Low Nitrogen Availability in Organic Fertilizers Limited Organic Watermelon Transplant Growth
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
2.2. Fertilizer Teatments
2.3. Chemical Properties of Liquid Fertilizers
2.4. Transplant Physiological and Morphological Measurements
2.5. Data Analyses
3. Results
3.1. Chemical Properties of Liquid Fertilizers
3.2. Shoot Growth
3.3. Root Growth
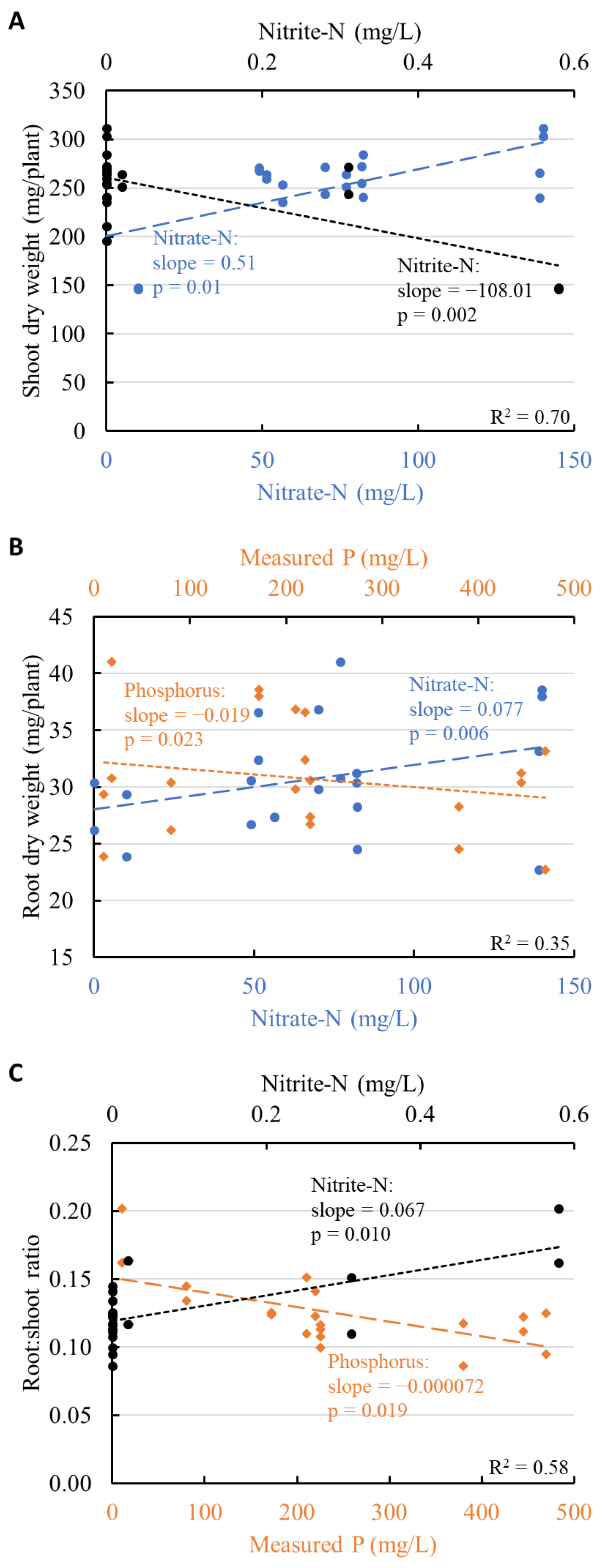
3.4. Mineral Nutrients and Plant Growth
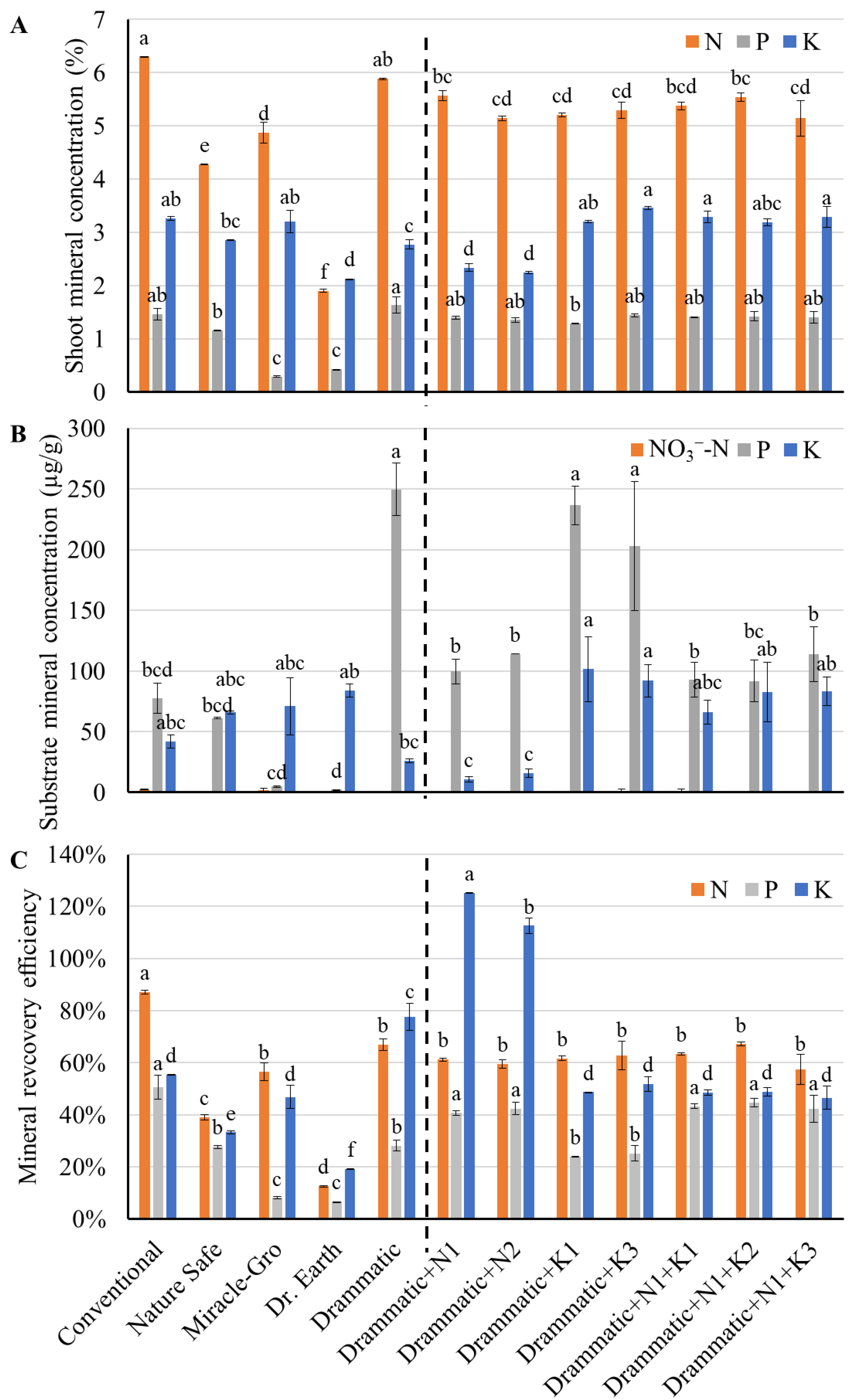
3.5. Mineral Concentrations of Shoot and Substrate
4. Discussion
4.1. Nitrogen and Phosphorus Availability and Mineralization Rates Varied Among Fertilizers
4.2. Plant Growth as Affected by Fertilizers
4.2.1. Shoot Growth Limited by Nitrogen Availability
4.2.2. Shoot Growth and Nitrite Toxicity
4.2.3. Shoot Growth Unaffected by Phosphorus and Potassium Concentration
4.2.4. Root Growth and Root:Shoot Ratio Weakly Affected by Mineral Availability
4.3. Limitations and Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grange, S.; Leskovar, D.I.; Pike, L.M.; Cobb, B.G. Seedcoat structure and oxygen-enhanced environments affect germination of triploid watermelon. J. Am. Soc. Hortic. Sci. 2003, 128, 253–259. [Google Scholar] [CrossRef]
- NeSmith, D. Root distribution and yield of direct seeded and transplanted watermelon. J. Am. Soc. Hortic. Sci. 1999, 124, 458–461. [Google Scholar] [CrossRef]
- Weston, L.A.; Zandstra, B.H. Effect of root container size and location of production on growth and yield of tomato transplants. J. Am. Soc. Hortic. Sci. 1986, 111, 498–501. [Google Scholar] [CrossRef]
- Janssen, M. Determinants of organic food purchases: Evidence from household panel data. Food Qual. Prefer. 2018, 68, 19–28. [Google Scholar] [CrossRef]
- Reganold, J.P.; Wachter, J.M. Organic agriculture in the twenty-first century. Nat. Plants 2016, 2, 15221. [Google Scholar] [CrossRef]
- Shahabi Ahangarkolaee, S.; Gorton, M. The effects of perceived regulatory efficacy, ethnocentrism and food safety concern on the demand for organic food. Int. J. Consum. Stud. 2021, 45, 273–286. [Google Scholar] [CrossRef]
- US Department of Agriculture, National Agricultural Statistics Service. 2021 Organic Survey. 2022. Available online: https://www.nass.usda.gov/Surveys/Guide_to_NASS_Surveys/Organic_Production/ (accessed on 26 August 2024).
- Bergstrand, K.-J. Organic fertilizers in greenhouse production systems—A review. Sci. Hortic. 2022, 295, 110855. [Google Scholar] [CrossRef]
- Chatzistathis, T.; Kavvadias, V.; Sotiropoulos, T.; Papadakis, I.E. Organic fertilization and tree orchards. Agriculture 2021, 11, 692. [Google Scholar] [CrossRef]
- Burnett, S.E.; Mattson, N.S.; Williams, K.A. Substrates and fertilizers for organic container production of herbs, vegetables, and herbaceous ornamental plants grown in greenhouses in the United States. Sci. Hortic. 2016, 208, 111–119. [Google Scholar] [CrossRef]
- Timsina, J. Can organic sources of nutrients increase crop yields to meet global food demand? Agronomy 2018, 8, 214. [Google Scholar] [CrossRef]
- Zikeli, S.; Deil, L.; Möller, K. The challenge of imbalanced nutrient flows in organic farming systems: A study of organic greenhouses in Southern Germany. Agric. Ecosyst. Environ. 2017, 244, 1–13. [Google Scholar] [CrossRef]
- Pang, X.; Letey, J. Organic farming challenge of timing nitrogen availability to crop nitrogen requirements. Soil Sci. Soc. Am. J. 2000, 64, 247–253. [Google Scholar] [CrossRef]
- He, H.; Peng, M.; Ru, S.; Hou, Z.; Li, J. A suitable organic fertilizer substitution ratio could improve maize yield and soil fertility with low pollution risk. Front. Plant Sci. 2022, 13, 988663. [Google Scholar] [CrossRef]
- Kozai, T.; Kubota, C.; Chun, C.; Afreen, F.; Ohyama, K. Necessity and concept of the closed transplant production system. In Transplant Production in the 21st Century, Proceedings of the International Symposium on Transplant Production in Closed System for Solving the Global Issues on Environmental Conservation, Food, Resources and Energy, Chiba, Japan, 28 February–2 March 2000; Springer: Berlin/Heidelberg, Germany, 2000; pp. 3–19. [Google Scholar]
- Shrefler, J.; Brandenberger, L.; Rebek, E.; Damicone, J.; Taylor, M.; Watermelon production. Oklahoma Cooperative Extension, Oklahoma State University, Oklahoma, United States of America, Fact Sheets, HLA-6236. 2015. Available online: http://osufacts.okstate.edu (accessed on 26 August 2024).
- McAvoy, T.; Coolong, T. Commercial Watermelon Production; The University of Georgia: Athens, GA, USA, 2017. Bulletin 996. Available online: https://extension.uga.edu/publications/detail.html?number=B996&title=commercial-watermelon-production (accessed on 26 August 2024).
- Zhang, Q.; Masabni, J.; Niu, G. Organic fertilizer type and dose affect growth, morphological and physiological parameters, and mineral nutrition of watermelon seedlings. PeerJ 2024, 12, e16902. [Google Scholar] [CrossRef]
- Wissuwa, M.; Gamat, G.; Ismail, A.M. Is root growth under phosphorus deficiency affected by source or sink limitations? J. Exp. Bot. 2005, 56, 1943–1950. [Google Scholar] [CrossRef]
- Niu, Y.F.; Chai, R.S.; Jin, G.L.; Wang, H.; Tang, C.X.; Zhang, Y.S. Responses of root architecture development to low phosphorus availability: A review. Ann. Bot. 2013, 112, 391–408. [Google Scholar] [CrossRef]
- Lopez, G.; Ahmadi, S.H.; Amelung, W.; Athmann, M.; Ewert, F.; Gaiser, T.; Gocke, M.I.; Kautz, T.; Postma, J.; Rachmilevitch, S. Nutrient deficiency effects on root architecture and root-to-shoot ratio in arable crops. Front. Plant Sci. 2023, 13, 1067498. [Google Scholar] [CrossRef]
- Caspersen, S.; Bergstrand, K.-J. Phosphorus restriction influences P efficiency and ornamental quality of poinsettia and chrysanthemum. Sci. Hortic. 2020, 267, 109316. [Google Scholar] [CrossRef]
- Henry, J.B.; McCall, I.; Jackson, B.; Whipker, B.E. Growth Response of Herbaceous Ornamentals to Phosphorus Fertilization. HortScience 2017, 52, 1362–1367. [Google Scholar] [CrossRef]
- Hochmuth, G.J.; Hanlon, E. Plant Tissue Analysis and Interpretation for Vegetable Crops in Florida: HS964/EP081 Rev. 11/2022; EDIS 2022 (6); University of Florida: Gainesville, FL, USA, 2022. [Google Scholar] [CrossRef]
- Gruber, B.D.; Giehl, R.F.; Friedel, S.; von Wirén, N. Plasticity of the Arabidopsis root system under nutrient deficiencies. Plant Physiol. 2013, 163, 161–179. [Google Scholar] [CrossRef]
- Altland, J.E. The pour-through procedure for monitoring container substrate chemical properties: A review. Horticulturae 2021, 7, 536. [Google Scholar] [CrossRef]
- Blok, C.; Streminska, M.; Vermeulen, T.; Klein, P. Organic fertilisers and nitrogen availability. Acta Hortic. 2017, 1168, 1–10. [Google Scholar] [CrossRef]
- Gaskell, M.; Smith, R. Nitrogen Sources for Organic Vegetable Crops. HortTechnology 2007, 17, 431–441. [Google Scholar] [CrossRef]
- Nygaard Sorensen, J.; Thorup-Kristensen, K. Plant-based fertilizers for organic vegetable production. J. Plant Nutr. Soil Sci. 2011, 174, 321–332. [Google Scholar] [CrossRef]
- Raviv, M.; Oka, Y.; Katan, J.; Hadar, Y.; Yogev, A.; Medina, S.; Krasnovsky, A.; Ziadna, H. High-nitrogen compost as a medium for organic container-grown crops. Bioresour. Technol. 2005, 96, 419–427. [Google Scholar] [CrossRef]
- Zhao, X.; Nechols, J.R.; Williams, K.A.; Wang, W.; Carey, E.E. Comparison of phenolic acids in organically and conventionally grown pac choi (Brassica rapa L. chinensis). J. Sci. Food Agric. 2009, 89, 940–946. [Google Scholar] [CrossRef]
- Miller, A.J.; Cramer, M.D. Root nitrogen acquisition and assimilation. Plant Soil 2005, 274, 1–36. [Google Scholar] [CrossRef]
- Ryan, M.H.; Kaur, P.; Nazeri, N.K.; Clode, P.L.; Keeble-Gagnère, G.; Doolette, A.L.; Smernik, R.J.; Van Aken, O.; Nicol, D.; Maruyama, H.; et al. Globular structures in roots accumulate phosphorus to extremely high concentrations following phosphorus addition. Plant Cell Environ. 2019, 42, 1987–2002. [Google Scholar] [CrossRef]
- Colla, G.; Rouphael, Y.; Mirabelli, C.; Cardarelli, M. Nitrogen-use efficiency traits of mini-watermelon in response to grafting and nitrogen-fertilization doses. J. Plant Nutr. Soil Sci. 2011, 174, 933–941. [Google Scholar] [CrossRef]
- Schultheis, J.R.; Dufault, R.J. Watermelon seedling growth, fruit yield, and quality following pretransplant nutritional conditioning. HortScience 1994, 29, 1264–1268. [Google Scholar] [CrossRef]
- Na, L.; Li, Z.; Xiangxiang, M.; Ara, N.; Jinghua, Y.; Mingfang, Z. Effect of Nitrate/Ammonium Ratios on Growth, Root Morphology and Nutrient Elements Uptake of Watermelon (Citrullus lanatus) Seedlings. J. Plant Nutr. 2014, 37, 1859–1872. [Google Scholar] [CrossRef]
- Oke, O.L. Nitrite Toxicity to Plants. Nature 1966, 212, 528. [Google Scholar] [CrossRef]
- Shen, Q.; Ran, W.; Cao, Z. Mechanisms of nitrite accumulation occurring in soil nitrification. Chemosphere 2003, 50, 747–753. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, J.L.; Lowe, R.H. Organic Matter and N Effects on Soil Nitrite Accumulation and Resultant Nitrite Toxicity to Tobacco Transplants. Agron. J. 1981, 73, 787–790. [Google Scholar] [CrossRef]
- Birch, P.; Eagle, D. Toxicity to seedlings of nitrite in sterilized composts. J. Hortic. Sci. 1969, 44, 321–330. [Google Scholar] [CrossRef]
- Bancroft, K.; Grant, I.F.; Alexander, M. Toxicity of NO2: Effect of nitrite on microbial activity in an acid soil. Appl. Environ. Microbiol. 1979, 38, 940–944. [Google Scholar] [CrossRef] [PubMed]
- Samater, A.H.; Van Cleemput, O.; Ertebo, T. Influence of the presence of nitrite and nitrate in soil on maize biomass production, nitrogen immobilization and nitrogen recovery. Biol. Fertil. Soils 1998, 27, 211–218. [Google Scholar] [CrossRef]
- Olaniyi, J. Growth and seed yield response of egusi melon to nitrogen and phosphorus fertilizers application. J. Sustain. Agric. 2008, 2, 255–260. [Google Scholar]
- Nawaz, M.A.; Chen, C.; Shireen, F.; Zheng, Z.; Sohail, H.; Afzal, M.; Ali, M.A.; Bie, Z.; Huang, Y. Genome-wide expression profiling of leaves and roots of watermelon in response to low nitrogen. BMC Genom. 2018, 19, 456. [Google Scholar] [CrossRef]
- Hermans, C.; Hammond, J.P.; White, P.J.; Verbruggen, N. How do plants respond to nutrient shortage by biomass allocation? Trends Plant Sci. 2006, 11, 610–617. [Google Scholar] [CrossRef]
- Shipley, B.; Meziane, D. The balanced-growth hypothesis and the allometry of leaf and root biomass allocation. Funct. Ecol. 2002, 16, 326–331. [Google Scholar] [CrossRef]
- Elmstrom, G. Watermelon root development affected by direct seeding and transplanting. HortScience 1973, 8, 134–136. [Google Scholar]
- Navarro-Morillo, I.; Navarro-Perez, V.; Perez-Millan, R.; Navarro-León, E.; Blasco, B.; Cámara-Zapata, J.M.; Garcia-Sanchez, F. Effects of root and foliar application of corn steep liquor on pepper plants: A physiological, nutritional, and morphological study. Horticulturae 2023, 9, 221. [Google Scholar] [CrossRef]
- Zhu, M.-M.; Liu, E.-Q.; Bao, Y.; Duan, S.-L.; She, J.; Liu, H.; Wu, T.-T.; Cao, X.-Q.; Zhang, J.; Li, B.; et al. Low concentration of corn steep liquor promotes seed germination, plant growth, biomass production and flowering in soybean. Plant Growth Regul. 2019, 87, 29–37. [Google Scholar] [CrossRef]
- Orsini, F.; Maggio, A.; Rouphael, Y.; De Pascale, S. “Physiological quality” of organically grown vegetables. Sci. Hortic. 2016, 208, 131–139. [Google Scholar] [CrossRef]
- Grunert, O.; Hernandez-Sanabria, E.; Vilchez-Vargas, R.; Jauregui, R.; Pieper, D.H.; Perneel, M.; Van Labeke, M.-C.; Reheul, D.; Boon, N. Mineral and organic growing media have distinct community structure, stability and functionality in soilless culture systems. Sci. Rep. 2016, 6, 18837. [Google Scholar] [CrossRef]
- Grunert, O.; Reheul, D.; Van Labeke, M.-C.; Perneel, M.; Hernandez-Sanabria, E.; Vlaeminck, S.E.; Boon, N. Growing media constituents determine the microbial nitrogen conversions in organic growing media for horticulture. Microb. Biotechnol. 2016, 9, 389–399. [Google Scholar] [CrossRef]
- Grunert, O.; Robles-Aguilar, A.A.; Hernandez-Sanabria, E.; Schrey, S.D.; Reheul, D.; Van Labeke, M.-C.; Vlaeminck, S.E.; Vandekerckhove, T.G.L.; Mysara, M.; Monsieurs, P.; et al. Tomato plants rather than fertilizers drive microbial community structure in horticultural growing media. Sci. Rep. 2019, 9, 9561. [Google Scholar] [CrossRef]
- Cannavo, P.; Recous, S.; Valé, M.; Bresch, S.; Paillat, L.; Benbrahim, M.; Guénon, R. Organic fertilization of growing media: Response of N mineralization to temperature and moisture. Horticulturae 2022, 8, 152. [Google Scholar] [CrossRef]
- Zhang, Q.; Masabni, J.; Niu, G. Microbial Biostimulants and Seaweed Extract Synergistically Influence Seedling Growth and Morphology of Three Onion Cultivars. Horticulturae 2024, 10, 800. [Google Scholar] [CrossRef]


| No. | Treatment | Fert 1 Rate (g/L) | Fert 2 Rate (g/L) | Fert 3 Rate (g/L) | N Rate (mg/L) | P Rate (mg/L) | K Rate (mg/L) |
|---|---|---|---|---|---|---|---|
| 1 | Conventional | 2.0 | - | - | 400 | 174 | 332 |
| 2 | Nature Safe | 5.7 | - | - | 400 | 174 | 332 |
| 3 | Miracle-Gro | 5.0 | - | - | 400 | 174 | 332 |
| 4 | Dr. Earth | 10.0 | - | - | 400 | 174 | 332 |
| 5 | Drammatic | 20.0 | - | - | 400 | 349 | 166 |
| 6 | Drammatic+N1 | 10.0 | 4.00 | - | 400 | 174 | 83 |
| 7 | Drammatic+N2 | 10.0 | 1.33 | - | 400 | 174 | 94 |
| 8 | Drammatic+K1 | 20.0 | 1.40 | - | 400 | 349 | 332 |
| 9 | Drammatic+K3 | 20.0 | 4.00 | - | 400 | 349 | 332 |
| 10 | Drammatic+N1+K1 | 10.0 | 4.00 | 1.40 | 400 | 174 | 332 |
| 11 | Drammatic+N1+K2 | 10.0 | 4.00 | 0.60 | 400 | 174 | 332 |
| 12 | Drammatic+N1+K3 | 10.0 | 4.00 | 4.00 | 400 | 174 | 332 |
| Treatment | EC (mS·cm−1) | pH | NO3−-N (mg·L−1) | NH4+-N (mg·L−1) | NO2−-N (mg·L−1) | Inorganic N (mg·L−1) | Measured P (mg·L−1) | %N to Label | %P to Label |
|---|---|---|---|---|---|---|---|---|---|
| Conventional | 1.98 | 6.27 | 140.0 | 3.9 | 0.00 | 143.9 | 172 | 36.0% | 98.6% |
| Nature Safe | 1.70 | 4.54 | 0.0 | 25.4 | 0.00 | 25.4 | 80 | 6.4% | 45.9% |
| Miracle-Gro | 2.10 | 6.71 | 77.0 | 13.0 | 0.02 | 90.0 | 18 | 22.5% | 10.3% |
| Dr. Earth | 1.71 | 7.15 | 10.1 | 4.8 | 0.58 | 15.5 | 10 | 3.9% | 5.7% |
| Drammatic | 2.61 | 4.58 | 139.0 | 84.0 | 0.00 | 223.0 | 470 | 55.8% | 134.7% |
| Drammatic+N1 | 1.67 | 4.88 | 56.4 | 52.5 | 0.00 | 108.9 | 225 | 27.2% | 129.0% |
| Drammatic+N2 | 1.66 | 5.48 | 70.0 | 37.5 | 0.31 | 107.8 | 210 | 27.0% | 120.4% |
| Drammatic+K1 | 3.22 | 4.58 | 81.9 | 77.0 | 0.00 | 158.9 | 445 | 39.7% | 127.6% |
| Drammatic+K3 | 3.36 | 5.39 | 82.2 | 52.0 | 0.00 | 134.2 | 380 | 33.6% | 108.9% |
| Drammatic+N1+K1 | 2.56 | 4.82 | 51.3 | 41.5 | 0.00 | 92.8 | 220 | 23.2% | 126.1% |
| Drammatic+N1+K2 | 2.34 | 4.84 | 48.9 | 55.5 | 0.00 | 104.4 | 225 | 26.1% | 129.0% |
| Drammatic+N1+K3 * | 2.38 | 5.76 | - | - | - | - | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Zhang, Q.; Masabni, J.; Niu, G. Low Nitrogen Availability in Organic Fertilizers Limited Organic Watermelon Transplant Growth. Horticulturae 2024, 10, 1140. https://doi.org/10.3390/horticulturae10111140
Liu J, Zhang Q, Masabni J, Niu G. Low Nitrogen Availability in Organic Fertilizers Limited Organic Watermelon Transplant Growth. Horticulturae. 2024; 10(11):1140. https://doi.org/10.3390/horticulturae10111140
Chicago/Turabian StyleLiu, Jun, Qianwen Zhang, Joseph Masabni, and Genhua Niu. 2024. "Low Nitrogen Availability in Organic Fertilizers Limited Organic Watermelon Transplant Growth" Horticulturae 10, no. 11: 1140. https://doi.org/10.3390/horticulturae10111140
APA StyleLiu, J., Zhang, Q., Masabni, J., & Niu, G. (2024). Low Nitrogen Availability in Organic Fertilizers Limited Organic Watermelon Transplant Growth. Horticulturae, 10(11), 1140. https://doi.org/10.3390/horticulturae10111140







