Effects of Ethylene Inhibitors on the Long-Term Maintenance of the Embryogenic Callus of Vitis vinifera L.
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Sterilization
2.2. Media and Culture Conditions
2.3. Ethylene Inhibitors
2.4. Statistical Analysis
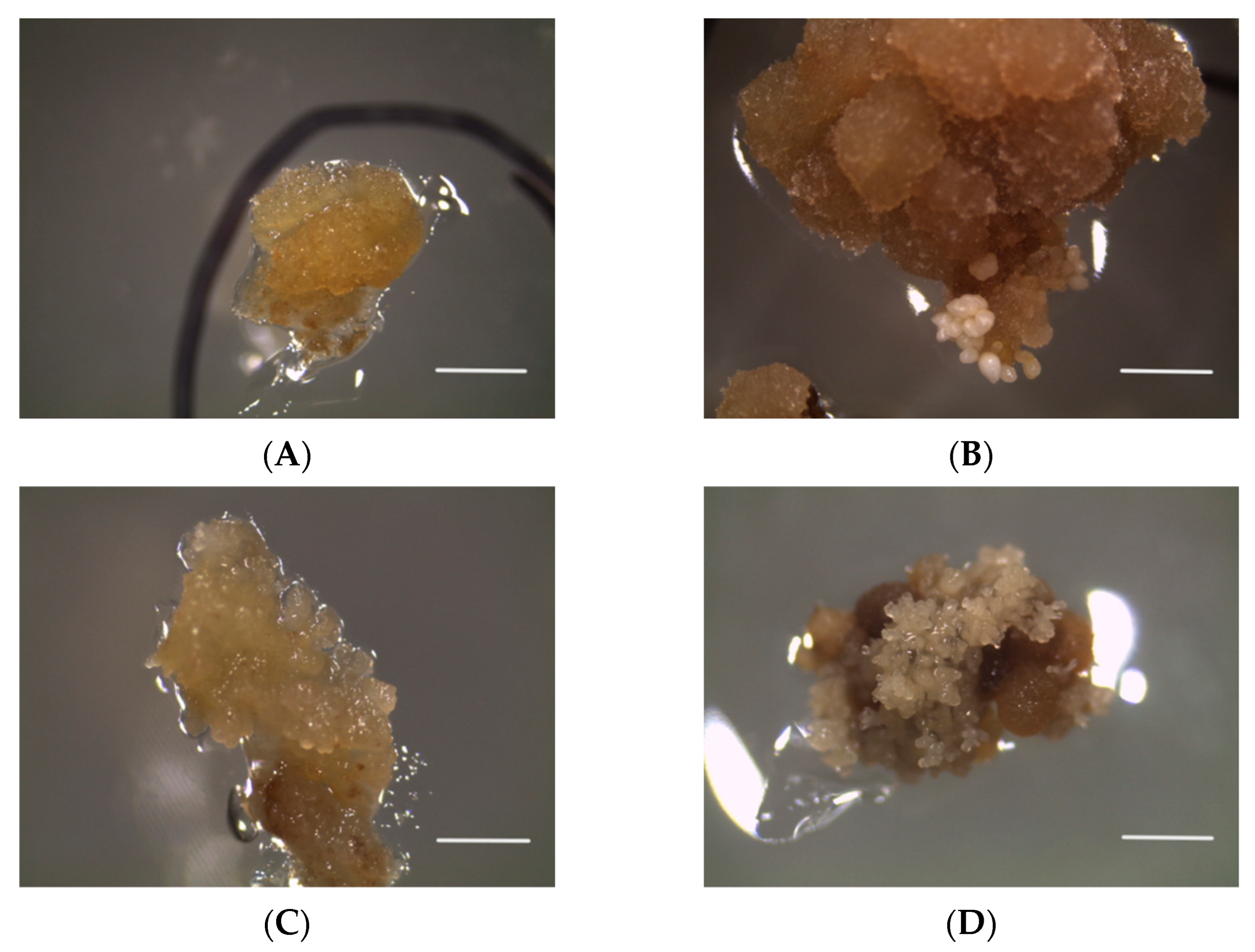
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- FAO. World Food and Agriculture—Statistical Yearbook 2021; FAO: Roma, Italy, 2021; ISBN 978-92-5-134332-6. [Google Scholar]
- Iocco, P.; Franks, T.; Thomas, M. Genetic Transformation of Major Wine Grape Cultivars of Vitis vinifera L. Transgenic Res. 2001, 10, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Franks, T.; Gang He, D.; Thomas, M. Regeneration of Transgenic Shape Vitis Vinifera L. Sultana Plants: Genotypic and Phenotypic Analysis. Mol. Breed 1998, 4, 321–333. [Google Scholar] [CrossRef]
- Capriotti, L.; Ricci, A.; Molesini, B.; Mezzetti, B.; Pandolfini, T.; Piunti, I.; Sabbadini, S. Efficient Protocol of de Novo Shoot Organogenesis from Somatic Embryos for Grapevine Genetic Transformation. Front. Plant Sci. 2023, 14, 1172758. [Google Scholar] [CrossRef] [PubMed]
- Ya, R.; Li, J.; Zhang, N.; Yu, Q.; Xu, W. Phenotypically Abnormal Cotyledonary Vitis Vinifera Embryos Differ in Anatomy, Endogenous Hormone Levels and Transcriptome Profiles. Tree Physiol. 2023, 43, 467–485. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.-N.; Lu, M.-J.; Zhou, M.; Wang, H.-Y.; Feng, J.-Y.; Wen, Y.-Q. Reduction of Pectin May Decrease the Embryogenicity of Grapevine (Vitis Vinifera) pro-Embryonic Masses after 10 Years of in Vitro Culture. Sci. Hortic. 2023, 309, 111690. [Google Scholar] [CrossRef]
- Domínguez, C.; Martínez, Ó.; Nieto, Ó.; Ferradás, Y.; González, M.V.; Rey, M. Involvement of Polyamines in the Maturation of Grapevine (Vitis vinifera L.‘Mencía’) Somatic Embryos over a Semipermeable Membrane. Sci. Hortic. 2023, 308, 111537. [Google Scholar] [CrossRef]
- Méndez-Hernández, H.A.; Ledezma-Rodríguez, M.; Avilez-Montalvo, R.N.; Juárez-Gómez, Y.L.; Skeete, A.; Avilez-Montalvo, J.; De-la-Peña, C.; Loyola-Vargas, V.M. Signaling Overview of Plant Somatic Embryogenesis. Front. Plant Sci. 2019, 10, 77. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Reddy, M. In Vitro Plant Propagation: A Review. J. For. Environ. Sci. 2011, 27, 61–72. [Google Scholar] [CrossRef]
- Pěnčík, A.; Turečková, V.; Paulišić, S.; Rolčík, J.; Strnad, M.; Mihaljević, S. Ammonium Regulates Embryogenic Potential in Cucurbita Pepo through pH-Mediated Changes in Endogenous Auxin and Abscisic Acid. Plant Cell Tissue Organ Cult. PCTOC 2015, 122, 89–100. [Google Scholar] [CrossRef]
- Loyola-Vargas, V.M.; Ochoa-Alejo, N. Somatic Embryogenesis. An Overview. In Somatic Embryogenesis. Fundamental Aspects and Applications; Loyola-Vargas, V.M., Ochoa-Alejo, N., Eds.; Springer: Berlin/Heidelberg, Germany, 2016; pp. 1–8. [Google Scholar]
- Preece, J.E.; Read, P.E. Environmental Management for Optimizing Micropropagation. In Proceedings of the I International Symposium on Acclimatization and Establishment of Micropropagated Plants 616, Sani-Halkidiki, Macedonia, Greece, 19–22 September 2001; pp. 49–58. [Google Scholar]
- Zhang, W.; Hu, W.; Wen, C.-K. Ethylene Preparation and Its Application to Physiological Experiments. Plant Signal. Behav. 2010, 5, 453–457. [Google Scholar] [CrossRef]
- Iqbal, N.; Khan, N.A.; Ferrante, A.; Trivellini, A.; Francini, A.; Khan, M.I.R. Ethylene Role in Plant Growth, Development and Senescence: Interaction with Other Phytohormones. Front. Plant Sci. 2017, 8, 235913. [Google Scholar] [CrossRef] [PubMed]
- George, E.F.; Hall, M.A.; De Klerk, G.-J. Plant Propagation by Tissue Culture: Volume 1. the Background; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2007; Volume 1, ISBN 1-4020-5005-4. [Google Scholar]
- Biddington, N.L. The Influence of Ethylene in Plant Tissue Culture. Plant Growth Regul. 1992, 11, 173–187. [Google Scholar] [CrossRef]
- Bashir, M.A.; Silvestri, C.; Salimonti, A.; Rugini, E.; Cristofori, V.; Zelasco, S. Can Ethylene Inhibitors Enhance the Success of Olive Somatic Embryogenesis? Plants 2022, 11, 168. [Google Scholar] [CrossRef]
- Bai, B.; Su, Y.H.; Yuan, J.; Zhang, X.S. Induction of Somatic Embryos in Arabidopsis Requires Local YUCCA Expression Mediated by the Down-Regulation of Ethylene Biosynthesis. Mol. Plant 2013, 6, 1247–1260. [Google Scholar] [CrossRef]
- Feher, A.; Pasternak, T.P.; Dudits, D. Transition of Somatic Plant Cells to an Embryogenic State. Plant Cell Tissue Organ Cult. 2003, 74, 201–228. [Google Scholar] [CrossRef]
- Dimasi-Theriou, K.; Economou, A.S. Ethylene Enhances Shoot Formation in Cultures of the Peach Rootstock GF-677 (Prunus Persica x P. Amygdalus). Plant Cell Rep. 1995, 15, 87–90. [Google Scholar] [CrossRef]
- Mohiuddin, A.K.M.; Chowdhury, M.K.U.; Abdullah, Z.C.; Napis, S. Influence of Silver Nitrate (Ethylene Inhibitor) on Cucumber in Vitro Shoot Regeneration. Plant Cell Tissue Organ Cult. 1997, 51, 75–78. [Google Scholar] [CrossRef]
- Mahmoud, L.M.; Grosser, J.W.; Dutt, M. Silver Compounds Regulate Leaf Drop and Improve in Vitro Regeneration from Mature Tissues of Australian Finger Lime (Citrus australasica). Plant Cell Tissue Organ Cult. PCTOC 2020, 141, 455–464. [Google Scholar] [CrossRef]
- Ptak, A.; Tahchy, A.E.; Wyżgolik, G.; Henry, M.; Laurain-Mattar, D. Effects of Ethylene on Somatic Embryogenesis and Galanthamine Content in Leucojum aestivum L. Cultures. Plant Cell Tissue Organ Cult. PCTOC 2010, 102, 61–67. [Google Scholar] [CrossRef]
- Navarro-García, N.; Martínez-Romero, D.; Pérez-Tornero, O. Assessment of the Impact of Ethylene and Ethylene Modulators in Citrus Limon Organogenesis. Plant Cell Tissue Organ Cult. PCTOC 2016, 127, 405–415. [Google Scholar] [CrossRef]
- Kumar, V.; Ramakrishna, A.; Ravishankar, G.A. Influence of Different Ethylene Inhibitors on Somatic Embryogenesis and Secondary Embryogenesis from Coffea Canephora P Ex Fr. Vitro Cell. Dev. Biol.-Plant 2007, 43, 602–607. [Google Scholar] [CrossRef]
- Abeles, F.B.; Morgan, P.W.; Saltveit, M.E., Jr. Ethylene in Plant Biology; Academic Press: Cambridge, MA, USA, 2012; ISBN 0-08-091628-7. [Google Scholar]
- Beyer, E. Silver Ion: A Potent Antiethylene Agent in Cucumber and Tomato1. HortScience 1976, 11, 195–196. [Google Scholar] [CrossRef]
- Spinoso-Castillo, J.L.; Chavez-Santoscoy, R.A.; Bogdanchikova, N.; Pérez-Sato, J.A.; Morales-Ramos, V.; Bello-Bello, J.J. Antimicrobial and Hormetic Effects of Silver Nanoparticles on in Vitro Regeneration of Vanilla (Vanilla Planifolia Jacks. Ex Andrews) Using a Temporary Immersion System. Plant Cell Tissue Organ Cult. PCTOC 2017, 129, 195–207. [Google Scholar] [CrossRef]
- Leslie, C.A.; Romani, R.J. Inhibition of Ethylene Biosynthesis by Salicylic Acid. Plant Physiol. 1988, 88, 833–837. [Google Scholar] [CrossRef]
- Gribaudo, I.; Gambino, G.; Vallania, R. Somatic Embryogenesis from Grapevine Anthers: The Optimal Developmental Stage for Collecting Explants. Am. J. Enol. Vitic. 2004, 55, 427–430. [Google Scholar] [CrossRef]
- Yim, B.; Mun, J.-H.; Jeong, Y.-M.; Hur, Y.Y.; Yu, H.-J. Flower and Microspore Development in’Campbell Early’(Vitis Labruscana) and’Tamnara’(V. Spp.) Grapes. Hortic. Sci. Technol. 2015, 33, 420–428. [Google Scholar] [CrossRef]
- Forleo, L.R.; D’Amico, M.; Basile, T.; Marsico, A.D.; Cardone, M.F.; Maggiolini, F.A.M.; Velasco, R.; Bergamini, C. Somatic Embryogenesis in Vitis for Genome Editing: Optimization of Protocols for Recalcitrant Genotypes. Horticulturae 2021, 7, 511. [Google Scholar] [CrossRef]
- Kansas State University. Available online: https://www.k-state.edu/wgrc/electronic_lab/aceto_stain.html (accessed on 29 April 2024).
- Onay, A.; Pirinç, V.; Tilkat, E.; Aktürk, Z.; Yildirim, H. Somatic Embryogenesis of Pistachio from Female Flowers. J. Hortic. Sci. Biotechnol. 2004, 79, 960–964. [Google Scholar] [CrossRef]
- Capriotti, L.; Limera, C.; Mezzetti, B.; Ricci, A.; Sabbadini, S. From Induction to Embryo Proliferation: Improved Somatic Embryogenesis Protocol in Grapevine for Italian Cultivars and Hybrid Vitis Rootstocks. Plant Cell Tissue Organ Cult. PCTOC 2022, 151, 221–233. [Google Scholar] [CrossRef]
- Torregrosa, L.; Vialet, S.; Adivèze, A.; Iocco-Corena, P.; Thomas, M.R. Grapevine (Vitis vinifera L.). Agrobacterium Protoc. 2015, 2, 177–194. [Google Scholar] [CrossRef]
- Nitsch, J.; Nitsch, C. Haploid Plants from Pollen Grains. Science 1969, 163, 85–87. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A Revised Medium for Rapid Growth and Bio Assays with Tobacco Tissue Cultures. Physiol. Plant 1962, 15, 473–497. [Google Scholar] [CrossRef]
- De Paoli, G.; Rossi, V.; Scorzoli, A. Micropropagazione Delle Piante Ortoflorofrutticole; Edagricole: Bologna, Italy, 1994. [Google Scholar]
- Torregrosa, L. A Simple and Efficient Method to Obtain Stable Embryogenic Cultures from Anthers of Vitis vinifera L. VITIS-GEILWEILERHOF 1998, 37, 91–92. [Google Scholar] [CrossRef]
- Martinelli, L.; Gribaudo, I.; Bertoldi, D.; Candioli, E.; Poletti, V. High Efficiency Somatic Embryogenesis and Plant Germination in Grapevine Cultivars Chardonnay and Brachetto a Grappolo Lungo. Vitis 2001, 40, 111–115. [Google Scholar] [CrossRef]
- López-Pérez, A.J.; Carreño, J.; Martínez-Cutillas, A.; Dabauza, M. High Embryogenic Ability and Plant Regeneration of Table Grapevine Cultivars (Vitis vinifera L.) Induced by Activated Charcoal. Vitis 2005, 44, 79–85. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.R-Project.Org/ (accessed on 1 March 2023).
- Fox, J.; Weisberg, S. An R Companion to Applied Regression, 3rd ed.; Sage: Thousand Oaks, CA, USA, 2019. [Google Scholar]
- Dinno, A. Conover.Test: Conover-Iman Test of Multiple Comparisons Using Rank Sums. R Package Version 1.1.6. 2024. Available online: https://cran.r-project.org/web/packages/conover.test/conover.test.pdf (accessed on 1 April 2024).
- Dinno, A.; Rank Sums. Dunn.Test: Dunn’s Test of Multiple Comparisons Using Rank Sums. R Package Version 1.3.5. 2017. Available online: https://cran.r-project.org/web/packages/dunn.test/dunn.test.pdf (accessed on 1 April 2024).
- Steinitz, B.; Barr, N.; Tabib, Y.; Vaknin, Y.; Bernstein, N. Control of in Vitro Rooting and Plant Development in Corymbia Maculata by Silver Nitrate, Silver Thiosulfate and Thiosulfate Ion. Plant Cell Rep. 2010, 29, 1315–1323. [Google Scholar] [CrossRef]
- Fortin, C.; Campbell, P.G. Thiosulfate Enhances Silver Uptake by a Green Alga: Role of Anion Transporters in Metal Uptake. Environ. Sci. Technol. 2001, 35, 2214–2218. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Gopal, J.; Sivanesan, I. Nanomaterials in Plant Tissue Culture: The Disclosed and Undisclosed. RSC Adv. 2017, 7, 36492–36505. [Google Scholar] [CrossRef]
- Lefevere, H.; Bauters, L.; Gheysen, G. Salicylic Acid Biosynthesis in Plants. Front. Plant Sci. 2020, 11, 338. [Google Scholar] [CrossRef]
- Luo, J.-P.; Jiang, S.-T.; Pan, L.-J. Enhanced Somatic Embryogenesis by Salicylic Acid of Astragalus Adsurgens Pall.: Relationship with H2O2 Production and H2O2-Metabolizing Enzyme Activities. Plant Sci. 2001, 161, 125–132. [Google Scholar] [CrossRef]
- Luo, J.-P.; Jia, J.-F.; Gu, Y.-H.; Liu, J. High Frequency Somatic Embryogenesis and Plant Regeneration in Callus Cultures of Astragalus Adsurgens Pall. Plant Sci. 1999, 143, 93–99. [Google Scholar] [CrossRef]
- Hatanaka, T.; Sawabe, E.; Azuma, T.; Uchida, N.; Yasuda, T. The Role of Ethylene in Somatic Embryogenesis from Leaf Discs of Coffea Canephora. Plant Sci. 1995, 107, 199–204. [Google Scholar] [CrossRef]
- Hutchinson, M.J.; Saxena, P.K. Acetylsalicylic Acid Enhances and Synchronizes Thidiazuron-Induced Somatic Embryogenesis in Geranium (Pelargonium x hortorum Bailey) Tissue Cultures. Plant Cell Rep. 1996, 15, 512–515. [Google Scholar] [CrossRef] [PubMed]


| Treatment | Total Vital Calluses (%) | Embryogenic Calluses (%) |
|---|---|---|
| Control | 79.6 ab | 38.2 bcd |
| C1C | 98.1 d | 22.6 abc |
| STS 20 | 89.2 bcd | 59.4 d |
| STS 40 | 100.0 d | 45.3 cd |
| STS 60 | 92.5 cd | 66.8 d |
| SA 25 | 87.9 abcd | 30.1 abcd |
| SA 50 | 78.3 a | 5.0 a |
| SA 75 | 84.9 abc | 8.6 ab |
| Treatment | Total Vital Calluses (%) | Embryogenic Calluses (%) |
|---|---|---|
| Control | 100.0 c | 0.0 n.s. |
| C1C | 86.7 bc | 10.7 n.s. |
| STS 20 | 16.1 ab | 37.5 n.s. |
| STS 40 | 2.8 a | 0.0 n.s. |
| STS 60 | 3.6 a | 0.0 n.s. |
| SA 25 | 100.0 c | 7.1 n.s. |
| SA 50 | 100.0 c | 0.0 n.s. |
| SA 75 | 65.6 ab | 12.5 n.s. |
| Treatment | Total Vital Calluses (%) | Embryogenic Calluses (%) |
|---|---|---|
| Control | 100.0 c | 72.5 bc |
| C1C | 68.5 a | 100.0 d |
| STS 20 | 100.0 c | 88.2 bcd |
| STS 40 | 92.4 b | 72.0 abc |
| STS 60 | 95.8 b | 100.0 d |
| SA 25 | 100.0 c | 38.8 a |
| SA 50 | 100.0 c | 54.3 ab |
| SA 75 | 54.8 a | 100.0 d |
| Treatment | Total Vital Calluses (%) | Embryogenic Calluses (%) |
|---|---|---|
| Control | 100.0 n.s. | 95.8 n.s. |
| C1C | 100.0 n.s. | 100.0 n.s. |
| STS 20 | 96.4 n.s. | 100.0 n.s. |
| STS 40 | 100.0 n.s. | 100.0 n.s. |
| STS 60 | 100.0 n.s. | 96.4 n.s. |
| SA 25 | 100.0 n.s. | 100.0 n.s. |
| SA 50 | 95.6 n.s. | 91.2 n.s. |
| SA 75 | 100.0 n.s. | 94.9 n.s. |
| Treatment | Total Vital Calluses (%) † | Embryogenic Calluses (%) ‡ |
|---|---|---|
| Control | 100.0 b | 0.0 n.s. |
| C1C | 100.0 b | 0.0 n.s. |
| STS 20 | 100.0 b | 9.8 n.s. |
| STS 40 | 100.0 b | 0.0 n.s. |
| STS 60 | 91.7 a | 0.0 n.s. |
| SA 25 | 100.0 b | 0.0 n.s. |
| SA 50 | 100.0 b | 3.1 n.s. |
| SA 75 | 100.0 b | 0.0 n.s. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Forleo, L.R.; Basile, T.; Suriano, B.; Maggiolini, F.A.M.; D’Amico, M.; Cardone, M.F.; Velasco, R.; Bergamini, C. Effects of Ethylene Inhibitors on the Long-Term Maintenance of the Embryogenic Callus of Vitis vinifera L. Horticulturae 2024, 10, 1038. https://doi.org/10.3390/horticulturae10101038
Forleo LR, Basile T, Suriano B, Maggiolini FAM, D’Amico M, Cardone MF, Velasco R, Bergamini C. Effects of Ethylene Inhibitors on the Long-Term Maintenance of the Embryogenic Callus of Vitis vinifera L. Horticulturae. 2024; 10(10):1038. https://doi.org/10.3390/horticulturae10101038
Chicago/Turabian StyleForleo, Lucia Rosaria, Teodora Basile, Bruna Suriano, Flavia Angela Maria Maggiolini, Margherita D’Amico, Maria Francesca Cardone, Riccardo Velasco, and Carlo Bergamini. 2024. "Effects of Ethylene Inhibitors on the Long-Term Maintenance of the Embryogenic Callus of Vitis vinifera L." Horticulturae 10, no. 10: 1038. https://doi.org/10.3390/horticulturae10101038
APA StyleForleo, L. R., Basile, T., Suriano, B., Maggiolini, F. A. M., D’Amico, M., Cardone, M. F., Velasco, R., & Bergamini, C. (2024). Effects of Ethylene Inhibitors on the Long-Term Maintenance of the Embryogenic Callus of Vitis vinifera L. Horticulturae, 10(10), 1038. https://doi.org/10.3390/horticulturae10101038








