Extension of Vase Life by Nano-Selenium in Rosa hybrida
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Measurement Indicators
- (1)
- Vase Life: The morphological changes in cut roses were observed daily. The vase life was calculated as the number of days from when the flower stem was inserted into the vase solution until 50% of the petals wilted and fell off. The average vase life (d) was recorded [20].
- (2)
- Petal moisture content: Measure the fresh weight and dry weight of the petals. The formula is
- (3)
- Soluble Sugar (SS) and Soluble Protein (SP) Content (μg/g) Analysis: Grind 0.3 g of petals in liquid nitrogen to a powder and resuspend in 5 mL of distilled water. Place the thoroughly mixed solution in a water bath at 85 °C for 30 min; then, collect the supernatant via centrifugation at 10,000× g for 10 min at 4 °C. Add distilled water to the supernatant to a final volume of 10 mL; then, determine the soluble sugar content using the anthrone sulfuric acid method [21] at 620 nm. Determine the soluble protein content using the Coomassie Brilliant Blue G-250 method [22]. Grind 0.3 g of petals in liquid nitrogen to a powder and resuspend in 3 mL of phosphate buffer (pH 7.0). Centrifuge the solution at 10,000× g for 15 min at 4 °C. Mix 0.1 mL of the supernatant with 4.9 mL of Coomassie Brilliant Blue G-250 solution (0.1 g/L). After incubating for 2 min, analyze the mixture at a wavelength of 595 nm.
- (4)
- Antioxidant Enzyme Activity (U/g FW) Analysis: Grind 0.3 g of petals in liquid nitrogen to a powder. SOD activity was measured using the photochemical reduction method with nitroblue tetrazolium (NBT) [23]. The absorbance of the sample was measured at 560 nm, and the enzyme amount that inhibited 50% of NBT photochemical reduction was considered 1 unit of SOD activity. POD activity was determined using the guaiacol method [23]. The change in absorbance at 470 nm within 60 s was recorded, where 0.01 represents 1 unit of POD activity. CAT activity was measured using the ultraviolet–visible spectrophotometry method [24], with the change in absorbance at 240 nm during a 60 s iodine titration, where 0.1 represents 1 unit of CAT activity.
- (5)
- Malondialdehyde (MDA) Content (μmol/g) Analysis: The malondialdehyde content was determined using the thiobarbituric acid (TBA) method [25]. Petals (0.3 g) were ground into a powder and extracted with 5% trichloroacetic acid (5 mL). The extract was centrifuged (2500× g at 4°C for 10 min), and then the supernatant (2 mL) was mixed with 0.7% TBA (2 mL) and incubated in a boiling water bath (100 °C) for 30 min. The solution was then centrifuged (10,000× g for 20 min), and the absorbance of the supernatant was measured at 450, 532, and 600 nm.
- (6)
- Hydrogen Peroxide (H₂O₂) (μg/g) Analysis: Hydrogen peroxide content was measured [26] as follows: Each petal powder sample (0.3 g) was homogenized in cold acetone (6 mL, 100%) and then centrifuged at 12,000× g for 10 min at 4 °C to obtain the supernatant. To 1 mL of the obtained extract, 0.2 mL of NH₄OH and 0.1 mL of 5% Ti (SO₄)₂ were added, followed by centrifugation at 3000× g for 10 min. The precipitate was dissolved in 4 mL of H₂SO₄. Finally, the optical density was measured at 412 nm using a spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA).
- (7)
- Total Phenolic Acid (μg/g) Analysis: Petals (0.3 g) were ground into powder in liquid nitrogen and then extracted with 80% methanol solvent (50 mL) by stirring at room temperature for 2 days. After removing the methanol, the obtained extract was stored at 4 °C. A 0.5 mL aliquot of the diluted extract (1:10 g/mL) or gallic acid (as a standard for phenolic compounds) was added to 4 mL of 1 M sodium carbonate and 5 mL of diluted Folin–Ciocalteu reagent (1:10). Finally, the optical density was measured at 765 nm to estimate the total phenolic content [27].
- (8)
- Total Flavonoid (mg/g) Analysis: Dissolve 0.3 g of petal powder in 5 mL of methanol solvent and sonicate at 40 °C for 45 min, then centrifuge at 1000× g for 10 min. Prepare a standard calibration curve using quercetin. Mix 0.6 mL of the standard quercetin solution or the extract with 0.6 mL of 2% aluminum chloride solution. After mixing, incubate the solution at room temperature for 60 min. Measure the absorbance of the reaction mixture at 420 nm using a UV–visible spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA) [28].
2.3. Data Statistics
3. Results
3.1. Changes of Different Concentrations of Nano-Se on the Vase Life of Cut Roses
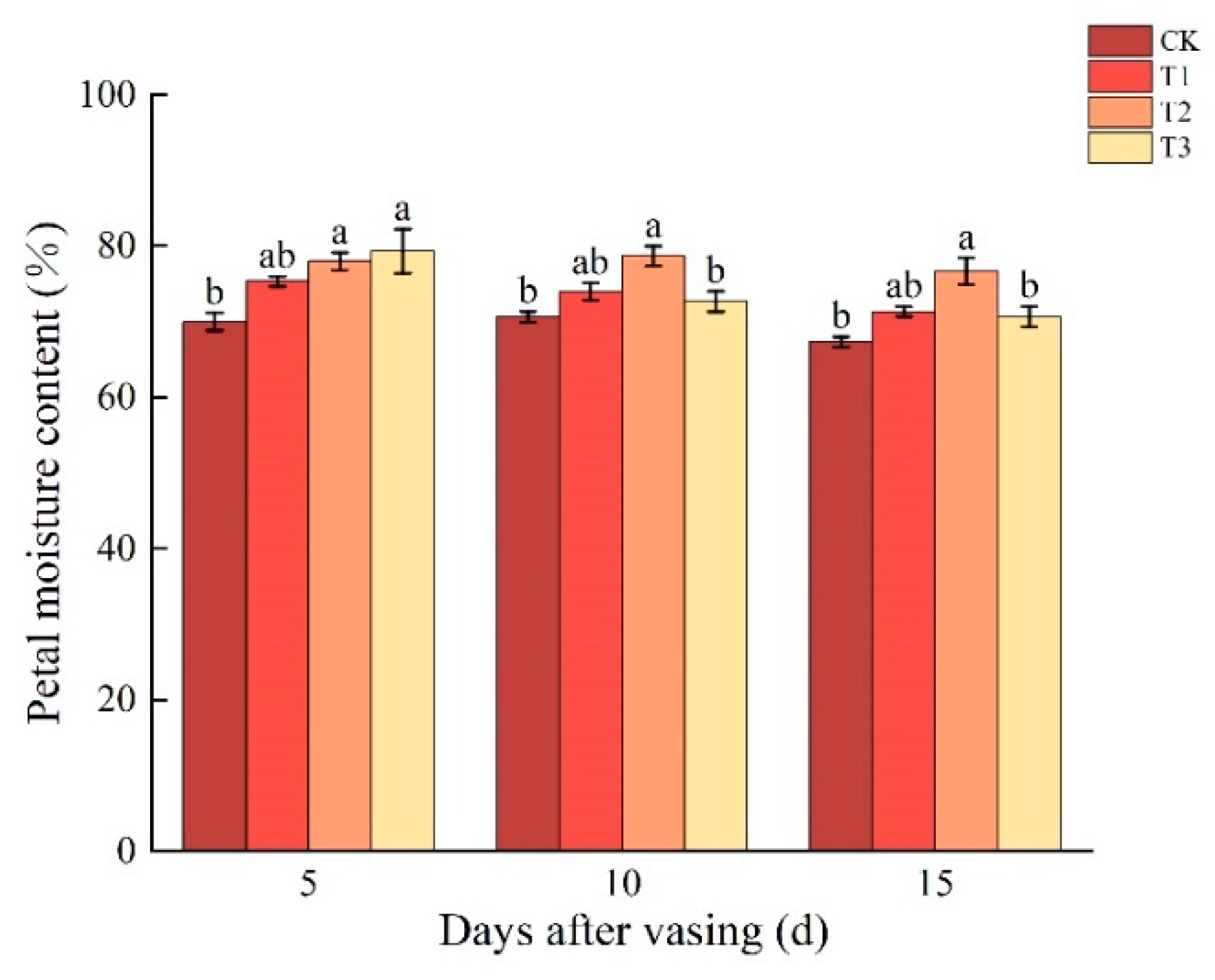
3.2. Changes in Petal Moisture Content of Cut Roses under Different Concentrations of Nano-Se
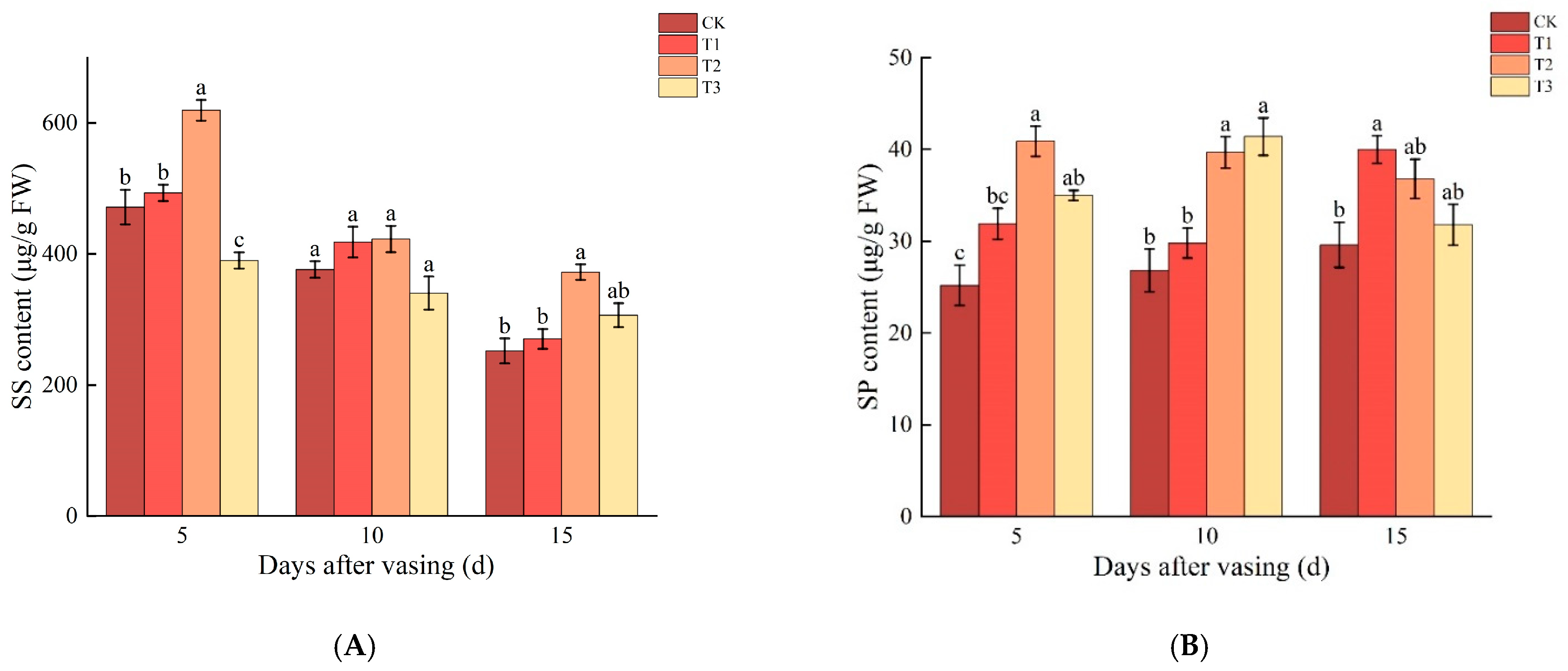
3.3. Changes in SS and SP Contents of Cut Roses under Different Concentrations of Nano-Se
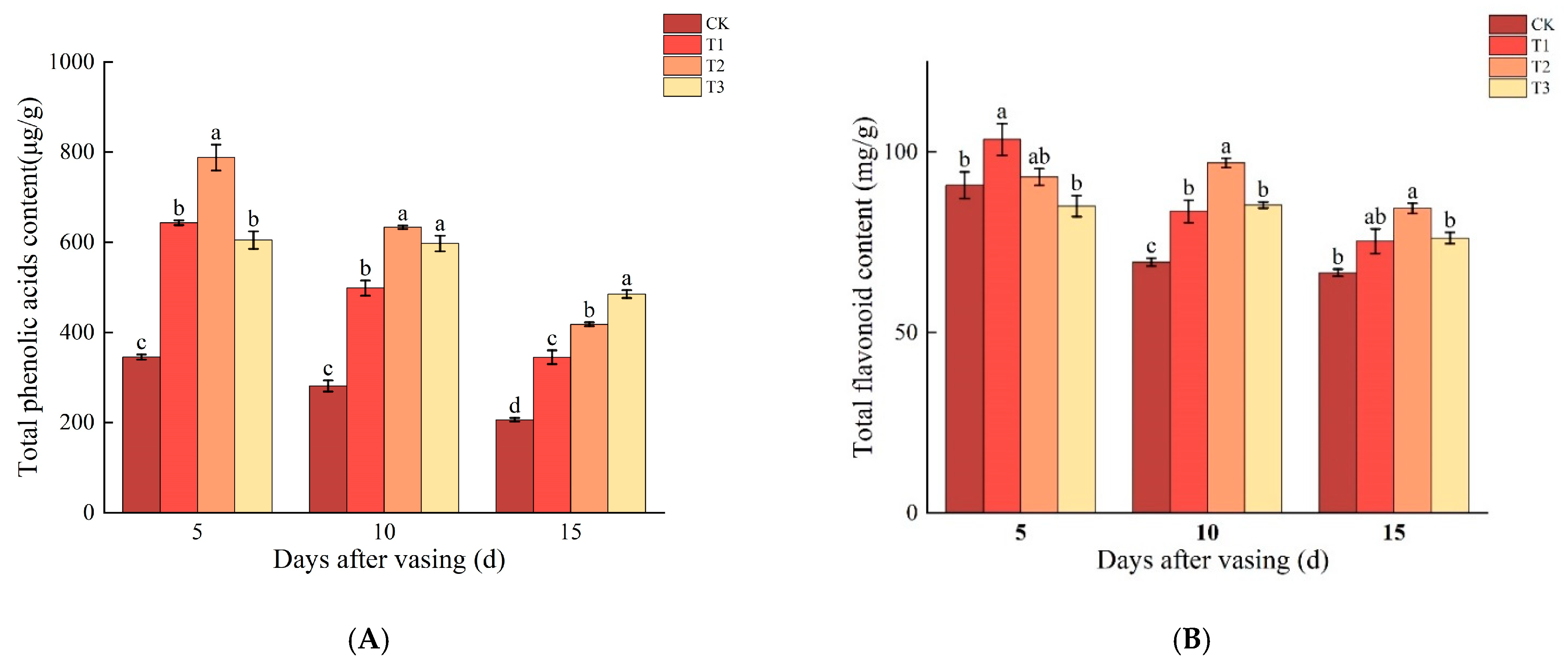
3.4. Changes in Total Phenolic Acid and Total Flavonoid Contents of Cut Roses under Different Concentrations of Nano-Se
3.5. Changes in POD, SOD, and CAT Activities of Cut Roses under Different Concentrations of Nano-Se
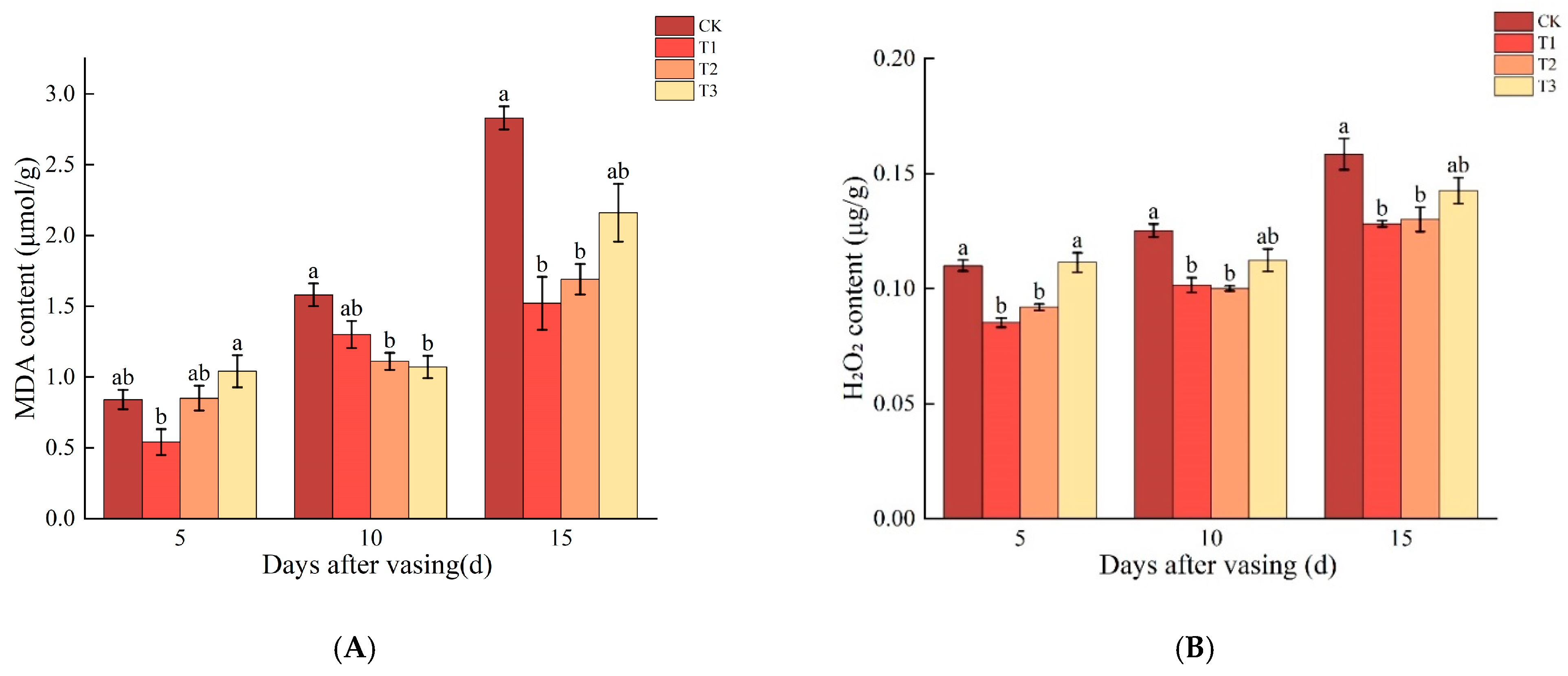
3.6. Changes in MDA and H2O2 Contents of Cut Roses under Different Concentrations of Nano-Se
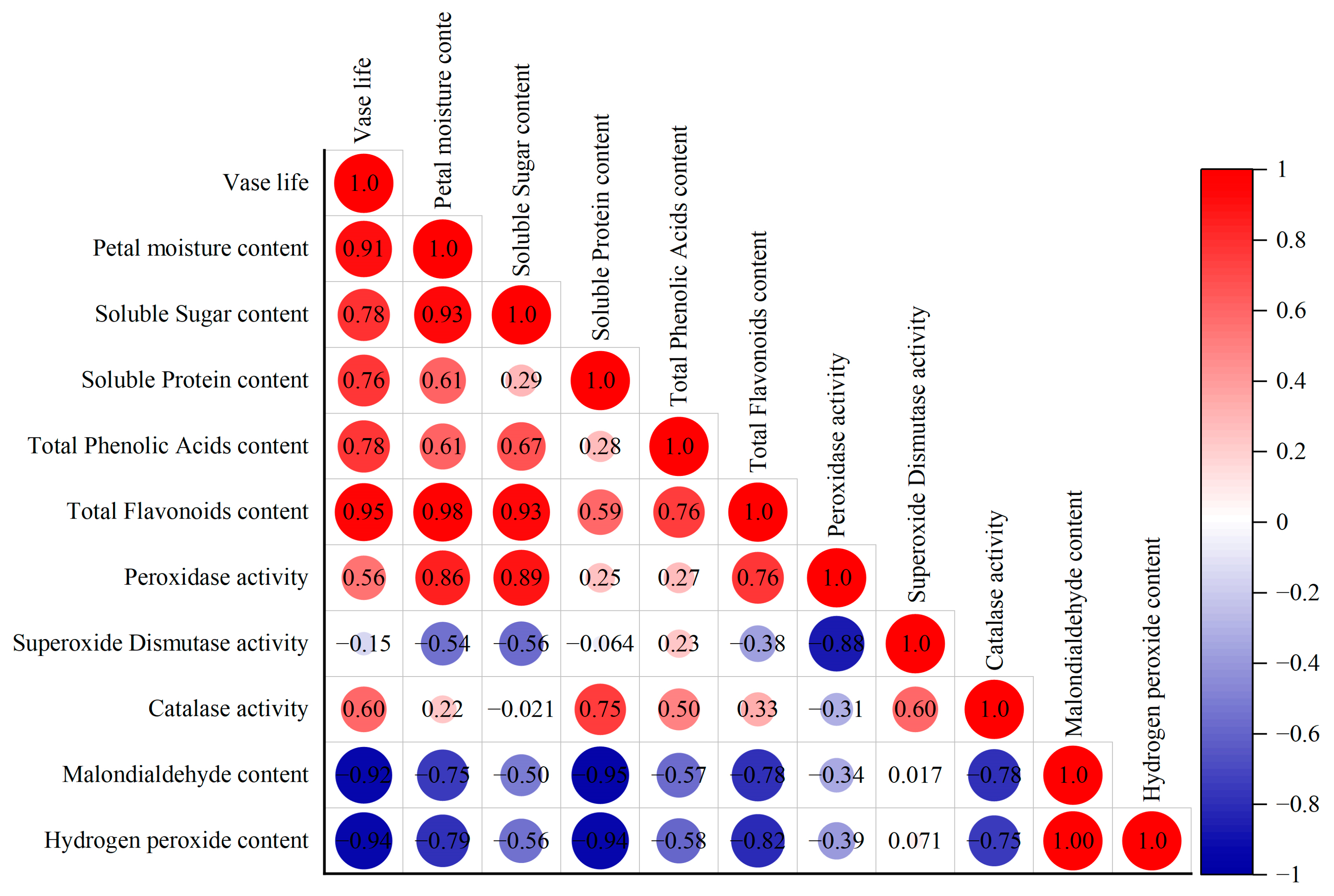
3.7. Correlation Analysis of Physiological Indicators
4. Discussion
4.1. An Appropriate Concentration of Nano-Se Can Significantly Extend the Vase Life of Cut Roses
4.2. Appropriate Concentrations of Nano-Se Delay the Loss of SS and SP Content in Cut Roses
4.3. Adaptability of Vase Roses to Selenium-Enriched Environments
4.4. Comprehensive Evaluation of Nano-Se Treatment on the Postharvest Quality of Cut Roses
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Elhindi, K.M. Evaluation of several holding solutions for prolonging vase-life and keeping quality of cut sweet pea flowers (Lathyrus odoratus L.). Saudi J. Biol. Sci. 2012, 19, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, I.; Dole, J.M.; Clark, E.M.R.; Blazich, F.A. Floral foam and/or conventional or organic preservatives affect the vase-life and quality of cut rose (Rosa × hybrida L.) stems. J. Hortic. Sci. Biotechnol. 2014, 89, 41–46. [Google Scholar] [CrossRef]
- Ma, Y. In Vitro Models for Nanotoxicity Testing; Wiley: West Sussex, UK, 2009. [Google Scholar]
- Domokos-Szabolcsy, E.; Marton, L.; Sztrik, A.; Babka, B.; Prokisch, J.; Fari, M. Accumulation of red elemental selenium nanoparticles and their biological effects in Nicotinia tabacum. Plant Growth Regul. 2012, 68, 525–531. [Google Scholar] [CrossRef]
- Djanaguiraman, M.; Belliraj, N.; Bossmann, S.H.; Prasad, P.V.V. High-Temperature Stress Alleviation by Selenium Nanoparticle Treatment in Grain Sorghum. ACS Omega 2018, 3, 2479–2491. [Google Scholar] [CrossRef] [PubMed]
- Bano, I.; Skalickova, S.; Sajjad, H.; Skladanka, J.; Horky, P. Uses of Selenium Nanoparticles in the Plant Production. Agronomy 2021, 11, 2229. [Google Scholar] [CrossRef]
- Bai, K.; Hong, B.; He, J.; Hong, Z.; Tan, R. Preparation and antioxidant properties of selenium nanoparticles-loaded chitosan microspheres. Int. J. Nanomed. 2017, 12, 4527–4539. [Google Scholar] [CrossRef]
- Alagawany, M.; Qattan, S.; Attia, Y.; El-Saadony, M.; Elnesr, S.; Mahmoud, M.; Madkour, M.; Abd El-Hack, M.; Reda, F.; Ebani, V. Use of Chemical Nano-Selenium as an Antibacterial and Antifungal Agent in Quail Diets and Its Effect on Growth, Carcasses, Antioxidant, Immunity and Caecal Microbes. Animals 2021, 11, 3027. [Google Scholar] [CrossRef]
- Vera, P.; Canellas, E.; Nerín, C. New antioxidant multilayer packaging with nanoselenium to enhance the shelf-life of market food products. Nanomaterials 2018, 8, 837. [Google Scholar] [CrossRef]
- Samynathan, R.; Venkidasamy, B.; Ramya, K.; Muthuramalingam, P.; Shin, H.; Kumari, P.S.; Thangavel, S.; Sivanesan, I. A Recent Update on the Impact of Nano-Selenium on Plant Growth, Metabolism, and Stress Tolerance. Plants 2023, 12, 853. [Google Scholar] [CrossRef]
- Foyer, C.; Descourvières, P.; Kunert, K. Protection against oxygen radicals: An important defence mechanism studied in transgenic plants. Plant Cell Environ. 2006, 17, 507–523. [Google Scholar] [CrossRef]
- Kaur, M.; Sharma, S.; Singh, D. Influence of selenium on carbohydrate accumulation in developing wheat grains. Commun. Soil Sci. Plant Anal. 2018, 49, 1650–1659. [Google Scholar] [CrossRef]
- Shahraki, B.; Bayat, H.; Aminifard, M.; Azarmi-Atajan, F. Effects of Foliar Application of Selenium and Nano-Selenium on Growth, Flowering, and Antioxidant Activity of Pot Marigold (Calendula officinalis L.) Under Salinity Stress Conditions. Commun. Soil Sci. Plant Anal. 2022, 53, 2749–2765. [Google Scholar] [CrossRef]
- Zhang, M.; Tang, S.; Huang, X.; Zhang, F.; Pang, Y.; Huang, Q.; Yi, Q. Selenium uptake, dynamic changes in selenium content and its influence on photosynthesis and chlorophyll fluorescence in rice (Oryza sativa L.). Environ. Exp. Bot. 2014, 107, 39–45. [Google Scholar] [CrossRef]
- Morales-Espinoza, M.C.; Cadenas-Pliego, G.; Pérez-Alvarez, M.; Hernández-Fuentes, A.D.; Cabrera de la Fuente, M.; Benavides-Mendoza, A.; Valdés-Reyna, J.; Juárez-Maldonado, A.; Morales-Espinoza, M.C.; Cadenas-Pliego, G.; et al. Se Nanoparticles Induce Changes in the Growth, Antioxidant Responses, and Fruit Quality of Tomato Developed under NaCl Stress. Molecules 2019, 24, 3030. [Google Scholar] [CrossRef]
- Manaf, H.H. Beneficial effects of exogenous selenium, glycine betaine and seaweed extract on salt stressed cowpea plant. Ann. Agric. Sci. 2016, 61, 41–48. [Google Scholar] [CrossRef]
- Turakainen, M.; Hartikainen, H.; Seppänen, M.M. Effects of selenium treatments on potato (Solanum tuberosum L.) growth and concentrations of soluble sugars and starch. J. Agric. Food Chem. 2004, 52, 5378–5382. [Google Scholar] [CrossRef]
- Li, D.; Zhou, C.; Zou, N.; Wu, Y.; Zhang, J.; An, Q.; Li, J.-Q.; Pan, C. Nanoselenium foliar application enhances biosynthesis of tea leaves in metabolic cycles and associated responsive pathways. Environ. Pollut. 2021, 273, 116503. [Google Scholar] [CrossRef]
- Ahmadi-Majd, M.; Mousavi-Fard, S.; Rezaei Nejad, A.; Fanourakis, D. Nano-Selenium in the holding solution promotes rose and carnation vase life by improving both water relations and antioxidant status. J. Hortic. Sci. Biotechnol. 2023, 98, 246–261. [Google Scholar] [CrossRef]
- Sánchez Díaz, J.M.; Jiménez-Becker, S.; Jamilena, M. A screening test for the determination of cut flower longevity and ethylene sensitivity of carnation. Hortic. Sci. 2017, 44, 14–20. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, Y.; Song, Y.; Zhu, J.; Shang, W.; Jiang, L.; Liu, W.; He, S.; Shen, Y.; Shi, L.; et al. ClO2 Prolongs the Vase Life of Paeonia lactiflora ‘Hushui Dangxia’ Cut Flowers by Inhibiting Bacterial Growth at the Stem Base. Horticulturae 2024, 10, 732. [Google Scholar] [CrossRef]
- van Doorn, W.G.; Sinz, A.; Tomassen, M.M. Daffodil flowers delay senescence in cut Iris flowers. Phytochemistry 2004, 65, 571–577. [Google Scholar] [CrossRef]
- Ul Haq, A.; Lone, M.; Farooq, S.; Parveen, S.; Altaf; Tahir, I.; Hefft, D.; Ahmad, A.; Ahmad, P. Nitric oxide effectively orchestrates postharvest flower senescence: A case study of Consolida ajacis. Funct. Plant Biol. 2021, 50, 97–107. [Google Scholar] [CrossRef]
- Labancová, E.; Vivodová, Z.; Šípošová, K.; Kollárová, K. Silicon Actuates Poplar Calli Tolerance after Longer Exposure to Antimony. Plants 2023, 12, 689. [Google Scholar] [CrossRef]
- Lou, X.; Anwar, M.; Wang, Y.; Zhang, H.; Ding, J. Impact of inorganic salts on vase life and postharvest qualities of the cut flower of Perpetual Carnation. Braz. J. Biol. 2020, 81, 228–236. [Google Scholar] [CrossRef]
- Mazrou, R.; Hassan, S.; Yang, M.; Hassan, F. Melatonin Preserves the Postharvest Quality of Cut Roses through Enhancing the Antioxidant System. Plants 2022, 11, 2713. [Google Scholar] [CrossRef]
- Chandra, S.; Khan, S.; Avula, B.; Lata, H.; Yang, M.H.; ElSohly, M.A.; Khan, I.A. Assessment of Total Phenolic and Flavonoid Content, Antioxidant Properties, and Yield of Aeroponically and Conventionally Grown Leafy Vegetables and Fruit Crops: A Comparative Study. Evid. Based Complement. Altern. Med. 2014, 2014, 253875. [Google Scholar] [CrossRef]
- McDonald, S.; Prenzler, P.D.; Antolovich, M.; Robards, K. Phenolic content and antioxidant activity of olive extracts. Food Chem. 2001, 73, 73–84. [Google Scholar] [CrossRef]
- Lu, N.; Wu, L.; Zhang, C.; Shan, C. Sodium selenite improves the vase life of Eustoma grandiflorum cut flowers. Hortic. Sci. 2022, 49, 248–256. [Google Scholar] [CrossRef]
- Cremonini, E.; Zonaro, E.; Donini, M.; Lampis, S.; Boaretti, M.; Dusi, S.; Lleo, M.; Vallini, G. Biogenic selenium nanoparticles: Characterization, antimicrobial activity and effects on human dendritic cells and fibroblasts. Microb. Biotechnol. 2016, 9, 758–771. [Google Scholar] [CrossRef]
- Jedrzejuk, A.; Rochala, J.; Zakrzewski, J.; Rabiza-Świder, J. Identification of xylem occlusions occurring in cut clematis (Clematis L., fam. Ranunculaceae Juss.) stems during their vase life. Sci. World J. 2012, 2012, 749281. [Google Scholar] [CrossRef]
- Zheng, M.; Guo, Y. Effects of neodymium on the vase life and physiological characteristics of Lilium casa Blanca petals. Sci. Hortic. 2019, 256, 108553. [Google Scholar] [CrossRef]
- Aryal, S.; Baniya, M.K.; Danekhu, K.; Kunwar, P.; Gurung, R.; Koirala, N. Total Phenolic Content, Flavonoid Content and Antioxidant Potential of Wild Vegetables from Western Nepal. Plants 2019, 8, 96. [Google Scholar] [CrossRef]
- Li, S.; Chen, Z.; Yang, F.; Yue, W. The age of vanadium-based nanozymes: Synthesis, catalytic mechanisms, regulation and biomedical applications. Chin. Chem. Lett. 2024, 35, 108793. [Google Scholar] [CrossRef]
- Hashemabadi, D. The role of silver nano-particles and silver thiosulfate on the longevity of cut carnation (Dianthus caryophyllus) flowers. J. Environ. Biol. Acad. Environ. Biol. India 2014, 35, 661–666. [Google Scholar]
- Saeed, T.; Hassan, I.; Abbasi, N.A.; Jilani, G. Effect of gibberellic acid on the vase life and oxidative activities in senescing cut gladiolus flowers. Plant Growth Regul. 2014, 72, 89–95. [Google Scholar] [CrossRef]
- Kazemi, M.; Shokri, K. Role of salicylic acid in decreases of membrane senescence in cut lisianthus flowers. World Appl. Sci. J. 2011, 13, 142–146. [Google Scholar]
- Zahedi, S.M.; Moharrami, F.; Sarikhani, S.; Padervand, M. Selenium and silica nanostructure-based recovery of strawberry plants subjected to drought stress. Sci. Rep. 2020, 10, 17672. [Google Scholar] [CrossRef]
- Hassan, F.A.S.; Fetouh, M.I. Does moringa leaf extract have preservative effect improving the longevity and postharvest quality of gladiolus cut spikes? Sci. Hortic. 2019, 250, 287–293. [Google Scholar] [CrossRef]
- Ahmadi-Majd, M.; Mousavi-Fard, S.; Rezaei Nejad, A.; Fanourakis, D. Carbon nanotubes in the holding solution stimulate flower opening and prolong vase life in carnation. Chem. Biol. Technol. Agric. 2022, 9, 15. [Google Scholar] [CrossRef]
- Far, A.S.; Mousavi-Fard, S.; Nejad, A.R.; Shahbazi, F.; Ahmadi-Majd, M.; Fanourakis, D. Nano Silver and melatonin effectively delay the senescence of cut carnation flowers under simulated vibrational stress. J. Hortic. Sci. Biotechnol. 2024, 99, 597–608. [Google Scholar]
- Ghazi, A.A.; El-Nahrawy, S.; El-Ramady, H.; Ling, W. Biosynthesis of Nano-Selenium and Its Impact on Germination of Wheat under Salt Stress for Sustainable Production. Sustainability 2022, 14, 1784. [Google Scholar] [CrossRef]
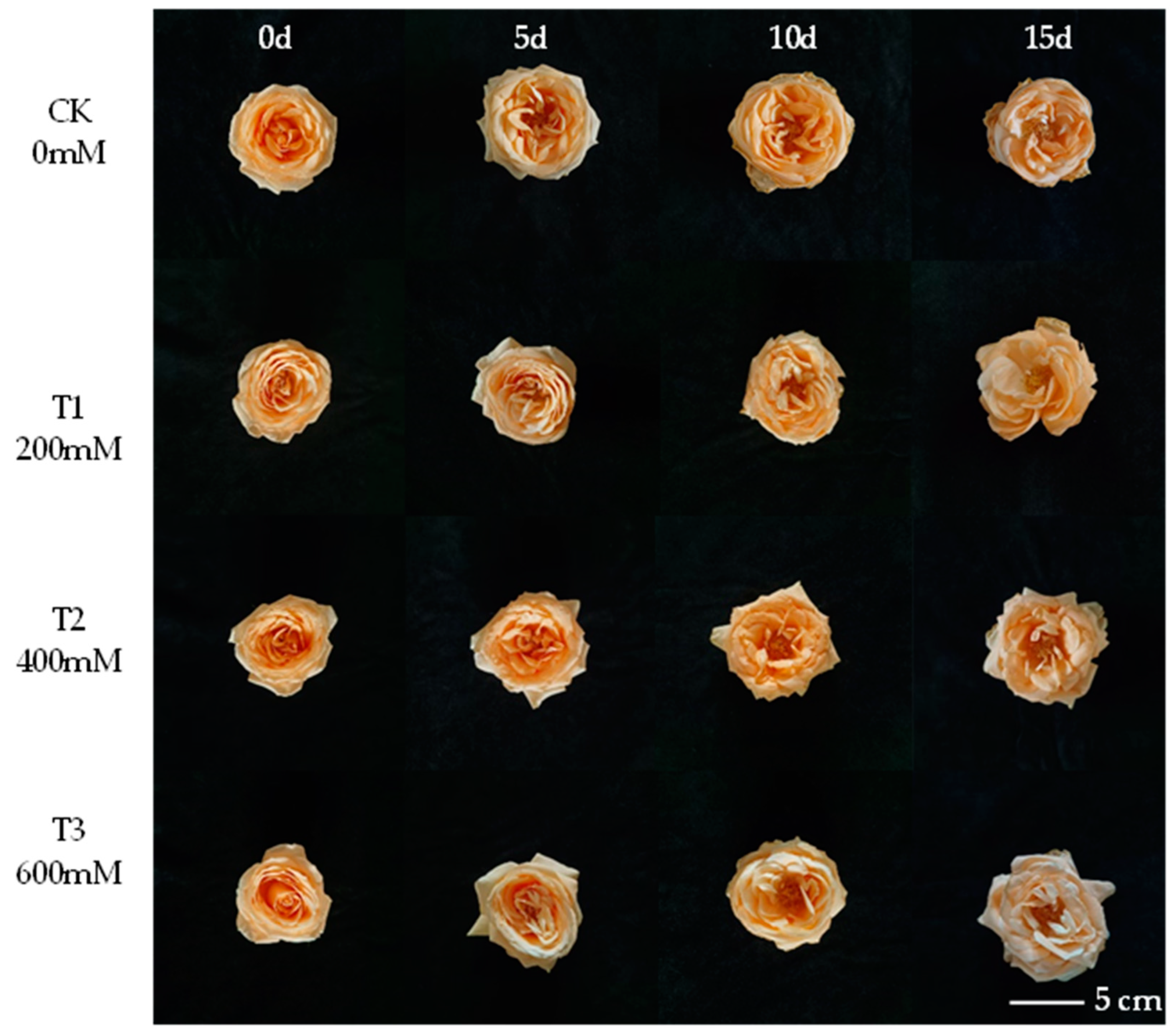

| Treatment | Vase Life (d) | The Extended Day (d) | Vase Life Termination Symptoms |
|---|---|---|---|
| CK | 13.0 ± 1.0 b | 0 | 50% wilted and the first petal falls off |
| T1 | 17.3 ± 1.5 a | 4.3 | 50% wilted and the first petal falls off |
| T2 | 18.7 ± 0.6 a | 5.7 | 50% wilted and the first petal falls off |
| T3 | 16.7 ± 1.5 a | 3.7 | 50% wilted and the first petal falls off |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Cai, Y.; Cai, D.; Xue, J.; Wang, D.; Xue, Y.; Wang, Q.; Xu, F. Extension of Vase Life by Nano-Selenium in Rosa hybrida. Horticulturae 2024, 10, 1071. https://doi.org/10.3390/horticulturae10101071
Wang Y, Cai Y, Cai D, Xue J, Wang D, Xue Y, Wang Q, Xu F. Extension of Vase Life by Nano-Selenium in Rosa hybrida. Horticulturae. 2024; 10(10):1071. https://doi.org/10.3390/horticulturae10101071
Chicago/Turabian StyleWang, Yiting, Yiling Cai, Dongbo Cai, Jia Xue, Dao Wang, Yansheng Xue, Qijian Wang, and Feng Xu. 2024. "Extension of Vase Life by Nano-Selenium in Rosa hybrida" Horticulturae 10, no. 10: 1071. https://doi.org/10.3390/horticulturae10101071
APA StyleWang, Y., Cai, Y., Cai, D., Xue, J., Wang, D., Xue, Y., Wang, Q., & Xu, F. (2024). Extension of Vase Life by Nano-Selenium in Rosa hybrida. Horticulturae, 10(10), 1071. https://doi.org/10.3390/horticulturae10101071






