A Model for Fat Content Detection in Walnuts Based on Near-Infrared Spectroscopy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material
2.2. Main Instruments
2.2.1. Method for Acquisition of the Initial Spectrum of Walnut Kernels
2.2.2. Method for Determining the Fat Content of Walnut Kernels
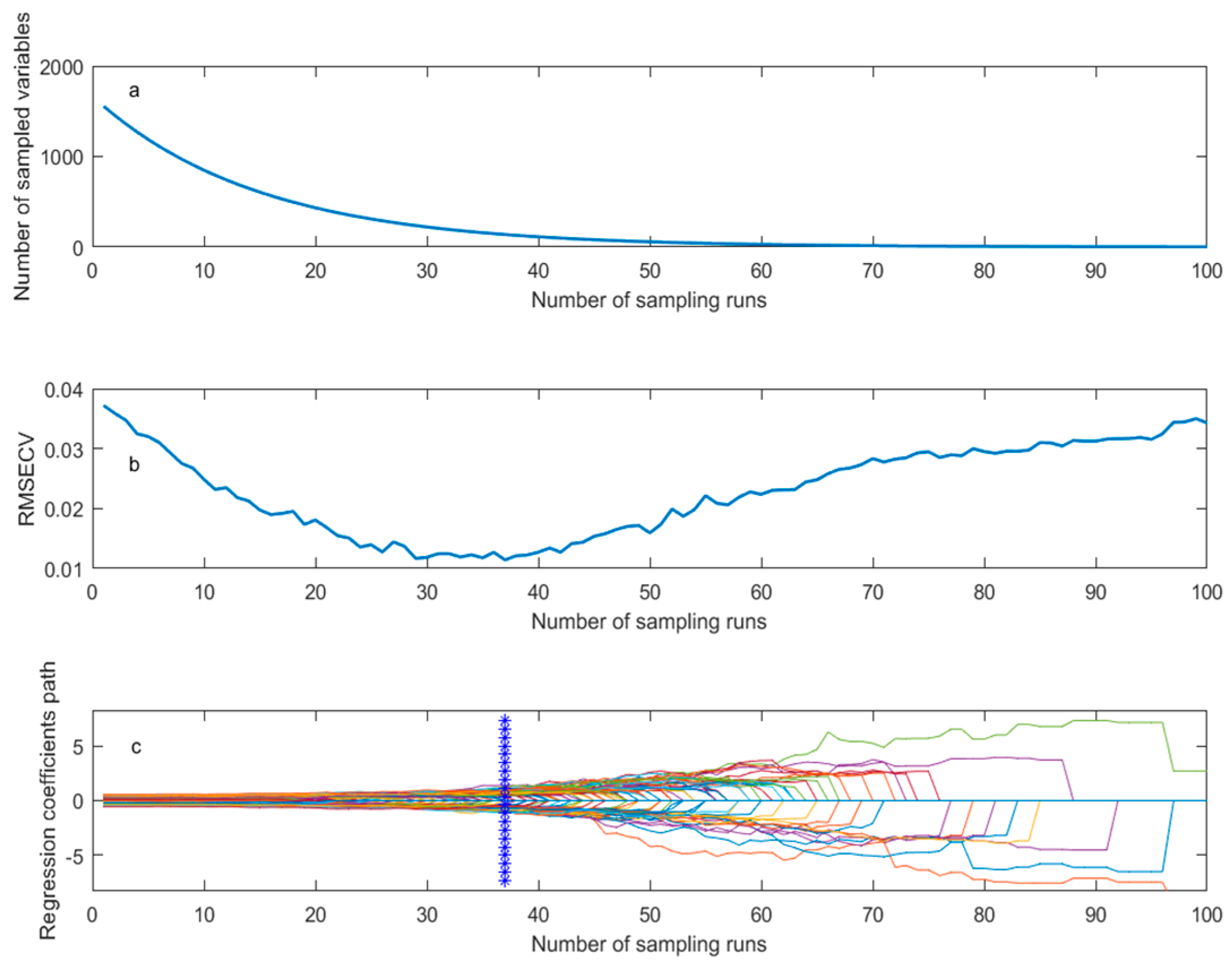
2.2.3. Spectral Preprocessing and Modeling Methods
2.2.4. Evaluation of Model Performance
2.2.5. Reject Abnormal Samples
2.2.6. Sample Division
3. Analysis of the Results
3.1. Near-Infrared Spectroscopy Analysis
3.2. Effects of Different Spectral Preprocessing Methods
3.3. Model Validation
4. Discussion
5. Summary
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, W.; Li, Y.; Tomasetto, F.; Yan, W.; Tan, Z.; Liu, J.; Jiang, J. Non-destructive Measurements of Toona sinensis Chlorophyll and Nitrogen Content Under Drought Stress Using Near Infrared Spectroscopy. Front. Plant Sci. 2022, 12, 809–828. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.Y.; Gao, J.; Huang, L.P.; Yang, L.P.; Wang, J.Y. Current Situation and Development Idea of Walnut Industry in Four Prefectures of Southern Xinjiang. North. Hortic. 2021, 13, 148–154. [Google Scholar]
- Nogales-Bueno, J.; Baca-Bocanegra, B.; Hernández-Hierro, J.M.; Garcia, R.; Barroso, J.M.; Heredia, F.J.; Rato, A.E. Assessment of Total Fat and Fatty Acids in Walnuts Using Near-Infrared Hyperspectral Imaging. Front. Plant Sci. 2021, 12, 729880. [Google Scholar] [CrossRef] [PubMed]
- Arndt, M.; Drees, A.; Ahlers, C.; Fischer, M. Determination of the Geographical Origin of Walnuts (Juglans regia L.) Using Near-Infrared Spectroscopy and Chemometrics. Foods 2020, 9, 1860. [Google Scholar] [CrossRef]
- Kusumiyati, J.S.H.; Sutari, W.; Mubarok, S.; Kurniasari, I. Non-destructive detection of two cucumber cultivars fruit quality using NIR Spectroscopy. Earth Environ. Sci. 2020, 583, 012002. [Google Scholar] [CrossRef]
- Tian, Z.; Tan, Z.; Li, Y.; Yang, Z. Rapid monitoring of flavonoid content in sweet tea (Lithocarpus litseifolius (Hance) Chun) leaves using NIR spectroscopy. Plant Methods 2022, 18, 44. [Google Scholar] [CrossRef]
- Nogales-Bueno, J.; Feliz, L.; Baca-Bocanegra, B.; Hernández-Hierro, J.M.; Heredia, F.J.; Barroso, J.M.; Rato, A.E. Comparative study on the use of three different near infrared spectroscopy recording methodologies for varietal discrimination of walnuts. Talanta 2020, 206, 120189. [Google Scholar] [CrossRef]
- Zareef, M.; Arslan, M.; Hassan, M.M.; Ali, S.; Qing, O.Y.; Li, H.; Wu, X.; Hashim, M.M.; Javaria, S.; Chen, Q.S. Application of benchtop NIR spectroscopy coupled with multivariate analysis for rapid prediction of antioxidant properties of walnut (Juglans regia). Food Chem. 2021, 359, 129928. [Google Scholar] [CrossRef]
- Zeng, P.; Li, X.; Wu, X.; Diao, Y.; Liu, Y.; Liu, P. Rapid Identification of Wild Gentiana Genus in Different Geographical Locations Based on FT-IR and an Improved Neural Network Structure Double-Net. Molecules 2022, 27, 5979. [Google Scholar] [CrossRef]
- Yi, J.; Sun, Y.; Zhu, Z.; Liu, N.; Lu, J. Near-infrared reflectance spectroscopy for the prediction of chemical composition in walnut kernel. Int. J. Food Prop. 2017, 20, 1633–1642. [Google Scholar] [CrossRef]
- Ma, W.Q.; Zhang, M.; Li, Z.X.; Yang, L.L. Detection and analysis of walnut kernel protein content based on near infrared spectroscopy. Trans. Chin. Soc. Agric. Mach. 2017, 48, 407–411. [Google Scholar]
- Ma, W.Q.; Zhang, M.; Li, Y.; Yang, L.L.; Zhu, Z.J.; Cui, K.B. Non-destructive detection for fat content of walnut kernels by near infrared spectroscopy. Trans. Chin. Soc. Agric. Mach. 2019, 50, 374–379. [Google Scholar]
- Li, C.J.; Zhang, S.; Sun, H.; Chen, C.; Xing, S. Study on a two-dimensional correlation visible–Near infrared spectroscopy kinetic model for the moisture content of fresh walnuts stored at room temperature. J. Food Process Eng. 2020, 43, 13551. [Google Scholar] [CrossRef]
- Wang, Q.P.; Li, Q.W.; Cui, B.S.; Su, J.H. Study on the prediction of oils of carya cathayensis sargent using near infrared reflectance spectroscopy. Southwest China J. Agric. Sci. 2009, 22, 873–875. [Google Scholar]
- Zhang, P.; Li, J.K.; Meng, X.J.; Zang, P.; Feng, X.Y.; Wang, B.G. Research on nondestructive measurement of soluble tannin content of astringent persimmon using visible and near infrared diffuse reflection spectroscopy. Spectrosc. Spectr. Anal. 2011, 31, 951–954. [Google Scholar]
- Li, Y.; Wei, Y.M.; Wang, F. Affecting factors on the accuracy of near-infrared spectroscopy analysis. J. Nucl. Agric. Sci. 2005, 19, 236–240. [Google Scholar]
- Ma, H.; Zhang, K.; Ji, J.Y.; Jin, X.; Zhao, K.X. Research on quantitative detection technology of freshness of Agaricus bisporus based on spectroscopic technology. Spectrosc. Spectr. Anal. 2021, 41, 3740–3746. [Google Scholar]
- Feng, Y.C.; Zhang, Q.; Hu, C.Q. Study on the selection of parameters for the evaluating drug NIR universal quantitative models. Spectrosc. Spectr. Anal. 2016, 36, 2447–2454. [Google Scholar]
- González-Casado, S.; López-Gámez, G.; Martín-Belloso, O.; Elez-Martínez, P.; Soliva-Fortuny, R. Pulsed light of near-infrared and visible light wavelengths induces the accumulation of carotenoids in tomato fruits during post-treatment time. Food Sci. 2022, 87, 3913–3924. [Google Scholar] [CrossRef]
- Kröncke, N.; Neumeister, M.; Benning, R. Near-Infrared Reflectance Spectroscopy for Quantitative Analysis of Fat and Fatty Acid Content in Living Tenebrio molitor Larvae to Detect the Influence of Substrate on Larval Composition. Insects 2023, 14, 114. [Google Scholar] [CrossRef]
- Ji, W.J.; Li, X.; Li, C.X. Using different data mining algorithms to predict soil organic matter based on visible-near infrared spectroscopy. Spectrosc. Spectr. Anal. 2012, 32, 2393–2398. [Google Scholar]
- Ma, S.; Tang, X.; Xu, Y. Nondestructive determination of pH value in beef using visible/near-infrared spectroscopy and genetic algorithm. Trans. Chin. Soc. Agric. Eng. 2012, 28, 263–268. [Google Scholar]
- Basile, T.; Marsico, A.D.; Perniola, R. Use of Artificial Neural Networks and NIR Spectroscopy for Non-Destructive Grape Texture Prediction. Foods 2022, 11, 281. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.J. Research on Construction and Optimization of Wheat Grain Protein Content Model Based on Near Infrared Spectroscopy Technology. Ph.D. Thesis, Shanxi Agricultural University, Jinzhong, China, 2021. [Google Scholar]
- Wang, Y.Q.; Liu, G.M.; Hu, L.P.; Zhao, X.Z.; Zhang, D.S.; He, H.J. Prediction of Anthocyanidins Content in Purple Chinese Cabbage Based on Visible/Near Infrared Spectroscopy. Foods 2023, 12, 1922. [Google Scholar] [CrossRef]
- Zhang, J. Research on strawberry brix detection by near-infrared spectroscopy based on SPXY-WT-CARS algorithm. Food Ferment. Sci. Andtechnol. 2020, 56, 136–139+142. [Google Scholar]
- Zhang, Z.W.; Wen, Z.Y.; Zeng, T.L.; Wei, K.L.; Liang, Y.Q. Miniature near-infrared fiber optic spectrometer for quantitative protein-fat detection in milk powder. Spectrosc. Spectr. Anal. 2013, 33, 1796–1800. [Google Scholar]
- Li, P.; Ma, J.; Zhong, N. Fourier transform near-infrared spectroscopy coupled with variable selection methods for fast determination of salmon fillets storage time. J. Mol. Struct. 2022, 1264, 133–223. [Google Scholar] [CrossRef]
- Tang, W.T.; Xu, J.F.; Hu, D.; Zhao, C. Determination of protein and fat content in pecan based on near infrared spectroscopy. Cereals Oils 2022, 35, 158–162. [Google Scholar]
- Rinnan, Å.; Van Den Berg, F.; Engelsen, S.B. Review of the most common pre-processing techniques for near-infrared spectra. TrAC Trends Anal. Chem. 2009, 28, 1201–1222. [Google Scholar] [CrossRef]
- Mishra, P.; Rutledge, D.N.; Roger, J.M. Chemometric pre-processing can negatively affect the performance of near-infrared spectroscopy models for fruit quality prediction. Talanta 2021, 229, 122303. [Google Scholar] [CrossRef]
- Zhang, H.L.; Sun, X.D.; Hao, Y.; Liu, Y.D. Determination of Soluble Solids and Total Acidity in Gannan Navel Orange by NearInfrared Diffuse Reflection Spectroscopy. Food Sci. 2011, 32, 151–154. [Google Scholar]
- Song, X.J.; Wang, H.J.; Zhang, D.J.; Yu, J.C.; Zhou, Y.; Yu, G. Study on Quality Detection of Fresh Cut Hami Melon by Near Infrared Spectroscopy. Farm. Prod. Process. 2018, 10, 53–54+79. [Google Scholar]
- Li, L.L.; Jin, H.L.; Cui, B.B.; Wang, X.J. Rapid determination of soybean protein and crude fat content by near-infrared transmittance spectroscopy. Cereals Oils 2014, 27, 57–60. [Google Scholar]
- Wang, C.X.; Cao, J.F.; Gu, Z.F.; Xu, M.X.; Wu, Q.Y. Rapid Nondestructive Test of Soybean Protein and Fat by Near Infrared Spectroscopy Combined with Different Model Methods. Soybean Sci. 2019, 38, 968–976. [Google Scholar]
- Duan, L.Y.; Hou, C.Y.; Li, Y.L.; Gao, H.L.; Lou, H.H.; Hou, Z.Y. Study on rapid determination of protein, fat and polysaccharide in fruit of Ligustrum lucidum. J. Henan Inst. Sci. Technol. (Nat. Sci. Ed.) 2018, 46, 54–58. [Google Scholar]
- Zhu, J.Y.; Zhu, Y.J.; Feng, G.H.; Zeng, M.F.; Liu, S.Q. Establishment of quantitative models for blueberry storage quality based on near infrared spectroscopy combined with extreme learning machine. Food Ferment. Ind. 2022, 48, 270–276. [Google Scholar]
- Li, L.; Huan, H.Y.; Zhao, S.; Hu, Y.L.; Yang, S.X. NIR spectral detection model of protein, fat, total sugar and moisture in rice. J. Chin. Cereals Oils Assoc. 2017, 32, 121–126. [Google Scholar]
- Lu, H.; Peng, B.Q.; Feng, X.Y.; Shen, X.F. Near-infrared spectroscopy of straight-chain starch, protein, fat and moisture content of rice Detection model optimization. China Rice 2020, 26, 55–59+63. [Google Scholar]




| Component | Maximum Value (%) | Minimum Value (%) | Mean Value (%) | Standard Deviation |
|---|---|---|---|---|
| Fat | 74.76 | 47.40 | 63.22 | 3.38 |
| Modeling Methods | Preprocessing Methods | Wavelength Variable | Correction Set | Test Set | RPD | ||
|---|---|---|---|---|---|---|---|
| R2 | RMSEC | R2 | RMSEP | ||||
| BP neural network | MSC | 86 | 0.66 | 2.40 | 0.67 | 2.30 | 1.43 |
| FD | 99 | 0.89 | 1.34 | 0.82 | 2.23 | 2.69 | |
| SD | 129 | 0.86 | 1.56 | 0.89 | 1.58 | 2.57 | |
| SNV | 99 | 0.62 | 2.54 | 0.55 | 2.65 | 1.28 | |
| MSC+FD | 106 | 0.86 | 1.51 | 0.82 | 1.69 | 2.50 | |
| SVR | MSC | 86 | 0.60 | 2.70 | 0.64 | 2.04 | 1.12 |
| FD | 99 | 0.88 | 1.81 | 0.86 | 2.08 | 1.44 | |
| SD | 129 | 0.90 | 1.76 | 0.83 | 1.70 | 1.70 | |
| SNV | 99 | 0.64 | 2.53 | 0.65 | 2.20 | 1.23 | |
| MSC+FD | 106 | 0.88 | 1.72 | 0.84 | 1.44 | 1.81 | |
| Pre-Processing Method | Wavelength Variable | Characteristic Wavelength/cm−1 | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SD + CARS | 129 | 4065 | 4077 | 4308 | 4432 | 4636 | 4648 | 4651 | 4663 | 4675 | 4736 | 4991 | 5149 | 5257 | 5272 | 5280 | 5334 |
| 5407 | 5577 | 5681 | 5704 | 5708 | 5758 | 5782 | 5824 | 5863 | 5866 | 5886 | 5994 | 6013 | 6052 | 6079 | 6090 | ||
| 6160 | 6163 | 6171 | 6495 | 6557 | 6665 | 6750 | 6811 | 6969 | 6996 | 7182 | 7224 | 7239 | 7255 | 7351 | 7417 | ||
| 7587 | 7617 | 7625 | 7629 | 7641 | 7652 | 7783 | 7853 | 7887 | 7918 | 7965 | 7980 | 8026 | 8107 | 8134 | 8219 | ||
| 8227 | 8273 | 8312 | 8354 | 8373 | 8416 | 8431 | 8458 | 8501 | 8508 | 8539 | 8616 | 8647 | 8659 | 8713 | 8724 | ||
| 8751 | 8782 | 8790 | 8832 | 8852 | 8867 | 8902 | 8906 | 8917 | 8929 | 8940 | 8952 | 8964 | 8987 | 9029 | 9033 | ||
| 9064 | 9068 | 9072 | 9199 | 9276 | 9307 | 9318 | 9326 | 9345 | 9368 | 9411 | 9434 | 9476 | 9484 | 9507 | 9523 | ||
| 9530 | 9538 | 9542 | 9631 | 9665 | 9669 | 9673 | 9696 | 9773 | 9777 | 9797 | 9808 | 9835 | 9866 | 9947 | 9955 | ||
| 9962 | |||||||||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, L.; Zhang, H.; Wang, Y.; Zhang, J.; Zhang, R.; Gao, S.; Dian, Y.; Bai, Z.; Feng, C.; Zhang, Z. A Model for Fat Content Detection in Walnuts Based on Near-Infrared Spectroscopy. Horticulturae 2024, 10, 1143. https://doi.org/10.3390/horticulturae10111143
Luo L, Zhang H, Wang Y, Zhang J, Zhang R, Gao S, Dian Y, Bai Z, Feng C, Zhang Z. A Model for Fat Content Detection in Walnuts Based on Near-Infrared Spectroscopy. Horticulturae. 2024; 10(11):1143. https://doi.org/10.3390/horticulturae10111143
Chicago/Turabian StyleLuo, Langqin, Honghua Zhang, Yu Wang, Jianliang Zhang, Rui Zhang, Shan Gao, Yuanyong Dian, Zijin Bai, Chunhui Feng, and Ze Zhang. 2024. "A Model for Fat Content Detection in Walnuts Based on Near-Infrared Spectroscopy" Horticulturae 10, no. 11: 1143. https://doi.org/10.3390/horticulturae10111143
APA StyleLuo, L., Zhang, H., Wang, Y., Zhang, J., Zhang, R., Gao, S., Dian, Y., Bai, Z., Feng, C., & Zhang, Z. (2024). A Model for Fat Content Detection in Walnuts Based on Near-Infrared Spectroscopy. Horticulturae, 10(11), 1143. https://doi.org/10.3390/horticulturae10111143








