological Analyses; Winterhalder, B., Smith, E., Eds.; University of Chicago Press: Chicago, IL, USA, 1981; pp. 99–125. Available online: https://archive.org/details/huntergathererfo0000unse/mode/2up (accessed on 15 July 2024).
- Pyke, G.H. Optimal foraging theory: A critical review. Annu. Rev. Ecol. Syst. 1984, 15, 523–575. [Google Scholar] [CrossRef]
- Gomes, L.; de Medeiros, P.; Prata, A. Wild food plants of Brazil: A theoretical approach to non-random selection. J. Ethnobiol. Ethnomed. 2023, 19, 28. [Google Scholar] [CrossRef]
- Haq, S.M.; Khoja, A.A.; Waheed, M.; Siddiqui, M.H.; Alamri, S.; Alfagham, A.T.; Al-Humaid, L.; Bussmann, R.W. Food ethnobotany of forest resource in the high-altitude Himalaya Mountains: Enhancing the food sovereignty of ethnic groups. For. Policy Econ. 2024, 164, 103247. [Google Scholar] [CrossRef]
- Ceccanti, C.; Landi, M.; Benvenuti, S.; Pardossi, A.; Guidi, L. Mediterranean Wild Edible Plants: Weeds or “New Functional Crops”? Molecules 2018, 23, 2299. [Google Scholar] [CrossRef] [PubMed]
- Guarrera, P.M.; Savo, V. Perceived health properties of wild and cultivated food plants in local and popular traditions of Italy: A review. J. Ethnopharmacol. 2013, 146, 659–680. [Google Scholar] [CrossRef] [PubMed]
- Abbet, C.; Mayor, R.; Roguet, D.; Spichiger, R.; Hamburger, M.; Potterat, O. Ethnobotanical survey on wild alpine food plants in Lower and Central Valais (Switzerland). J. Ethnopharmacol. 2014, 151, 624–634. [Google Scholar] [CrossRef] [PubMed]
- Rivera, D.; Obón, C.; Inocencio, C.; Heinrich, M.; Verde, A.; Fajardo, J.; Palazón, J.A. Gathered Food Plants in the Mountains of Castilla–La Mancha (Spain): Ethnobotany and Multivariate Analysis. Econ. Bot. 2007, 61, 269–289. [Google Scholar] [CrossRef]
- Obón, C.; Martínez, R.; Giner, J.F.; Rivera, D. Las plantas comestibles recolectadas en la provincia de Alicante, estudio comparativo entre la Marina Alta y el Bajo Segura. In Salut, Alimentació I Cultura Popular al Pais Valencia; Guillem, X., García, G., Eds.; CEIC Alfons el Vell: Gandía, Spain, 2009; pp. 279–294. [Google Scholar]
- Rivera, D.; Verde, A.; Fajardo, J.; Inocencio, C.; Obón, C.; Heinrich, M. Guía Etnobotánica de los Alimentos Locales Recolectados en la Provincia de Albacete; Instituto de Estudios Albacetenses “Don Juan Manuel”: Albacete, Spain, 2006; pp. 1–470. [Google Scholar]
- Rivera, D.; Cobon Heinrich, M.; Inocencio, C.; Verde, A.; Fajardo, J. Gathered Mediterranean Food Plants—Ethnobotanical Investigations and Historical Development. In Local Mediterranean Food Plants and Nutraceuticals; Heinrich, M., Muller, W., Galli, C., Eds.; Karger: Basel, Switzerland, 2006; pp. 18–74. [Google Scholar]
- Rivera, D.; Alcaraz, F.; Obón, C. Wild and Cultivated Plants Used as Food and Medicine by the Cimbrian Ethnic Minority in the Alps. Acta Hortic. 2012, 955, 31–39. [Google Scholar] [CrossRef]
- Obón, C.; Rivera, D.; Alcaraz, F. Wild and Cultivated Plants Used as Food and Medicine by the Mòcheni Ethnic Minority in the Alps. Acta Hortic. 2012, 955, 113–118. [Google Scholar] [CrossRef]
- Leonti, M.; Nebel, S.; Rivera, D.; Heinrich, M. Wild Gathered Food Plants in the European Mediterranean: A Comparative Analysis. Econ. Bot. 2006, 60, 130–142. [Google Scholar] [CrossRef]
- Baldi, A.; Bruschi, P.; Campeggi, S.; Egea, T.; Rivera, D.; Obón, C.; Lenzi, A. The Renaissance of Wild Food Plants: Insights from Tuscany (Italy). Foods 2022, 11, 300. [Google Scholar] [CrossRef]
- Obón, C.; Nicolás, C.; Rivera, D. Estudio de las plantas comestibles silvestres del municipio de Murcia. In Actas del III Congreso de la Naturaleza de la Región de Murcia; García, P., Ed.; ANSE-CEMACAM: Murcia, Spain, 2007; pp. 97–105. [Google Scholar]
- Levadoux, L. Les populations sauvages et cultivées des Vitis vinifera L; Institut national de la recherche agronomique: Paris, France, 1956; Volume 1, pp. 59–118. [Google Scholar]
- Rivera, D.; Verde, A.; Fajardo, J.; Obón, C.; Inocencio, C.; Valdés, A. Modelos etnobiológicos como alternativa al control de malas hierbas con agricultura biológica, los criptocultivos. In La Malherbología en los Nuevos Sistemas de Producción Agraria; Mansilla, J.A., Artigao Monreal, J.A., Eds.; Sociedad Española de Malherbología: Albacete, Spain, 2007; pp. 149–154. [Google Scholar]
- Gastwirth, J.L. Estimation of the Lorenz Curve and Gini Index. Rev. Econ. Stat. 1972, 54, 306–316. [Google Scholar] [CrossRef]
- Davies, J.; Hoy, M. Making inequality comparisons when Lorenz curves intersect. Am. Econ. Rev. 1995, 85, 980–986. [Google Scholar]
- Dagum, C. The generation and distribution of income, the Lorenz curve and the Gini ratio. Économie Appliquée 1980, 33, 327–367. [Google Scholar] [CrossRef]
- OpenAI. ChatGPT, GPT-4, AI Assistant. Available online: https://www.openai.com/chatgpt (accessed on 15 July 2024).
- Gemini. Bard, AI Assistant. Available online: https://gemini.google.com/app (accessed on 15 July 2024).
- Perplexity, AI Assistant. Available online: https://www.perplexity.ai/ (accessed on 15 July 2024).
- Mistral, AI Assistant. Available online: https://chat.mistral.ai/chat (accessed on 15 July 2024).
- Jones, E.T.; McLain, R.J.; Weigand, J. Nontimber Forest Products in The United States; University Press of Kansas: Lawrence, KS, USA, 2021; pp. 1–445. [Google Scholar]
- Milla, R.; Bastida, J.M.; Turcotte, M.M.; Jones, G.; Violle, C.; Osborne, C.P.; Chacón-Labella, J.; Sosinski, Ê.E., Jr.; Kattge, J.; Laughlin, D.C.; et al. Phylogenetic patterns and phenotypic profiles of the species of plants and mammals farmed for food. Nat. Ecol. Evol. 2018, 2, 1808–1817. [Google Scholar] [CrossRef]
- Heywood, V.H. Use and Potential of Wild Plants in Farm Households; FAO: Rome, Italy, 1999; pp. 1–113. [Google Scholar]
- Etkin, N.L. The cult of the wild. In Eating on the Wild Side: The Pharmacologic, Ecologic, and Social Implications of Using Noncultigens; Etkin, N.L., Ed.; The University of Arizona Press: Tucson, AZ, USA, 1994; pp. 1–21. [Google Scholar]
- Harlan, J.R. Genetic Resources in Wild Relatives of Crops. Crop Sci. 1976, 16, 329–333. [Google Scholar] [CrossRef]
- Sõukand, R.; Kalle, R. Changes in the Use of Wild Food Plants in Estonia18th—21st Century; Springer Nature: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Peters, C.R.; O’Brien, E.M.; Box, E.O. Plant types and seasonality of wild-plant foods, Tanzania to southwestern Africa: Resources for models of the natural environment. J. Hum. Evol. 1984, 13, 397–414. [Google Scholar] [CrossRef]
- Head, L. The social dimensions of invasive plants. Nat. Plants 2017, 3, 1–7. [Google Scholar] [CrossRef]
- Gioria, M.; Hulme, P.E.; Richardson, D.M.; Pyšek, P. Why are invasive plants successful? Annu. Rev. Plant Biol. 2023, 74, 635–670. [Google Scholar] [CrossRef]
- Müller-Schärer, H.; Schaffner, U.; Steinger, T. Evolution in invasive plants: Implications for biological control. Trends Ecol. Evol. 2004, 19, 417–422. [Google Scholar] [CrossRef]
- Leonti, M. The relevance of quantitative ethnobotanical indices for ethnopharmacology and ethnobotany. J. Ethnopharmacol. 2022, 288, 115008. [Google Scholar] [CrossRef]
- Tardío, J.; Pardo-de-Santayana, M. Cultural importance indices: A comparative analysis based on the useful wild plants of southern cantabria (northern Spain). Econ. Bot. 2008, 62, 24–39. [Google Scholar] [CrossRef]
- Medeiros, M.F.T.; Silva, O.S.; Albuquerque, U.P. Quantification in ethnobotanical research: An overview of indices used from 1995 to 2009. Sitientibus Série Ciências Biológicas 2011, 11, 211–230. [Google Scholar] [CrossRef]
- Piketty, T. Capital in the Twenty-First Century; Harvard University Press: Cambridge, MA, USA, 2014. [Google Scholar]
- Chotikapanich, D. Modeling Income Distributions and Lorenz Curves; Springer Nature: Berlin/Heidelberg, Germany, 2008. [Google Scholar]
- Duan, C.; Chen, B. Analysis of global energy consumption inequality by using Lorenz curve. Energy Procedia 2018, 152, 750–755. [Google Scholar] [CrossRef]
- GBIF—Global Biodiversity Information Facility. Free and Open Access to Biodiversity Data. Available online: https://www.gbif.org/ (accessed on 11 September 2024).
- Pandey, R.; Kumar Khatri, S.; Kumar Singh, N.; Verma, P. Artificial Intelligence and Machine Learning for EDGE Computing; Elsevier—Academic Press: Amsterdam, The Netherlands, 2022. [Google Scholar] [CrossRef]
- Lauriola, I.; Lavelli, A.; Aiolli, F. An introduction to deep learning in natural language processing: Models, techniques, and tools. Neurocomputing 2022, 470, 443–456. [Google Scholar] [CrossRef]
- Bird, S. NLTK: The Natural Language Toolkit. In Proceedings of the COLING/ACL 2006 Interactive Presentation Sessions, Sydney, Australia, 17–18 July 2006; Curran, J., Ed.; Association for Computational Linguistics: Sydney, Australia, 2006; pp. 69–72. Available online: https://aclanthology.org/P06-4018.pdf (accessed on 14 September 2024).
- Vasiliev, Y. Natural Language Processing with Python and Spacy: A Practical Introduction; No Starch Press: San Francisco, CA, USA, 2020; pp. 1–216. [Google Scholar]
- Yilmaz, Z.A.; Wang, S.; Yang, W.; Zhang, H.; Lin, J. Applying BERT to document retrieval with birch. In Proceedings of the 2019 Conference on Empirical Methods in Natural Language Processing and the 9th International Joint Conference on Natural Language Processing (EMNLP-IJCNLP): System Demonstrations, Hong Kong, China, 3–7 November 2019; Padó, S., Huang, R., Eds.; Association for Computational Linguistics: Hong Kpong, China, 2019; pp. 19–24. Available online: https://aclanthology.org/D19-3004.pdf (accessed on 14 September 2024).
- Singh, V.; Kumar, A. Advances in Bioinformatics; Springer: Singapore, 2021. [Google Scholar] [CrossRef]
- McGinnis, S.; Madden, T.L. BLAST: At the core of a powerful and diverse set of sequence analysis tools. Nucleic Acids Res. 2004, 32 (Suppl. S2), W20–W25. [Google Scholar] [CrossRef]
- Uffelmann, E.; Huang, Q.Q.; Munung, N.S.; De Vries, J.; Okada, Y.; Martin, A.R.; Martin, H.; Lappalainen, T.; Posthuma, D. Genome-wide association studies. Nat. Rev. Methods Primers 2021, 1, 59. [Google Scholar] [CrossRef]
- Kazi, L.; Cherkashin, E.; Ristevski, B. IX International Conference, Applied Internet and Information Technologies, AIIT2019, Proceedings; University of Novi Sad, Technical faculty “Mihajlo Pupin”: Zrenjanin, Republic of Serbia, 2019; Available online: https://eprints.uklo.edu.mk/id/eprint/8749/1/Proceedings_AIIT2019.pdf (accessed on 14 September 2024).
- Zhai, Z.; Martínez, J.F.; Beltran, V.; Martínez, N.L. Decision support systems for agriculture 4.0: Survey and challenges. Comput. Electron. Agric. 2020, 170, 105256. [Google Scholar] [CrossRef]
- Pang, B.; Nijkamp, E.; Wu, Y.N. Deep learning with TensorFlow: A review. J. Educ. Behav. Stat. 2020, 45, 227–248. [Google Scholar] [CrossRef]
- Raschka, S.; Liu, Y.H.; Mirjalili, V. Machine Learning with PyTorch and Scikit-Learn: Develop Machine Learning and Deep Learning Models with Python; Packt Publishing Ltd.: Birmingham, UK, 2022; pp. 1–216. [Google Scholar]
- Karmaker, S.K.; Hassan, M.M.; Smith, M.J.; Xu, L.; Zhai, C.; Veeramachaneni, K. AutoML to date and beyond: Challenges and opportunities. ACM Comput. Surv. (CSUR) 2021, 54, 1–36. [Google Scholar] [CrossRef]
- AutoML.org. Freiburg-Hannover-Tübingen. AutoML. Available online: https://www.automl.org/automl/ (accessed on 25 July 2024).
- H2O. H2O Danube. Available online: https://h2o.ai/ (accessed on 25 July 2024).
- Islam, M.; Jin, S. An Overview of Data Visualization. In Proceedings of the 2019 International Conference on Information Science and Communications Technologies (ICISCT), Tashkent, Uzbekistan, 4–6 November 2019; pp. 1–7. [Google Scholar] [CrossRef]
- Matplotlib. Matplotlib: Visualization with Python. Available online: https://matplotlib.org/ (accessed on 25 July 2024).
- Waskom, M. Seaborn: Statistical Data Visualization. Available online: https://seaborn.pydata.org/ (accessed on 25 July 2024).
- Plotly. Plotly.py. Available online: https://github.com/plotly/plotly.py (accessed on 25 July 2024).
- Gonçalves, C.T.; Gonçalves, M.J.A.; Campante, M.I. Developing Integrated Performance Dashboards Visualisations Using Power BI as a Platform. Information 2023, 14, 614. [Google Scholar] [CrossRef]
- Romano, P.; Giugno, R.; Pulvirenti, A. Tools and collaborative environments for bioinformatics research. Brief. Bioinform. 2011, 12, 549–561. [Google Scholar] [CrossRef] [PubMed]
- GitHub. Let’s Build from Here. The World’s Leading AI-Powered Developer Platform. Available online: https://github.com/ (accessed on 25 July 2024).
- Jupyter. Jupyter Notebooks. Free Software, Open Standards, and Web Services for Interactive Computing Across all Programming Languages. Available online: https://jupyter.org/ (accessed on 25 July 2024).
- Li, L.; Liu, L.; Peng, Y.; Su, Y.; Hu, Y.; Zou, R. Integration of multimodal data for large-scale rapid agricultural land evaluation using machine learning and deep learning approaches. Geoderma 2023, 439, 116696. [Google Scholar] [CrossRef]
- Xie, L.; Draizen, E.J.; Bourne, P. Harnessing big data for systems pharmacology. Annu. Rev. Pharmacol. Toxicol. 2017, 57, 245–262. [Google Scholar] [CrossRef]
- Choi, R.Y.; Coyner, A.S.; Kalpathy-Cramer, J.; Chiang, M.F.; Campbell, J. Introduction to machine learning, neural networks, and deep learning. Transl. Vis. Sci. Technol. 2020, 9, 14. [Google Scholar] [CrossRef] [PubMed]
- Min, B.; Ross, H.; Sulem, E.; Veyseh, A.P.B.; Nguyen, T.H.; Sainz, O.; Aguirre, E.; Heinz, I.; Roth, D. Recent advances in natural language processing via large pre-trained language models: A survey. ACM Comput. Surv. 2023, 56, 1–40. [Google Scholar] [CrossRef]
- Blockeel, H.; Devos, L.; Frénay, B.; Nanfack, G.; Nijssen, S. Decision trees: From efficient prediction to responsible AI. Front. Artif. Intell. 2023, 6, 1124553. [Google Scholar] [CrossRef] [PubMed]
- Antoniadis, A.; Lambert-Lacroix, S.; Poggi, J.M. Random forests for global sensitivity analysis: A selective review. Reliab. Eng. Syst. Saf. 2021, 206, 107312. [Google Scholar] [CrossRef]
- Gawlikowski, J.; Tassi, C.R.N.; Ali, M.; Lee, J.; Humt, M.; Feng, J.; Kruspe, A.; Triebel, R.; Jung, P.; Roscher, R.; et al. A survey of uncertainty in deep neural networks. Artif. Intell. Rev. 2023, 56 (Suppl. S1), 1513–1589. [Google Scholar] [CrossRef]
- Dong, X.; Yu, Z.; Cao, W.; Shi, Y.; Ma, Q. A survey on ensemble learning. Front. Comput. Sci. 2020, 14, 241–258. [Google Scholar] [CrossRef]
- Marcílio, W.E.; Eler, D.M. From explanations to feature selection: Assessing SHAP values as feature selection mechanism. In Proceedings of the 2020 33rd SIBGRAPI Conference on Graphics, Patterns and Images (SIBGRAPI), Porto de Galinhas, Brazil, 7–10 November 2020; IEEE: Piscataway, NJ, USA, 2020; pp. 340–347. [Google Scholar] [CrossRef]
- Visani, G.; Bagli, E.; Chesani, F.; Poluzzi, A.; Capuzzo, D. Statistical stability indices for LIME: Obtaining reliable explanations for machine learning models. J. Oper. Res. Soc. 2022, 73, 91–101. [Google Scholar] [CrossRef]
- POWO. Plant of the World Online. Available online: https://powo.science.kew.org/ (accessed on 18 September 2024).
- Google Scholar. Google Scholar. Available online: https://scholar.google.com/ (accessed on 18 September 2024).
- Web of Science. Web of Science. Available online: https://www.webofscience.com/wos/woscc/basic-search (accessed on 18 September 2024).
- PubMed. National Library of Medicine. Available online: https://pubmed.ncbi.nlm.nih.gov/ (accessed on 18 September 2024).
- Molina, M.; Pardo-de-Santayana, M.; Tardío, J. Natural production and cultivation of Mediterranean wild edibles. In Mediterranean Wild Edible Plants; Sánchez-Mata, M.C., Tardío, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2016; pp. 81–107. Available online: https://link.springer.com/chapter/10.1007/978-1-4939-3329-7_5 (accessed on 14 September 2024).
- Mayer-Chissick, U.; Lev, E. Wild edible plants in Israel tradition versus cultivation. In Medicinal and Aromatic Plants of the Middle-East, Medicinal and Aromatic Plants of the World 2; Yaniv, Z., Dudai, N., Eds.; Springer: Dordrecht, The Netherlands, 2014; pp. 9–26. [Google Scholar] [CrossRef]
- Singh, N.; Pandey, R.; Chandraker, S.K.; Pandey, S.; Malik, S.; Patel, D. Use of Wild Edible Plants Can Meet the Needs of Future Generation. In Agro-Biodiversity and Agri-Ecosystem Management; Kumar, P., Tomar, R.S., Bhat, J.A., Dobriyal, M., Rani, M., Eds.; Springer: Singapore, 2022; pp. 341–366. [Google Scholar] [CrossRef]
- Bacchetta, L.; Visioli, F.; Cappelli, G.; Caruso, E.; Martin, G.; Nemeth, E.; Bacchetta, G.; Bedini, G.; Wezel, A.; van Asseldonk, T.; et al. A Manifesto for the Valorization of Wild Edible Plants. J. Ethnopharmacol. 2016, 191, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Arreola, J.; Franco, J.A.; Martínez-Sánchez, J.J. Fertilization strategies for Silene vulgaris (Caryophyllaceae) production, a wild species with alimentary use. HortScience 2004, 39, 796D. Available online: https://journals.ashs.org/hortsci/view/journals/hortsci/39/4/article-p796D.xml (accessed on 25 July 2024). [CrossRef]
- Arreola, J.; Franco, J.A.; Vicente, M.J.; Martinez-Sanchez, J.J. Effect of nursery irrigation regimes on vegetative growth and root development of Silene vulgaris after transplantation into semi-arid conditions. J. Hortic. Sci. Biotechnol. 2006, 81, 583–592. [Google Scholar] [CrossRef]
- Paschoalinotto, B.H.; Polyzos, C.; Rouphael, A.; Dias, M.I.B.; Petropoulos, S.A. Domestication of wild edible species: The response of Scolymus hispanicus plants to different fertigation regimes. Horticulturae 2023, 9, 103. [Google Scholar] [CrossRef]
- Papadimitriou, D.M.; Daliakopoulos, I.N.; Kontaxakis, E.; Sabathianakis, M.; Manios, T.; Savvas, D. Effect of moderate salinity on Golden Thistle (Scolymus hispanicus L.) grown in a soilless cropping system. Sci. Hortic. 2022, 303, 111182. [Google Scholar] [CrossRef]
- Paoletti, A.; Benincasa, P.; Famiani, F.; Rosati, A. Spear yield and quality of wild asparagus (Asparagus acutifolius L.) as an understory crop in two olive systems. Agrofor. Syst. 2023, 97, 1361–1373. [Google Scholar] [CrossRef]
- Ford-Lloyd, B.V.; Schmidt, M.; Armstrong, S.J.; Barazani, O.Z.; Engels, J.; Hadas, R.; Hammer, K.; Kell, S.P.; Kang, D.; Khoshbakht, K.; et al. Crop wild relatives—Undervalued, underutilized and under threat? BioScience 2011, 61, 559–565. [Google Scholar] [CrossRef]
- Nair, K.P. Utilizing crop wild relatives to combat global warming. Adv. Agron. 2019, 153, 175–258. [Google Scholar] [CrossRef]
- Bohra, A.; Kilian, B.; Sivasankar, S.; Caccamo, M.; Mba, C.; McCouch, S.R.; Varshney, R.K. Reap the crop wild relatives for breeding future crops. Trends Biotechnol. 2022, 40, 412–431. [Google Scholar] [CrossRef]




| Basic Codes | Basic Categories | Notes |
|---|---|---|
| A | Post-cultivated wild plants | Remains of perennial crops in abandoned fields occupied by natural vegetation |
| B | Sub-spontaneous wild plants | Natural habitats suitable for the species and close to crop fields |
| C | Spontaneous wild plants | Natural habitats |
| D | Colonial wild plants (subset of spontaneous) | Natural habitats |
| E | Autochthonous or indigenous wild plants (subset of spontaneous) | Natural habitats |
| F | Hybrid mixed wild plants | Natural habitats |
| G 1 | Domesticated cultivated plants | Cropland |
| Coded relationships | Relationships between Categories 2 | |
| C = D ∪ E | Spontaneous wild plants are the union of colonial and autochthonous wild plants | |
| D ∩ E = ∅ | Colonial and autochthonous wild plants are mutually exclusive | |
| A ∩ B = ∅ | Post-cultivated and sub-spontaneous wild plants are mutually exclusive | |
| A ∩ C = ∅ | Post-cultivated and spontaneous wild plants are mutually exclusive | |
| B ∩ C = ∅ | Sub-spontaneous wild plants and spontaneous wild plants and are mutually exclusive. | |
| A ∪ B ⊆ G 1 | All post-cultural and Sub-spontaneous wild plants are domesticated plants, despite their wild appearance. | |
| F = (G 1 ∩ E) ∪ (E ∩ D) | Relationship of hybrid wild plants with other categories | |
| Theme | Features | Weed 1 | Invasive 1 |
|---|---|---|---|
| Reproductive Strategy | Exhibit a high reproductive capacity (R-strategists), allowing them to establish and spread rapidly in various environments. | Yes | Yes |
| Ability to Compete | Can outcompete native or desirable plants for resources such as light, water, and nutrients. | Yes | Yes |
| Adaptability | Tendency to be highly adaptable to different environmental conditions, enabling them to thrive in a variety of habitats | Yes | Yes |
| Habitat Occupancy | Primarily associated with disturbed or cultivated areas, competing with crops or desirable plants in human-altered landscapes | Yes | Not |
| Ecological Impact | Often considered nuisances in agricultural settings, impacting crop yields and quality and sometimes damage infrastructure | Yes | Not |
| Human Perception | Generally perceived as unwanted plants in cultivated areas | Yes | Not |
| Management Approach | Control measures often include herbicide application, cultivation practices, or manual removal, including collection as food for humans or livestock | Yes | Not |
| Ecological Impact | Can have broader ecological impacts, leading to the decline or displacement of native species and disruption of ecosystem functions. They may alter habitat structures, nutrient cycles, and community compositions, affecting the overall biodiversity. | Not | Yes |
| Human Perception | These are also generally perceived as unwanted plants, but in natural environments. The preservation of native biodiversity is a fundamental goal of conservation biology. Invasive species can contribute to the decline of native species, making their management essential for maintaining healthy ecosystems | Not | Yes |
| Management Approach | Management may involve a more comprehensive ecological approach, considering the restoration of native habitats and the prevention of further spread. | Not | Yes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rivera, D.; Rivera-Obón, D.-J.; Palazón, J.-A.; Obón, C. From Weeds to Feeds: Exploring the Potential of Wild Plants in Horticulture from a Centuries-Long Journey to an AI-Driven Future. Horticulturae 2024, 10, 1021. https://doi.org/10.3390/horticulturae10101021
Rivera D, Rivera-Obón D-J, Palazón J-A, Obón C. From Weeds to Feeds: Exploring the Potential of Wild Plants in Horticulture from a Centuries-Long Journey to an AI-Driven Future. Horticulturae. 2024; 10(10):1021. https://doi.org/10.3390/horticulturae10101021
Chicago/Turabian StyleRivera, Diego, Diego-José Rivera-Obón, José-Antonio Palazón, and Concepción Obón. 2024. "From Weeds to Feeds: Exploring the Potential of Wild Plants in Horticulture from a Centuries-Long Journey to an AI-Driven Future" Horticulturae 10, no. 10: 1021. https://doi.org/10.3390/horticulturae10101021
APA StyleRivera, D., Rivera-Obón, D.-J., Palazón, J.-A., & Obón, C. (2024). From Weeds to Feeds: Exploring the Potential of Wild Plants in Horticulture from a Centuries-Long Journey to an AI-Driven Future. Horticulturae, 10(10), 1021. https://doi.org/10.3390/horticulturae10101021









