Comparative Analysis of the Effects of Crude Metabolic Extracts of Three Biocontrol Bacteria on Microbial Community Structure Provides a New Strategy for the Biological Control of Apple Replant Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. The Preparation of Crude Metabolic Extracts
2.3. Antagonism Tests of Cell-Free Culture Filtrates
2.4. Potting Experiment
2.4.1. Biomass
2.4.2. Soil DNA Extraction
2.4.3. Quantitative PCR (qPCR) Analysis
2.4.4. Analysis of Soil Microbial Community Structure
2.5. Field Experiment
2.6. UHPLC-Q Exactive LC-MS/MS Extensive Untargeted Metabolism Analysis
2.7. Effects of Three Major Secondary Metabolites on Apple Seedlings and Pathogenic Fusarium
2.7.1. Growth-Promoting Effect
2.7.2. Antifungal Effects
2.8. Statistical Analysis
3. Results
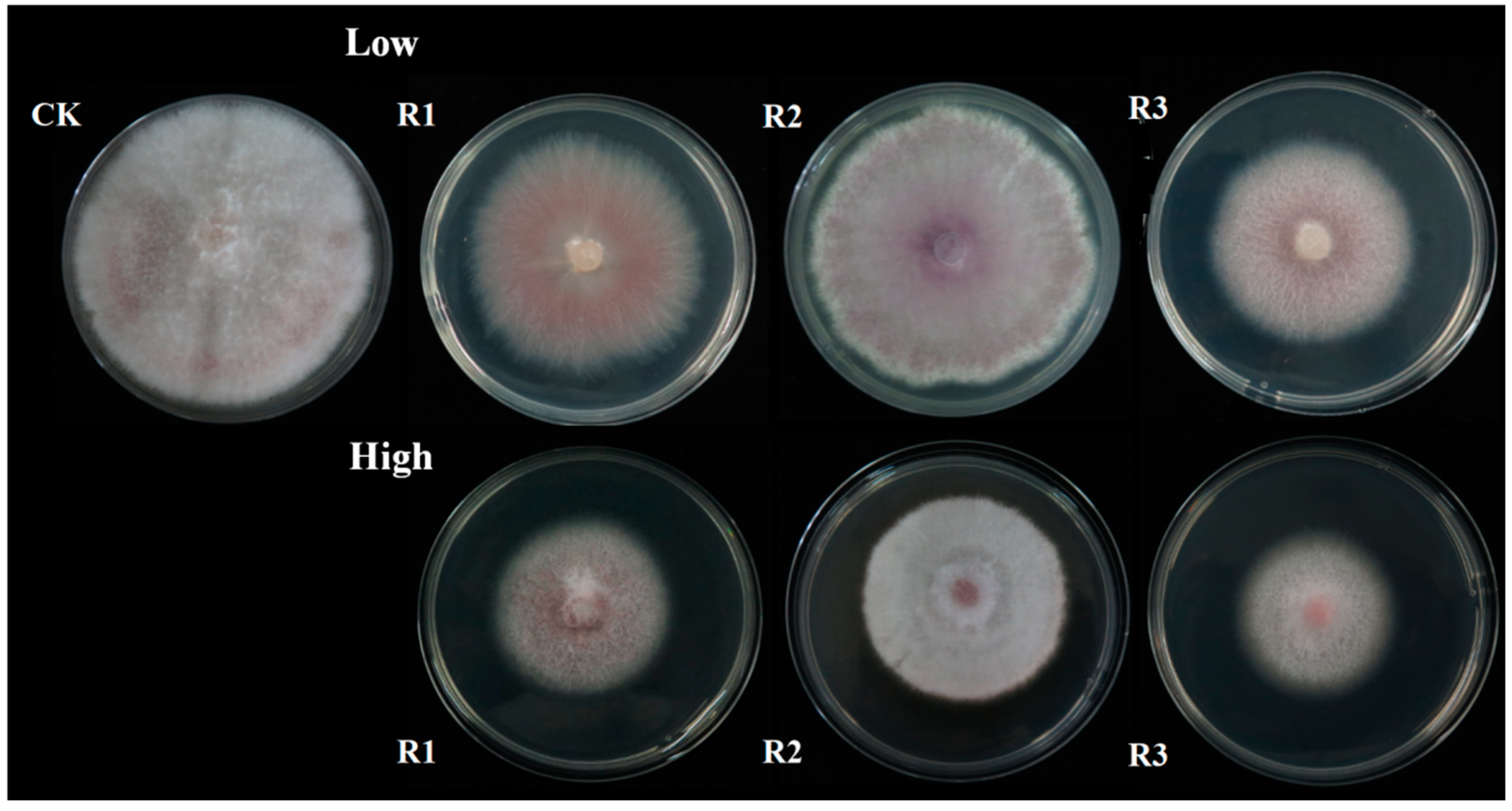
3.1. Effect of CFCF on the Growth of Apple Seedlings in the Pot Experiment
3.2. Effect of CFCF on the Growth of Grafted Seedlings in the Field Experiment
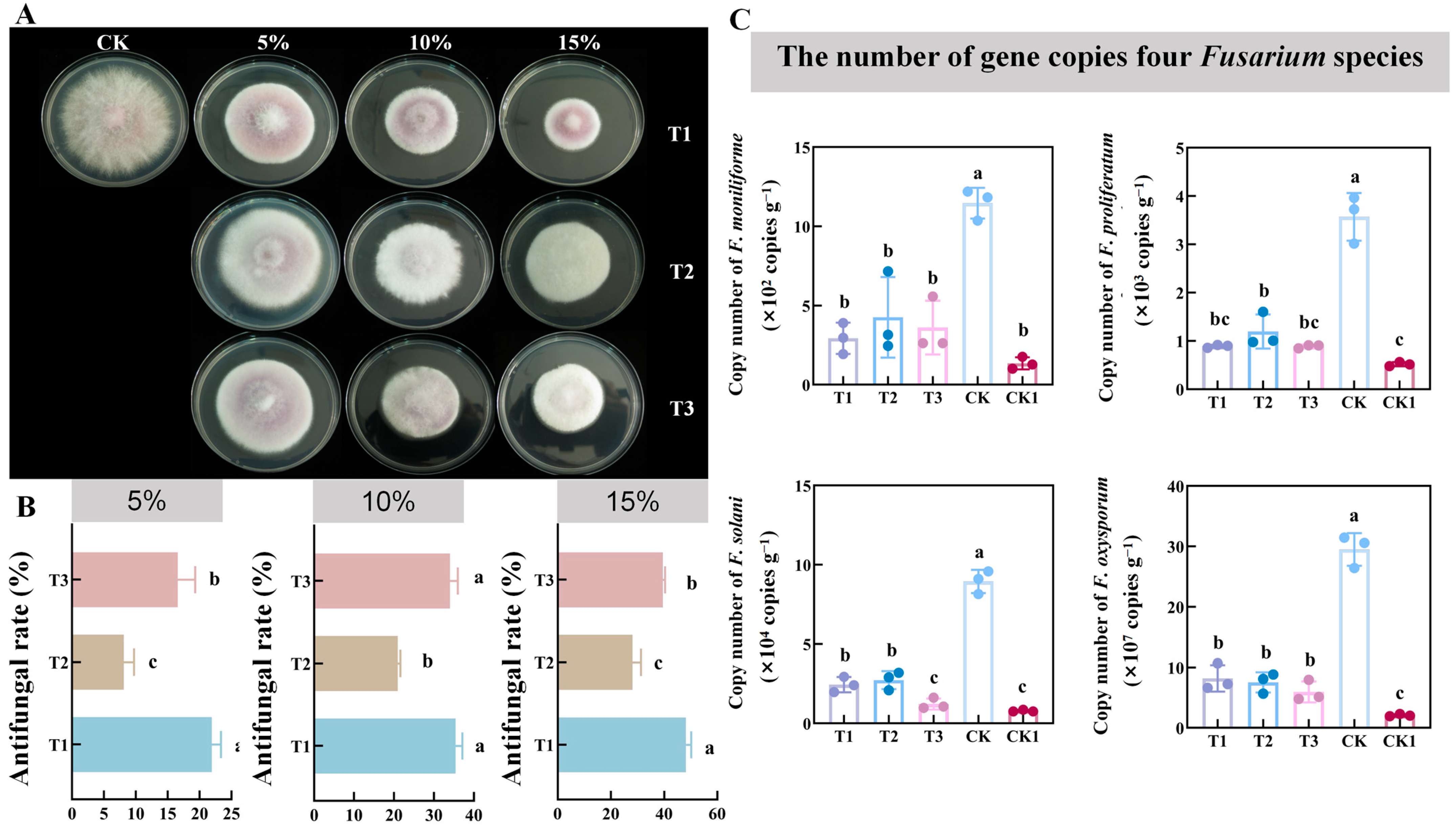
3.3. Antifungal Effect of CFCF on Fusarium oxysporum
3.4. Effect of CFCF on the Number of Gene Copies of Four Fusarium Species in the Soil
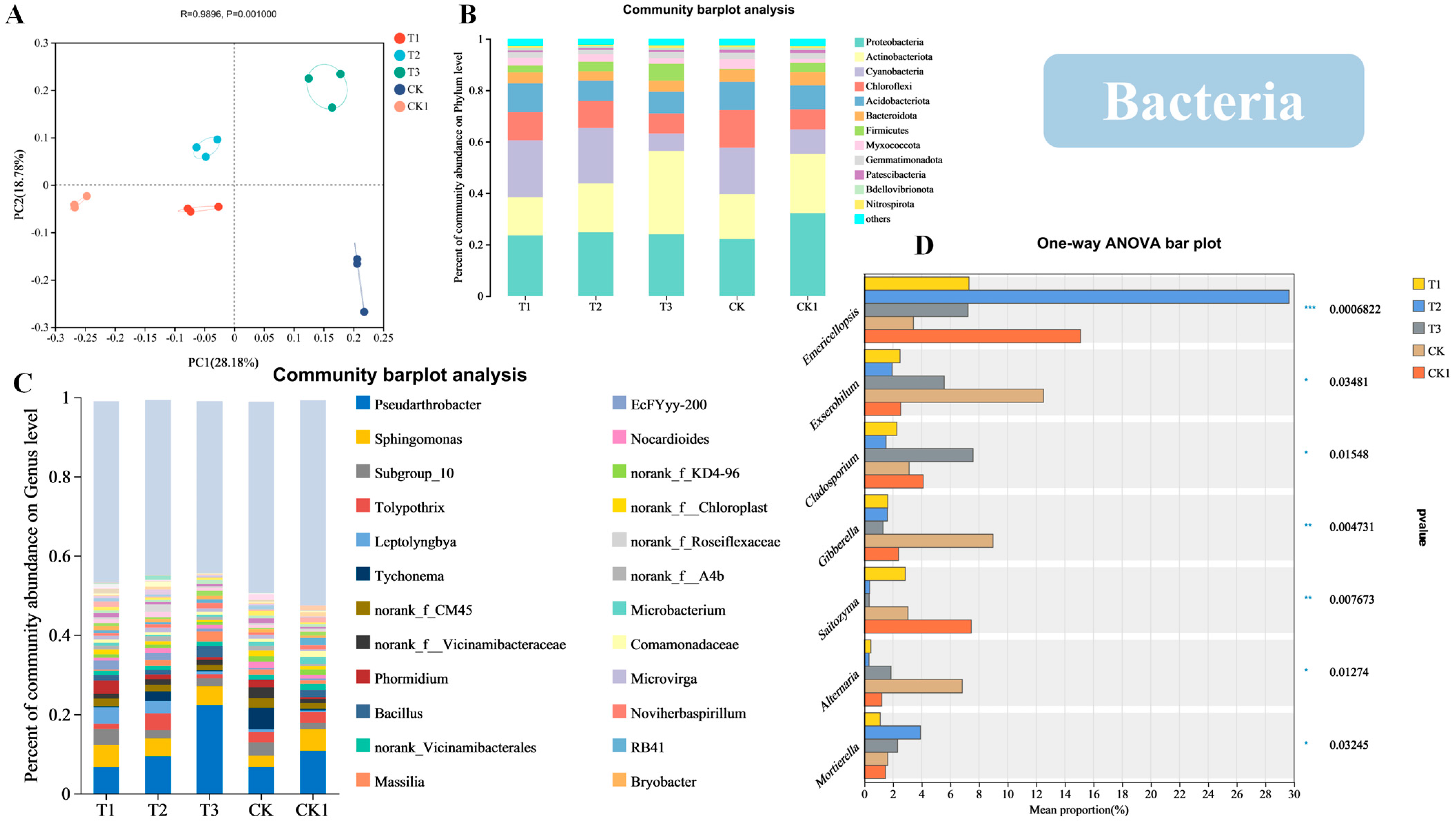
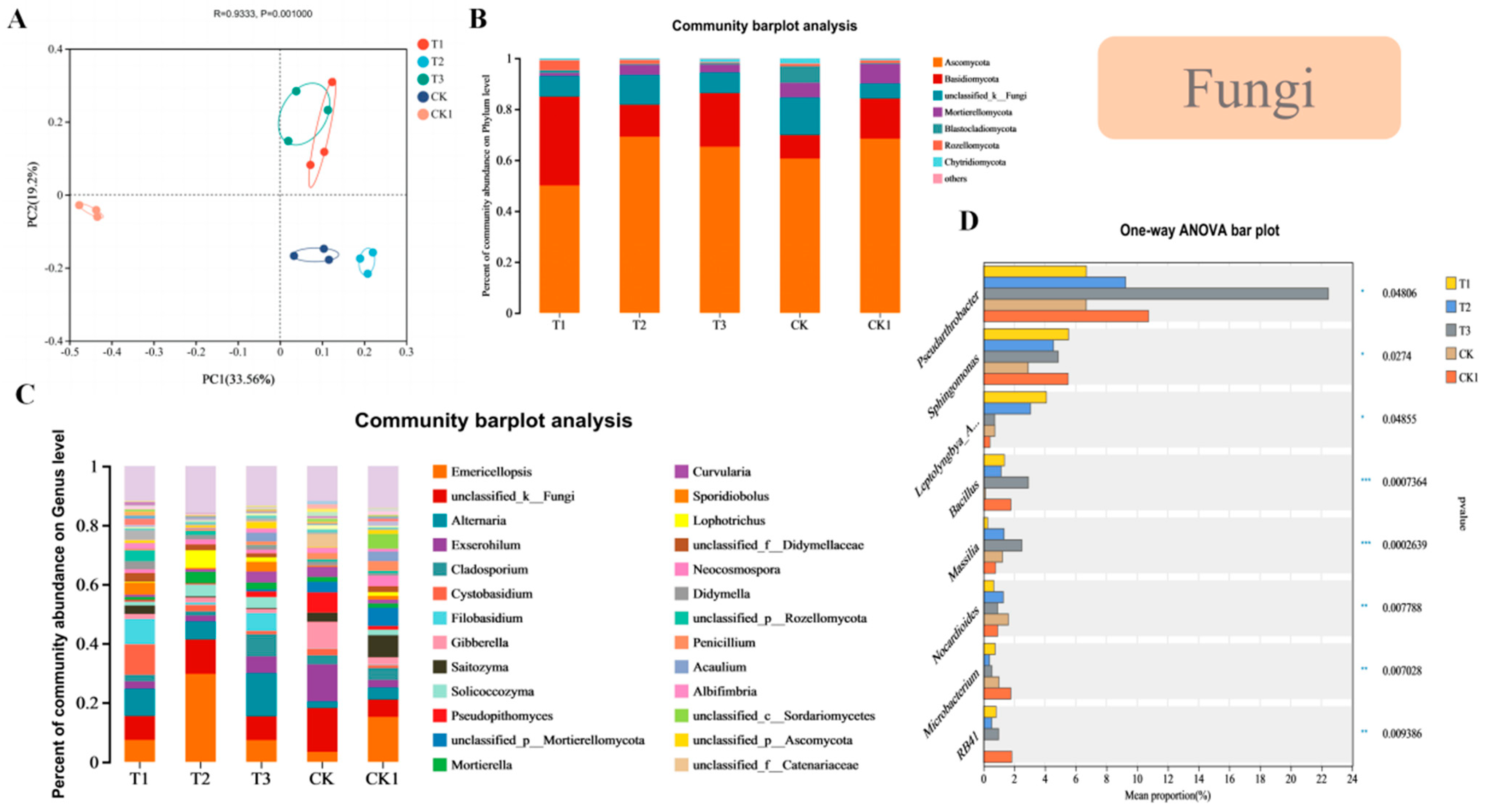
3.5. Effects of Different CFCFs on the Soil Microbial Community Structure
3.6. PCoA Analysis of the Soil Microbial Communities
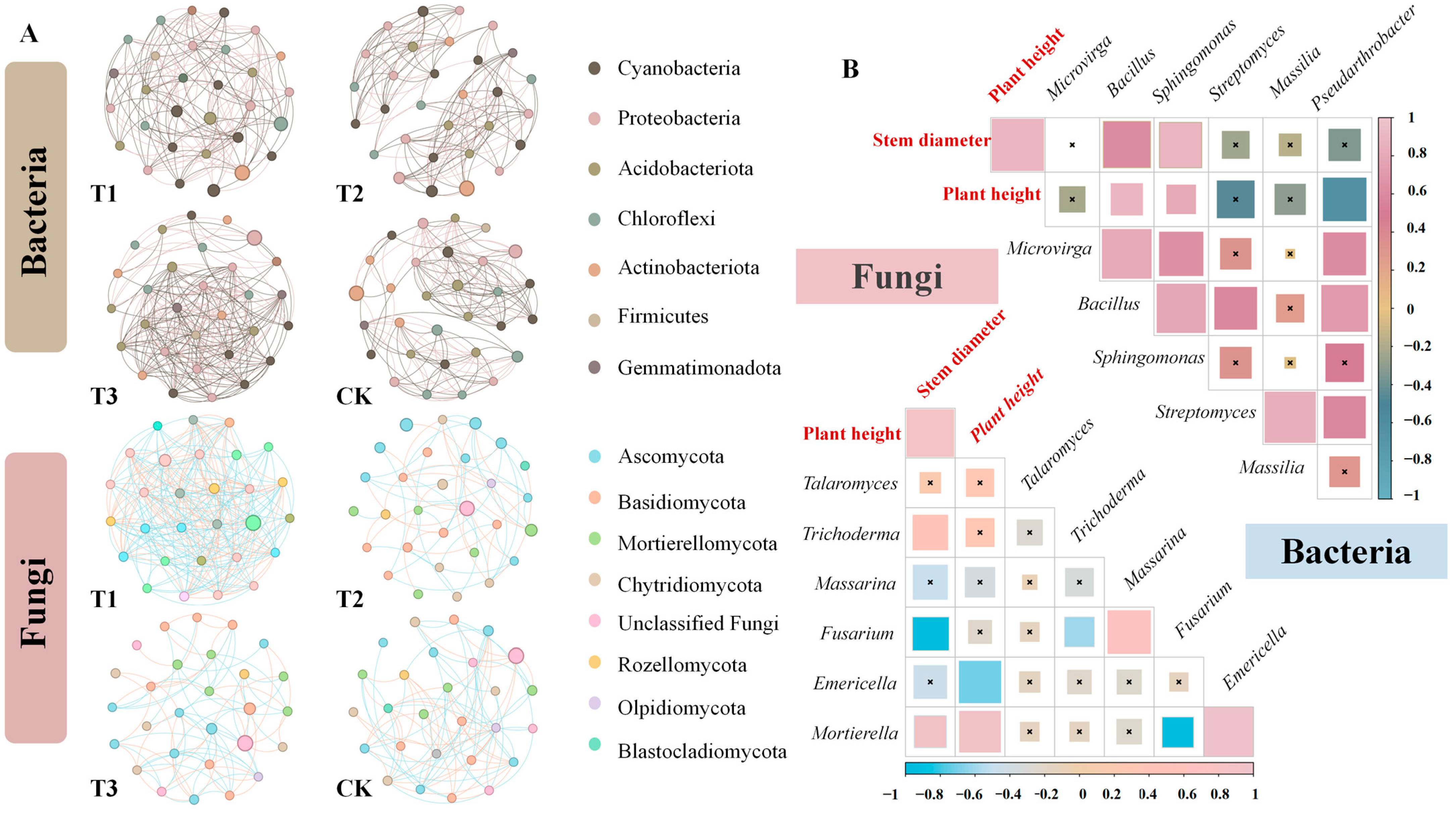
3.7. Co-Occurrence Network Analysis of the Soil Microorganisms Treated with Crude Metabolic Extracts from Biocontrol Bacteria
3.8. Correlation Analysis between the Plant Seedling Biomass and Key Microorganisms in the Rhizosphere Soil
3.9. LC-MS/MS Broadly Untargeted Metabolomic Analysis of L. reuteri
3.10. Effects of Three Main Secondary Metabolite Compounds on the Growth of M9T337
3.11. Inhibitory Effects of Three Main Secondary Metabolite Compounds on F. oxysporum
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Na, W.; Wolf, J.; Fusuo, Z. Towards sustainable intensification of apple production in China—Yield gaps and nutrient use efficiency in apple farming systems. J. Integr. Agric. 2016, 15, 716–725. [Google Scholar] [CrossRef]
- Sun, Y.; Lu, Y.; Wang, Z.; Li, M. Production efficiency and change characteristics of China’s apple industry in terms of planting scale. PLoS ONE 2021, 16, e0254820. [Google Scholar] [CrossRef] [PubMed]
- Lidia, N.; Heribert, I.; Pertot, I.; Blaž, S. Reanalysis of microbiomes in soils affected by apple replant disease (ARD): Old foes and novel suspects lead to the proposal of extended model of disease development. Appl. Soil Ecol. 2018, 129, 24–33. [Google Scholar] [CrossRef]
- Traud, W.; Kornelia, S.; Wulf, A.; Gerhard, B.; Gisela, G.-S.; Xorla, K.; Rainer, M.; Stefanie, R.; Michaela, S.; Doris, V.; et al. Apple replant disease: Causes and mitigation strategies. Curr. Issues Mol. Biol. 2019, 30, 89–106. [Google Scholar] [CrossRef]
- Chen, Y.; Du, J.; Li, Y.; Tang, H.; Yin, Z.; Yang, L.; Ding, X. Evolutions and managements of soil microbial community structure drove by continuous cropping. Front. Microbiol. 2022, 13, 839494. [Google Scholar] [CrossRef]
- Liu, X.; Xu, S.; Wang, X.; Xin, L.; Wang, L.; Mao, Z.; Chen, X.; Wu, S. MdBAK1 overexpression in apple enhanced resistance to replant disease as well as to the causative pathogen Fusarium oxysporum. Plant Physiol. Biochem. 2022, 179, 144–157. [Google Scholar] [CrossRef]
- Wang, G.; Yin, C.; Pan, F.; Wang, X.; Xiang, L.; Wang, Y.; Wang, J.; Tian, C.; Chen, J.; Mao, Z. Analysis of the fungal community in apple replanted soil around Bohai Gulf. Hortic. Plant J. 2018, 4, 175–181. [Google Scholar] [CrossRef]
- Xu, X.; Jiang, W.; Wang, G.; Ding, F.; Li, Q.; Wang, R.; Chen, X.; Shen, X.; Yin, C.; Mao, Z. Analysis of soil fungal community in aged apple orchards in Luochuan County, Shaanxi Province. Agriculture 2022, 13, 63. [Google Scholar] [CrossRef]
- Duan, Y.; Jiang, W.; Zhang, R.; Chen, R.; Chen, X.; Yin, C.; Mao, Z. Discovery of Fusarium proliferatum f. sp. Malus domestica causing apple replant disease in China. Plant Dis. 2022, 106, 2958–2966. [Google Scholar] [CrossRef]
- Alicia, B.-S.; Maik, L.; Doris, V.; Søren, J.S.; Traud, W.; Kornelia, S.; Samuel, J. Exploring microbial determinants of apple replant disease (ARD): A microhabitat approach under split-root design. FEMS Microbiol. Ecol. 2020, 96, fiaa211. [Google Scholar] [CrossRef]
- Mark, M.; Manici, L. Apple replant disease: Role of microbial ecology in cause and control. Annu. Rev. Phytopathol. 2012, 50, 45–65. [Google Scholar] [CrossRef]
- Milan, P.; Samuel, C.H.; Fulya, B.-G. Methods for management of soilborne diseases in crop production. Agriculture 2020, 10, 16. [Google Scholar] [CrossRef]
- Wang, H.; Tang, W.; Mao, Y.; Ma, S.; Chen, X.; Shen, X.; Yin, C.; Mao, Z. Isolation of Trichoderma virens 6PS-2 and its effects on Fusarium proliferatum f. sp. Malus domestica MR5 related to apple replant disease (ARD) in China. Hortic. Plant J. 2022, in press. [Google Scholar] [CrossRef]
- Julia, R.B. Getting the drift-methyl bromide application and adverse birth outcomes in an agricultural area. Environ. Health Perspect. 2013, 121, a198. [Google Scholar] [CrossRef][Green Version]
- Ravinder, S.; Joginder, P.; Sheetal, R.; Mohinder, K. Suppression of soil borne fungal pathogens associated with apple replant disease by cyclic application of native strains of Pseudomonas aeruginosa. J. Appl. Nat. Sci. 2017, 9, 2105–2109. [Google Scholar] [CrossRef]
- Hausmann, B.; Knorr, K.H.; Schreck, K.; Tringe, S.G.; del Rio, T.G.; Loy, A.; Pester, M. Consortia of low-abundance bacteria drive sulfate reduction-dependent degradation of fermentation products in peat soil microcosms. ISME J. 2016, 10, 2365–2375. [Google Scholar] [CrossRef]
- Abdel-Gayed, M.A.; Abo-Zaid, G.A.; Mohamed, M.S.R.; Hafez, E.E. Fermentation, formulation and evaluation of PGPR Bacillus subtilis isolate as a bioagent for reducing occurrence of peanut soil-borne diseases. J. Integr. Agric. 2019, 18, 2080–2092. [Google Scholar] [CrossRef]
- Ramalingam, R.; Abeer, H. Bacillus: A Biological Tool for Crop Improvement through Bio-Molecular Changes in Adverse Environments. Front. Physiol. 2017, 8, 667. [Google Scholar] [CrossRef]
- Komal, S.; Neha, C.; Anukool, V.; Virendra Singh, R.; Sanjiv, S. Fenugreek associated bacterium Priestia endophytica SK1 induces defense response against fusarium wilt by accumulation of secondary metabolites. S. Afr. J. Bot. 2023, 160, 229–234. [Google Scholar] [CrossRef]
- Duan, Y.; Chen, J.; He, W.; Chen, J.; Pang, Z.; Hu, H.; Xie, J. Fermentation optimization and disease suppression ability of a Streptomyces ma. FS-4 from banana rhizosphere soil. BMC Microbiol. 2020, 20, 24. [Google Scholar] [CrossRef]
- Duan, Y.; Chen, R.; Zhang, R.; Jiang, W.; Chen, X.; Yin, C.; Mao, Z. Isolation, identification, and antibacterial mechanisms of Bacillus amyloliquefaciens QSB-6 and its effect on plant roots. Front. Microbiol. 2021, 12, 746799. [Google Scholar] [CrossRef] [PubMed]
- Geng, W.; Lv, Y.; Duan, Y.; Wang, H.; Jiang, W.; Zhang, R.; Chen, R.; Chen, X.; Shen, X.; Yin, C.; et al. Preparation of composite microbial culture and its biocontrol effect on apple replant disease. Sci. Hortic. 2022, 303, 111236. [Google Scholar] [CrossRef]
- Zhang, R.; Huang, J.; Duan, Y.; Wang, H.; Wang, M.; Chen, X.; Shen, X.; Yin, C.; Mao, Z. The fermentation products of Penicillium D12 and Lactobacillus reuteri promoted the growth of Pingyi sweet tea seedlings and improved the soil biological environment of continuous cultivation. J. Plant Nutr. Fertil. 2022, 28, 344–356. [Google Scholar]
- Gu, Y.; Meng, D.; Yang, S.; Xiao, N.; Li, Z.; Liu, Z.; Li, L.; Zeng, X.; Zeng, S.; Yin, H. Invader-resident community similarity contribute to the invasion process and regulate biofertilizer effectiveness. J. Clean. Prod. 2019, 241, 118278. [Google Scholar] [CrossRef]
- Wang, J.; Raza, W.; Jiang, G.; Yi, Z.; Fields, B.; Greenrod, S.; Friman, V.P.; Jousset, A.; Shen, Q.; Wei, Z. Bacterial volatile organic compounds attenuate pathogen virulence via evolutionary trade-offs. ISME J. 2023, 17, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Jiang, W.; Chen, R.; Wang, H.; Duan, Y.; Chen, X.; Shen, X.; Yin, C.; Mao, Z. Quicklime and superphosphate alleviating apple replant disease by improving acidified soil. ACS Omega 2022, 7, 7920–7930. [Google Scholar] [CrossRef]
- Chen, P.; Wang, Y.; Liu, Q.; Zhang, Y.; Li, X.; Li, H.; Li, W. Phase changes of continuous cropping obstacles in strawberry (Fragaria × ananassa Duch.) production. Appl. Soil Ecol. 2020, 155, 103626. [Google Scholar] [CrossRef]
- Ali, A.; Elrys, A.S.; Liu, L.; Iqbal, M.; Zhao, J.; Huang, X.; Cai, Z. Cover plants-mediated suppression of Fusarium Wilt and root-knot incidence of cucumber is associated with the changes of rhizosphere fungal microbiome structure-under plastic shed system of north China. Front. Microbiol. 2022, 13, 697815. [Google Scholar] [CrossRef]
- Manici, L.; Markus, K.; Franke-Whittle, I.H.; Thomas, R.; Gerhard, B.; Nicoletti, F.; Caputo, F.; Topp, A.; Heribert, I.; Andreas, N. Relationship between root-endophytic microbial communities and replant disease in specialized apple growing areas in Europe. Appl. Soil Ecol. 2013, 72, 207–214. [Google Scholar] [CrossRef]
- Yared Tesfai, T.; Mark, M.; Iwan, F.L.; McLeod, A. A multi-phasic approach reveals that apple replant disease is caused by multiple biological agents, with some agents acting synergistically. Soil Biol. Biochem. 2021, 43, 1917–1927. [Google Scholar] [CrossRef]
- Wang, X.; Wang, G.; Liu, Y.; Chen, X.; Shen, X.; Yin, C.; Mao, Z. Correlation analysis of apple replant disease and soil fungal community structure in the Northwest Loess Plateau area. Acta Hortic. Sin. 2018, 45, 855–864. [Google Scholar] [CrossRef]
- Senka, Č.; Manupriyam, D.; Marian, M.; Guillem, S.; Vladimir, S.; Nicolas, C.; Hans-Joachim, R.; Shinichi, S.; van der Peter, M. Niche availability and competitive loss by facilitation control proliferation of bacterial strains intended for soil microbiome interventions. Nat. Commun. 2024, 15, 2557. [Google Scholar] [CrossRef]
- Zhou, D.; Wang, X.; Anjago, W.M.; Li, J.; Li, W.; Li, M.; Jiu, M.; Zhang, Q.; Zhang, J.; Deng, S.; et al. Borrelidin-producing and root-colonizing Streptomyces rochei is a potent biopesticide for two soil-borne oomycete-caused plant diseases. Biol. Control 2024, 188, 105411. [Google Scholar] [CrossRef]
- Evans, W.; Jochen, S.; Altus, V.; Frank, R. Phenolics mediate suppression of Fusarium oxysporum f. sp. cubense TR4 by legume root exudates. Rhizosphere 2022, 21, 100459. [Google Scholar] [CrossRef]
- Drira, M.; Jihen, E.; Hajer Ben, H.; Faiez, H.; Christine, G.; Christophe, R.; Didier Le, C.; Philippe, M.; Slim, A.; Imen, F. Optimization of exopolysaccharides production by Porphyridium sordidum and their potential to induce defense responses in Arabidopsis thaliana against Fusarium oxysporum. Biomolecules 2021, 11, 282. [Google Scholar] [CrossRef]
- Wogene, S.; Tibor, J.; Zoltán, M. Unveiling the significance of rhizosphere: Implications for plant growth, stress response, and sustainable agriculture. Plant Physiol. Biochem. 2024, 206, 108290. [Google Scholar] [CrossRef]
- Hu, Q.; Tan, L.; Gu, S.; Xiao, Y.; Xiong, X.; Zeng, W.; Feng, K.; Wei, Z.; Deng, Y. Network analysis infers the wilt pathogen invasion associated with non-detrimental bacteria. NPJ Biofilms Microbiomes 2020, 6, 8. [Google Scholar] [CrossRef]
- Li, H.; Li, C.; Song, X.; Liu, Y.; Gao, Q.; Zheng, R.; Li, J.; Zhang, P.; Liu, X. Impacts of continuous and rotational cropping practices on soil chemical properties and microbial communities during peanut cultivation. Sci. Rep. 2022, 12, 2758. [Google Scholar] [CrossRef]
- Yifan, Z.; Étienne, G.; Jong-Duk, P.; Mohammad, R.S. The small-molecule language of dynamic microbial interactions. Annu. Rev. Microbiol. 2022, 76, 641–660. [Google Scholar] [CrossRef]
- Sui, J.; Yang, J.; Li, C.; Zhang, L.; Hua, X. Effects of a microbial restoration substrate on plant growth and rhizosphere microbial community in a continuous cropping poplar. Microorganisms 2023, 11, 486. [Google Scholar] [CrossRef]
- Cheng, F.; Li, G.; Peng, Y.; Wang, A.; Zhu, J. Mixed bacterial fermentation can control the growth and development of Verticillium dahliae. Biotechnol. Biotechnol. Equip. 2020, 34, 58–69. [Google Scholar] [CrossRef]
- Joanna, Ś.; Agnieszka, K.; Attila, S.; Maria Swiontek, B. Pseudomonas sivasensis 2RO45 inoculation alters the taxonomic structure and functioning of the canola rhizosphere microbial community. Front. Microbiol. 2023, 14, 1168907. [Google Scholar] [CrossRef]
- Wang, F.; Wei, Y.; Yan, T.; Wang, C.; Chao, Y.; Jia, M.; An, L.; Sheng, H. Sphingomonas sp. Hbc-6 alters physiological metabolism and recruits beneficial rhizosphere bacteria to improve plant growth and drought tolerance. Front. Plant Sci. 2022, 13, 1002772. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Delgado-Baquerizo, M.; Yuan, M.M.; Ding, J.; Yergeau, E.; Zhou, J.; Crowther, T.W.; Liang, Y. Home-based microbial solution to boost crop growth in low-fertility soil. New Phytol. 2023, 239, 752–765. [Google Scholar] [CrossRef]
- Xiong, W.; Jousset, A.; Guo, S.; Karlsson, I.; Zhao, Q.; Wu, H.; Kowalchuk, G.A.; Shen, Q.; Li, R.; Geisen, S. Soil protist communities form a dynamic hub in the soil microbiome. ISME J. 2017, 12, 634–638. [Google Scholar] [CrossRef]



| Microbial Species | Treatment | Shannon | Simpson | ACE | Chao |
|---|---|---|---|---|---|
| Fungi | T1 | 3.75 ± 0.14 bc | 0.09 ± 0.01 c | 506.47 ± 9.26 c | 573.85 ± 13.61 bc |
| T2 | 3.57 ± 0.09 c | 0.12 ± 0.01 b | 592.40 ± 22.87 b | 594.86 ± 19.44 b | |
| T3 | 4.07 ± 0.07 b | 0.05 ± 0.00 d | 755.84 ± 32.66 a | 685.31 ± 43.43 a | |
| CK | 4.45 ± 0.08 a | 0.04 ± 0.00 d | 559.07 ± 25.68 bc | 647.67 ± 13.99 ab | |
| CK1 | 2.96 ± 0.04 d | 0.19 ± 0.02 a | 486.69 ± 18.99 c | 508.34 ± 7.20 c | |
| Bacteria | T1 | 6.20 ± 0.05 b | 0.05 ± 0.01 a | 3235.27 ± 184.56 c | 3157.08 ± 125.27 c |
| T2 | 6.04 ± 0.14 b | 0.01 ± 0.00 b | 4013.57 ± 52.35 ab | 3456.98 ± 76.87 b | |
| T3 | 6.81 ± 0.06 a | 0.01 ± 0.00 b | 4178.50 ± 72.46 a | 3862.23 ± 53.86 a | |
| CK | 6.19 ± 0.05 b | 0.02 ± 0.00 b | 3720.47 ± 44.58 b | 3454.59 ± 30.55 b | |
| CK1 | 5.45 ± 0.18 c | 0.05 ± 0.00 a | 2939.46 ± 31.21 c | 3308.99 ± 58.72 bc |
| Treatment | Fresh Weight (g) | Dry Weight (g) | ||
|---|---|---|---|---|
| Low | High | Low | High | |
| R1 | 2.54 ± 0.04 b | 2.41 ± 0.05 b | 0.45 ± 0.02 b | 0.40 ± 0.05 b |
| R2 | 2.70 ± 0.01 a | 2.87 ± 0.02 a | 0.65 ± 0.02 a | 0.74 ± 0.03 a |
| R3 | 2.39 ± 0.04 b | 2.13 ± 0.07 c | 0.48 ± 0.02 b | 0.34 ± 0.03 b |
| CK | 1.96 ± 0.08 c | 1.96 ± 0.08 d | 0.22 ± 0.02 c | 0.22 ± 0.02 c |
| Treatment | Antifungal Effects (%) | |
|---|---|---|
| Low | High | |
| R1 | 25.34 ± 0.19 b | 50.54 ± 0.19 b |
| R2 | 10.45 ± 0.17 c | 38.30 ± 0.23 c |
| R3 | 40.00 ± 0.23 a | 74.21 ± 0.09 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lv, J.; Jiang, W.; Xu, Z.; Wang, G.; Li, X.; Wu, X.; Ding, F.; Liu, Y.; Chen, X.; Yin, C.; et al. Comparative Analysis of the Effects of Crude Metabolic Extracts of Three Biocontrol Bacteria on Microbial Community Structure Provides a New Strategy for the Biological Control of Apple Replant Disease. Horticulturae 2024, 10, 1035. https://doi.org/10.3390/horticulturae10101035
Lv J, Jiang W, Xu Z, Wang G, Li X, Wu X, Ding F, Liu Y, Chen X, Yin C, et al. Comparative Analysis of the Effects of Crude Metabolic Extracts of Three Biocontrol Bacteria on Microbial Community Structure Provides a New Strategy for the Biological Control of Apple Replant Disease. Horticulturae. 2024; 10(10):1035. https://doi.org/10.3390/horticulturae10101035
Chicago/Turabian StyleLv, Jinhui, Weitao Jiang, Zihui Xu, Gongshuai Wang, Xiaoxuan Li, Xinyu Wu, Fengxia Ding, Yusong Liu, Xuesen Chen, Chengmiao Yin, and et al. 2024. "Comparative Analysis of the Effects of Crude Metabolic Extracts of Three Biocontrol Bacteria on Microbial Community Structure Provides a New Strategy for the Biological Control of Apple Replant Disease" Horticulturae 10, no. 10: 1035. https://doi.org/10.3390/horticulturae10101035
APA StyleLv, J., Jiang, W., Xu, Z., Wang, G., Li, X., Wu, X., Ding, F., Liu, Y., Chen, X., Yin, C., & Mao, Z. (2024). Comparative Analysis of the Effects of Crude Metabolic Extracts of Three Biocontrol Bacteria on Microbial Community Structure Provides a New Strategy for the Biological Control of Apple Replant Disease. Horticulturae, 10(10), 1035. https://doi.org/10.3390/horticulturae10101035







