Differentiating Leaf Structures and Physiological Responses to Freezing Stress of Mangrove Kandelia obovata from Different Provenances
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Experimental Design
2.2. Determination of Leaf Anatomy
2.3. Determination of Leaf Physiological Changes
2.4. Statistical Analysis
3. Results
3.1. Comparison of Morphology in Different K. obovata Provenances under Freezing Stress
3.2. Effects of Freezing Stress on the Anatomical Structure of K. obovata Leaves
3.3. The Physiological Response of K. obovata to Freezing Stress
3.4. Principal Component Analysis and Membership Function Analysis of K. obovata from Different Provenances
4. Discussion
4.1. Differential Anatomical Variations in the Leaf Structure of K. obovata from Different Provenances and Their Responses to Freezing Stress
4.2. Temporal Variations in the Physiological Status of K. obovata from Different Provenances under Cold Stress
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, Y.S.; Gu, J.D. Ecological responses, adaptation and mechanisms of mangrove wetland ecosystem to global climate change and anthropogenic activities. Int. Biodeterior. Biodegrad. 2021, 162, 105248. [Google Scholar] [CrossRef]
- Kathiresan, K. Mangroves: Types and Importance, 1st ed.; Mangroves: Ecology, Biodiversity and Management; Springer: Singapore, 2021; pp. 1–31. [Google Scholar]
- Chen, Q.X.; Zheng, J.; Wang, J.W. Artifcial Mangrove in Zhejiang, Province, 5th ed.; China Forestry Publishing House: Beijing, China, 2015; pp. 105–128. [Google Scholar]
- Liu, X.; Lu, X.; Yang, S.; Liu, Y.; Wang, W.; Wei, X.; Ji, H.; Zhang, B.; Xin, W.; Wen, J.; et al. Role of exogenous abscisic acid in freezing tolerance of mangrove Kandelia obovata under natural frost condition at near 32°N. BMC Plant Biol. 2022, 22, 593. [Google Scholar] [CrossRef]
- Halbritter, A.H.; Fior, S.; Keller, I.; Billeter, R.; Edwards, P.J.; Holderegger, R.; Karrenberg, S.; Pluess, A.R.; Widmer, A.; Alexander, J.A. Trait differentiation and adaptation of plants along elevation gradients. J. Evol. Biol. 2018, 31, 784–800. [Google Scholar] [CrossRef]
- Lu, W.X.; Zhang, B.H.; Zhang, Y.Y.; Yang, S.C. Differentiation of cold tolerance in an artificial population of a mangrove species, Kandelia obovata, is associated with geographic origins. Front. Plant Sci. 2022, 12, 695746. [Google Scholar] [CrossRef] [PubMed]
- Ni, X.; Sun, L.; Cai, Q.; Ma, S.; Feng, Y.; Sun, Y.; An, L.; Ji, C. Variation and determinants of leaf anatomical traits from boreal to tropical forests in eastern China. Ecol. Indic. 2022, 140, 108992. [Google Scholar] [CrossRef]
- Xu, G.; He, M.; Zhao, D.; Lyu, D.; Qin, S. Physiological and structural changes in apple tree branches of different varieties during dormancy. Horticulturae 2023, 9, 947. [Google Scholar] [CrossRef]
- Domínguez, E.; Cuartero, J.; Heredia, A. An overview on plant cuticle biomechanics. Plant Sci. 2011, 181, 77–84. [Google Scholar] [CrossRef]
- Ramírez-Valiente, J.A.; Sánchez-Gómez, D.; Aranda, I.; Valladares, F. Phenotypic plasticity and local adaptation in leaf ecophysiological traits of 13 contrasting cork oak populations under different water availabilities. Tree Physiol. 2010, 30, 618–627. [Google Scholar] [CrossRef]
- Pritzkow, C.; Williamson, V.; Szota, C.; Trouvé, R.; Arndt, S.K. Phenotypic plasticity and genetic adaptation of functional traits influences intra-specific variation in hydraulic efficiency and safety. Tree Physiol. 2020, 40, 215–229. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Liu, Y.; Yang, S.; Wang, J.; Lu, X.; Wei, X.; Wang, W.; Wang, J.; Chen, Q. Development and characterization of 29 InDel markers from the mangrove Kandelia obovata genome using a resequencing dataset. Conserv. Genet. Resour. 2022, 14, 263–266. [Google Scholar] [CrossRef]
- Hu, M.J.; Sun, W.H.; Tsai, W.C.; Xiang, S.; Lai, X.K.; Chen, D.Q.; Liu, X.D.; Wang, Y.F.; Le, Y.X.; Chen, S.M.; et al. Chromosome-scale assembly of the Kandelia obovata genome. Hortic. Res. 2020, 7, 75. [Google Scholar] [CrossRef] [PubMed]
- Ruan, Y.; Huang, B.; Lai, S.; Wan, Y.; Li, J.; Huang, S.; Liao, P. Population genetic structure, local adaptation, and conservation genetics of Kandelia obovata. Tree Genet. Genomes 2013, 9, 913–925. [Google Scholar] [CrossRef]
- Chen, Q.; Zheng, J.; Zhou, W.; Wang, J.; Yang, S.; Li, X.; Lu, X.; Liu, X. A new Kandelia obovata cultivar ‘Longgang’. Acta Hortic. Sin. 2019, 46, 2927–2928. [Google Scholar]
- Hu, Y.; Yang, L.; Gao, C.; Liao, D.; Long, L.; Qiu, J.; Wei, H.; Deng, Q.; Zhou, Y. A comparative study on the leaf anatomical structure of Camellia oleifera in a low-hot valley area in Guizhou Province, China. PLoS ONE 2022, 17, e0262509. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.M.; Xu, Q.L.; Li, Q.; Zhang, H.; Xiao, J. Physiological responses of the two blueberry cultivars to inoculation with an arbuscular mycorrhizal fungus under low-temperature stress. J. Plant Nutr. 2017, 40, 2562–2570. [Google Scholar] [CrossRef]
- Shi, L.; Dong, X.; Fu, H.; Chai, X.; Bao, S.; Ren, Y.; Hu, K.; Li, Q.; Chen, Z. Differences in physiological characteristics of green prickly ash germplasm resources in response to low-temperature Stress. Horticulturae 2023, 9, 1242. [Google Scholar] [CrossRef]
- Jakhar, S.; Mukherjee, D. Chloroplast pigments, proteins, lipid peroxidation and activities of antioxidative enzymes during maturation and senescence of leaves and reproductive organs of Cajanus cajan L. Physiol. Mol. Biol. Plants 2014, 20, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Moon, Y.E.; Han, S.G.; Yun, S.K.; Joa, J.H.; Park, J.S. Impact of cold stress on physiological responses and fruit quality of shiranuhi mandarin in response to cold conditions. Horticulturae 2023, 9, 906. [Google Scholar] [CrossRef]
- Schreiber, S.G.; Hamann, A.; Hacke, U.G.; Thomas, B.R. Sixteen years of winter stress: An assessment of cold hardiness, growth performance and survival of hybrid poplar clones at a boreal planting site. Plant Cell Environ. 2013, 36, 419–428. [Google Scholar] [CrossRef]
- Kaur, R.; Kaur, N.; Singh, H. Pericarp and pedicel anatomy in relation to fruit cracking in lemon (Citrus limon L. Burm.). Sci. Hortic. 2019, 246, 462–468. [Google Scholar] [CrossRef]
- Cao, H.; Huang, H.; Lei, X.; Zhang, D.; Zhang, R. Difference of the leaf anatomical structure of coconut varieties under low temperature treatments. Chin. J. Trop. Crops 2014, 35, 2420–2425. [Google Scholar]
- Duan, W.J.; Shen, Y.F.; Cao, Z.H.; Shu, Q.L.; Hu, J.J.; Li, C.S. Effect of foliar fertilizer on leaf anatomy structure and photosynthetic characteristics of Camellia oleifera container seedlings. J. Northwest A F Univ. 2015, 43, 92–97. [Google Scholar]
- Liao, W.T.; Wang, R.H.; Zhong, F.X.; Li, T.; Chen, C.X. Comparison of photosynthetic and leaf anatomical characteristics of five excellent Camellia oleifera clones. Non-Wood For. Res. 2015, 33, 56–61. [Google Scholar]
- Zhang, Y.; Chen, C.; Jin, Z.; Yang, Z.; Li, Y. Leaf anatomy, photosynthesis, and chloroplast ultrastructure of heptacodium miconioides seedlings reveal adaptation to light environment. Environ. Exp. Bot. 2022, 195, 104780. [Google Scholar] [CrossRef]
- Domínguez, J.A.; Heredia-Guerrero, A.A. The biophysical design of plant cuticles: An overview. New Phytol. 2011, 189, 938–949. [Google Scholar] [CrossRef] [PubMed]
- Matas, A.J.; López-Casado, G.; Cuartero, J.; Heredia, A. Relative humidity and temperature modify the mechanical properties of isolated tomato fruit cuticles. Am. J. Bot. 2005, 92, 462–468. [Google Scholar] [CrossRef]
- Luis, Z.G.; Bezerra, K.M.G.; Pereira, J.E.S. Adaptability and leaf anatomical features in oil palm seedlings produced by embryo rescue and pre-germinated seeds. Plant Physiol. 2010, 22, 209–215. [Google Scholar] [CrossRef]
- Theocharis, A.; Clément, C.; Barka, E.A. Physiological and molecular changes in plants grown at low temperatures. Planta 2012, 235, 1091–1105. [Google Scholar] [CrossRef]
- Kurbidaeva, A.S.; Novokreshchenova, M.G. Genetic control of plant resistance to cold. Russ. J. Genet. 2011, 47, 646–661. [Google Scholar] [CrossRef]
- Fei, J.; Wang, Y.S.; Cheng, H.; Sun, F.L.; Sun, C.C. Comparative physiological and proteomic analyses of mangrove plant Kandelia obovata under cold stress. Ecotoxicology 2021, 30, 1826–1840. [Google Scholar] [CrossRef]
- Peng, Y.L.; Wang, Y.S.; Fei, J.; Sun, C.C.; Cheng, H. Ecophysiological differences between three mangrove seedlings (kandelia obovata, aegiceras corniculatum, and avicennia marina) exposed to chilling stress. Ecotoxicology 2015, 24, 7–8. [Google Scholar] [CrossRef]
- Provart, N.J.; Gil, P.; Chen, W.Q.; Han, B.; Chang, H.S.; Wang, X.; Zhu, T. Gene expression phenotypes of Arabidopsis associated with sensitivity to low temperatures. Plant Physiol. 2003, 132, 893–906. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Xu, J.J.; Li, J.M.; Hu, X.H. NO is involved in JA− and H2O2− mediated ALA-induced oxidative stress tolerance at low temperatures in tomato. Environ. Exp. Bot. 2019, 161, 334–343. [Google Scholar] [CrossRef]
- Orabi, S.A.; Dawood, M.G.; Salman, S.R. Comparative study between the physiological role of hydrogen peroxide and salicylic acid in alleviating the harmful effect of low temperature on tomato plants grown under sand-ponic culture. Sci. Agric. 2015, 9, 49–59. [Google Scholar]
- Korn, M.; Peterek, S.; Mock, H.P.; Heyer, A.G.; Hincha, D.K. Heterosis in the freezing tolerance, and sugar and flavonoid contents of crosses between Arabidopsis thaliana accessions of widely varying freezing tolerance. Plant. Cell Environ. 2008, 31, 813–827. [Google Scholar] [CrossRef]
- Schulz, E.; Tohge, T.; Zuther, E.; Fernie, A.R.; Hincha, D.K. Natural variation in flavonol and anthocyanin metabolism during cold acclima-tion in Arabidopsis thaliana accessions. Plant Cell Environ. 2015, 38, 1658–1672. [Google Scholar] [CrossRef]
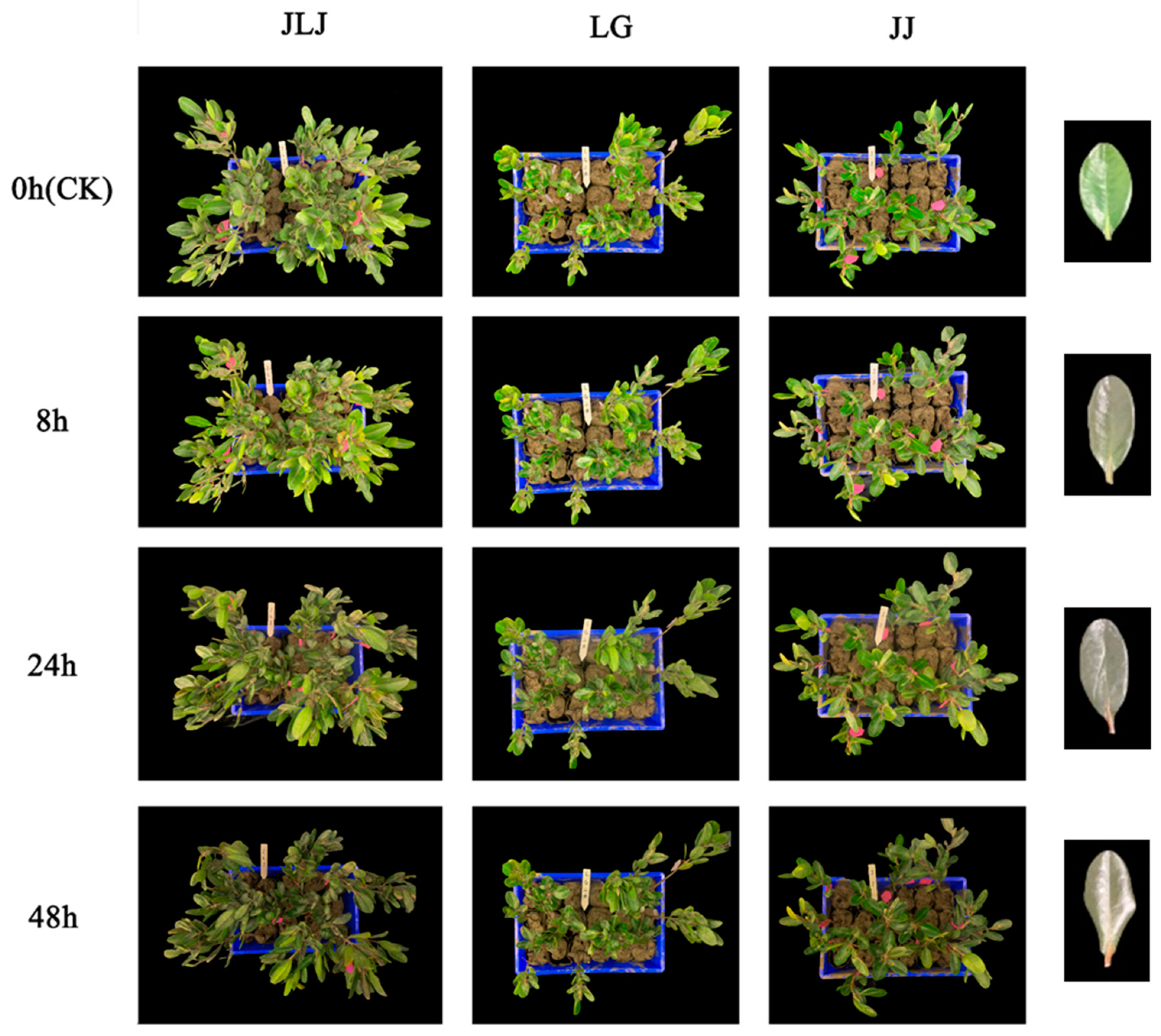


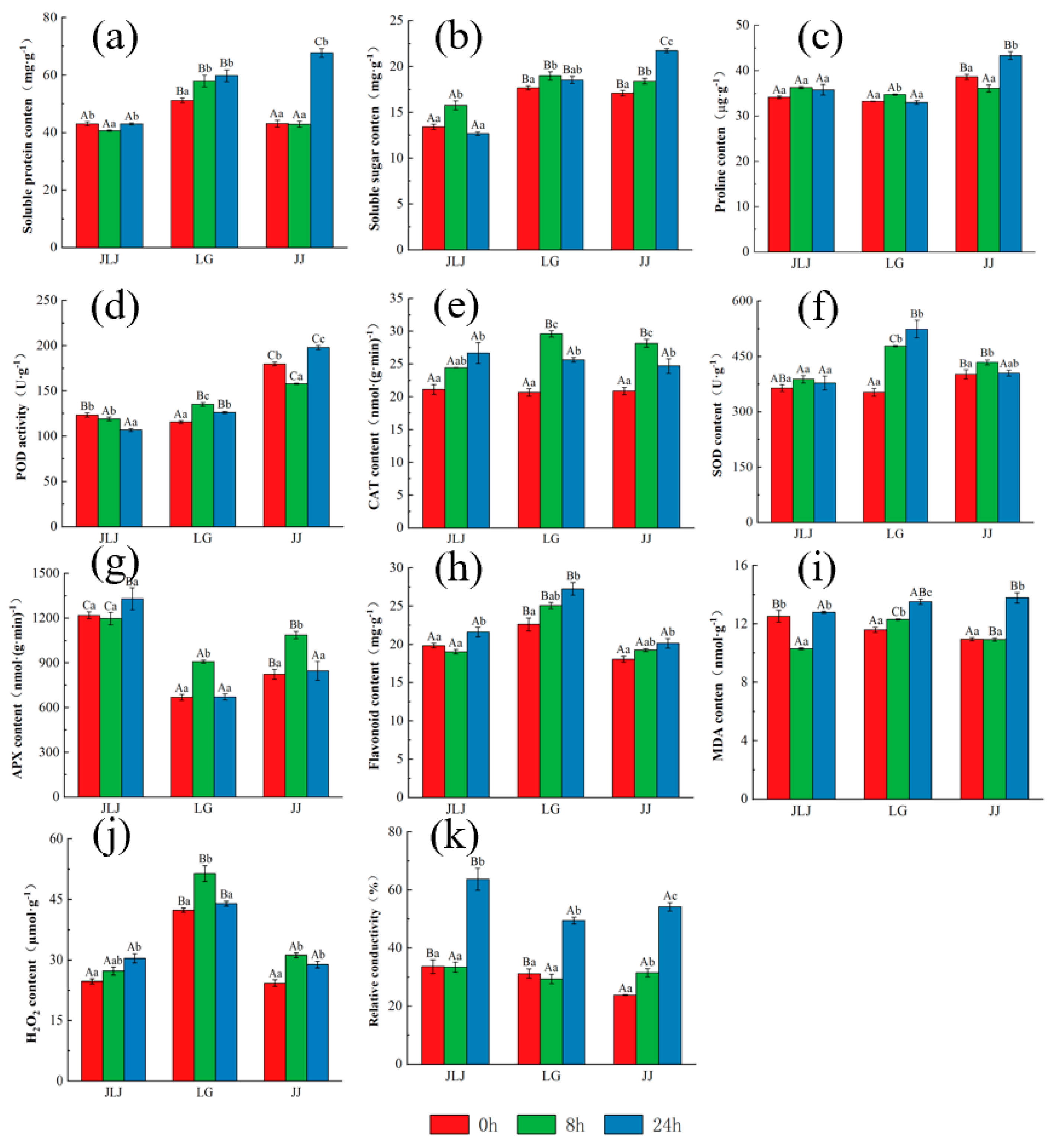
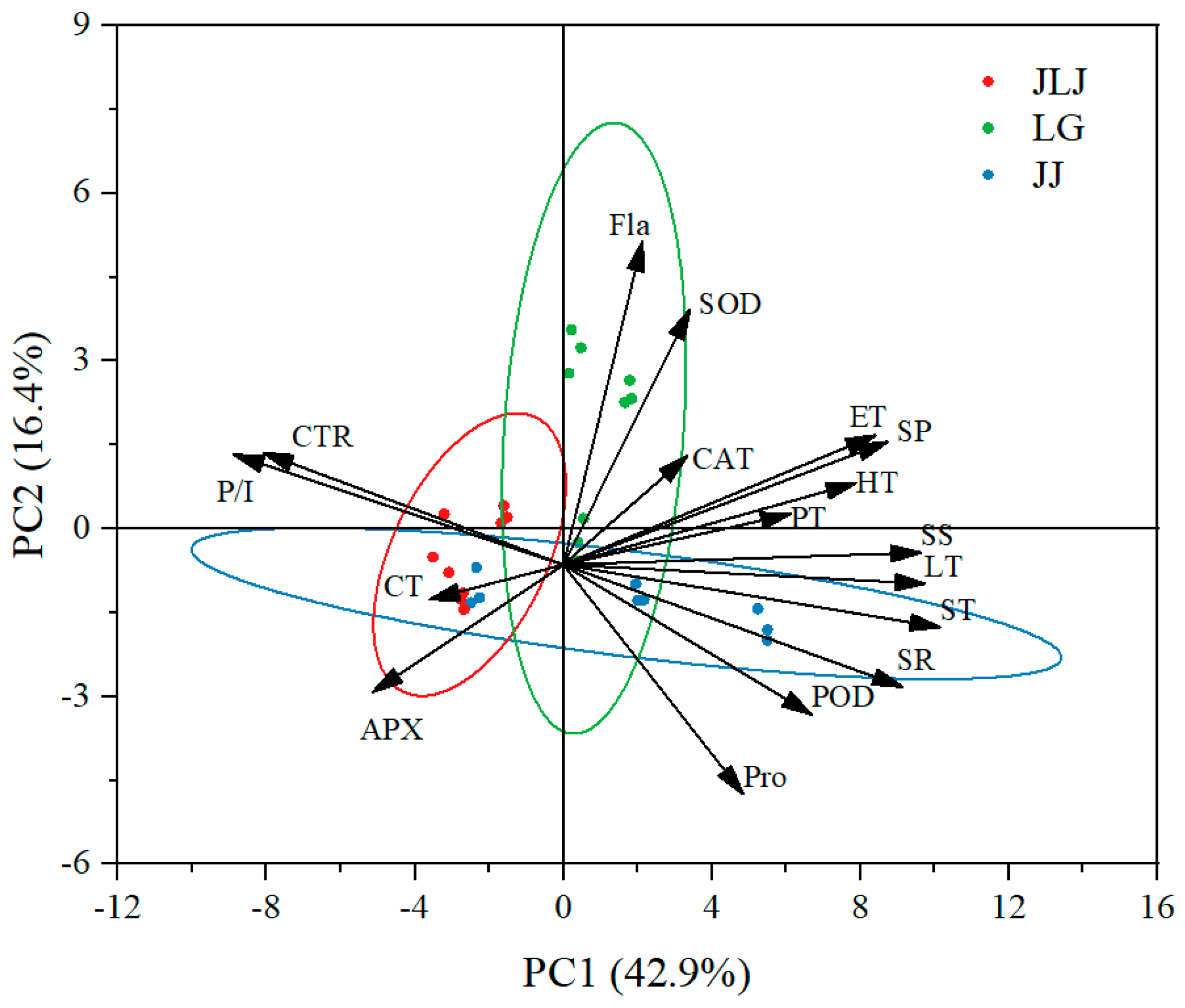
| Provenance | Geographical Coordinates | Annual Minimum Temperature (°C) | Annual Maximum Temperature (°C) | Hypocotyl Length (cm) | Hypocotyl Ground Diameter (mm) | Hypocotyl Weight (g) |
|---|---|---|---|---|---|---|
| JLJ | 24°25′ N, 117°55′ E | 5 | 40 | 22.16 ± 0.58 | 11.99 ± 0.86 | 14.92 ± 1.19 |
| LG | 27°34′ N, 120°36′ E | −4 | 38 | 17.03 ± 0.13 | 9.75 ± 0.09 | 7.83 ± 0.12 |
| JJ | 28°67′N, 121°43′ E | −5 | 39 | 17.53 ± 0.09 | 9.91 ± 0.21 | 7.93 ± 0.15 |
| Provenance | Item | 0 h (CK) | 8 h | 24 h | 48 h |
|---|---|---|---|---|---|
| JLJ | LT/µm | 512.45 ± 8.80 c | 449.90 ± 10.85 ab | 437.22 ± 12.33 a | 474.27 ± 13.83 b |
| CT/µm | 7.23 ± 0.23 a | 7.78 ± 0.28 ab | 8.11 ± 0.31 b | 8.21 ± 0.33 b | |
| ET/µm | 48.25 ± 1.57 b | 44.82 ± 0.84 ab | 47.11 ± 1.29 ab | 43.73 ± 1.34 a | |
| HT/µm | 47.9 ± 1.78 b | 42.14 ± 1.24 a | 42.74 ± 2.8 ab | 55.63 ± 1.94 c | |
| PT/µm | 175.46 ± 6.27 c | 144.06 ± 3.56 b | 139.25 ± 6.35 b | 117.63 ± 5.03 a | |
| ST/µm | 233.61 ± 10.62 bc | 211.11 ± 9.74 ab | 200.01 ± 7.95 a | 249.07 ± 13.16 c | |
| CTR/% | 34.36 ± 1.3 b | 32.16 ± 0.79 b | 31.86 ± 1.21 b | 25.05 ± 1.21 a | |
| SR/% | 45.39 ± 1.44 a | 46.6 ± 1.18 a | 45.73 ± 1.26 a | 52.07 ± 1.49 b | |
| P/I | 0.78 ± 0.05 b | 0.7 ± 0.03 b | 0.71 ± 0.05 b | 0.5 ± 0.04 a | |
| LG | LT/µm | 507.94 ± 11.28 bc | 510.79 ± 18.58 c | 472.61 ± 11.69 ab | 455.51 ± 10.20 a |
| CT/µm | 8.86 ± 0.31 b | 8.32 ± 0.21 b | 6.78 ± 0.56 a | 8.54 ± 0.29 b | |
| ET/µm | 47.69 ± 2.06 a | 51.58 ± 1.71 a | 47.66 ± 1.05 a | 48.09 ± 1.14 a | |
| HT/µm | 45.6 ± 2.12 a | 48.98 ± 2.82 a | 46.18 ± 2.26 a | 49.25 ± 1.44 a | |
| PT/µm | 157.48 ± 5.97 b | 162.18 ± 6.85 b | 152.07 ± 4.26 b | 129.59 ± 7.44 a | |
| ST/µm | 248.32 ± 10.09 b | 239.73 ± 12.17 ab | 219.93 ± 9.29 a | 220.04 ± 7.05 a | |
| CTR/% | 31.06 ± 1.13 ab | 31.83 ± 0.82 b | 32.29 ± 0.81 b | 28.32 ± 1.36 a | |
| SR/% | 48.7 ± 1.3 a | 46.6 ± 1.06 a | 46.32 ± 1.01 a | 48.36 ± 1.29 a | |
| P/I | 0.66 ± 0.04 a | 0.69 ± 0.03 a | 0.71 ± 0.03 a | 0.6 ± 0.04 a | |
| JJ | LT/µm | 439.85 ± 9.38 a | 525.99 ± 10.29 b | 551.99 ± 25.18 b | 448.44 ± 20.38 a |
| CT/µm | 8.12 ± 0.3 c | 7.51 ± 0.35 bc | 6.93 ± 0.2 b | 5.61 ± 0.17 a | |
| ET/µm | 45.24 ± 2.87 a | 48.82 ± 5.9 ab | 50 ± 1.37 b | 46.87 ± 1.7 ab | |
| HT/µm | 46.61 ± 1.38 a | 45.63 ± 1.34 a | 49.56 ± 1.53 a | 48.39 ± 2.28 a | |
| PT/µm | 144.57 ± 5.60 ab | 159.65 ± 3.73 b | 163.63 ± 8.64 b | 136.64 ± 9.23 a | |
| ST/µm | 195.32 ± 5.28 a | 264.38 ± 9.98 b | 281.87 ± 17.99 b | 210.93 ± 10.24 a | |
| CTR/% | 32.77 ± 0.84 b | 30.46 ± 0.77 ab | 29.75 ± 1.01 a | 30.13 ± 0.75 a | |
| SR/% | 44.44 ± 0.93 a | 50.06 ± 1.06 b | 50.63 ± 1.34 b | 46.99 ± 0.61 a | |
| P/I | 0.75 ± 0.03 b | 0.62 ± 0.03 a | 0.6 ± 0.03 a | 0.64 ± 0.02 a |
| Membership Function Value | Provenance | ||
|---|---|---|---|
| JLJ | LG | JJ | |
| LT | 0.255 | 0.522 | 0.599 |
| CT | 0.446 | 0.580 | 0.354 |
| ET | 0.282 | 0.615 | 0.472 |
| HT | 0.286 | 0.644 | 0.690 |
| PT | 0.378 | 0.497 | 0.461 |
| ST | 0.226 | 0.470 | 0.599 |
| SR | 0.237 | 0.447 | 0.636 |
| CTR | 0.666 | 0.435 | 0.285 |
| P/I | 0.758 | 0.508 | 0.357 |
| SP | 0.059 | 0.578 | 0.390 |
| SS | 0.141 | 0.632 | 0.707 |
| Pro | 0.235 | 0.065 | 0.616 |
| MDA | 0.452 | 0.622 | 0.456 |
| H2O2 | 0.117 | 0.797 | 0.141 |
| REC | 0.496 | 0.323 | 0.319 |
| POD | 0.106 | 0.207 | 0.787 |
| CAT | 0.382 | 0.519 | 0.437 |
| SOD | 0.140 | 0.577 | 0.354 |
| APX | 0.877 | 0.122 | 0.378 |
| Fla | 0.231 | 0.754 | 0.119 |
| Mean value | 0.338 | 0.496 | 0.458 |
| Order of stress resistance | 3 | 1 | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xin, W.; An, X.; Liu, H.; Liu, S.; Yang, S.; Wei, X.; Zhao, J.; Lin, R.; Liu, X.; Chen, Q. Differentiating Leaf Structures and Physiological Responses to Freezing Stress of Mangrove Kandelia obovata from Different Provenances. Horticulturae 2024, 10, 182. https://doi.org/10.3390/horticulturae10020182
Xin W, An X, Liu H, Liu S, Yang S, Wei X, Zhao J, Lin R, Liu X, Chen Q. Differentiating Leaf Structures and Physiological Responses to Freezing Stress of Mangrove Kandelia obovata from Different Provenances. Horticulturae. 2024; 10(2):182. https://doi.org/10.3390/horticulturae10020182
Chicago/Turabian StyleXin, Wenzhen, Xia An, Huizi Liu, Shuangshuang Liu, Sheng Yang, Xin Wei, Jiali Zhao, Renan Lin, Xing Liu, and Qiuxia Chen. 2024. "Differentiating Leaf Structures and Physiological Responses to Freezing Stress of Mangrove Kandelia obovata from Different Provenances" Horticulturae 10, no. 2: 182. https://doi.org/10.3390/horticulturae10020182
APA StyleXin, W., An, X., Liu, H., Liu, S., Yang, S., Wei, X., Zhao, J., Lin, R., Liu, X., & Chen, Q. (2024). Differentiating Leaf Structures and Physiological Responses to Freezing Stress of Mangrove Kandelia obovata from Different Provenances. Horticulturae, 10(2), 182. https://doi.org/10.3390/horticulturae10020182





