In Vitro Propagation Journey of Ornamental Gladiolus (Gladiolus Species): A Systematic Review Analysis Based on More Than 50 Years Research
Abstract
1. Introduction
2. Gladiolus Cultivation
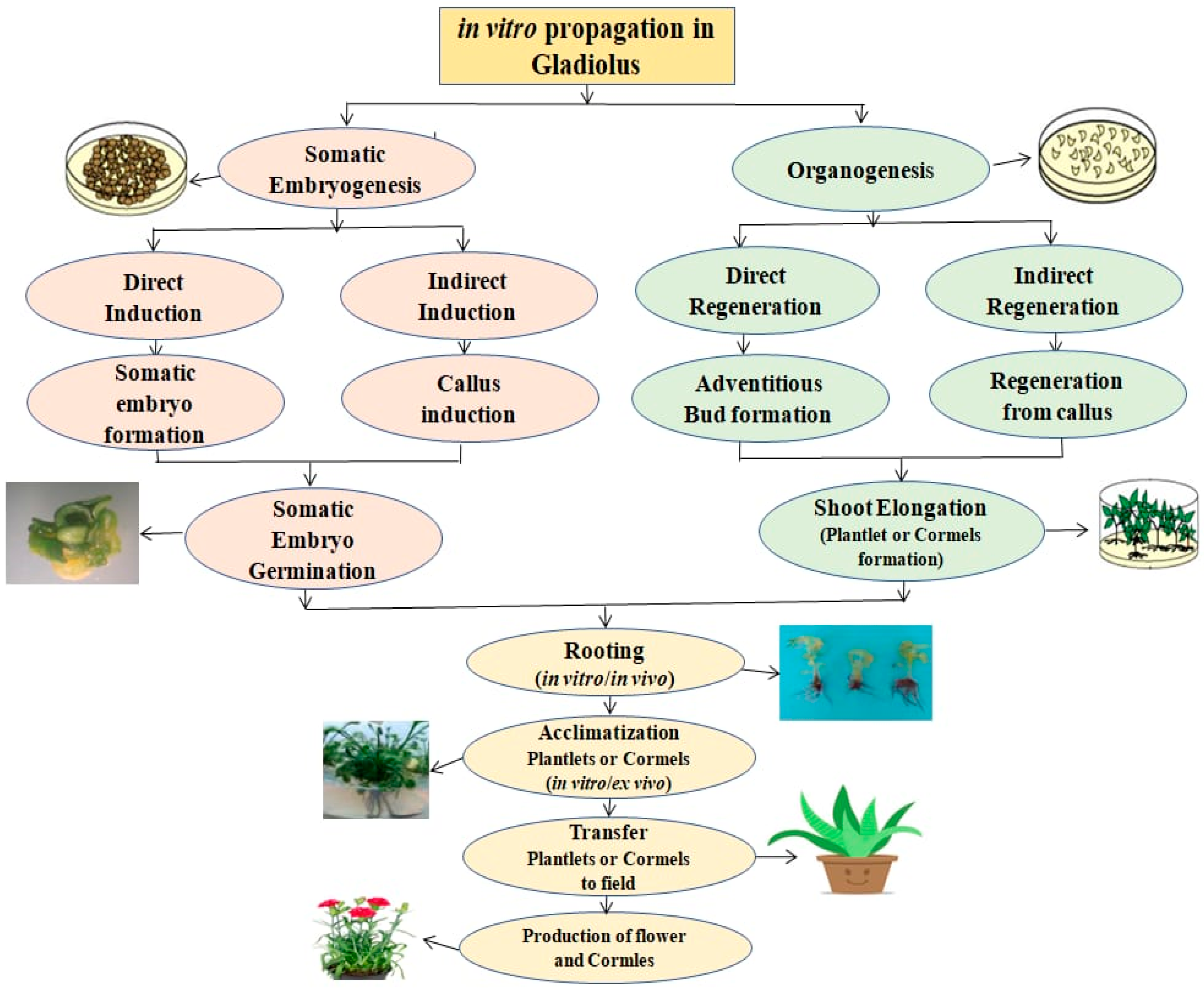
3. Gladiolus In Vitro Propagation: Advantages and Applications
3.1. Establishment and Regeneration: Shoot Tip Culture, Direct and Indirect Organogenesis
3.1.1. Organogenesis
Direct Organogenesis
Indirect Organogenesis
3.2. In Vitro Establishment, Multiplication or Proliferation of Ornamental Gladiolus (Gladiolus Species)
3.2.1. Genotypes/Species/Cultivars
3.2.2. The Kind and Selection of Explants
3.2.3. Mother Plant Sanitation Conditions and Sterilization of Explants
3.2.4. Effect of Plant Growth Regulators
3.2.5. Shoot Multiplication
Genotypes
Media and Additives
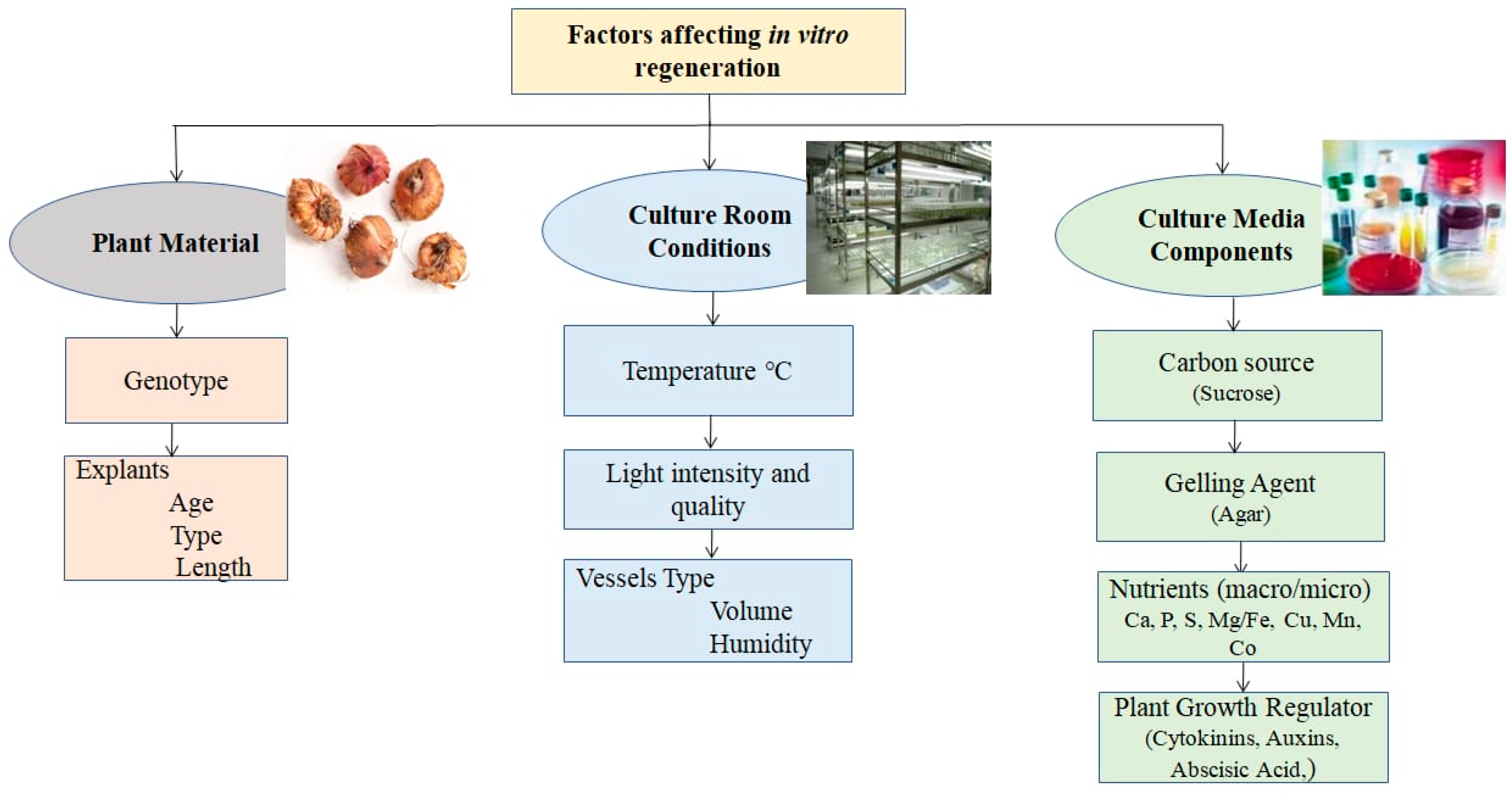
3.2.6. Cultural Conditions
3.2.7. Explants Size
3.2.8. Callus Quality and Age
4. Somatic Embryogenesis
4.1. Factors Affecting Cell Suspension Culture via Somatic Embryogenesis
4.2. Genotypes/Cultivars/Species
4.3. Media and Additives
4.4. Types of Explants
4.5. Growth Regulators
4.6. Culture Conditions
5. Rooting, Elongation and Microtuberization
5.1. In Vitro Rooting of Microshoots
5.2. Rooting, Elongation, and Microtuberization
5.2.1. Genotypes/Cultivars/Species
5.2.2. Microshoot Age and Size
5.2.3. Media and Additives
5.2.4. Growth Regulators
5.2.5. Cultural Conditions
5.2.6. Corm and Cormel Formation In Vitro
5.2.7. Factors Influencing Cormel Formation In Vitro
Cultivars/Genotypes/Species
Explant Type
Media and Additives
Growth Regulators
Cultural Conditions
Acclimatization
Ex Vitro Acclimatization
6. Difficulty and Challenges
7. Insights and New Technologies for In Vitro Culture of Gladiolus
8. Conclusions and Future Prospects
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumar, M.; Chaudhary, V.; Sirohi, U.; Srivastav, A.L. Economically viable flower drying techniques to sustain flower industry amid COVID-19 pandemic. Environ. Dev. Sustain. 2023, 1–46. [Google Scholar] [CrossRef]
- Pragya; Ranjan, J.K.; Attri, B.; Das, H.K.; Ahmed, N. Performance of gladiolus genotypes for cut flower and corm production under high altitude of Uttarakhand. Indian J. Hortic. 2010, 67, 386–390. [Google Scholar]
- Manning, J.; Goldblatt, P. The Iris Family: Natural History and Classification; Timber Press: Portland, OR, USA, 2008; pp. 138–142. [Google Scholar]
- Anonymous. Gladiolus; Technical Bulletin no. 14; Division of Floriculture and Landscaping, IARI: New Delhi, India, 2002; p. 100. [Google Scholar]
- Delpierre, G.R.; Du Plessis, N.M. The Winter-Growing Gladiolus of South Africa; Tafelberg: Cape Town, South Africa, 1974; p. 71. [Google Scholar]
- Raghava, S.P.S. Genetic Improvement of Gladiolus in India. In Proceedings of the National Conference on Gladiolus, Lucknow, India, 24–25 January 2000; NBRI: Lucknow, India, 2000; p. 1. [Google Scholar]
- Kadam, J.J.; Agale, R.C.; Rite, S.C.; Pandav, S.M. Exploration of fungicides and phyto-extract against Fusarium oxysporum f. sp. gladioli causing corm rot of gladiolus. Discov. Agric. 2014, 2, 61–64. [Google Scholar]
- Hussain, I.; Muhammad, A.; Rashid, H.; Quraishi, A. In vitro multiplication of gladiolus. Plant Tissue Cult. 2001, 11, 121–126. [Google Scholar]
- Memon, N.; Qasim, M.; Jaskani, M.J.; Ahmad, R. In vitro cormel production of gladiolus. Pak. J. Agric. Sci. 2010, 47, 115–123. [Google Scholar]
- Kamo, K.; Rajasekaran, K.; Cary, J. Growth characteristics of micropropagated, regenerated and transgenic gladiolus plants. J. Appl. Hortic. 2014, 16, 193–198. [Google Scholar] [CrossRef]
- Mujib, A.; Ali, M.; Tonk, D.; Zafar, N. Nuclear 2C DNA and genome size analysis in somatic embryo regenerated gladiolus plants using flow cytometry. Adv. Hortic. Sci. 2017, 31, 165–174. [Google Scholar]
- Kumar, A.; Kumar, A.; Sharma, V.; Mishra, A.; Singh, S.; Kumar, P. In vitro regeneration of gladiolus (Gladiolus hybrida L.): Optimization of growth media and assessment of genetic fidelity. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 2900–2909. [Google Scholar] [CrossRef]
- Ziv, M.; Halevy, A.H.; Shilo, R. Organs and plantlets regeneration of Gladiolus through tissue culture. Ann. Bot. 1970, 34, 671–676. [Google Scholar] [CrossRef]
- Simonsen, J.; Hildebrandt, A.C. In vitro growth and differentiation of gladiolus plants from callus cultures. Can. J. Bot. 1971, 49, 1817–1819. [Google Scholar] [CrossRef]
- Hussey, G. Totipotency in tissue explants and callus of some members of the Liliaceae, Iridaceae, and Amaryllideceae. J. Exp. Biol. 1975, 26, 253–256. [Google Scholar]
- Hussey, G. In vitro propagation of gladiolus by precocious axillary shoot formation. Sci. Hortic. 1977, 6, 287–296. [Google Scholar] [CrossRef]
- Hussey, G. In vitro propagation of some members of the Liliaceae, Iridaceae and Amaryllideace. Acta Hortic. 1977, 78, 303–309. [Google Scholar] [CrossRef]
- Ziv, M. Transplanting gladiolus plants propagated in vitro. Sci. Hortic. 1979, 11, 257–260. [Google Scholar] [CrossRef]
- Logan, A.E.; Zettler, F.W. Rapid in vitro propagation of virus-indexed gladioli. Acta Hortic. 1985, 164, 169–180. [Google Scholar] [CrossRef]
- Begum, S.; Haddiuzaman, S. In vitro rapid shoot proliferation and corm development in Gladiolus grandiflorus cv. Redbrand. Plant Tissue Cult. 1995, 5, 7–12. [Google Scholar]
- Dantu, P.K.; Bhojwani, S.S. In vitro corm formation and field evaluation of corm-derived plants of gladiolus. Sci. Hortic. 1995, 61, 115–129. [Google Scholar] [CrossRef]
- Kumar, A.; Sood, A.; Palni, L.M.S.; Gupta, A.K. In vitro propagation of Gladiolus hybridus Hort.: Synergistic effect of heat shock and sucrose on morphogenesis. Plant Cell Tissue Organ Cult. 1999, 57, 105–112. [Google Scholar] [CrossRef]
- Ahmad, T.; Ahmad, M.S.; Nasir, I.A.; Riazuddin, S. In vitro production of cormlets in gladiolus. Pak. J. Biol. Sci. 2000, 3, 819–821. [Google Scholar] [CrossRef][Green Version]
- Babu, P.; Chawla, H.S. In vitro regeneration and Agrobacterium mediated transformation in gladiolus. J. Hortic. Sci. Biotechnol. 2000, 75, 400–404. [Google Scholar] [CrossRef]
- Boonvanno, K.; Kanchanapoom, K. In vitro propagation of gladiolus. Suranaree J. Sci. Technol. 2000, 7, 25–29. [Google Scholar]
- Ziv, M.; Lilien-Kipnis, H. Bud regeneration from inflorescence explants for rapid propagation of geophytes in vitro. Plant Cell Rep. 2000, 19, 845–885. [Google Scholar] [CrossRef]
- Priyakumari, I.; Sheela, V.L. Micropropagation of gladiolus cv. ‘Peach Blossom’ through enhanced release axillary buds. J. Trop. Agric. 2005, 43, 47–50. [Google Scholar]
- Aftab, F.; Alam, M.; Afrasiab, H.; Afrasiab, H. In vitro shoot multiplication and callus induction in Gladiolus hybridus Hort. Pak. J. Bot. 2008, 40, 517–522. [Google Scholar]
- Memon, N. In vitro propagation of gladiolus plantlets and cormels. J. Hortic. Sci. Ornam. Plants 2012, 4, 280–291. [Google Scholar]
- Saha, B.; Datta, A.K.; Datta, S.; da Silva, J.A.T. In vitro corm development, field evaluation and determination of genetic stability of corm-derived elite Gladiolus germplasm. Floric. Ornam. Biotechnol. 2013, 7, 82–85. [Google Scholar]
- Memon, N.; Jasakni, M.J.; Qasim, M.; Sharif, N. Cormel formation in gladiolus through tissue culture. Pak. J. Agric. Sci. 2014, 51, 475–482. [Google Scholar]
- Nezamabad, P.S.; Habibi, M.K.; Dizadji, A.; Kalantari, S. Elimination of bean yellow mosaic virus through thermotherapy combined with meristem-tip culture in gladiolus corms. J. Crop Prot. 2015, 4, 533–543. [Google Scholar]
- Kundang, N.; Kumar, V.; Kumar, S. Effect of macronutrients and plant growth hormones for the in vitro formation of cormlets of Gladiolus pacifica. CIB-Tech. J. Biotechnol. 2017, 6, 10–16. [Google Scholar]
- Tripathi, M.K.; Malviya, R.K.; Vidhyashankar, M.; Patel, R.P. Effect of plant growth regulators on in vitro morphogenesis in gladiolus (Gladiolus hybridus Hort.) from cultured corm slice. Int. J. Agric. Technol. 2017, 13, 583–599. [Google Scholar]
- Malviya, R.K.; Tripathi, M.; Vidhyashankar, M.; Patel, R.P.; Ahuja, A. Effect of different phytohormones on plant regeneration of gladiolus (Gladiolus hybridus Hort.) from cultured cormel. Asian J. Microbiol. Biotechnol. Environ. Sci. 2018, 19, 155–165. [Google Scholar]
- Devi, P.; Kumar, P.; Sengar, R.S.; Yadav, M.K.; Kumar, M.; Singh, S.K.; Singh, S. In-vitro multiple shoots production from cormel shoot buds in gladiolus (Gladiolus hybrida). Int. J. Curr. Microbiol. Appl. Sci. 2019, 8, 1345–1350. [Google Scholar] [CrossRef]
- Budiarto, K.; Rosario, T.L. Evaluation of culture media for in vitro conservation of gladiolus cultivars. AGRIVITA J. Agric. Sci. 2020, 42, 205–213. [Google Scholar] [CrossRef]
- Deshmukh, V.D.; Kharde, A.V.; Talekar, B.K. Interactive effects of ba and iaa on shoot proliferation of gladiolus (Gladiolus grandiflorus) var. white prosperity. J. Orient. Res. Madras 2021, 90, 12–19. [Google Scholar]
- Isah, T.; Qurratul; Umar, S. Influence of silver nitrate and copper sulphate on somatic embryogenesis, shoot morphogenesis, multiplication and associated physiological biochemical changes in Gladiolus hybridus L. Res. Sq. 2022, 149, 563–587. [Google Scholar]
- Poothong, S.; Duangkon, N.; Sukanthamala, S. The effects of sucrose, activated charcoal, and coconut dust on in vitro corm induction of Gladiolus hybrida under aseptic technique. Health Sci. Sci. Technol. Rev. 2023, 16, 28–39. [Google Scholar]
- Tripathi, M.K.; Tiwari, S.; Tripathi, N.; Malviya, R.K.; Bhatt, D. In vitro morphogenesis in gladiolus (Gladiolus hybridus Hort.) from corm slice. Mol. Biol. Plant Physiol. 2023, 33, 73. [Google Scholar]
- Tripathi, M.K.; Tripathi, N.; Tiwari, S.; Malviya, R.K.; Tiwari, P.N.; Tiwari, S. Plant regeneration from cultured cormel explants in gladiolus (Gladiolus hybridus HORT.). Mol. Biol. Plant Physiol. 2023, 33, 139. [Google Scholar]
- Kumar, M.; Sirohi, U.; Yadav, M.K.; Chaudhary, V. In vitro culture technology and advanced biotechnology tools toward improvement in gladiolus (Gladiolus species): Present scenario and future prospects. Mol. Biotechnol. 2023, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Elsheikh, A.M.; Daffalla, H.M.; Khalfala, M.M. In Vitro micropropagation of the ornamental plant Dieffenbachia—A review. Univers. J. Plant Sci. 2013, 1, 91–99. [Google Scholar] [CrossRef]
- Azmi, N.S.; Ahmad, R.; Ibrahim, R. Effects of red and blue (RB) LED on the in vitro growth of Rosa kordesii in multiplication phase. In Proceedings of the 2nd International Conference on Agriculture and Biotechnology, Singapore, 12–14 March 2014. [Google Scholar]
- Chandra, S.; Bandopadhyay, R.; Kumar, V.; Chandra, R. Acclimatization of tissue cultured plantlets: From laboratory to land. Biotechnol. Lett. 2010, 32, 1199–1205. [Google Scholar] [CrossRef]
- Preece, J.E.; Sutter, E.G. Acclimatization of micropropagated plants to the greenhouse and field. In Micropropagation; Debergh, P.C., Zimmerman, R.H., Eds.; Kluwer Academic Publishers: New York, NY, USA, 1991; pp. 71–93. [Google Scholar]
- Roberts, A.V.; Smith, E.F.; Mottley, J. The preparation of micropropagated plantlets for transfer to soil without acclimatization. In Methods in Molecular Biology; Pollard, J.W., Walker, J.M., Eds.; Springer: New York, NY, USA, 1990; Volume 6, pp. 227–236. [Google Scholar]
- Ascough, G.D.; Erwin, J.E.; Van Staden, J. Micropropagation of Iridaceae—A review. Plant Cell Tissue Organ Cult. 2009, 97, 1–19. [Google Scholar] [CrossRef]
- Datta, S.K. Breeding of Ornamentals: Gladiolus. LS—Int. J. Life Sci. 2020, 9, 115–133. [Google Scholar] [CrossRef]
- Memon, N.; Qasim, M.; Jaskani, M.J.; Awan, F.S.; Khan, A.I.; Sadia, B.; Hussain, Z. Assessment of somaclonal variation in in vitro propagated cormels of gladiolus. Pak. J. Bot. 2012, 44, 769–776. [Google Scholar]
- Bajaj, Y.P.S.; Sidhu, M.M.S.; Gill, A.P.S. Some factors affecting the in vitro propagation of Gladiolus. Sci. Hortic. 1983, 18, 269–275. [Google Scholar] [CrossRef]
- Pragya; Singh, S.K.; Misra, R.L.; Ranjan, J.K. In vitro shoot regeneration from cormel derived callus of gladiolus and bio-hardening of plantlets. Indian J. Biotechnol. 2012, 11, 99–104. [Google Scholar]
- Kabir, M.H.; Mamun, A.N.K.; Yesmin, F.; Subramaniam, S. In vitro propagation of Gladiolus dalenii from the callus through the culture of corm slices. J. Phytol. 2014, 6, 40–45. [Google Scholar]
- Kamo, K. A cultivar comparison of plant regeneration from suspension cells, callus, and cormel slices of gladiolus. In Vitro Cell. Dev. Biol.-Plant 1995, 31, 113–115. [Google Scholar] [CrossRef]
- Pathania, N.S.; Misra, R.L.; Raghava, S.P.S. Precocious shoot proliferation and microcorm production in gladiolus through tissue culture. J. Ornam. Hortic. 2001, 4, 69–73. [Google Scholar]
- Roy, S.K.; Gangopadhyay, G.; Bandyopadhyay, T.; Modak, B.K.; Datta, S.; Mukherjee, K.K. Enhancement of in vitro micro corm production in gladiolus using alternative matrix. Afr. J. Biotechnol. 2006, 5, 1204–1209. [Google Scholar]
- Nhut, D.T.; da Silva, J.A.T.; Huyen, P.X.; Paek, K.Y. The importance of explant source on regeneration and micropropagation of Gladiolus by liquid shake culture. Sci. Hortic. 2004, 102, 407–414. [Google Scholar] [CrossRef]
- Prasad, V.S.S.; Gupta, S.D. In vitro shoot regeneration of gladiolus in semi-solid agar versus liquid cultures with support systems. Plant Cell Tissue Organ Cult. 2006, 87, 263–271. [Google Scholar] [CrossRef]
- Sheena, A.; Sheela, V.L. Effects of the growth retardant triadimefon on the ex vitro establishment of gladiolus (Gladiolus grandiflorus L.) cv. Vinks Glory. Plant Tissue Cult. Biotechnol. 2010, 20, 171–178. [Google Scholar] [CrossRef]
- Kamo, K. Effect of phytohormones on plant regeneration from callus of gladiolus cultivar “Jenny Lee”. In Vitro Cell. Dev. Biol. 1994, 30, 26–31. [Google Scholar] [CrossRef]
- Goo, D.H.; Joung, H.Y.; Kim, K.W. Differentiation of gladiolus plantlets from callus and subsequent flowering. Acta Hort 2003, 620, 339–342. [Google Scholar] [CrossRef]
- Emek, Y.; Erdag, B. In vitro propagation of Gladiolus anatolicus (Boiss.) Stapf. Pak. J. Bot. 2007, 39, 23–30. [Google Scholar]
- Sinha, P.; Roy, S.K. Plant regeneration through in vitro cormel formation from callus culture of Gladiolus primulinus Baker. Plant Tissue Cult. 2002, 12, 139–145. [Google Scholar]
- Stefaniak, B. Somatic embryogenesis and plant regeneration of gladiolus (Gladiolus hort.). Plant Cell Rep. 1994, 13, 386–389. [Google Scholar] [CrossRef]
- Mokshin, E.V.; Lukatkin, A.S.; Teixeira da Silva, J.A. Micropropagation of selected cultivars of lilium and gladiolus. Floric. Ornam. Plant Biotechnol. 2006, 2, 533–539. [Google Scholar]
- Thun, V.; Goo, D.H.; Kim, M.H.; Byun, M.S.; Kim, K.W. Effect of in vitro culture environments and culture methods on cormlet formation of gladiolus. Hortic. Environ. Biotechnol. 2008, 49, 114–120. [Google Scholar]
- Budiarto, K. In vitro regeneration of three gladiolus cultivars using cormel explants. J. ILMU DASAR 2009, 10, 109–113. [Google Scholar]
- El-Kazzaz, A.A.; El-Bagoury, H.; Mahmoud, I.; El Tantawy, A.A.; Al-Ansary, A.A. Production of gladiolus germplasm via in vitro tissue cultures. J. Duhok Univ. 2012, 15, 440–447. [Google Scholar]
- Haouala, F.; Chaieb, E. Effects of explant position and polarity on callus induction and shoot regeneration of gladiolus (Gladiolus hybridus Hort). Floric. Ornam. Biotechnol. 2012, 6, 133–139. [Google Scholar]
- Beura, S.; Singh, R.; Jagadiv, P.N. In vitro cloning of gladiolus cv. American Beauty. J. Ornam. Hortic. 2005, 8, 268–271. [Google Scholar]
- Remotti, P.C.; Löffler, H.J. Callus induction and plant regeneration from gladiolus. Plant Cell Tissue Organ Cult. 1995, 42, 171–178. [Google Scholar] [CrossRef]
- Zaidi, N.; Khan, N.H.; Zafar, F.; Zafar, S.I. Bulbous and cormous monocotyledonous ornamental plants in vitro. Sci. Vis. 2000, 6, 58–73. [Google Scholar]
- Cardoso, J.C.; Teixeira Da Silva, J.A. Micropropagation of gerbera using chlorine dioxide (ClO2) to sterilize the culture medium. In Vitro Cell. Dev. Biol. Plant 2012, 48, 362–368. [Google Scholar] [CrossRef]
- Belanekar, S.B.; Nadkarni, H.R.; Sawant, S.S.; Gokhale, N.B. Micropropagation in gladiolus (Gladiolus grandiflorus L.) var. White Friendship. J. Maharashtra Agric. Univ. 2010, 35, 66–68. [Google Scholar]
- Te-Chato, S.; Naksombut, S.; Boonsiri, J. Effect of variety and explant on callus formation and micropropagation of anthurium. Warasan Songkhla Nakharin J. Sci. Technol. 2002, 24, 569–578. [Google Scholar]
- George, E.F.; Hall, M.A.; De Klerk, G.J. Plant tissue culture procedure-background. In Plant Propagation by Tissue Culture; Springer: Dordrecht, The Netherlands, 2008; pp. 1–28. [Google Scholar]
- George, E.F.; Debergh, P.C. Micropropagation: Uses and methods. In Plant Propagation by Tissue Culture; Springer: Dordrecht, The Netherlands, 2008; pp. 29–64. [Google Scholar]
- Gupta, S.D.; Datta, S. Antioxidant enzyme activities during in vitro morphogenesis of gladiolus and the effect of application of antioxidants on plant regeneration. Biol. Plant. 2003, 47, 179–183. [Google Scholar] [CrossRef]
- Mateen, R.M. Development and optimization of micro-propagation, in vitro methodology for gladiolus. BioScientific Rev. 2019, 1, 21–36. [Google Scholar] [CrossRef]
- Choudhary, D.; Agarwal, G.; Singh, V.P.; Arora, A. In vitro micropropagation of Gladiolus grandiflora (var. Snow Princess) flower from cormel explant. Indian J. Plant Physiol. 2010, 15, 90–93. [Google Scholar]
- Dharmasena, P.A.I.U.; Karunananda, D.P.; Eeswara, J.P. Effect of gibberellic acid (GA3) and sugar on in vitro cormlet formation, multiplication and ex vitro sprouting of Gladiolus hybrida variety Princess Lee. Trop. Agric. Res. 2011, 23, 1–10. [Google Scholar] [CrossRef]
- Jala, A. Potential of benzyl adenine, naphthalene acetic acid and sucrose concentration on growth, development, and regeneration of new shoot and cormel on gladiolus. Am. Trans. Eng. Appl. Sci. 2013, 2, 277–285. [Google Scholar]
- González-Pérez, E.; Juárez-Muñoz, J.; Ayala-Garay, O.J.; Yáñez-Morales, M.; De, J. Ex vitro acclimatization of gladiolus plantlets. Propag. Ornam. Plants 2014, 14, 125–132. [Google Scholar]
- Akhare, A.A.; Dhumale, D.B.; Sakhare, S.B.; Ekta, S. Remove from marked records in vitro establishment of gladiolus cv. White Friendship and Fidelio using axillary buds as explant. Asian J. Hortic. 2008, 3, 149–152. [Google Scholar]
- Suzuki, K.; Takatsu, Y.; Gonai, T.; Kasumi, M. Plant regeneration and chromosome doubling of wild Gladiolus species. Acta Hortic. 2005, 673, 175–181. [Google Scholar] [CrossRef]
- Torabi-Giglou, M.; Hajieghrari, B. In vitro study on regeneration of Gladiolus grandiflorus corm calli as affected by plant growth regulators. Pak. J. Biol. Sci. 2008, 11, 1147–1154. [Google Scholar] [CrossRef][Green Version]
- Ojha, A.; Sharma, V.; Sharma, V.N.; Rawat, A. Effect of different carbohydrates sources on the formation of cormlets of economic important plant: Gladiolus pacifica. Int. J. Biotechnol. Biochem. 2010, 6, 485–491. [Google Scholar]
- Rakosy-Tican, E.; Bors, B.; Szatmari, A. In vitro culture and medium-term conservation of the rare wild species Gladiolus imbricatus. Afr. J. Biotechnol. 2012, 11, 14703–14712. [Google Scholar]
- Bera, A.K.; Maity, T.R.; Samanta, A.; Dolai, A.; Saha, B.; Datta, S. Enhancement of in vitro corm production in gladiolus by periodically replacement of liquid media using coir matrix. J. Appl. Hortic. 2015, 17, 222–224. [Google Scholar] [CrossRef]
- Aslam, F.; Habib, S.; Naz, S. Effect of different phytohormones on plant regeneration of Amaryllis hippeastrum. Pak. J. Sci. 2012, 64, 54–58. [Google Scholar]
- Memon, N.; Qasim, M.; Jaskani, M.J.; Khooharo, A.A.; Hussain, Z.; Ahmad, I. Comparison of various explants on the basis of efficient shoot regeneration in gladiolus. Pak. J. Bot. 2013, 45, 877–885. [Google Scholar]
- Devi, K.L.; Maitra, S.; Mandal, M.; Thokchom, R. In-vitro Response of Gladiolus (var. American Beauty) towards Plant Growth Regulators Using Cormel Explants. Int. J. Environ. Clim. Chang. 2023, 13, 388–395. [Google Scholar] [CrossRef]
- ASalih, E.E.; AlSalihy, A.A. Using of UV light type C and growth regulators in propagation and cormels formation in Gladiolus spp. Ann. Rom. Soc. Cell Biol. 2021, 25, 8486–8497. [Google Scholar]
- Kamo, K.; Joung, Y.H. Gladiolus. In Biotechnology in Agriculture and Forestry; Pua, E.C., Davey, M.R., Eds.; Transgenic Crops VI; Springer: Berlin/Heidelberg, Germany, 2007; Volume 61, pp. 289–298. [Google Scholar]
- Dantu, P.K.; Bhojwani, S.S. In vitro propagation and corm formation in gladiolus. Gartenbauwissenschaft 1987, 52, 90–93. [Google Scholar]
- Ziv, M.; Ronen, G.; Raviv, M. Proliferation of meristematic clusters in disposable presterilized plastic bio-reactors for large-scale micropropagation of plants. In Vitro Cell. Dev. Biol. Plant 1998, 34, 152–158. [Google Scholar] [CrossRef]
- Ziv, M. The effect of growth retardants on shoot proliferation and morphologenesis in liquid cultured Gladiolus plants. Acta Hortic. 1990, 280, 207–214. [Google Scholar] [CrossRef]
- Ziv, M. Morphogenic control of plants micropropagated in bioreactor cultures and its possible impact on acclimatization. Acta Hortic. 1992, 319, 119–124. [Google Scholar] [CrossRef]
- Ziv, M.; Lilien-Kipnis, H. Gladiolus. In Handbook of Plant Cell Culture; Ammirato, Ed.; McGraw-Hill: New York, NY, USA, 1990; Volume 5, pp. 461–478. [Google Scholar]
- Sutter, E.G. Micropropagation of Ixia viridifolia and a Gladiolus × Homoglossum hybrid. Sci. Hortic. 1986, 29, 181–189. [Google Scholar] [CrossRef]
- Dutta Gupta, S.; Prasad, V.S.S. Shoot multiplication kinetics and hyperhydric status of regenerated shoots of gladiolus in agar-solidified and matrix-supported liquid cultures. Plant Biotechnol. Rep. 2010, 4, 85–94. [Google Scholar] [CrossRef]
- Lipsky, A.K.; Kataeva, N.V.; Butenko, R.G. Comparison of parameters of the gladiolus bud cultures grown in the bio-reactor at different regimes. Acta Hortic. 1997, 447, 671–673. [Google Scholar] [CrossRef]
- Eide, A.K.; Munster, C.; Heyerdahl, P.H.; Lyngved, R.; Olsen, O.A.S. Liquid culture systems for plant propagation. Acta Hortic. 2003, 625, 173–185. [Google Scholar] [CrossRef]
- Rout, G.R.; Mohapatra, A.; Jain, S.M. Tissue culture of ornamental pot plant: A critical review on present scenario and future prospects. Biotechnol. Adv. 2006, 24, 531–560. [Google Scholar] [CrossRef] [PubMed]
- Ziv, M. Simple bio-reactors for mass propagation of plants. Plant Cell Tissue Organ Cult. 2005, 81, 277–285. [Google Scholar] [CrossRef]
- Dogra, N.; Dhatt, K.K. In vitro production of cormels in Gladiolus hybridus through gamma rays. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 1308–1318. [Google Scholar] [CrossRef]
- Erdag, B.B.; Calmaz Emek, Y.; Aktas, L.Y. In vitro somatic embryogenesis from cormel derived callus cultures of Gladiolus anatolicus (Boiss.) Stapf. Propag. Ornam. Plants 2009, 9, 176–180. [Google Scholar]
- Rajasekharan, P.E.; Rao, T.M.; Janakiram, T.; Ganeshan, S. Freeze preservation of gladiolus pollen. Euphytica 1994, 80, 105–109. [Google Scholar] [CrossRef]
- DNagaraju, V.; Bhowmik, G.; Parthasarathy, V.A. Effect of paclobutrazol and sucrose on in vitro cormel formation in gladiolus. Acta Bot. Croat. 2002, 61, 27–33. [Google Scholar]
- Ruffoni, B.; Savona, M.; Barberini, S. Biotechnological support for the development of new Gladiolus Hybrids. Floric. Ornam. Biotechnol. 2012, 6, 45–52. [Google Scholar]
- Kumar, A.; Palni, L.M.S.; Sood, A.; Sharma, M.; Palni, U.T.; Gupta, A.K. Heat-shock induced somatic embryogenesis in callus cultures of gladiolus in the presence of high sucrose. J. Hortic. Sci. Biotechnol. 2002, 77, 73–78. [Google Scholar] [CrossRef]
- Dantu, P.K.; Bhojwan, S.S. In vitro propagation of gladiolus: Optimisation of conditions for shoot multiplication. J. Plant Biochem. Biotechnol. 1992, 1, 115–118. [Google Scholar] [CrossRef]
- Steinitz, B.; Cohen, A.; Goldberg, Z.; Kochba, M. Precocious gladiolus corm formation in liquid shake cultures. Plant Cell Tissue Organ Cult. 1991, 26, 63–70. [Google Scholar] [CrossRef]
- De Bruyn, M.H.; Ferreira, D.I. In vitro corm production of Gladiolus dalenii and G. tristis. Plant Cell Tissue Organ Cult. 1992, 31, 123–128. [Google Scholar] [CrossRef]
- Bettaieb, T. Régénération in vitro de Variants Somaclonaux de Glaïeul (Gladiolus grandiflorus Hort.) Tolérants aux Basses Températures. Ph.D. Dissertation, Instituut Voor Tropische Geneeskunde, Antwerpen, Belgium, 2003. [Google Scholar]
- Kamo, K.; Gera, A.; Cohen, J.; Hammond, J.; Blowers, A.; Smith, F.; Van Eck, J. Transgenic gladiolus plants transformed with the bean yellow mosaic virus coat-protein gene in either sense or antisense orientation. Plant Cell Rep. 2005, 23, 654–663. [Google Scholar] [CrossRef] [PubMed]
- Kamo, K.; Van Eck, J. Effect of bialaphos and phosphinothricin on plant regeneration from long and short-term callus cultures of gladiolus. In Vitro Cell. Dev. Biol.-Plant 1997, 33, 180–183. [Google Scholar] [CrossRef]
- Zimmerman, J.L. Somatic embryogenesis: A model for early development in higher plants. Plant Cell 1993, 5, 1411–1423. [Google Scholar] [CrossRef] [PubMed]
- Nic-Can, G.I.; Galaz-Ávalos, R.M.; De-la-Peña, C.; Alcazar-Magaña, A.; Wrobel, K.; Loyola-Vargas, V.M. Somatic embryogenesis: Identified factors that lead to embryogenic repression. A case of species of the same genus. PLoS ONE 2015, 10, e0126414. [Google Scholar] [CrossRef] [PubMed]
- Kamo, K.; Chen, J.; Lawson, R. The establishment of cell suspension cultures of gladiolus that regenerate plants. In Vitro Cell. Dev. Biol. 1990, 26, 425–430. [Google Scholar] [CrossRef]
- Remotti, P.C. Primary and secondary embryogenesis from cell suspension cultures of gladiolus. Plant Sci. 1995, 107, 205–214. [Google Scholar] [CrossRef]
- Wu, J.; Liu, C.; Seng, S.; Khan, M.A.; Sui, J.; Gong, B.; Liu, C.; Wu, C.; Zhong, X.; He, J.; et al. Somatic embryogenesis and Agrobacterium-mediated transformation of Gladiolus hybridus cv. ‘Advance Red’. Plant Cell Tissue Organ Cult. 2015, 120, 717–728. [Google Scholar] [CrossRef]
- Nasir, I.A.; Riazuddin, S. In vitro selection for Fusarium wilt resistance in gladiolus. J. Integr. Plant Biol. 2008, 50, 601–612. [Google Scholar] [CrossRef]
- Lilien-Kipnis, H.; Kochba, M. Mass propagation of new Gladiolus hybrids. Acta Hortic. 1987, 212, 631–638. [Google Scholar] [CrossRef]
- Fridborg, S.L.; Ericsson, T. Effect of activated charcoal on growth and morphogenesis in cell cultures. Physiol. Plant. 1975, 34, 306–308. [Google Scholar] [CrossRef]
- Hussain, S.C.; Geetha, C.K.; Rajeevan, P.K.; Valsalakumari, P.K. Plant regeneration from root derived callus in gladiolus. J. Ornam. Hortic. 1994, 2, 46–50. [Google Scholar]
- Ziv, M. Enhanced shoot and cormlet proliferation in liquid cultured gladiolus buds by growth retardants. Plant Cell Tissue Organ Cult. 1989, 17, 101–110. [Google Scholar] [CrossRef]
- Kumar, A.; Palni, L.M.S.; Sood, A. Factors affecting in vitro formation of cormlets in Gladiolus hybridus Hort. and their field performance. Acta Physiol. Plant. 2011, 33, 509–515. [Google Scholar] [CrossRef]
- Sen, J.; Sen, S. Two-step bud culture technique for a high frequency regeneration of gladiolus corms. Sci. Hortic. 1995, 64, 133–138. [Google Scholar] [CrossRef]
- Al-Juboory, K.H.; Shibli, R.A.; Skiryn, R. Organogenesis and cormel production from callus culture of gladiolus cv. Balady. Mutah. J. Res. Stud. 1997, 12, 143–160. [Google Scholar] [CrossRef]
- Arora, J.S.; Singh, K.; Grewal, H.S.; Gosal, S.S.; Chanana, Y.R. In vitro cormel production from nodal buds and cormel tips in gladiolus. In Plant Tissue Culture; Islam, A.S., Ed.; Oxford and IBH Publishing Co. Pvt. Ltd.: New Delhi, India; Calcutta, India, 1996; pp. 50–53. [Google Scholar]
- Kim, K.W.; Han, S.Y. Cormlet formation in gladiolus shoot base by growth retardants in vitro. J. Korean Soc. Hortic. Sci. 1993, 34, 136–144. [Google Scholar]
- Mares, D.J.; Sowokinos, J.R.; Hawker, J.S. Carbohydrate metabolism in developing potato tubers. In Potato Physiology; Li, P.H., Ed.; Academic Press: Orlando, FL, USA, 1985; pp. 279–327. [Google Scholar]
- Goo, D.H.; Kim, K.W. Influence of sucrose, ABA and daylength on cormlet formation of gladiolus in vitro: Histological observation. J. Korean Soc. Hortic. Sci. 1994, 35, 400–405. [Google Scholar]
- Grewal, H.S.; Gosal, S.S.; Arora, J.S.; Singh, K. Mass propagation of carnation, chrysanthemum and gladiolus through tissue culture. In Proceedings of the International Horticulture Congress (23rd), Florence, Italy, 30 August 1990. [Google Scholar]
- Kumar, A.; Palni, L.M.S. Changes in endogenous polyamines during in vitro cormlet formation in Gladiolus hybridus Hort. Sci. Hortic. 2013, 162, 260–264. [Google Scholar] [CrossRef]
- Martin-Tanguy, J. Metabolism and function of polyamines in plants: Recent development (new approaches). Plant Growth Regul. 2001, 34, 135–148. [Google Scholar] [CrossRef]
- Kaur-Sawhney, R.; Tiburcio, A.F.; Altabella, T.; Galston, A.W. Polyamines in plants: An overview. J. Cell Mol. Biol. 2003, 2, 1–12. [Google Scholar]
- Steinitz, B.; Lilien-Kipnis, H. Control of gladiolus corm and cormel formation in tissue culture. J. Plant Physiol. 1989, 135, 495–500. [Google Scholar] [CrossRef]
- Ginzburg, C.; Ziv, M. Hormonal regulation of cormel formation in gladiolus stolons grown in vitro. Ann. Bot. 1973, 37, 219–224. [Google Scholar] [CrossRef]
- Courduroux, J.C. Etude du mechanisme physiologique de la tuberization chez le topinambour (Helianthus tuberosus L.). Ann. Sci. Nat. Bot. 1967, 8, 215–356. [Google Scholar]
- El-Antalby, H.M.M.; Wareing, P.F.; Hillmarn, J. Some physiological responses to d,1 abscisin (dormin). Planta 1967, 73, 74–90. [Google Scholar] [CrossRef]
- Ziv, M.; Ariel, T. Bud proliferation and plant regeneration in liquid-cultured philodendron treated with ancymidol and paclobutrazol. J. Plant Growth Regul. 1991, 10, 53. [Google Scholar] [CrossRef]
- Steinitz, B.; Yhel, H. In vitro propagation of Narcissus tazetta. HortScience 1982, 17, 333–334. [Google Scholar] [CrossRef]
- Gosal, S.S.; Grewal, H.S. Tissue culture propagation: Problems and potentials, In Horticulture-New Technologies and Applications; Prakash, J., Pierik, R.L.M., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1991; pp. 197–200. [Google Scholar]
- Memon, N.; Qasim, M.; Jaskani, M.J.; Ahmad, R.; Anwar, R. Effect of various corm sizes on the vegetative, floral and corm yield attributes of gladiolus. Pakastan J. Agric. Sci. 2009, 46, 13–19. [Google Scholar]
- Shillo, R.; Halevy, A.H. The effect of various environmental factors on flowering of gladiolus. IV. Interaction of environmental factors general discussion. Sci. Hortic. 1976, 4, 157–162. [Google Scholar] [CrossRef]
- Bose, T.K.; Jana, B.K.; Mukhopadhyay, T.P. A note on the effect of day length on growth and flowering in Hippeastrum. Indian J. Hortic. 1983, 40, 110–112. [Google Scholar]
- Park, I.S.; Choi, J.D.; Byun, M.S.; Goo, D.H.; Kim, K.W. Effects of liquid shaking culture and growth retardant on cormlet formation of gladiolus ‘Spic & Span’ in vitro. J. Korean Soc. Hortic. Sci. 2001, 42, 215–218. [Google Scholar]
- Huylenbroeck, J.M.V.; Debergh, P.C. Physiological aspects of micropropagated plantlets. Plant Tissue Cult. Biotechnol. 1986, 23, 136–141. [Google Scholar]
- Desjardins, Y. Factors affecting CO2 fixation in striving to optimize photoautotrophy in micropropagated plants. Plant Tissue Cult. Biotechnol. 1995, 1, 13–25. [Google Scholar]
- Jager, A.K.; McAlister, B.G.; Van Staden, J. In vitro culture of Gladiolus carneus. S. Afr. J. Bot. 1998, 64, 146–147. [Google Scholar] [CrossRef][Green Version]
- Cantor, M.; Tolety, J. Gladiolus. In Wild Crop Relatives: Genomic and Breeding Resources; Kole, C., Ed.; Springer: Berlin/Heidelberg, Germany, 2011; Volume 10, pp. 133–159. [Google Scholar]
- González-Pérez, E.; Aya la-Garay, O.J.; Carrillo-Salazar, J.A.; García de los Santos, G.; Yáñez-Morales, M.J.; Juárez-Muñoz, J. A study of development flower quality and fertilization in gladiolus (Gladiolus grandiflorus Hort.). Rev. Fitotec. Mex. 2011, 34, 277–283. [Google Scholar]
- Memon, N.U.N.; Wahocho, N.A.; Miano, T.F.; Leghari, M.H. Propagation of gladiolus corms and cormels: A review. Afr. J. Biotechnol. 2016, 15, 1699–1710. [Google Scholar]
- Misra, R.L. Propagation of gladiolus through sprouts-An entirely new method in gladiolus propagation. In Floriculture-Technology, Trades and Trends; Prakash, J., Bhandary, K.R., Eds.; Oxford and IBH Publishing Co. Pvt. Ltd.: New Delhi, India, 1994; pp. 67–70. [Google Scholar]
- Kaur, K.; Jhanji, S.; Kaur, G. Assessment of priming of cormels with plant growth substances on vegetative growth and cormel-associated traits in gladiolus. Ann. Plant Soil Res. 2023, 25, 304–309. [Google Scholar]
- Teixeira da Silva, J.A. Thin cell layer technology in ornamental plant micropropagation and biotechnology. Afr. J. Biotechnol. 2003, 2, 683–691. [Google Scholar]
- Riaz, T.; Khan, S.K.; Javaid, A. Screening of gladiolus germplasm for agronomic performance and resistance against corm rot disease. Afr. J. Biotechnol. 2010, 9, 6701–6707. [Google Scholar]
- Tomiozzo, R.; Uhlmann, L.O.; Becker, C.C.; Schwab, N.T.; Streck, N.A.; Balest, D.S. How to produce gladiolus corms? Ornam. Hortic. 2019, 25, 299–306. [Google Scholar] [CrossRef]
- Malik, S.; Kumar, M.; Singh, M.K. Dormancy in gladiolus: The cause and remedy—A review. J. Plant Dev. Sci. 2009, 1, 45–47. [Google Scholar]
- Mangal, M.; Bhardwaj, S.V.; Handa, A.; Jindal, K.K. production of virus tested gladioli through in vitro and in vivo techniques. Acta Hortic. 2003, 624, 511–514. [Google Scholar] [CrossRef]
- Shabbir, A.; Hameed, N.; Ali, A.; Bajwa, R. Effect of different cultural conditions on micropropagation of rose (Rose indica L.). Pak. J. Bot. 2009, 41, 2877–2882. [Google Scholar]
- Madhavan, S.; Balasubramanian, V.; Selvarajan, R. Viruses infecting bulbous ornamental plants and their diagnosis and management. In Virus Diseases of Ornamental Plants; Springer: Singapore, 2021; pp. 277–299. [Google Scholar]
- Abdalla, N.; El-Ramady, H.; Seliem, M.K.; El-Mahrouk, M.E.; Taha, N.; Bayoumi, Y.; Shalaby, T.A.; Dobránszki, J. An academic and technical overview on plant micropropagation challenges. Horticulturae 2022, 8, 677. [Google Scholar] [CrossRef]
- Pospisilova, J.; Solarova, J.; Catsky, J. Photosynthetic responses to stresses during in vitro cultivation. Photosynthetica 1992, 26, 3–18. [Google Scholar]
- Urban, J.; Ingwers, M.W.; McGuire, M.A.; Teskey, R.O. Increase in leaf temperature opens stomata and decouples net photosynthesis from stomatal conductance in Pinus taeda and Populus deltoides x nigra. J. Exp. Bot 2017, 68, 1757–1767. [Google Scholar] [CrossRef]
- Askari, N.; Aliniaeifard, S.; Visser, R.G. Low CO2 levels are detrimental for in vitro plantlets through disturbance of photosynthetic functionality and accumulation of reactive oxygen species. Horticulturae 2022, 8, 44. [Google Scholar] [CrossRef]
- De Klerk, G.J. Micropropagation of bulbous crops: Technology and present state. Floric. Ornam. Biotechnol. 2012, 6, 1–8. [Google Scholar]
- Smulders, M.J.M.; De Klerk, G.J. Epigenetics in plant tissue culture. Plant Growth Regul. 2011, 63, 137–146. [Google Scholar] [CrossRef]
- Jackson, M.B. Ethylene and responses of plants to soil waterlogging and submergence. Annu. Rev. Plant Physiol. 1985, 36, 146–174. [Google Scholar] [CrossRef]
- Lilien-Kipnis, H.; Azizbekova, N.; Ziv, M. Scaled-up proliferation and regeneration of Nerine in liquid cultures Part II. Ontogeny of somatic embryos and bulblet regeneration. Plant Cell Tissue Organ Cult. 1994, 39, 117–123. [Google Scholar] [CrossRef]
- Ziv, M. Morphogenesis of Gladiolus buds in bioreactors for scaled-up propagation of geophytes. In Progress in Plant Cellular and Molecular Biology; Nijkamp, H.J.J., Van Der Plas, L.H.W., Van Aartrijk, J., Eds.; Kluwer: Dordrecht, The Netherlands, 1990; pp. 119–124. [Google Scholar]
- Ziv, M.; Kahany, S.; Lilien-Kipnis, H. Scaled-up proliferation and regeneration of Nerine in liquid cultures. Part I. The induction and maintenance of proliferating meristematic clusters by paclobutrazol in bioreactors. Plant Cell Tissue Organ Cult. 1994, 39, 109–115. [Google Scholar] [CrossRef]
- De Klerk, G.J. Why plants grow in tissue culture? Prophyta Annu. 2010, 2010, 42–44. [Google Scholar]
- Chen, C.C. Humidity in plant tissue culture vessels. Biosyst. Eng. 2004, 88, 231–241. [Google Scholar] [CrossRef]
- Tanaka, K.; Fujiwara, K.; Kozai, T. Effects of relative humidity in the culture vessel on the transpiration and net photosynthetic rates of potato plantlets in vitro. Acta Hortic. 1992, 319, 59–64. [Google Scholar] [CrossRef]
- De Klerk, G.J. How to measure somaclonal variation: A review. Acta Bot. Neerl. 1990, 39, 129–144. [Google Scholar] [CrossRef]
- D’Arth, S.M.; Simpson, S.I.; Seelye, J.F.; Jameson, P.E. Bushiness and cytokinin sensitivity in micropropagated Zantedeschia. Plant Cell Tissue Organ Cult. 2002, 70, 113–118. [Google Scholar] [CrossRef]
- Naik, P.K.; Nayak, S. Different modes of plant regeneration and factors affecting in vitro bulblet production in Ornithogalum virens. Sci. Asia 2005, 31, 409–414. [Google Scholar] [CrossRef]
- Paek, K.Y.; Murthy, H.N. High frequency of bulblet regeneration from bulb scale sections of Fritillaria thunbergii. Plant Cell Tissue Organ Cult 2002, 68, 247–252. [Google Scholar] [CrossRef]
- Chokheli, V.A.; Dmitriev, P.A.; Rajput, V.D.; Bakulin, S.D.; Azarov, A.S.; Varduni, T.V.; Stepanenko, V.V.; Tarigholizadeh, S.; Singh, R.K.; Verma, K.K.; et al. Recent development in micropropagation techniques for rare plant species. Plants 2020, 9, 1733. [Google Scholar] [CrossRef]
- Murthy, H.N.; Joseph, K.S.; Paek, K.Y.; Park, S.Y. Bioreactor systems for micropropagation of plants: Present scenario and future prospects. Front. Plant Sci. 2023, 14, 1159588. [Google Scholar] [CrossRef]
- Cerasela, P.; Giancarla, V.; Camen, D.; Andreea, P.; Eleonora, N.; Alina, S.; Gorinoiu, G. Evaluation of somaclonal variation in Gladiolus grandifloras through molecular markers ISSR. J. Hortic. For. Biotechnol. 2018, 22, 100–104. [Google Scholar]
- Kamo, K.; Jordan, R.; Guaragna, M.A.; Hsu, H.T.; Ueng, P. Resistance to cucumber mosaic virus in gladiolus plants transformed with either a defective replicase or coat protein subgroup II gene from cucumber mosaic virus. Plant Cell Rep. 2010, 29, 695–704. [Google Scholar] [CrossRef]
- Kaur, C.; Kumar, S.; Raj, S.K.; Chauhan, P.S.; Sharma, N. Characterization of a new isolate of bean yellow mosaic virus group-IV associated with mosaic disease of gladiolus in India. J. Plant Pathol. Microbiol. 2015, 6, 10. [Google Scholar] [CrossRef]
- Kaur, C.; Raj, R.; Srivastava, A.; Kumar, S.; Raj, S.K. Sequence analysis of six full-length bean yellow mosaic virus genomes reveals phylogenetic diversity in India strains, suggesting subdivision of phylogenetic group-IV. Arch. Virol. 2018, 163, 235–242. [Google Scholar] [CrossRef]
- Rao, T.M.; Negi, S.S.; Swamy, R.D. Isolation of leaf mesophyll protoplasts in gladiolus. Indian J. Hortic. 1991, 48, 79–82. [Google Scholar]
- Taylor, W.; Bhatti, S.; Long, D.; Sauve, R. In vitro culture of gladiolus. SNA Res. Conf. 2000, 45, 328–330. [Google Scholar]
- Ratilal, P.H. Tissue Culture Plant and Synthetic Seed Production of Gladiolus Variety Amarican Beauty. Master’s Thesis, Department of Floriculture and Landscaping Department, Aspee College of Horticulture and Forestry Gujarat Agriculture University, Navsari, India, 2009. [Google Scholar]
- Sharma, S.; Shahzad, A.; Da Silva, J.A.T. Synseed technology—A complete synthesis. Biotechnol. Adv. 2013, 31, 186–207. [Google Scholar] [CrossRef]
- Pathania, N.S.; Misra, R.L. In vitro mutagenesis studies in gladiolus for induction of resistance to Fusarium oxysporum fsp gladioli. Acta Hortic. 2003, 624, 487–494. [Google Scholar] [CrossRef]
- Kamo, K.; Blowers, A.; Smith, F.; Van Eck, J.; Lawson, R. Stable transformation of gladiolus using suspension cells and callus. J. Am. Soc. Hortic. Sci. 1995, 120, 347–352. [Google Scholar] [CrossRef]
- Kamo, K.; Blowers, A.; Smith, F.; Van Eck, J. Stable transformation of gladiolus by particle gun bombardment of cormels. Plant Sci. 1995, 110, 105–111. [Google Scholar] [CrossRef]
- Lakshman, D.K.; Pandey, R.; Kamo, K.; Bauchan, G.; Mitra, A. Genetic transformation of Fusarium oxysporum f spgladioli with Agrobacterium to study pathogenesis in gladiolus. Eur. J. Plant Pathol. 2012, 133, 729–738. [Google Scholar] [CrossRef]
- Bull, T.; Michelmore, R. Molecular determinants of in vitro plant regeneration: Prospects for enhanced manipulation of lettuce (Lactuca sativa L.). Front. Plant Sci. 2022, 13, 1211. [Google Scholar] [CrossRef]
- Bednarek, P.T.; Orłowska, R. Plant tissue culture environment as a switch-key of (epi)genetic changes. Plant Cell Tissue Organ Cult 2020, 140, 245–257. [Google Scholar] [CrossRef]
- Ghosh, A.; Igamberdiev, A.U.; Debnath, S.C. Tissue culture-induced DNA methylation in crop plants: A review. Mol. Biol. Rep. 2021, 48, 823–841. [Google Scholar] [CrossRef]
- Kamo, K.; Aebig, J.; Guaragna, M.A.; James, C.; Hsu, H.T.; Jordan, R. Gladiolus plants transformed with single-chain variable fragment antibodies to cucumber mosaic virus. Plant Cell Tissue Organ Cult. 2012, 110, 13–21. [Google Scholar] [CrossRef]
- Kamo, K.; Lakshman, D.; Pandey, R.; Guaragna, M.A.; Okubara, P.; Rajasekaran, K.; Cary, J.; Jordan, R. Resistance to Fusarium oxysporum f sp gladioli in transgenic gladiolus plants expressing either a bacterial chloroperoxidase or fungal chitinase genes. Plant Cell Tissue Organ Cult. 2016, 124, 541–553. [Google Scholar] [CrossRef]
- Loyola-Vargas, V.M.; Avilez-Montalvo, R.N. Plant Tissue Culture: A Battle Horse in the Genome Editing Using CRISPR/Cas9. Methods Mol. Biol. 2018, 1815, 131–148. [Google Scholar] [PubMed]
- Subburaj, S.; Chung, S.J.; Lee, C.; Ryu, S.M.; Kim, D.H.; Kim, J.S.; Bae, S.; Lee, G.J. Site-directed mutagenesis in Petunia x hybrida protoplast system using direct delivery of purified recombinant Cas9 ribonucleoproteins. Plant Cell Rep. 2016, 35, 1535–1544. [Google Scholar] [CrossRef]
- Zhang, B.; Yang, X.; Yang, C.; Li, M.; Guo, Y. Exploiting the CRISPR/Cas9 system for targeted genome mutagenesis in petunia. Sci. Rep. 2016, 6, 20315. [Google Scholar] [CrossRef]
- López-Ruiz, B.A.; Juárez-González, V.T.; Luján-Soto, E.; Dinkova, T.D. The role of small RNAs in plant somatic embryogenesis. In Epigenetics in Plants of Agronomic Importance: Fundamentals and Applications; Alvarez-Venegas, R., De-la-Peña, C., Casas-Mollano, J., Eds.; Springer: Cham, Switzerland, 2019; pp. 311–338. [Google Scholar]
- Kanwal, S.; Guo, X.; Ward, C.; Volpe, G.; Qin, B.; Esteban, M.A.; Bao, X. Role of long non-coding RNAs in reprogramming to induced pluripotency. Genom. Proteom. Bioinf. 2020, 18, 16–25. [Google Scholar] [CrossRef]
- Cordeiro, D.; Canhoto, J.; Correia, S. Regulatory non-coding RNAs: Emerging roles during plant cell reprogramming and in vitro regeneration. Front. Plant Sci. 2022, 13, 1049631. [Google Scholar] [CrossRef]
- Luo, Y.C.; Zhou, H.; Li, Y.; Chen, J.Y.; Yang, J.H.; Chen, Y.Q.; Qu, L.H. Rice embryogenic calli express a unique set of microRNAs, suggesting regulatory roles of microRNAs in plant post-embryogenic development. FEBS Lett. 2006, 580, 5111–5116. [Google Scholar] [CrossRef]
- Gao, Y.; Li, D.; Zhang, L.L.; Borthakur, D.; Li, Q.S.; Ye, J.H.; Zheng, X.-Q.; Lu, J.-L. MicroRNAs and their targeted genes associated with phase changes of stem explants during tissue culture of tea plant. Sci. Rep. 2019, 9, 20239. [Google Scholar] [CrossRef]
- Juárez-González, V.T.; López-Ruiz, B.A.; Baldrich, P.; Luján-Soto, E.; Meyers, B.C.; Dinkova, T.D. The explant developmental stage profoundly impacts small RNA-mediated regulation at the dedifferentiation step of maize somatic embryogenesis. Sci. Rep. 2019, 9, 14511. [Google Scholar] [CrossRef]
- Zhang, S.; Zhou, J.; Han, S.; Yang, W.; Li, W.; Wei, H.; Wei, H.; Li, X.; Qi, L. Four abiotic stress-induced miRNA families differentially regulated in the embryogenic and non-embryogenic callus tissues of Larix leptolepis. Biochem. Biophys. Res. Commun. 2010, 398, 355–360. [Google Scholar] [CrossRef]
- Wu, X.M.; Kou, S.J.; Liu, Y.L.; Fang, Y.N.; Xu, Q.; Guo, W.W. Genomewide analysis of small RNAs in nonembryogenic and embryogenic tissues of citrus: microRNA- and siRNA-mediated transcript cleavage involved in somatic embryogenesis. Plant Biotechnol. J. 2015, 13, 383–394. [Google Scholar] [CrossRef]
- López-Ruiz, B.A.; Juárez-González, V.T.; Sandoval-Zapotitla, E.; Dinkova, T.D. Development-related miRNA expression and target regulation during staggered in vitro plant regeneration of tuxpeño VS-535 maize cultivar. Int. J. Mol. Sci. 2019, 20, 2079. [Google Scholar] [CrossRef]
- Cao, H.; Zhang, X.; Ruan, Y.; Zhang, L.; Cui, Z.; Li, X.; Jia, B. miRNA expression profiling and zeatin dynamic changes in a new model system of in vivo indirect regeneration of tomato. PLoS ONE 2020, 15, e0237690. [Google Scholar] [CrossRef] [PubMed]
- Chávez-Hernández, E.C.; Alejandri-Ramírez, N.D.; Juárez-González, V.T.; Dinkova, T.D. Maize miRNA and target regulation in response to hormone depletion and light exposure during somatic embryogenesis. Front. Plant Sci. 2015, 6, 555. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.M.; Liu, M.Y.; Ge, X.X.; Xu, Q.; Guo, W.W. Stage and tissue-specific modulation of ten conserved miRNAs and their targets during somatic embryogenesis of Valencia sweet orange. Planta 2011, 233, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Yu, W.; Hou, L.; Wang, X.; Zheng, F.; Wang, W.; Liang, D.; Yang, H.; Jin, Y.; Xie, X. Analysis of miRNAs and their targets during adventitious shoot organogenesis of Acacia crassicarpa. PLoS ONE 2014, 9, e93438. [Google Scholar] [CrossRef]
| Genotype | Mode of Regeneration | Response | Reference |
|---|---|---|---|
| White Friendship, Traderhorn, and Peter Pears | Indirect organogenesis | White Friendship produced more calluses | [31] |
| Blue Isle, Jenny Lee, Peter Pears, and Rosa Supreme | Somatic embryogenesis | Blue Isle and Rosa Supreme produced compact calluses; no callus produced in Jenny Lee | [65] |
| Black Rock, Oscar, Andrew First Called, and Azure Shore | Direct organogenesis | Oscar showed the highest regeneration frequency | [66] |
| Adlib Scarlet, May Queen, Pacific Pink, Sharone, and White Race | Direct organogenesis | Adlib Scarlet and Sharone produced better results | [67] |
| Kaifa, Clara, and Nabila | Direct organogenesis | Nabila showed the best results in regard to the shoot development | [68] |
| Peter Pears, Rose Supreme, and White Prosperity | Indirect organogenesis | Rose Supreme induced a higher percentage of calluses | [69] |
| ChaCha and Priscilla | Indirect organogenesis | The callus frequency was 93.3 and 100% for the cultivars ‘Priscilla’ and ‘ChaCha’, respectively | [70] |
| Explants | Mode of Regeneration | Response | Reference |
|---|---|---|---|
| Nodal cultures from different stages of flower spike, whole cormels of various size, cormel sprouts, and cormel segments | Direct organogenesis | The cormel sprouts’ shoots showed the better reaction | [9] |
| Terminal and axillary buds of cormlets and nodal buds | Direct organogenesis | Cormel tips responded more favorably than axillary buds | [23] |
| Cormel slices of top, middle, and bottom, and in vitro derived bisected shoot tips | Indirect organogenesis | The shoot induction response was better on the top slice | [24] |
| Cormel, meristem, and leaf explants | Direct organogenesis | Cormel explants were found to be a more effective source of shoot multiplication when compared to meristems | [28] |
| The transverse cormel slices viz. top, medium, and bottom | Indirect organogenesis | The bottom slice of the cormel produced more callus growth | [31] |
| Basal leaves and cormel slices | Indirect organogenesis | Basal leaves showed a higher frequency of calluses in comparison to cormel slices | [61] |
| Young leaf, basal portion of leaf, and corm slices | Somatic embryogenesis | There were some calluses on the young leaf bases, while the basal portion of the leaf induced more calluses | [65] |
| Apical and axillary gladiolus buds | Direct organogenesis | Axillary buds showed the best shoot multiplication | [66] |
| Apical, middle and basal parts of leaves and petals | Indirect organogenesis | Apical buds, leaves, and flower stalks exhibited excellent callus development (100%), whereas petals had a low callus formation ability (10%), and no callus formation was observed on floral stems, bracts, or floral spikes | [70] |
| Axillary bud, single elongated bud | Direct organogenesis | Single elongated buds showed the best results | [71] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, M.; Chaudhary, V.; Sirohi, U.; Singh, J.; Yadav, M.K.; Prakash, S.; Kumar, A.; Kumar, V.; Pal, V.; Chauhan, C.; et al. In Vitro Propagation Journey of Ornamental Gladiolus (Gladiolus Species): A Systematic Review Analysis Based on More Than 50 Years Research. Horticulturae 2024, 10, 148. https://doi.org/10.3390/horticulturae10020148
Kumar M, Chaudhary V, Sirohi U, Singh J, Yadav MK, Prakash S, Kumar A, Kumar V, Pal V, Chauhan C, et al. In Vitro Propagation Journey of Ornamental Gladiolus (Gladiolus Species): A Systematic Review Analysis Based on More Than 50 Years Research. Horticulturae. 2024; 10(2):148. https://doi.org/10.3390/horticulturae10020148
Chicago/Turabian StyleKumar, Mukesh, Veena Chaudhary, Ujjwal Sirohi, Jitender Singh, Manoj Kumar Yadav, Satya Prakash, Arvind Kumar, Vipin Kumar, Virendra Pal, Chetan Chauhan, and et al. 2024. "In Vitro Propagation Journey of Ornamental Gladiolus (Gladiolus Species): A Systematic Review Analysis Based on More Than 50 Years Research" Horticulturae 10, no. 2: 148. https://doi.org/10.3390/horticulturae10020148
APA StyleKumar, M., Chaudhary, V., Sirohi, U., Singh, J., Yadav, M. K., Prakash, S., Kumar, A., Kumar, V., Pal, V., Chauhan, C., Kaushik, K., Shukla, D., Motla, R., Kumar, S., & Malik, S. (2024). In Vitro Propagation Journey of Ornamental Gladiolus (Gladiolus Species): A Systematic Review Analysis Based on More Than 50 Years Research. Horticulturae, 10(2), 148. https://doi.org/10.3390/horticulturae10020148







