Reducing Splitting of ‘Murcott’ Tangor Fruit (Citrus reticulate × Citrus sinensis) through Foliar Application of Forchlorfenuron (CPPU)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Field Experiment
2.2.1. Fruit Splitting Calculation
2.2.2. Fruit Size
2.2.3. Fruit Fresh Weight
2.2.4. Peel Strength
2.2.5. Peel Thickness
2.2.6. Total Soluble Solid (TSS), Titratable Acidity (TA), and TSS to TA Ratio
2.3. Peel Tissue Sectioning
2.4. Quantitative Analysis of Sectioned Tissue
2.5. Statistical Analysis
3. Results
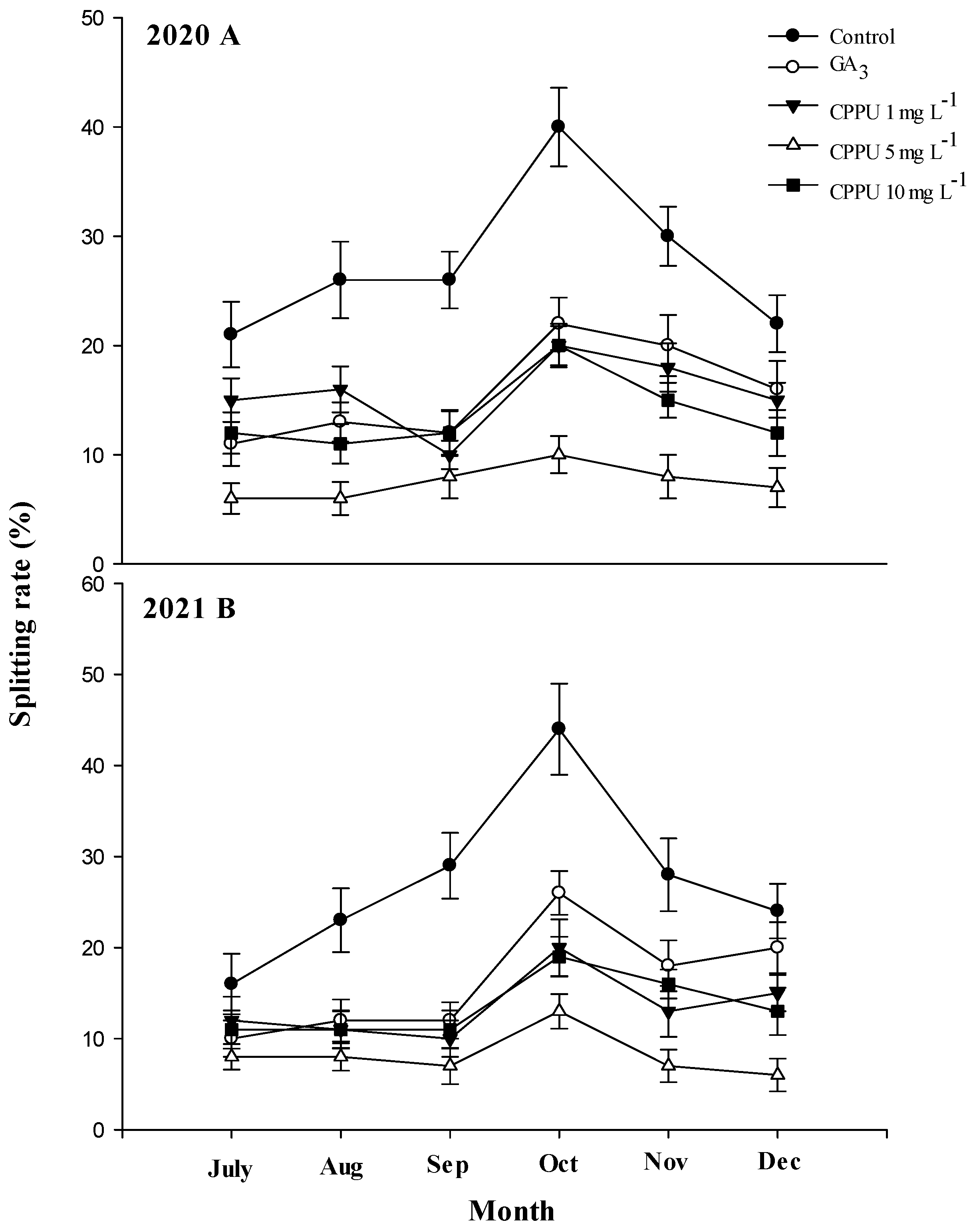
3.1. Fruit Splitting Rate
3.2. Physical Characteristics
3.3. Chemical Characteristics
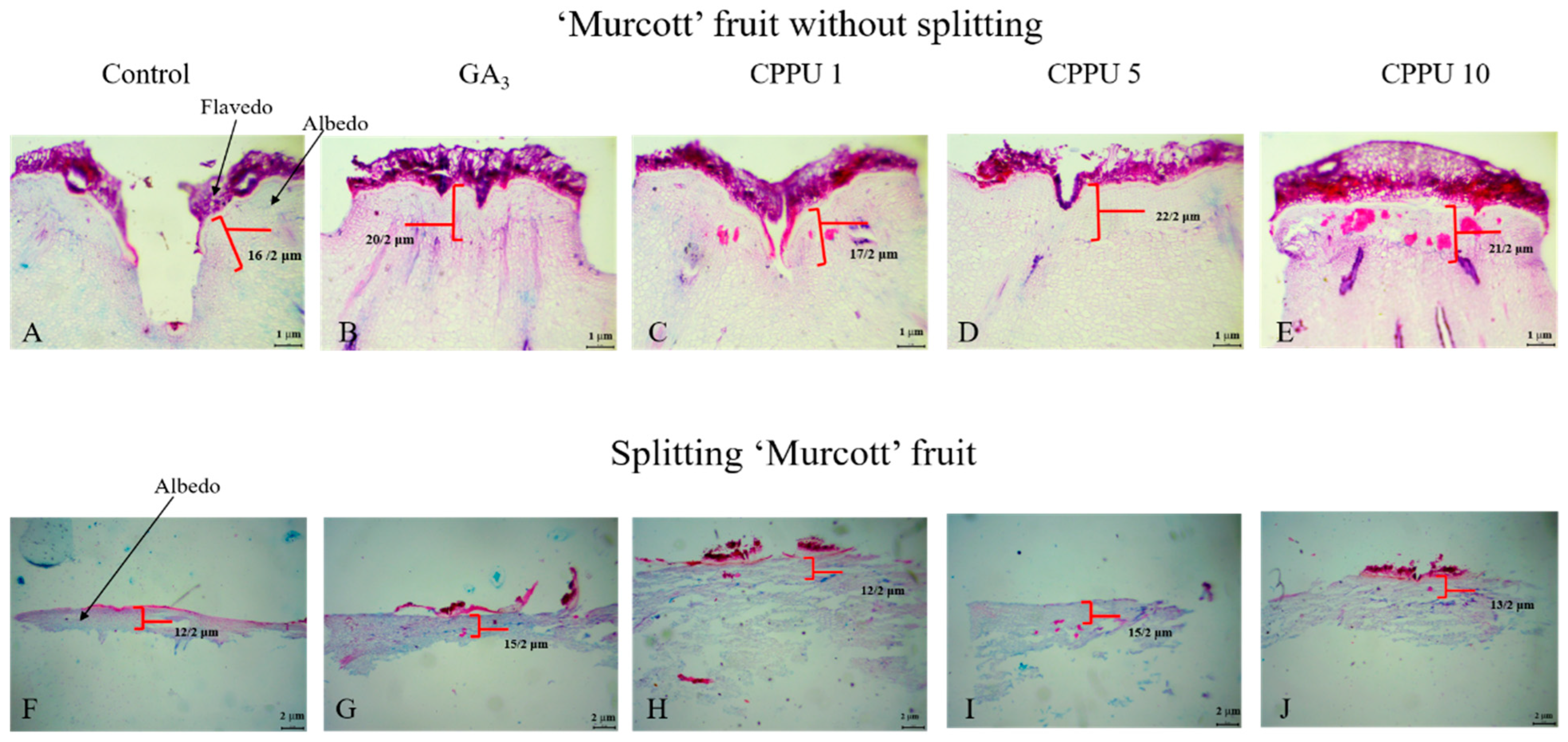
3.4. Tissue Section
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rabe, E.; Van der Walt, W.; Kleynhans, S. Factors Influencing Fruit Splitting in Ellendale; Annual Report S.A. Co-Op. Citrus Exchange Ltd.: Nelspruit, South Africa, 1989. [Google Scholar]
- Mupambi, G. Studies to Reduce the Size of the Navel-End Opening of Navel Oranges. Ph.D. Dissertation, University Stellenbosch, Stellenbosch, South Africa, 2010. [Google Scholar]
- De Cicco, V.; Intrigliolo, F.; Ippolito, A.; Vanadia, S.; Guiffrida, A. Factors in Navelina orange splitting. Proc. Int. Soc. Citricult. 1988, 1, 535–540. [Google Scholar]
- Bower, J.P.; Gilfillan, I.M.; Skinner, H. Fruit splitting in ‘Valencia’ and its relationship to the pectin status of the rind. Proc. Int. Soc. Citricult. 1992, 1, 511–514. [Google Scholar]
- Goldschmidt, E.E.; Galili, D. Fruit splitting in ‘Murcott’ tangerines: Control by reduced water supply. Proc. Int. Soc. Citricult. 1992, 2, 657–660. [Google Scholar]
- Almela, V.; Zaragoza, S.; Primo-Millo, E.; Agusti, M. Hormonal control of splitting in ‘Nova’ mandarin fruit. J. Hort. Sci. 1994, 69, 969–973. [Google Scholar]
- Sdoodee, S.; Chiarawipa, R. Fruit splitting occurrence of Shogun mandarin (Citrus reticulata Blanco cv. Shogun) in southern Thailand and alleviation by calcium and boron sprays. Songklanakarin J. Sci. Technol. 2005, 27, 719–730. [Google Scholar]
- Rabe, E.; Van Rensburg, P.J.J.; Van Der Walt, H.; Bower, J. Factors influencing preharvest fruit splitting in Ellendale (C. reticulata). HortScience 1990, 25, 1135f. [Google Scholar] [CrossRef]
- Barry, G.H.; Bower, J.P. Manipulation of fruit set and stylar-end fruit split in ‘Nova’ mandarin hybrid. Sci. Hortic. 1997, 70, 243–250. [Google Scholar] [CrossRef]
- García-Luis, A.; Duarte, A.M.M.; Porras, I.; García-Lidon, A.; Guardiola, J.L. Fruit splitting in ‘Nova’ hybrid mandarin in relation to the anatomy of the fruit and fruit set treatments. Sci. Hortic. 1994, 57, 215–231. [Google Scholar] [CrossRef]
- Rabe, E.; Van Rensburg, P.J.J. Gibberellic acid sprays, girdling, flower thinning and potassium applications affect fruit splitting and yield in the ‘Ellendale’ tangor. J. Hortic. Sci. 1996, 71, 195–203. [Google Scholar] [CrossRef]
- García-Luis, A.; Duarte, A.M.M.; Kanduser, M.; Guardiola, J.L. The anatomy of fruit in relation to the propensity of citrus fruit to split. Sci. Hortic. 2001, 87, 33–52. [Google Scholar] [CrossRef]
- Coit, J. Citrus Fruits; Macmillan: New York, NY, USA, 1915; p. 520. [Google Scholar]
- Greenberg, J.; Kaplan, I.; Fainzack, M.; Egozi, Y.; Giladi, B. Effects of auxin sprays on yield, fruit size, splitting and the incidence of creasing of ‘Nova’ mandarin. Acta Hortic. 2006, 727, 249–254. [Google Scholar] [CrossRef]
- Sandhu, S.; Bal, J.S. Quality Improvement in Lemon (Citrus Limon (L.) Burm.) through Integrated Management of Fruit Cracking. Afr. J. Agric. Res. 2013, 8, 3552–3557. [Google Scholar]
- Erner, Y.; Goren, R.; Monselise, S.P. Reduction of peel roughness of ‘Shamouti’ orange with growth regulators. J. Am. Soc. Hortic. Sci. 1976, 101, 513–515. [Google Scholar] [CrossRef]
- Stander, O.P.J.; Theron, K.I.; Cronjé, P.J.R. Foliar 2,4-D Application after physiological fruit drop reduces fruit splitting of Mandarin. HortTechnology 2014, 24, 717–723. [Google Scholar] [CrossRef]
- Kopečný, D.; Briozzo, P.; Popelková, H.; Šebela, M.; Končitíková, R.; Spíchal, L.; Nisler, J.; Madzak, C.; Frébort, I.; Laloue, M. Phenyl-and benzylurea cytokinins as competitive inhibitors of cytokinin oxidase/dehydrogenase: A structural study. Biochimie 2010, 92, 1052–1062. [Google Scholar] [CrossRef]
- Cruz-Castillo, J.G.; Baaldicchi, A.; Frioni, T.; Marocchi, F.; Moscatello, S.; Proietti, S.; Battistelli, A.; Famiani, F. Pre-anthesis CPPU low dosage application increases ‘Hayward’ kiwifruit weight without affecting the other qualitative and nutritional characteristics. Food Chem. 2014, 158, 224–228. [Google Scholar] [CrossRef]
- Jiang, Y.L.; Chen, L.Y.; Lee, T.C.; Chang, P.T. Improving postharvest storage of Fresh red-fleshed pitaya (Hylocereus polyrhizus sp.) fruit by pre-harvest application of CPPU. Sci. Hortic. 2020, 273, 109646. [Google Scholar] [CrossRef]
- Khaimov, A.; Mizrahi, Y. Effects of day-length, radiation, flower thinning and growth regulators on flowering of the vine cacti Hylocereus undatus and Selenicereus megalanthus. J. Hortic. Sci. Biotechnol. 2006, 60, 371–383. [Google Scholar] [CrossRef]
- Chang, P.T.; Hsieh, C.C.; Jiang, Y.L. Responses of ‘Shih Huo Chuan’ pitaya (Hylocereus polyrhizus (Weber) Britt. & Rose) to different degrees of shading nets. Sci. Hortic. 2016, 198, 154–162. [Google Scholar]
- Atkinson, J.A.; Wells, D.M. An updated protocol for high throughput plant tissue sectioning. Front. Plant Sci. 2017, 8, 1721. [Google Scholar] [CrossRef]
- Greenberg, J.; Holtzman, S.; Fainzack, M.; Egozi, Y.; Giladi, B.; Oren, Y.; Kaplan, I. Effects of NAA and GA3 sprays on fruit size and the incidence of creasing of ‘Washington’ navel orange. Acta Hortic. 2010, 884, 273–279. [Google Scholar] [CrossRef]
- Cline, J.A.; Trought, M. Effect of gibberellic acid on fruit cracking and quality of Bing and Sam sweet cherries. Can. J. Plant Sci. 2007, 87, 545–550. [Google Scholar] [CrossRef]
- Zoffoli, J.P.; Latorre, B.A.; Naranjo, P. Preharvest applications of growth regulators and their effect on postharvest quality of table grapes during cold storage. Postharvest Biol. Technol. 2009, 51, 183–192. [Google Scholar] [CrossRef]
- Marzouk, H.A.; Kassem, H.A. Improving yield, quality, and shelf life of Thompson seedless grapevine by preharvest foliar applications. Sci. Hortic. 2011, 130, 425–430. [Google Scholar] [CrossRef]
- Sahu, P.S.A.; Sahu, A. Effect of pre-harvest sprays of forchlorfenuron and boron on fruit cracking and quality of pomegranate (Punica granatum L.) cv. Kandhari. Int. J. Chem. Stud. 2018, 6, 2998–3002. [Google Scholar]
- Retamales, J.B.; Lobos, G.A.; Romero, S.; Godoy, R.; Moggia, C. Repeated applications of CPPU on highbush blueberry cv. Duke increase yield and enhance fruit quality at harvest and during postharvest. Chil. J. Agri. Res. 2014, 74, 157–161. [Google Scholar] [CrossRef]
- Cruz-Castillo, J.G.; Woolley, D.; Lawes, G. Kiwifruit size and CPPU response are influenced by the time of anthesis. Sci. Hortic. 2002, 95, 23–30. [Google Scholar] [CrossRef]
- Kim, J.; Takami, Y.; Mizugami, T.; Beppu, K.; Fukuda, T.; Kataoka, I. CPPU application on size and quality of hardy kiwifruit. Sci. Hortic. 2006, 110, 219–222. [Google Scholar] [CrossRef]
- Fahima, A.; Levinkron, S.; Maytal, Y.; Hugger, A.; Lax, I.; Huang, X.; Eyal, Y.; Lichter, A.; Goren, M.; Stern, R.A.; et al. Cytokinin treatment modifies litchi fruit pericarp anatomy leading to reduced susceptibility to post-harvest pericarp browning. Plant Sci. 2019, 283, 41–50. [Google Scholar] [CrossRef]
- Liu, X.S.; Luo, Y.C.; Wang, H.C.; Huang, X.M. Post-bloom CPPU application is effective at improving fruit set and suppressing coloration but ineffective at increasing fruit size in litchi. Horticulturae 2022, 8, 1096. [Google Scholar] [CrossRef]
- Albacete, A.; Cantero-Navarro, E.; Balibrea, M.E.; Grosskinsky, D.K.; De la Cruz Gonzalez, M.; Martinez-Adujar, C.; Smigocki, A.C.; Roitsch, T.; Perez-Alfocea, F. Hormonal and metabolic regulation of tomato fruit sink activity and yield under salinity. J. Exp. Bot. 2014, 65, 6081–6095. [Google Scholar] [CrossRef] [PubMed]
- Antognozzi, E.; Battistelli, A.; Famiani, F.; Moscatello, S.; Stanica, F.; Tombesi, A. Influence of CPPU on carbohydrate accumulation and metabolism in fruits of Actinidia deliciosa (A. Chev.). Sci. Hortic. 1996, 65, 37–47. [Google Scholar] [CrossRef]
- Zeng, H.; Yang, W.; Lu, C.; Lin, W.; Zou, M.; Zhang, H.; Wan, J.; Huang, X. Effect of CPPU on carbohydrate and endogenous hormone levels in young macadamia fruit. PLoS ONE 2016, 11, e0158705. [Google Scholar] [CrossRef] [PubMed]
- Patterson, K.; Mason, K.; Gould, K. Effects of CPPU (N-(2-chloro-4-pyridyl)-N′-phenylurea) on fruit growth, maturity, and storage quality of kiwifruit. N. Z. J. Crop Hortic. Sci. 1993, 21, 253–261. [Google Scholar] [CrossRef]
- Wu, L.; Lan, J.; Xiang, X.; Jin, Z.; Khan, S.; Liu, Y. Transcriptome sequencing and endogenous phytohormone analysis reveal new insights in CPPU controlling fruit development in kiwifruit (Actinidia chinensis). PLoS ONE 2020, 15, e0240355. [Google Scholar] [CrossRef]
- Devoghalaere, F.; Doucen, T.; Guitton, B.; Keeling, J.; Payne, W.; Ling, T.J. A genomics approach to understanding the role of auxin in apple (Malus × domestica) fruit size control. BMC Plant Biol. 2012, 12, 7. [Google Scholar] [CrossRef]
- Kumar, R.; Khurana, A.; Sharma, A.K. Role of plant hormones and their interplay in development and ripening of fleshy fruits. J. Exp. Bot. 2014, 65, 4561–4575. [Google Scholar] [CrossRef]
- Martí, C.; Orzáez, D.; Ellul, P.; Moreno, V.; Carbonell, J.; Granell, A. Silencing of DELLA induces facultative parthenocarpy in tomato fruits. Plant J. 2007, 52, 865–876. [Google Scholar] [CrossRef]
- Mignolli, F.; Vidoz, M.L.; Picciarelli, P.; Mariotti, L. Gibberellins modulate auxin responses during tomato (Solanum lycopersicum L.) fruit development. Physiol. Plant. 2019, 165, 768–779. [Google Scholar] [CrossRef]


| Treatment | Fresh Weight (g) z | Fruit Length (mm) | Fruit Width (mm) | Peel Strength (kg·cm−2) | Peel Thickness (mm) | TSS (°Brix) | TA (%) | TSS/TA |
|---|---|---|---|---|---|---|---|---|
| 2020 | ||||||||
| Control | 106.7 ± 7.4 b y | 49.8 ± 1.0 b | 63.7 ± 1.7 b | 1.2 ± 0.2 b | 1.9 ± 0.3 b | 9.2 ± 0.1 a | 1.1 ± 0.2 a | 8.6 ± 0.9 a |
| GA3 | 117.3 ± 11.7 ab | 49.2 ± 2.0 ab | 64.4 ± 2.0 ab | 1.6 ± 0.2 ab | 2.4 ± 0.2 ab | 8.9 ± 0.3 a | 1.1 ± 0.2 a | 8.0 ± 1.2 a |
| CPPU 1 | 114.7 ± 9.5 ab | 50.9 ± 1.4 a | 65.0 ± 2.0 ab | 1.6 ± 0.1 ab | 2.3 ± 0.3 ab | 8.4 ± 0.2 a | 1.1 ± 0.1 a | 7.9 ± 1.3 a |
| CPPU 5 | 132.3 ± 10.7 a | 52.5 ± 1.8 a | 67.3 ± 1.7 a | 2.0 ± 0.2 a | 2.8 ± 0.2 a | 9.4 ± 0.1 a | 1.1 ± 0.1 a | 8.5 ± 1.0 a |
| CPPU 10 | 123.7 ± 8.2 ab | 51.9 ± 0.5 a | 65.9 ± 1.2 ab | 1.8 ± 0.2 a | 2.5 ± 0.2 a | 9.7 ± 0.1 a | 1.2 ± 0.1 a | 8.1 ± 1.0 a |
| 2021 | ||||||||
| Control | 104.7 ± 9.3 b | 48.3 ± 1.2 b | 62.3 ± 2.0 a | 1.4 ± 0.2 b | 1.5 ± 0.2 b | 10.4 ± 0.2 a | 1.1 ± 0.1 a | 9.4. ± 1.0 a |
| GA3 | 120.7 ± 10.8 ab | 53.9 ± 1.0 a | 64.8 ± 2.4 a | 1.6 ± 0.2 ab | 1.8 ± 0.2 ab | 8.8 ± 0.2 a | 1.0 ± 0.1 a | 8.6 ± 1.0 a |
| CPPU 1 | 115.3 ± 6.6 ab | 49.7 ± 0.6 b | 59.6 ± 2.3 b | 1.80 ± 0.3 a | 2.0 ± 0.3 ab | 9.0 ± 0.2 a | 1.0 ± 0.1 a | 8.9 ± 1.1 a |
| CPPU 5 | 129.3 ± 9.8 a | 52.9 ± 0.8 a | 66.8 ± 2.3 a | 2.1 ± 0.1 a | 2.7 ± 0.5 a | 9.6 ± 0.1 a | 1.2 ± 0.1 a | 8.2 ± 1.0 a |
| CPPU 10 | 105.3 ± 8.5 b | 47.4 ± 0.8 b | 57.8 ± 1.1 b | 2.0 ± 0.2 a | 2.5 ± 0.1 a | 9.0 ± 0.1 a | 1.1 ± 0.1 a | 8.0 ± 1.2 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, Y.-L.; Chang, P.-T. Reducing Splitting of ‘Murcott’ Tangor Fruit (Citrus reticulate × Citrus sinensis) through Foliar Application of Forchlorfenuron (CPPU). Horticulturae 2024, 10, 1023. https://doi.org/10.3390/horticulturae10101023
Jiang Y-L, Chang P-T. Reducing Splitting of ‘Murcott’ Tangor Fruit (Citrus reticulate × Citrus sinensis) through Foliar Application of Forchlorfenuron (CPPU). Horticulturae. 2024; 10(10):1023. https://doi.org/10.3390/horticulturae10101023
Chicago/Turabian StyleJiang, Yi-Lu, and Pai-Tsang Chang. 2024. "Reducing Splitting of ‘Murcott’ Tangor Fruit (Citrus reticulate × Citrus sinensis) through Foliar Application of Forchlorfenuron (CPPU)" Horticulturae 10, no. 10: 1023. https://doi.org/10.3390/horticulturae10101023
APA StyleJiang, Y.-L., & Chang, P.-T. (2024). Reducing Splitting of ‘Murcott’ Tangor Fruit (Citrus reticulate × Citrus sinensis) through Foliar Application of Forchlorfenuron (CPPU). Horticulturae, 10(10), 1023. https://doi.org/10.3390/horticulturae10101023




