Enhanced Preservation of Bioactives in Wild Garlic (Allium ursinum L.) through Advanced Primary Processing
Abstract
:1. Introduction
2. Material and Methods
2.1. Plant Material
2.2. Storage of Wild Garlic Leaves
2.3. Drying Method Optimization
2.3.1. Convective Drying
2.3.2. Vacuum Drying
2.3.3. Lyophilization
2.4. Color Determination
2.5. Determination of Bioactive Compounds
2.5.1. Total Phenolic Content Determination
2.5.2. Alliinase Activity Determination
2.5.3. Determination of Total Thiosulfinates
2.5.4. Determination of the Content of L-Ascorbic Acid
2.5.5. Determination of Total Chlorophyll and Carotenoids Content
2.6. Sensory Analysis of Fresh Wild Garlic Leaves
2.7. Statistical Analysis
3. Results and Discussion
3.1. Storage of Fresh Wild Garlic Leaves
3.1.1. Effects of Storing Condition on Color and Bioactive Compounds
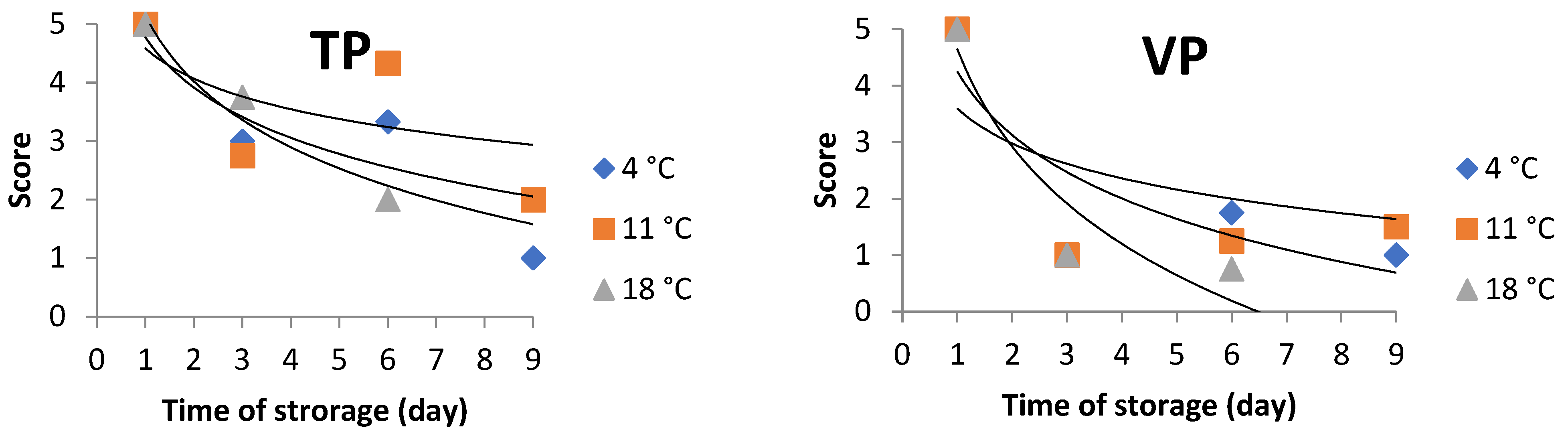
3.1.2. Effects of Storing Condition on Sensorial Attributes
3.2. Changes in Selected Parameters after Drying
3.2.1. Effects of Drying on Moisture Content and Drying Time
3.2.2. Effects of Drying on Color Parameters
3.2.3. Effects of Drying on Bioactive Compounds
3.3. Utilization of Wild Garlic Waste Generated during Storage
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cvetanović Kljakić, A.; Stupar, A.; Terzić, M.; Božunović, J.; Gašić, U.; Zengin, G.; Yildiztugay, E. Chemical Profiling and Biological Activities of Opopanax Hispidus Extracts: A Comparative Insight on Conventional and Green Extraction Technologies. Sustain. Chem. Pharm. 2023, 33, 101122. [Google Scholar] [CrossRef]
- Filipčev, B. The Effects of Aromatic Plants and Their Extracts in Food Products. In Feed Additives; Academic Press: Cambridge, MA, USA, 2020; pp. 279–294. [Google Scholar] [CrossRef]
- Stanisavljević, N.; Soković Bajić, S.; Jovanović, Ž.; Matić, I.; Tolinački, M.; Popović, D.; Popović, N.; Terzić-Vidojević, A.; Golić, N.; Beškoski, V.; et al. Antioxidant and Antiproliferative Activity of Allium ursinum and Their Associated Microbiota During Simulated in Vitro Digestion in the Presence of Food Matrix. Front. Microbiol. 2020, 11, 601616. [Google Scholar] [CrossRef] [PubMed]
- Pavlović, D.R.; Veljković, M.; Stojanović, N.M.; Gočmanac-Ignjatović, M.; Mihailov-Krstev, T.; Branković, S.; Sokolović, D.; Marčetić, M.; Radulović, N.; Radenković, M. Influence of Different Wild-Garlic (Allium ursinum) Extracts on the Gastrointestinal System: Spasmolytic, Antimicrobial and Antioxidant Properties. J. Pharm. Pharmacol. 2017, 69, 1208–1218. [Google Scholar] [CrossRef] [PubMed]
- Burton, G.P.; Prescott, T.A.K.; Fang, R.; Lee, M.A. Regional Variation in the Antibacterial Activity of a Wild Plant, Wild Garlic (Allium ursinum L.). Plant Physiol. Biochem. 2023, 202, 107959. [Google Scholar] [CrossRef] [PubMed]
- Rankovic, M.; Krivokapic, M.; Bradic, J.; Petkovic, A.; Zivkovic, V.; Sretenovic, J.; Jeremic, N.; Bolevich, S.; Kartashova, M.; Jeremic, J.; et al. New Insight Into the Cardioprotective Effects of Allium ursinum L. Extract against Myocardial Ischemia-Reperfusion Injury. Front. Physiol. 2021, 12, 690696. [Google Scholar] [CrossRef] [PubMed]
- Stupar, A.; Šarić, L.; Vidović, S.; Bajić, A.; Kolarov, V.; Šarić, B. Antibacterial Potential of Allium ursinum Extract Prepared by the Green Extraction Method. Microorganisms 2022, 10, 1358. [Google Scholar] [CrossRef] [PubMed]
- Tomšik, A.; Pavlić, B.; Vladić, J.; Cindrić, M.; Jovanov, P.; Sakač, M.; Mandić, A.; Vidović, S.; Pavli, B.; Vladi, J.; et al. Subcritical Water Extraction of Wild Garlic (Allium ursinum L.) and Process Optimization by Response Surface Methodology. J. Supercrit. Fluids 2017, 128, 79–88. [Google Scholar] [CrossRef]
- Stupar, A.; Vidović, S.; Vladić, J.; Radusin, T.; Mišan, A. A Sustainable Approach for Enhancing Stability and Bioactivity of Allium ursinum Extract for Food Additive Applications. Separations 2024, 11, 81. [Google Scholar] [CrossRef]
- Filipčev, B.; Kojić, J.; Miljanić, J.; Šimurina, O.; Stupar, A.; Škrobot, D.; Travičić, V.; Pojić, M. Wild Garlic (Allium ursinum) Preparations in the Design of Novel Functional Pasta. Foods 2023, 12, 4376. [Google Scholar] [CrossRef]
- Voća, S.; Žlabur, J.Š.; Uher, S.F.; Peša, M.; Opačić, N.; Radman, S. Neglected Potential of Wild Garlic (Allium ursinum L.)—Specialized Metabolites Content and Antioxidant Capacity of Wild Populations in Relation to Location and Plant Phenophase. Horticulturae 2021, 8, 24. [Google Scholar] [CrossRef]
- Rao, K.S.; Haran, R.H.; Rajpoot, V.S. Value Addition: A Novel Strategy for Quality Enhancement of Medicinal and Aromatic Plants. J. Appl. Res. Med. Aromat. Plants 2022, 31, 100415. [Google Scholar] [CrossRef]
- Ziv, C.; Fallik, E. Postharvest Storage Techniques and Quality Evaluation of Fruits and Vegetables for Reducing Food Loss. Agronomy 2021, 11, 1133. [Google Scholar] [CrossRef]
- Vallino, M.; Faccio, A.; Zeppa, G.; Dolci, P.; Cerutti, E.; Zaquini, L.; Faoro, F.; Balestrini, R. Impact of Drying Temperature on Tissue Anatomy and Cellular Ultrastructure of Different Aromatic Plant Leaves. Plant Biosyst.-Int. J. Deal. All Asp. Plant Biol. 2022, 156, 847–854. [Google Scholar] [CrossRef]
- Zaman, F.Q.; Jaffel, K.; Abdelmageed, A.H.A. The Effects of Post-Harvest Drying Period on the Yield and Chemical Composition of Leaf Essential Oil from Cymbopogon Citratus (DC.) Stapf. J. Essent. Oil Bear. Plants 2022, 25, 571–580. [Google Scholar] [CrossRef]
- Erceg, T.; Šovljanski, O.; Stupar, A.; Ugarković, J.; Aćimović, M.; Pezo, L.; Tomić, A.; Todosijević, M. A Comprehensive Approach to Chitosan-Gelatine Edible Coating with β-Cyclodextrin/Lemongrass Essential Oil Inclusion Complex—Characterization and Food Application. Int. J. Biol. Macromol. 2023, 228, 400–410. [Google Scholar] [CrossRef] [PubMed]
- Jangi, F.; Ebadi, M.T.; Ayyari, M. Qualitative Changes in Hyssop (Hyssopus officinalis L.) as Affected by Cold Plasma, Packaging Method and Storage Duration. J. Appl. Res. Med. Aromat. Plants 2021, 22, 100289. [Google Scholar] [CrossRef]
- Erceg, T.; Vukić, N.; Šovljanski, O.; Stupar, A.; Šergelj, V.; Aćimović, M.; Baloš, S.; Ugarković, J.; Šuput, D.; Popović, S.; et al. Characterization of Films Based on Cellulose Acetate/Poly(Caprolactone Diol) Intended for Active Packaging Prepared by Green Chemistry Principles. ACS Sustain. Chem. Eng. 2022, 10, 9141–9154. [Google Scholar] [CrossRef]
- Jyotshna; Nupur, S.; Akhilesh, K.Y.; Karuna, S.; Madan, M.G.; Raj, K.L. Impact of Postharvest Processes on Major Phenolic Constituents and Antioxidant Potentials of Different Ocimum Species. J. Appl. Res. Med. Aromat. Plants 2018, 10, 9–15. [Google Scholar] [CrossRef]
- Marchioni, I.; Pistelli, L.L.; Ferri, B.; Copetta, A.; Ruffoni, B.; Pistelli, L.L.; Najar, B. Phytonutritional Content and Aroma Profile Changes During Postharvest Storage of Edible Flowers. Front. Plant Sci. 2020, 11, 590968. [Google Scholar] [CrossRef]
- Tomšik, A.; Radojčin, M.; Stamenković, Z.; Kevrešan, Ž.; Mastilović, J.; Pavkov, I.; Vidović, S. Convective Drying Kinetics of and Preservation of Functional Ingredients of Wild Garlic (Allium ursinum L.) in Dependence of Drying Temperature. In Proceedings of the III International Congress on Food Technology, Quality and Safety, Novi Sad, Serbia, 25–27 October 2016. [Google Scholar]
- Pavkov, I.; Radojčin, M.; Stamenković, Z.; Kešelj, K.; Tylewicz, U.; Sipos, P.; Ponjičan, O.; Sedlar, A. Effects of Osmotic Dehydration on the Hot Air Drying of Apricot Halves: Drying Kinetics, Mass Transfer, and Shrinkage. Processes 2021, 9, 202. [Google Scholar] [CrossRef]
- Han, J.; Lawson, L.; Han, G.; Han, P. A Spectrophotometric Method for Quantitative Determination of Allicin and Total Garlic Thiosulfinates. Anal. Biochem. 1995, 225, 157–160. [Google Scholar] [CrossRef] [PubMed]
- Stojanović, Z.S.; Uletilović, D.D.; Kravić, S.; Kevrešan, Ž.S.; Grahovac, N.L.; Lončarević, I.S.; Đurović, A.D.; Marjanović Jeromela, A.M. Comparative Study of the Nutritional and Chemical Composition of New Oil Rape, Safflower and Mustard Seed Varieties Developed and Grown in Serbia. Plants 2023, 12, 2160. [Google Scholar] [CrossRef]
- Melgarejo, P.; Calín-Sánchez, Á.; Carbonell-Barrachina, Á.A.; Martínez-Nicolás, J.J.; Legua, P.; Martínez, R.; Hernández, F. Antioxidant Activity, Volatile Composition and Sensory Profile of Four New Very-Early Apricots (Prunus armeniaca L.). J. Sci. Food Agric. 2014, 94, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Serea, C.; Barna, O.; Manley, M.; Kidd, M. Effect of Storage Temperature on the Ascorbic Acid Content, Total Phenolic Content and Antioxidant Activity in Lettuce (Lactuca sativa L.). J. Anim. Plant Sci. 2014, 24, 1173–1177. [Google Scholar]
- Toledo, M.E.A.; Ueda, Y.; Imahori, Y.; Ayaki, M. L-Ascorbic Acid Metabolism in Spinach (Spinacia oleracea L.) during Postharvest Storage in Light and Dark. Postharvest Biol. Technol. 2003, 28, 47–57. [Google Scholar] [CrossRef]
- Wang, M.; Xu, J.; Ding, Z.; Xie, J. Prolong the Postharvest Shelf Life of Spinach through the Antioxidative Ability of Melatonin. Food Chem. X 2023, 19, 100769. [Google Scholar] [CrossRef] [PubMed]
- Ghidelli, C.; Pérez-Gago, M.B. Recent Advances in Modified Atmosphere Packaging and Edible Coatings to Maintain Quality of Fresh-Cut Fruits and Vegetables. Crit. Rev. Food Sci. Nutr. 2018, 58, 662–679. [Google Scholar] [CrossRef]
- Lachowicz, S.; Kolniak-Ostek, J.; Oszmiański, J.; Wiśniewski, R. Influence of Maturity on the Content of Phenolic Compounds of Alium Ursinum L. J. Food Process. Preserv. 2016, 41, e13089. [Google Scholar] [CrossRef]
- Pinto, T.; Aires, A.; Cosme, F.; Bacelar, E.; Morais, M.C.; Oliveira, I.; Ferreira-Cardoso, J.; Anjos, R.; Vilela, A.; Gonçalves, B. Bioactive (Poly)Phenols, Volatile Compounds from Vegetables, Medicinal and Aromatic Plants. Foods 2021, 10, 106. [Google Scholar] [CrossRef]
- Bernaert, N.; De Clercq, H.; Van Bockstaele, E.; De Loose, M.; Van Droogenbroeck, B. Antioxidant Changes during Postharvest Processing and Storage of Leek (Allium ampeloprasum Var. porrum). Postharvest Biol. Technol. 2013, 86, 8–16. [Google Scholar] [CrossRef]
- Mahmutovic, O.; Tahirovic, I.; Copra, A.; Memic, M.; Ibragic, S.; Karic, L. Correlation of Total Secondary Sulfur Compounds, Total Phenols and Antioxidant Capacity in the Ramsons and Garlic. Br. J. Pharm. Res. 2014, 4, 2662–2669. [Google Scholar] [CrossRef]
- Alexieva, J.; Mihaylova, D.; Popova, A. Antioxidant capacity and thin layer chromatography of ethanol extracts of Allium ursinum L. and Allium bulgaricum L. Sci. Bull. 2014, XVIII, 91–96. [Google Scholar]
- Mihaylova, D.S.; Lante, A.; Tinello, F.; Krastanov, A.I. Study on the Antioxidant and Antimicrobial Activities of Allium ursinum L. Pressurised-Liquid Extract. Nat. Prod. Res. 2014, 28, 2000–2200. [Google Scholar] [CrossRef] [PubMed]
- Nataša, S. Sekundarni Biomolekuli U Vrstama Allium Sect. Codonoprasum Rchb.—Biološke Aktivnosti, Fitohemijski I Hemotaksonomski Aspekti. Ph.D. Thesis, University of Novi Sad, Novi Sad, Serbia, 2009. [Google Scholar]
- Horničkova, J.; Kubec, R.; Cejpek, K.; Velišek, J.; Ovesna, J.; Stavelikova, H. Profiles of S-Alk(En)Ylcysteine Sulfoxides in Various Garlic Genotypes. Czech J. Food Sci. 2010, 28, 298–308. [Google Scholar] [CrossRef]
- Løkke, M.M.; Seefeldt, H.F.; Skov, T.; Edelenbos, M. Color and Textural Quality of Packaged Wild Rocket Measured by Multispectral Imaging. Postharvest Biol. Technol. 2013, 75, 86–95. [Google Scholar] [CrossRef]
- Løkke, M.M.; Edelenbos, M.; Larsen, E.; Feilberg, A. Investigation of Volatiles Emitted from Freshly Cut Onions (Allium cepa L.) by Real Time Proton-Transfer Reaction-Mass Spectrometry (PTR-MS). Sensors 2012, 12, 16060–16076. [Google Scholar] [CrossRef]
- Ben Haj Said, L.; Najjaa, H.; Farhat, A.; Neffati, M.; Bellagha, S.; Ben, L.; Said, H.; Najjaa, H.; Farhat, A. Thin Layer Convective Air Drying of Wild Edible Plant (Allium roseum) Leaves: Experimental Kinetics, Modeling and Quality. J. Food Sci. Technol. 2015, 52, 3739–3749. [Google Scholar] [CrossRef]
- Calín-Sánchez, Á.; Figiel, A.; Wojdyło, A.; Szarycz, M.; Carbonell-Barrachina, Á.A. Drying of Garlic Slices Using Convective Pre-Drying and Vacuum-Microwave Finishing Drying: Kinetics, Energy Consumption, and Quality Studies. Food Bioprocess Technol. 2014, 7, 398–408. [Google Scholar] [CrossRef]
- Zhou, L.; Guo, X.; Bi, J.; Yi, J.; Chen, Q.; Wu, X.; Zhou, M. Drying of Garlic Slices (Allium sativum L.) and its Effect on Thiosulfinates, Total Phenolic Compounds and Antioxidant Activity during Infrared Drying. J. Food Process. Preserv. 2017, 41, e12734. [Google Scholar] [CrossRef]
- Hossain, M.B.; Barry-Ryan, C.; Martin-Diana, A.B.; Brunton, N.P. Effect of Drying Method on the Antioxidant Capacity of Six Lamiaceae Herbs. Food Chem. 2010, 123, 85–91. [Google Scholar] [CrossRef]
- Chan, E.W.C.W.C.; Lim, Y.Y.Y.; Wong, S.K.K.; Lim, K.K.K.; Tan, S.P.P.; Lianto, F.S.S.; Yong, M.Y.Y. Effects of Different Drying Methods on the Antioxidant Properties of Leaves and Tea of Ginger Species. Food Chem. 2009, 113, 166–172. [Google Scholar] [CrossRef]
- Wang, J.; Cao, Y.; Sun, B.; Wang, C.; Mo, Y. Effect of Ultrasound on the Activity of Alliinase from Fresh Garlic. Ultrason. Sonochem. 2011, 18, 534–540. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xu, S.-Y.; Sun, D.-W. Preparation of Garlic Powder with High Allicin Content by Using Combined Microwave–Vacuum and Vacuum Drying as Well as Microencapsulation. J. Food Eng. 2007, 83, 76–83. [Google Scholar] [CrossRef]
- Pezzutti, A.; Crapiste, G.H. Sorptional Equilibrium and Drying Characteristics of Garlic. J. Food Eng. 1997, 31, 113–123. [Google Scholar] [CrossRef]
- Ratti, C.; Araya-Farias, M.; Mendez-Lagunas, L.; Makhlouf, J. Drying of Garlic (Allium sativum) and Its Effect on Allicin Retention. Dry. Technol. 2007, 25, 349–356. [Google Scholar] [CrossRef]
- Méndez Lagunas, L.L.; Castaigne, F. Effect of Temperature Cycling on Allinase Activity in Garlic. Food Chem. 2008, 111, 56–60. [Google Scholar] [CrossRef]
- Mitra, J.; Shrivastava, S.L.; Srinivasa Rao, P. Process Optimisation of Vacuum Drying of Onion Slices. Czech J. Food Sci. 2011, 29, 586–594. [Google Scholar] [CrossRef]

| Color Properties | |||||||||||
| L* | a* | b* | C* | h* | |||||||
| Fresh wild garlic leaves | 37.0 a | −14.6 cd | 17.8 abc | 23.0 abc | 129.5 ef | ||||||
| APPLIED PACKING METHOD | |||||||||||
| T (°C) | TS (days) | TP | VP | TP | VP | TP | VP | TP | VP | TP | VP |
| 4 | 3 | 38.0 abcd | 37.6 abc | −15.8 abc | −14.6 cd | 19.4 abcd | 18.0 abcd | 25.0 abcd | 23.2 abc | 129.3 ef | 129.2 cde |
| 4 | 6 | 39.0 bcd | 37.6 abcd | −14.8 bcd | −14.3 d | 18.8 abcd | 16.9 a | 23.9 abcd | 22.2 c | 128.4 bcdef | 130.3 f |
| 4 | 9 | 38.5 abcd | 37.8 abcd | −15.8 ab | −14.9 bcd | 20.6 de | 18.9 abcd | 26.0 de | 24.0 abcd | 127.7 bcde | 128.3 bcd |
| 11 | 3 | 38.4 abcd | 39.6 d | −15.3 abcd | −15.0 bcd | 19.3 abcd | 20.1 bcde | 24.6 abcd | 25.1 bcde | 128.6 cdef | 127.1 bcd |
| 11 | 6 | 38.4 abcd | 39.4 cd | −14.4 d | −15.4 abcd | 17.6 ab | 18.8 abcd | 22.7 abcd | 24.3 abcd | 129.5 ef | 129.6 ef |
| 11 | 9 | 39.5 cd | 39.0 bcd | −16.4 a | −15.4 abcd | 22.2 e | 20.5 cde | 27.6 ab | 25.6 cde | 126.5 b | 127.0 bc |
| 18 | 3 | 37.9 abcd | 38.2 abcd | −14.4 d | −14.9 bcd | 17.9 abcd | 18.2 abcd | 23.0 abc | 23.6 | 129.1 def | 129.5 ef |
| 18 | 6 | 45.2 e | 39.2 cd | −15.0 bcd | −14.3 d | 24.5 f | 17.5 ab | 29.1 f | 22.6 | 123.2 a | 129.3 ef |
| 18 | 9 | - | 37.9 abcd | - | −15.2 abcd | - | 19.1 abcd | - | 24.4 | - | 128.8 cdef |
| Packing method | * | NS | ** | ** | ** | ||||||
| Temperature | ** | NS | NS | NS | NS | ||||||
| Time of storage | * | ** | ** | ** | ** | ||||||
| PM × T | * | NS | NS | NS | * | ||||||
| PM × TS | ** | NS | * | NS | ** | ||||||
| T × TS | ** | NS | ** | ** | ** | ||||||
| PM × T × TS | ** | NS | ** | ** | ** | ||||||
| BIOACTIVE COMPOUNDS | |||||||||||||
| TCH | TC | TPC | AA | TTC | AAC | ||||||||
| (mg/100 g) | (mg/100 g) | (mg GAE/100 g) | (mg PA/100 g) | (mg AC/100 g) | (mg AAC/100 g) | ||||||||
| Fresh wild garlic leaves | 106.5 abcde | 20.25 cdef | 162.9 egh | 23.8 bc | 4.04 b | 4.04 b | |||||||
| APPLIED PACKING METHOD | |||||||||||||
| T (°C) | TS (days) | TP | VP | TP | VP | TP | VP | TP | VP | TP | VP | TP | VP |
| 4 | 3 | 126.6 hi | 103.9 abcd | 22.96 i | 18.32 abcd | 129.3 b | 139.8 bc | 30.6 bc | 31.8 ijk | 2.75 a | 4.29 b | 59.5 h | 58.8 h |
| 4 | 6 | 137.5 j | 108.2 bcdef | 22.92 i | 18.81 abcde | 180.4 ijk | 161.5 efg | 24.5 ghijk | 30.2 ghij | 2.87 ad | 6.09 e | 65.9 i | 52.1 g |
| 4 | 9 | 119.2 fgh | 109.9 cdef | 21.36 fghi | 20.70 efgh | 173.1 ghi | 168.5 fgh | 23.2 bcd | 28.5 efghi | 2.85 cd | 4.80 b | 56.7 h | 45.9 f |
| 11 | 3 | 103.1 abcd | 100.4 abc | 18.35 abcd | 18.53 abcd | 129.8 b | 143.5 cd | 25.6 bcde | 27.5 defg | 3.99 bcd | 6.56 e | 58.1 gh | 43.8 f |
| 11 | 6 | 132.8 ij | 113.7 defg | 22.37 ghi | 19.40 abcd | 186.1 jk | 176.5 hij | 31.0 hijk | 35.0 l | 5.85 e | 12.19 i | 46.2 f | 14.1 b |
| 11 | 9 | 97.5 ab | 95.3 a | 17.25 a | 18.12 bcdef | 158.2 ef | 169.4 fghi | 26.3 bcde | 33.9 kl | 4.58 b | 14.22 j | 28.3 gh | 21.6 c |
| 18 | 3 | 124.1 ghi | 102.0 abcd | 21.28 fghi | 17.80 ab | 152.7 de | 139.3 cd | 31.4 ijkl | 30.6 ghijk | 7.93 f | 9.51 h | 55.3 c | 30.0 d |
| 18 | 6 | 104.5 abcd | 103.0 abcd | 17.99 bcdef | 17.04 a | 190.0 k | 206.8 l | 33.4 jkl | 27.7 defgh | 8.15 fg | 13.90 j | 20.3 f | 5.0 a |
| 18 | 9 | - | 116.9 efgh | - | 22.46 hi | - | 189.0 k | - | 29.8 fghi | - | 12.24 i | - | 7.7 a |
| Packing method | ** | ** | NS | ** | ** | ** | |||||||
| Temperature | ** | ** | ** | ** | ** | ** | |||||||
| Time of storage | ** | NS | ** | ** | ** | ** | |||||||
| PM × T | ** | ** | ** | NS | ** | ** | |||||||
| PM × TS | ** | ** | NS | ** | ** | ** | |||||||
| T × TS | ** | ** | ** | ** | ** | ** | |||||||
| PM × T × TS | ** | * | ** | ** | ** | ** | |||||||
| Color Properties | Moisture Content (%) | Drying Time (h) | ||||||||
| L | a | b | ||||||||
| Lyophilization | ||||||||||
| 38.03 a | −14.50 ab | 17.23 ab | 5.46 d | 48 i | ||||||
| T (°C) | CD | VD | CD | VD | CD | VD | CD | VD | CD | VD |
| 40 | 39.33 cd | 38.37 ab | −15.52 a | −14.50 bc | 19.47 b | 18.21 ab | 9.03 d | 7.03 b | 9.0 h | 6.0 g |
| 50 | 36.81 a | 38.12 a | −13.21 c | −14.22 bc | 15.05 a | 17.39 a | 8.20 dc | 6.2 a | 4.5 f | 3.75 ef |
| 60 | 37.84 ab | 39.69 c | −14.92 ab | −15.14 b | 18.04 b | 19.86 c | 7.53 bc | 5.76 a | 2.75 d | 2.25 c |
| 70 | 38.53 bc | 41.76 d | −15.20 ab | −17.6 a | 18.81 b | 22.42 d | 7.78 b | 6.19 a | 1.75 b | 1.25 a |
| Drying method | ** | ** | ** | ** | ** | |||||
| Temperature | ** | ** | ** | ** | ** | |||||
| D × T | ** | ** | ** | ** | ** | |||||
| TPC | TTC | AA | ||||
| (mg GAE/100 g) | (mg AC/100 g) | (mg PA/100 g) | ||||
| Lyophilization | ||||||
| 10.90 b | 12.96 c | 7.71 f | ||||
| T (°C) | CD | VD | CD | VD | CD | VD |
| 40 | 10.21 b | 9.81 ab | 12.51 c | 9.01 b | 5.62 e | 3.82 ab |
| 50 | 8.71 ab | 9.57 ab | 8.75 b | 8.45 b | 4.70 c | 3.78 ab |
| 60 | 9.21 ab | 8.91 ab | 6.25 a | 9.04 b | 4.33 bc | 3.67 a |
| 70 | 7.89 a | 9.17 ab | 5.97 a | 8.48 b | 4.98 d | 3.96 ab |
| Drying method | ** | ** | ** | |||
| Temperature | ** | ** | ** | |||
| D × T | * | ** | ** | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stupar, A.; Kevrešan, Ž.; Bajić, A.; Tomić, J.; Radusin, T.; Travičić, V.; Mastilović, J. Enhanced Preservation of Bioactives in Wild Garlic (Allium ursinum L.) through Advanced Primary Processing. Horticulturae 2024, 10, 316. https://doi.org/10.3390/horticulturae10040316
Stupar A, Kevrešan Ž, Bajić A, Tomić J, Radusin T, Travičić V, Mastilović J. Enhanced Preservation of Bioactives in Wild Garlic (Allium ursinum L.) through Advanced Primary Processing. Horticulturae. 2024; 10(4):316. https://doi.org/10.3390/horticulturae10040316
Chicago/Turabian StyleStupar, Alena, Žarko Kevrešan, Aleksandra Bajić, Jelena Tomić, Tanja Radusin, Vanja Travičić, and Jasna Mastilović. 2024. "Enhanced Preservation of Bioactives in Wild Garlic (Allium ursinum L.) through Advanced Primary Processing" Horticulturae 10, no. 4: 316. https://doi.org/10.3390/horticulturae10040316
APA StyleStupar, A., Kevrešan, Ž., Bajić, A., Tomić, J., Radusin, T., Travičić, V., & Mastilović, J. (2024). Enhanced Preservation of Bioactives in Wild Garlic (Allium ursinum L.) through Advanced Primary Processing. Horticulturae, 10(4), 316. https://doi.org/10.3390/horticulturae10040316







