Abstract
Chuan chenpi is obtained by aging the peel of Citrus reticulata cv. ‘Dahongpao’, a traditional Chinese citrus variety. Chenpi has been used in traditional Chinese medicine since ancient times. It is believed that the longer the ripening period, the better the health properties. The composition of the metabolome of Chuan chenpi and how different aging periods affect it are not known. Current analysis was performed using Chuan chenpi aged for one (CR1), five (CR5) and ten (CR10) years. Initially, the total flavonoid and phenolic content were quantified, and then the global metabolomic profiles of CR1, CR5 and CR10 were studied. The total flavonoid and phenolic content increased significantly in CR5 compared to CR1 and then decreased in CR10. The metabolomic analyses led to the identification of 781 compounds belonging to more than 19 classes. Flavonoids and phenolic acids accounted for almost half (~48%) of the Chuan chenpi metabolome. Other major classes included amino acids (~8%), alkaloids (7.17%), organic acids (~7%), sugars (5.5%), nucleotides and derivatives (~5%), free fatty acids (3.33%) and other classes. The metabolite diversity of glycerol esters, terpenoids and stilbenes was constant during the three storage periods, whereas those of lignans, vitamins, coumarins, lipids and free fatty acids showed slight variations. The subclass distribution of phenylpropanoids, quinones, sphingolipids, and organic acids showed a decrease in diversity from CR1 to CR5, with CR10 showing a further decrease or remaining constant. Amino acids and derivatives, phenolic acids and flavonoids showed an increasing trend in the number of metabolites over the storage period. Compared to CR5, CR10 showed a higher number of differentially accumulated metabolites; in particular, flavonoids, phenolic acids and organic acids showed increased accumulation in CR10. In conclusion, the metabolome of Chuan chenpi is rich in flavonoids and phenols. Aging significantly affects the metabolome composition. Both CR5 and CR10 may be useful materials for health studies depending on the objectives of pharmacological use.
1. Introduction
Dried citrus peel, commonly known as chenpi, is used in traditional medicine in Asian countries, especially in China, Japan and Korea. In Guangdong, China, the peel of Citrus reticulata cv. Chachiensis and Tanaka are usually dried and processed into “Guang chenpi”, a commodity whose extracts have been frequently used in thousands of traditional Chinese medicine prescriptions. The peel of other cultivars such as Dahongpao from southwestern Sichuan and Chongqing, China, are processed into “Sichuan dried tangerine peel” known as Chuan chenpi. Notably, it has been mentioned as top-grade medicine in Shennong’s Classic of Materia Medica as well as several other classical manuscripts including Materia Medical Usage, Materia Medical of Decoction and Li Shizhen’s Compendium of Materia Medica [1]. Modern research has explored that chenpi’s ability to prevent obesity and type 2 diabetes [2] can alter colonic microbiota in high-fat-induced obese mice [3], reduce lipogenesis in differentiating 3T3-L1 adipocytes [4] and prevent chronic obstructive pulmonary disease [5] and acetaminophen-induced hepatotoxicity [6]. In general, chenpi has been associated with several health-beneficial properties, such as antioxidant, anti-obesity, anti-cancer, anti-viral, anti-inflammatory, etc. [7,8,9].
Recent reports have revealed the presence of more than 140 chemical components in chenpi. The main components of chenpi are flavonoids (flavone O-glycosides, flavonoid aglycones, polymethoxyflavones), volatile oils, alkaloids, organic acids, polysaccharides and other compounds, e.g., β-sitosterol [1]. Among these, flavonoids are the most important in distinguishing aged chenpi [10]. Moreover, flavonoids are also reported to be the major bioactive constituents in chenpi [11]. Among the reported compounds, phenolic acids, flavonol glycosides, fatty acids, and alkyl glycosides can be used as marker compounds to distinguish the storage time of chenpi [12]. Furthermore, chenpi is known for its richness in essential nutrients vital for humans, including inositol, vitamin B, vitamin C, thymol, β-glutinosterol and pectin. Additionally, tangerine peels contain trace elements like selenium and copper [13].
Historic evidence suggests that the longer the peel is aged, the higher are the health benefits [14]. Generally, the aging time ranges from one year to more than a decade; up to 30 years of aging has been reported [15]. This belief also affects the market value of the chenpi. For example, a well-preserved chenpi for three decades can cost up to CNY 10,000 (>$1500) [16]. Several studies over the past decade using modern tools have concluded that the health benefits increased with the aging time [17,18,19,20]. However, this is not universal for all the health beneficial compounds present in chenpi. For example, polymethoxyflavones and hesperidin content decreased with aging time [12]. In terms of polysaccharides, the extraction rate can reach its maximum for the chenpi aged for five years and then decrease. These changes were also related to the degradation and utilisation of polysaccharides by microorganisms [21]. These results suggest that there are certain factors that may influence the individual metabolite content during the aging period. Studies have explored that there is a strong association between the dominant microbial species, i.e., bacteria and fungi, with the active ingredients in chenpi [22]. Additionally, the temperature and humidity during storage time are critical to prevent mildew [1]. Heat treatment may also affect the total phenolic content and resultantly the bioactivity of chenpi [19]. Moreover, studies conducted on C. reticulata peels from different varieties/cultivars and growing areas have shown marked differences in the composition of the extracts [10,22,23]. In this regard, it is important to determine the metabolome composition of the cultivars and varieties (Citrus reticulata cv. ‘Dahongpao’) whose peels are preserved and used as chenpi [24]. However, the composition of its metabolome is not known. Moreover, how different aging periods can influence the Chuan chenpi metabolomic composition is important data that can help pharmaceutical industry and traditional Chinese medicine practitioners to choose the material with required metabolites in relation to the aging time. Therefore, authentic Dahongpao red tangerine was used as the raw material, and traditional Chuan chenpi preparation technology was employed to detect metabolic substances in Chuan chenpi after 1 year, 5 years and 10 years. Our results provide data for the pharmacological research and application development of Chuan chenpi. This is also of great significance for the processing and application of red tangerine and improving the added value of agricultural products.
2. Material and Methods
2.1. Preparation of Chuan Chenpi
The Chuan chenpi samples from Chongqing Three Gorges Vocational College were obtained from the raw material Citrus reticulata cv. ‘Dahongpao’, grown in the ancient red orange characteristic ecological agricultural park in Wanzhou District, Chongqing, in the eastern part of Sichuan Basin (30°24′ N; 107°55′ E). The site has a typical subtropical humid monsoon climate with four distinct seasons, a very long summer and an average annual sunshine duration of 1484.4 h. The annual average temperature and precipitation are 18.2 °C and 1243 mm, respectively.
To ensure quality, fresh red oranges of uniform size, free from damage or decay, were carefully selected. Preparation involves a thorough process of cleaning and slicing the peel into three equal parts using a small knife, joined at the base. The sliced peels are then laid flat on wire mesh and placed in a pre-set oven at 50℃ for complete drying. After drying, the Chuan chenpi was stored under standard conditions (temperature: 10–35 °C, humidity: 30–65%) for periods of 1 year (CR1), 5 years (CR5) and 10 years (CR10). The entire procedure was performed in triplicate groups for comprehensive testing and analysis.
2.2. Physiochemical Analysis
Total flavonoids were quantified by the NaNO2-AlCl3-NaOH method using a biochemical kit (NMKD0120, Norminkoda Biotechnology Co., Ltd., Wuhan, China) [25]. Similarly, total phenolics were determined by the Folin–Ciocalteu method according to the protocol provided in the biochemical kit (NMKD0119, Norminkoda Biotechnology Co., Ltd., Wuhan, China) [26].
2.3. Metabolomics Analysis
2.3.1. Sample Preparation and Extraction
The first step involved the use of the Scientz-100F vacuum freeze dryer (Scientz-100F; Ningbo Scientz Biotechnology, Ningbo, China) for freeze-drying biological materials. After freeze-drying, the samples were subjected to comminution in a mixer mill with a zirconia bead (MM 400; Retsch GmbH, Haan, Germany ) at 30 Hz for 90 s. A solution consisting of 1.2 mL of 70% methanol was then mixed with lyophilised powder (100 mg). The resulting mixture was vortexed six times for 30 s every 30 min. The final step in this phase was to store the sample overnight at 4 °C. Before subjecting the samples to ultra-performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS) analysis, the extracts were filtered using a SCAA-104 filter with a pore size of 0.22 μm (ANPEL, Shanghai, China). This filtration step was performed after centrifugation at 12,000 rpm for 10 min.
2.3.2. Ultra-Performance Liquid Chromatography (UPLC) Conditions
UPLC analysis was performed using the Shim-pack UFLC-SHIMADZU CBM-A system. The analysis was performed on a Waters ACQUITY UPLC HSS T3 C18 column with dimensions of 1.8 µm, 2.1 mm × 100 mm. The column was maintained at a constant temperature of 40 °C throughout the analysis. The flow rate (0.4 mL/min) was adjusted, and a 2 μL injection volume was used. The solvent system used was acetonitrile (0.1% formic acid)/water. The gradient programme started with a ratio of 95:5 v/v at the beginning (0 min), switched to 5:95 v/v at 10.0 min, returned to the initial ratio of 95:5 v/v at 11.1 min and maintained this composition until 15.0 min.
2.3.3. Electrospray Ionisation Quadrupole Linear Ion Trap Tandem Mass Spectrometry (ESI-Q TRAP-MS/MS)
The ESI-Q TRAP-MS/MS analysis was carried out using the QTRAP UPLC/MS/MS system (AB4500). The system incorporated an ESI Turbo Ion Spray interface and operated in both positive and negative ion modes. AB Sciex software (Analyst 1.6.3) was used for system management. The operating parameters for the gas settings included in the ESI source were configured at 50 psi for Ion Source Gas-I, 60 psi for Ion Source Gas-II and 25.0 psi for the curtain gas. In addition, the source temperature was maintained at 550 °C. An ion spray voltage (IS) of 5500 V was set in the positive ion mode and −4500 V in the negative ion mode. In addition, a high-level setting was used in collision activated dissociation (CAD). MRM studies included QQQ scans with nitrogen (a medium collision gas), and unique MRM transitions were optimised based on the metabolites eluted at each interval. As part of the tuning/mass calibration procedure, polypropylene glycol solution (10 μmol/L) was used in the QQQ mode, and 100 μmol/L polypropylene glycol solution was used in the LIT mode.
Unsupervised principal component analysis (PCA) was performed using the ‘prcomp’ function of the R programming environment (www.r-project.org; accessed on 10 December 23). R’s ‘cor’ function was used to calculate Pearson correlation coefficients (PCC) between samples, and the results were plotted as heat maps. Hierarchical clustering analysis was performed in R using the ComlexHeatmap package. Differential metabolites were found based on absolute Log2 fold change (|Log2 FC| ≥ 1.0) and variable importance in projection (VIP) scores (VIP > 1). The KEGG Compound database (http://www.kegg.jp/kegg/compound/; accessed on 10 December 23) and the Pathway database (http://www.kegg.jp/kegg/pathway.html; accessed on 10 December 23) were used for metabolite structure annotation and mapping, respectively. Student’s t-test was used to compare the content of individual and combination metabolites within a class or pathway [27].
3. Results
3.1. Effect of Storage Time on Peel Morphology and Phenolic Compounds of Citrus reticulata cv. Dahongpao Orange Peels
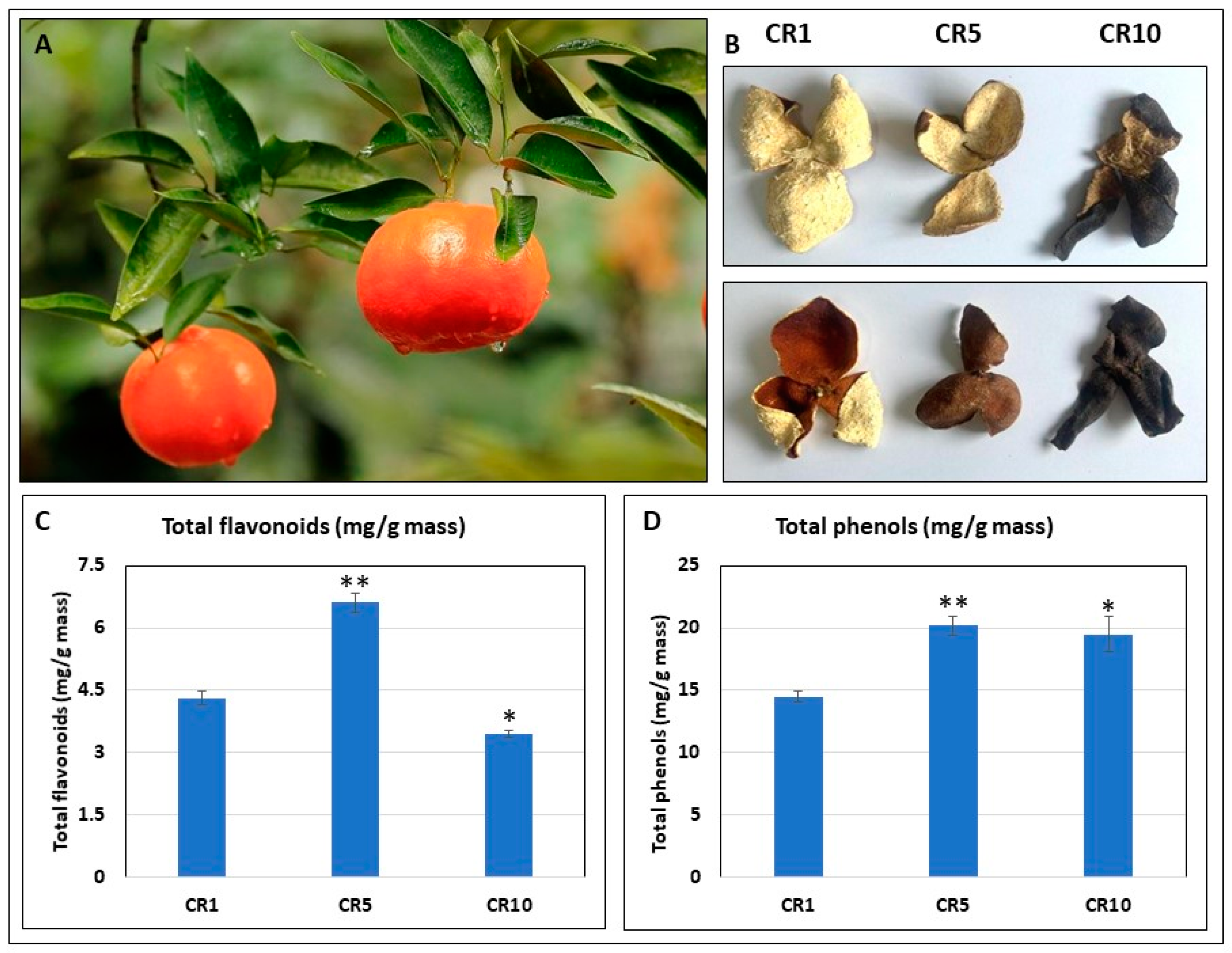
The current study investigated the variations in morphological and phenolic compounds within Sichuan chenpi orange peels, focussing on the raw material derived from Dahongpao red oranges. The study examines peel samples stored for different periods of time: 1 year (CR1), 5 years (CR5) and 10 years (CR10). A significant colour change was observed on the inside and outside of CR1, CR5 and CR10 (Figure 1A,B). The red colour of CR1 becomes dark red (CR5) and then blackish (CR10). Total phenolics increased in both CR5 and CR10 compared to CR1. Interestingly, the total flavonoid content was higher in CR5 compared to CR1 and CR10 (Figure 1C,D). This suggests that five years of storage may be optimal for achieving higher flavonoid contents in Chuan chenpi.
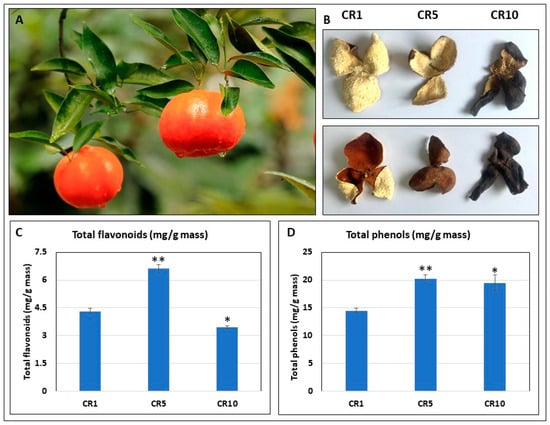
Figure 1.
Morphological and phenolic variations in Sichuan chenpi orange peels. (A) Chuan chenpi raw material Citrus reticulata cv. ‘Dahongpao’ from Wanzhou, Chongqing. (B) Chuan chenpi stored for 1, 5 and 10 years (CR1, CR5, CR10). Panels i and ii represent the inner and outer sides of CR1, CR5 and CR10 samples, respectively. (C) Total flavonoids and (D) total phenolics in CR1, CR5 and CR10. CR1, CR5 and CR10 represent sample storage time for 1 year, 5 years and 10 years, respectively. The errors bars on bar plots represent standard deviation (n = 3). Asterisks (*, **) represent t-test statistics at 95% and 99% confidence levels.
3.2. Metabolomics Statistics of Chuan Chenpi Metabolites during Different Storage Periods
Total ion current (TIC) plots of mass spectra obtained in positive and negative ion detection modes were examined for the QC samples. The TIC profiles of the metabolites were highly overlapping, with consistent retention times (RT) and peak intensities between the QC samples. The high overlap of the spectra indicates that the assay has good signal stability and provides reliable data results.
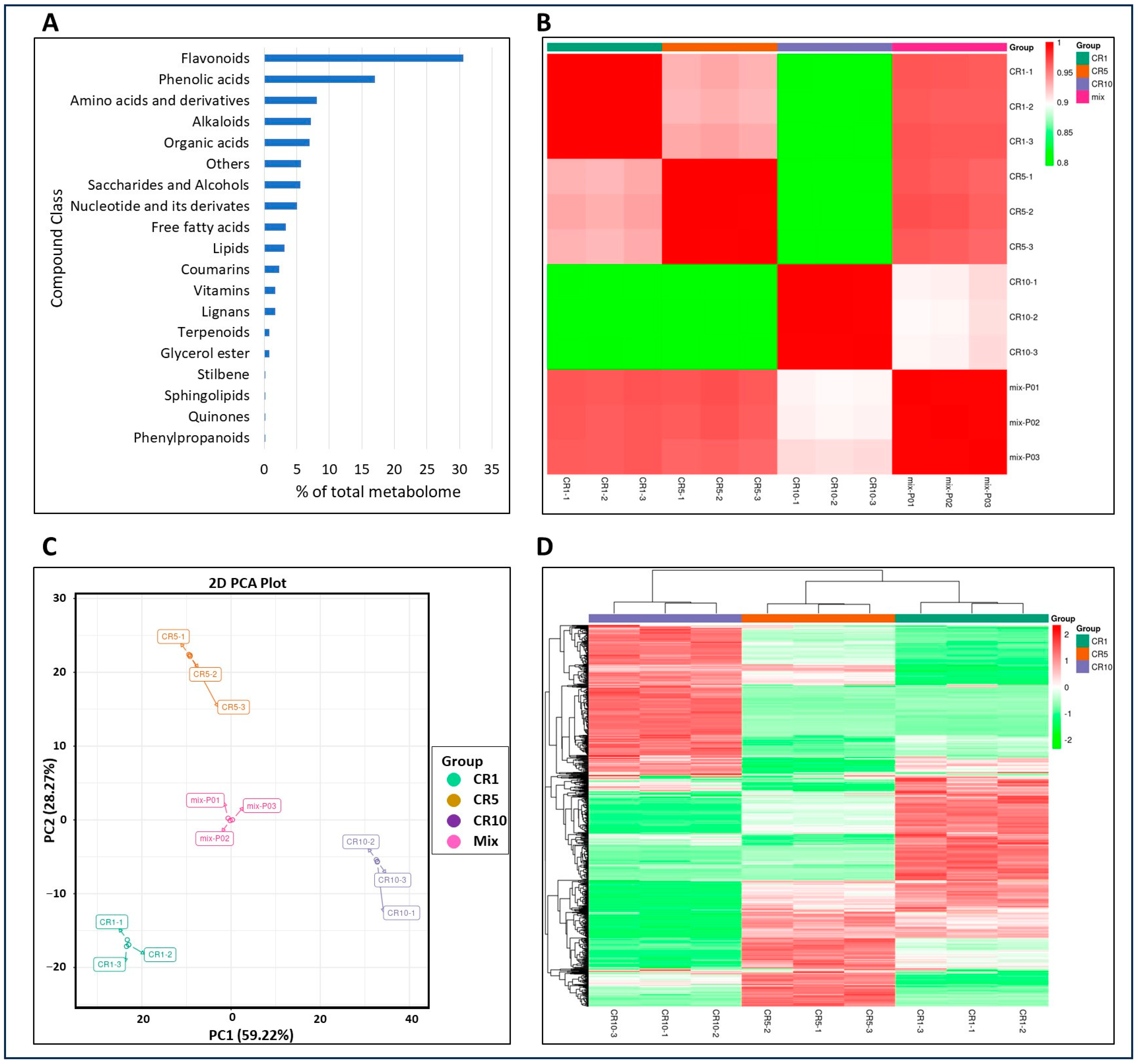
In the current study, a total of 781 metabolites were identified in Chuan chenpi samples CR1, CR5 and CR10. The majority (~48%) of these metabolites were flavonoids and phenolic acids (30.6% flavonoids, 17.03% phenolic acids). However, other classes of metabolites were also present: 8.06% amino acids and derivatives, 7.17% alkaloids, 6.91% organic acids, 5.63% others, 5.5% saccharides and alcohols, 4.99% nucleotides and their derivatives, 3.33% free fatty acids, 3.07% lipids, 2.3% coumarins, 1.67% vitamins and 1.67% lignans. Less than 1% of the metabolites belong to phenylpropanoids, quinones, sphingolipids, stilbenes, glycerol esters and terpenoids (Figure 2A). There was a very high correlation between replicates (more than 79%; Figure 2B; Supplementary Table S1), and PCA showed a clear grouping of metabolites (Figure 2C). This suggests that metabolites in CR1, CR5, and CR10 show significant variation. Clustering analysis further confirmed the distribution of metabolites in distinct groups within and between CR1, CR5 and CR10 (Figure 2D).
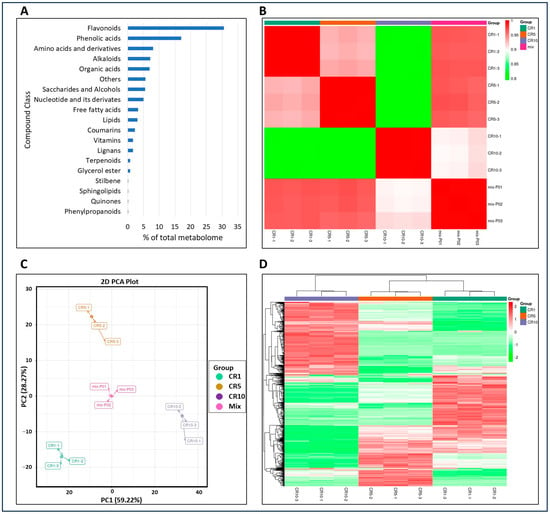
Figure 2.
Metabolomic analysis of Chuan chenpi. (A) Percentage of metabolite classes in the total number of identified metabolites, (B) Pearson’s correlation coefficient, (C) principal component analysis, and (D) clustering of metabolite intensity mass spectrometry data of each group of samples. CR1, CR5 and CR10 represent sample storage time for 1 year, 5 years and 10 years, respectively.
3.3. Differential Metabolic Profiles of Chuan Chenpi Dried Peels Subjected to 1, 5 and 10 Years of Storage
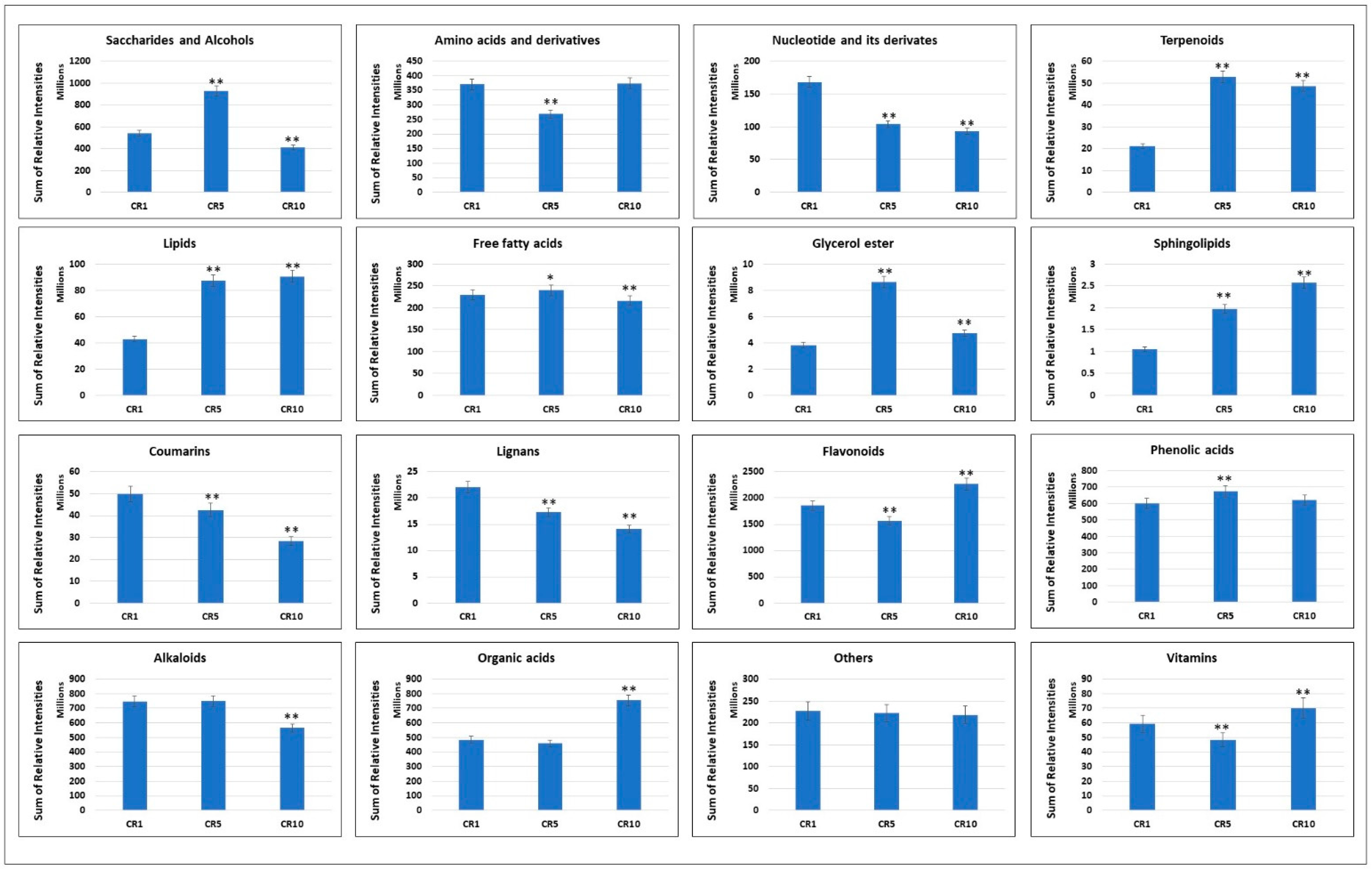
Metabolomic analysis of Sichuan chenpi peel samples stored for 1 year (CR1), 5 years (CR5) and 10 years (CR10) revealed significant variations in the total concentrations of different metabolite classes (Figure 3). For alkaloids, CR1 and CR5 showed comparable concentrations, but CR10 showed a significant decrease. Amino acids and derivatives showed a decreasing trend from CR1 to CR5, followed by a slight increase in CR10. Coumarins, flavonoids and phenolic acids showed a similar pattern, with CR10 showing a decrease in concentration compared to CR1 and CR5. Free fatty acids, glycerol esters, lignans, lipids, nucleotides and their derivatives, organic acids, saccharides and alcohols, sphingolipids, stilbenes, terpenoids and vitamins showed different trends in the three samples. In particular, some classes, such as saccharides and alcohols, organic acids and flavonoids, showed an increasing trend in concentration over the storage period, while others, such as nucleotide and its derivatives and stilbene, showed a decreasing trend. These results suggest that storage time affects the metabolite composition of Sichuan chenpi peels, with potential implications for their medicinal or culinary use.
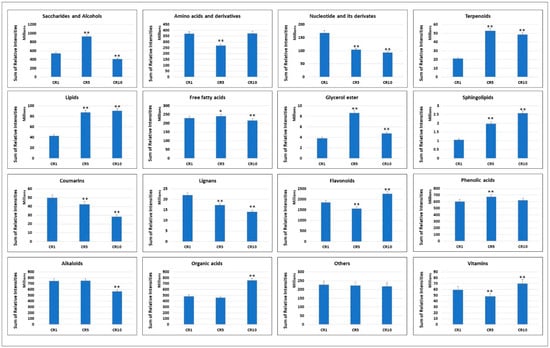
Figure 3.
Variations in the accumulation profiles of major metabolite groups after one, five and ten years of Chuan chenpi storage. The bars represent the sum of the relative intensities of compounds included in each group in CR1, CR5 and CR10. The errors bars on bar plots represent standard deviation. Asterisks (*, **) represent t-test statistics at 95% and 99% confidence levels.
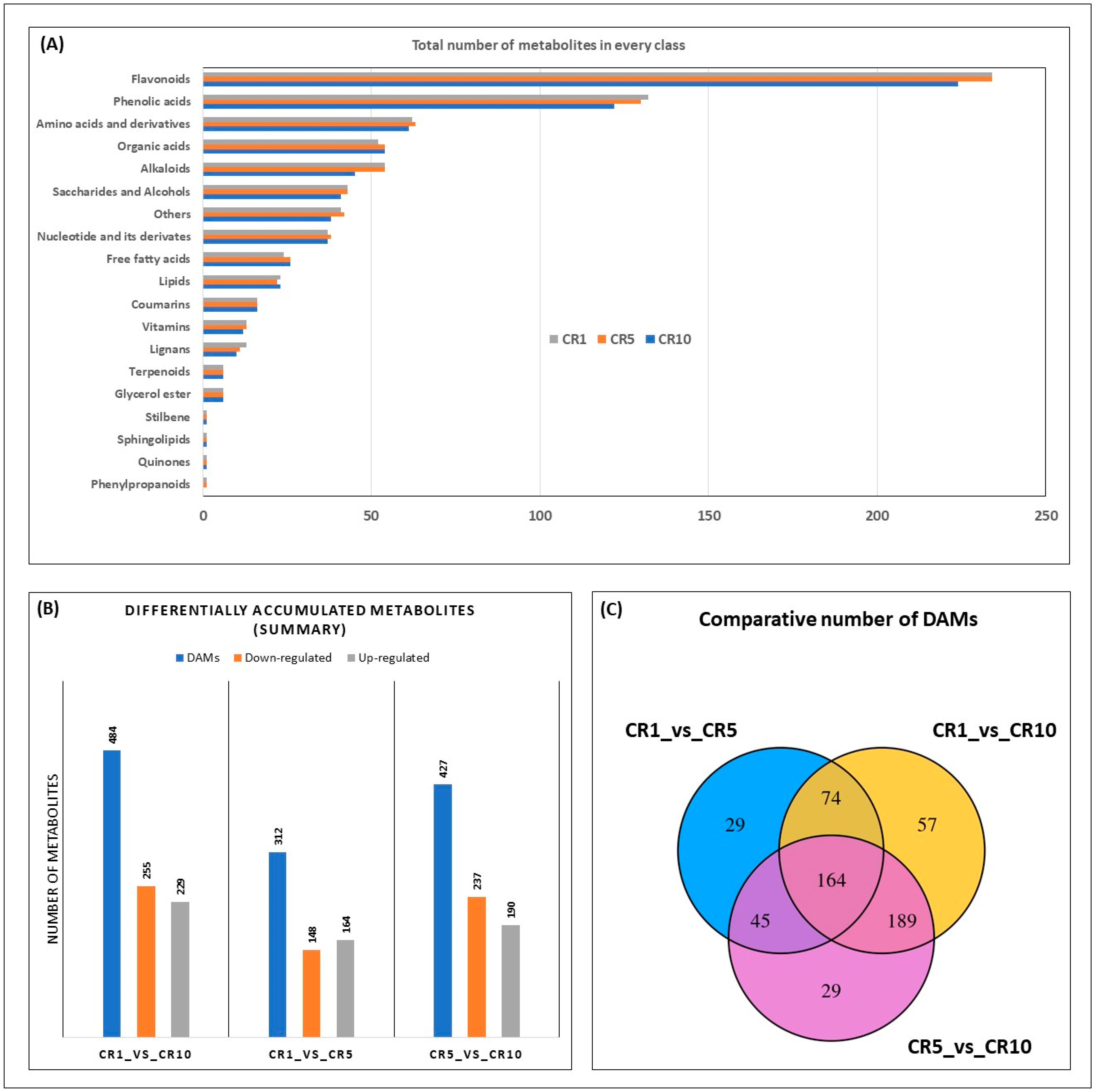
Analysis of the number of metabolites within each metabolite class provides additional insight into compositional changes over time (Figure 4A). Notably, the metabolite diversity within several classes, including glycerol esters, terpenoids and stilbenes, remained constant throughout CR1, CR5 and CR10, suggesting stability of these components during storage. Conversely, the number of metabolites in lignans, vitamins, coumarins, lipids and free fatty acids showed slight variations, indicating possible changes in specific chemical constituents within these classes. The subclass distribution in phenylpropanoids, quinones, sphingolipids and organic acids showed a decrease in diversity from CR1 to CR5, with CR10 showing a further decrease or remaining constant. Interestingly, amino acids and derivatives, phenolic acids and flavonoids showed an increasing trend in the number of metabolites over the storage period. These results highlight the dynamic nature of the Sichuan chenpi metabolome during prolonged storage (Figure 4A).
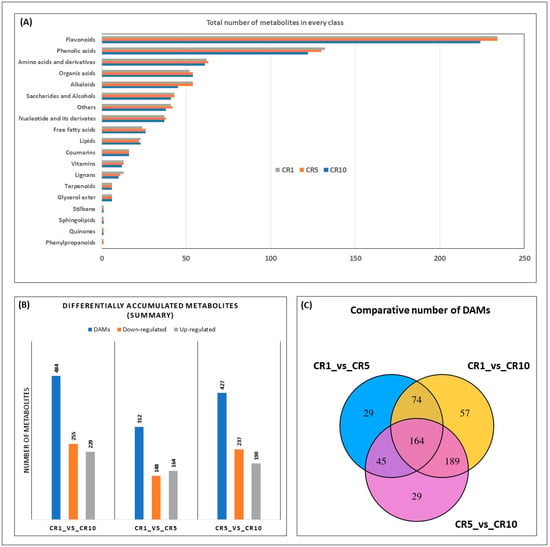
Figure 4.
Differentially accumulated metabolites in CR1, CR5 and CR10. (A) Total number of metabolites in each metabolite subgroup. (B) Summary of differentially up- and downregulated DAMs. (C) Venn diagram of common and unique DAMs. CR1, CR5 and CR10 represent sample storage time for 1 year, 5 years and 10 years, respectively.
The following criteria were used for the screening of differentially accumulated metabolites (DAMS): (1) selecting metabolites with a fold change ≥2 and ≤0.5; (2) selecting metabolites with a VIP value of ≥1. As a result, a total of 484, 312 and 427 DAMs were identified among CR1_vs._CR10, CR1_vs._CR5 and CR5_vs._CR10, respectively (Figure 4B). Although there were 164 DAMs common to all stages, 57, 29 and 29 DAMs were unique to CR1_vs._CR10, CR1_vs._CR5 and CR5_vs._CR10, respectively (Figure 4C). These distinct DAMs are potential candidates for selective markers for the respective stage of Chuan chenpi.
3.3.1. Metabolome Changes between CR1 and CR10
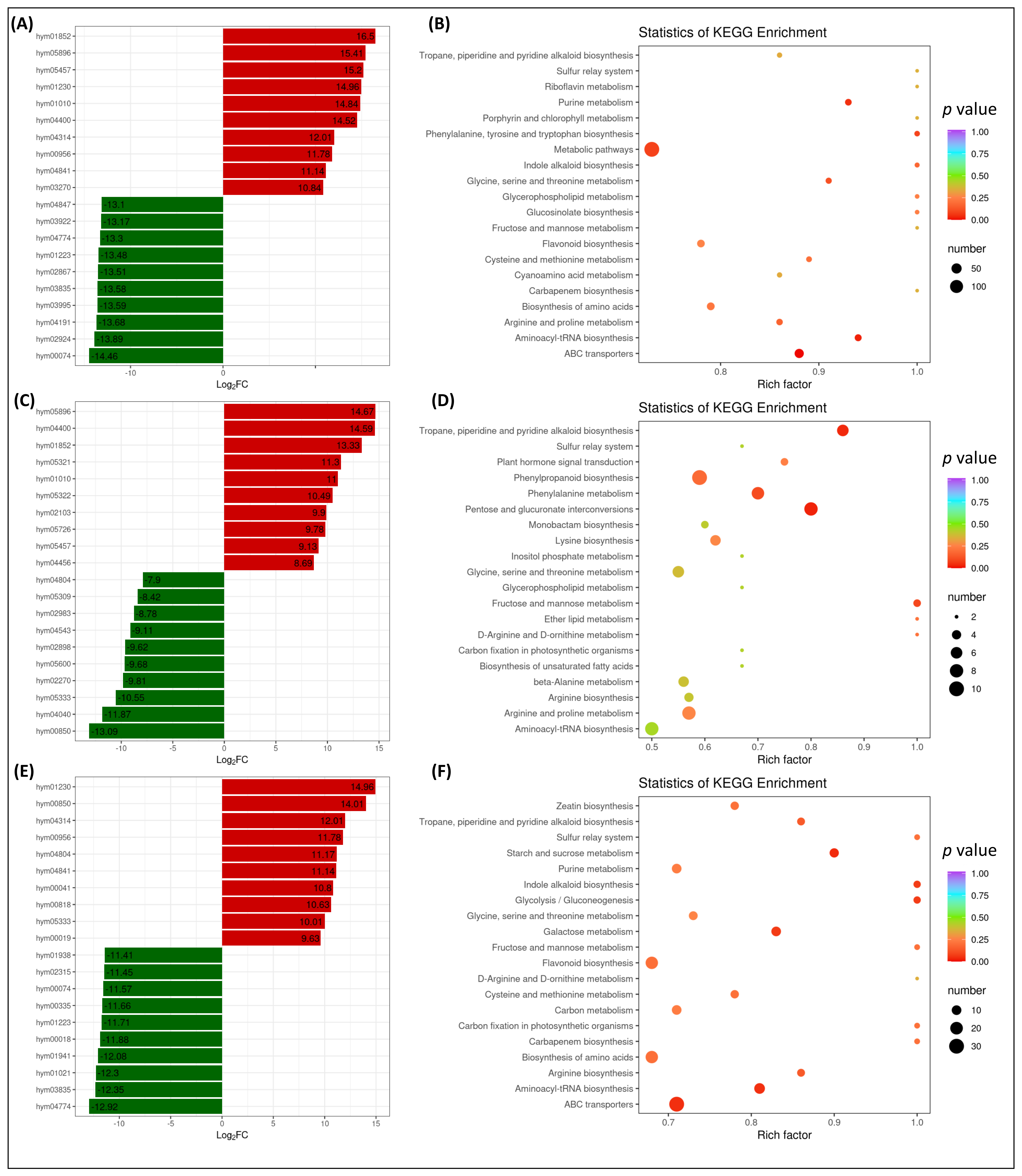
Comparison of DAMs in CR1 and CR10 revealed that 229 DAMs were up-accumulated, and 255 DAMs were down-accumulated in CR10 compared to CR1 (Figure 4B). The comparative analysis revealed several exclusive DAMs in CR5 or CR10 (Supplementary Table S1). After 10 years of storage, citrus peels show a remarkable up-accumulation of several metabolites. These metabolites include lysine butyrate, α-linolenic acid, luteolin 7-O-glucoside (cynaroside), N6-succinyladenosine, γ-linolenic acid, pinocembrin (dihydrochrysin), pyrogallic acid, xanthotoxol and others (Figure 5A, Supplementary Table S1). This accumulation suggests dynamic changes in the metabolic profile of citrus peels during prolonged storage. In particular, the presence of amino acids (lysine), fatty acids (α-linolenic acid, γ-linolenic acid), flavonoids (luteolin and its derivatives) and various organic acids indicates changes in pathways related to amino acid metabolism, lipid metabolism and secondary metabolite biosynthesis.
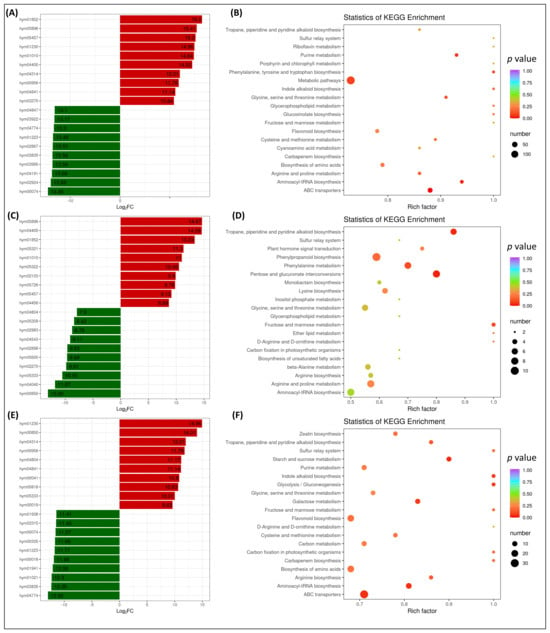
Figure 5.
Time-dependent metabolomic changes in Chuan chenpi. (A) Top and bottom 10 most differentially accumulated metabolites in Chuan chenpi after ten years (CR1 vs. CR10) and (B) scatter plot showing the pathways in which the DAMs were significantly enriched after ten years of storage (CR1 vs. CR10). (C) Top and bottom 10 most differentially accumulated metabolites in Chuan chenpi after five years (CR1 vs. CR5), and (D) scatter plot showing the pathways in which the DAMs were significantly enriched after five years of storage (CR1 vs. CR5). (E) Top and bottom 10 most differentially accumulated metabolites in Chuan chenpi between five and ten years (CR5 vs. CR10), and (F) scatter plot showing the pathways in which the DAMs were significantly enriched between five and ten years (CR5 vs. CR10).
In contrast, the down-accumulated DAMs encompass alkaloids (benzoylputrescine, N-methyltryptamine, agmatine, N-feruloylputrescine, tryptamine, putrescine, 3-indoleacrylic acid and others), indicating potential shifts in nitrogen metabolism and secondary metabolite biosynthesis. Additionally, alterations are observed in amino acids and derivatives, such as acetyltryptophan, H-homoarg-OH, L-homocystine, L-asparagine, oxidised glutathione, L-(+)-arginine and L-proline, implying potential effects on protein metabolism. Flavonoids, such as chrysoeriol-o-malonylhexoside, eriodictyol-7-O-glucoside, selgin-o-malonylhexoside, kaempferol-3-O-galactoside (trifolin) and hesperidin also show down-accumulation, suggesting changes in secondary metabolism pathways.
There were 116 DAMs representing flavonoids (63 up-accumulated). The up-accumulation of different flavonoids reveals a rich reservoir of bioactive compounds with potential health benefits. Luteolin-7-o-glucoside (cynaroside), pinocembrin (dihydrochrysin) and delphinidin-3-o-arabinoside are known for their antioxidant properties [28], offering protection against oxidative stress and inflammation. Kaempferol-3-O-galactoside (Trifolin) and hesperetin have been associated with cardiovascular health and may help to prevent heart-related problems [29]. Isosakuranetin (4′-methylnaringenin) and homoeriodictyol may have anti-inflammatory properties [30], while galangin-7-glucoside, syringetin and norwogonin are thought to have antimicrobial effects [31]. The presence of various flavonoids, including apigenin-7-o-β-D-glucoside, 6-hydroxyluteolin and 5,7,4′-trihydroxyisoflavone-7-o-galactoside, suggests a diverse range of compounds with potential anti-cancer and neuroprotective properties [32]. In addition, the flavonoids naringenin, eriodictyol and naringenin chalcone are associated with anti-inflammatory and immunomodulatory effects [33]. These flavonoids, together with others such as genistein, dihydroquercetin (taxifolin) and quercetin 3-O-glucoside (isotrifoliin), contribute to the overall health-promoting properties of citrus peels and could potentially be exploited for therapeutic applications in the areas of cardiovascular health, anti-inflammatory interventions and antioxidant support.
Among the organic acids that have increased in concentration (21 out of 28 DAMs), phosphoenolpyruvic acid, 2-hydroxy-(4-hydroxyphenyl)propanoic acid, α-hydroxyisobutyric acid and quinic acid are notable contributors. These acids play a crucial role in metabolic processes and may have implications for energy metabolism and antioxidant defences. In addition, the increase in the levels of 2,3-dihydroxybenzoic acid, D-galacturonic acid (Gal A) and phenylpyruvic acid suggests an increase in the antioxidant potential and structural integrity of citrus peels. On the contrary, certain organic acids, such as transcitridic acid, dodecanedioic acid and sodium valproate, showed a decrease in accumulation.
KEGG enrichment analysis identified the most enriched pathways as the sulphur relay system, riboflavin metabolism, purine metabolism, phenylalanine, tyrosine and tryptophan biosynthesis, indole alkaloid biosynthesis, glycerophospholipid metabolism and flavonoid biosynthesis (Figure 5B).
3.3.2. Metabolome Changes between CR1 and CR5
Comparison of DAMs in CR1 and CR5 revealed that 164 DAMs were up-accumulated, and 148 DAMs were down-accumulated in CR5 compared to CR1 (Supplementary Table S2, Figure 4B). The comparative analysis revealed several exclusive DAMs in CR1 or CR5 (Supplementary Table S1). After five years of storage, dry citrus peels undergo significant changes in the accumulation of metabolites, revealing a dynamic response to prolonged storage conditions. In particular, α-linolenic acid and γ-linolenic acid, both free fatty acids, show a remarkable up-regulation with log2FC values of 14.67 and 14.59, respectively, suggesting a potential role in lipid metabolism and oxidative stress response. Lysine butyrate, an amino acid derivative, shows a significant upregulation with a log2FC of 13.33, suggesting possible changes in amino acid metabolism. On the other hand, several metabolites show a downregulation after five years of storage. These include olivil diglucoside, apigenin-6-C-(2-β-D-glucosyl)-α-L-arabinoside, ixerisoside-D and others, with significant negative log2FC values, reflecting potential degradation or transformation processes. The downregulation of 2-methoxybenzoic acid, lysoPE 18:1 (2n isomer) and 4-o-caffeoylquinic acid highlights the changes in phenolic acids and lipid components (Supplementary Table S2, Figure 5C).
Of the 62 differentially accumulated phenolic acids, 33 were up-accumulated. 1-Feruloyl-sn-glycerol (log2fold 9.90) was one of the top 10 most accumulated metabolites. After 5 years of storage, citrus peels also showed a significant up-accumulation of 3,5-dicaffeoylquinic acid, caffeic acid and protocatechuic acid. These phenolic compounds contribute to the enhanced antioxidant and potentially health-promoting properties of dried citrus peels (Supplementary Table S2).
There were 71 DAMs representing flavonoids. Among the up-accumulated DAMs (23), phloretin, luteolin-7-o-glucoside (cynaroside) and delphinidin-3-o-arabinoside were the most up-accumulated (log2FC 9.78, 9.13 and 8.7, respectively). However, all other up-accumulated DAMs were in the range of 1.01 to 1.78 log2FC, including hesperetin. In contrast, 48 flavonoids were down-accumulated. The trend of down-accumulation of flavonoids in CR5 may indicate degradation or decomposition processes during prolonged storage. Several factors such as exposure to light, air and temperature changes can contribute to the degradation of bioactive compounds, including flavonoids. These compounds are known to be sensitive to environmental conditions. The down-accumulation observed for certain flavonoids may indicate a loss of these compounds over time, potentially affecting the health benefits associated with citrus peel.
Interestingly, of the 17 differentially accumulated organic acids, 13 were up-accumulated. The increased levels of organic acids such as 2-hydroxy-(4-hydroxyphenyl)propanoic acid, phosphoenolpyruvic acid and D-galacturonic acid, among others, may contribute to the antioxidant and antimicrobial properties of orange peels. These organic acids are essential for maintaining the acidic environment in the peel, which can have preservative effects and inhibit the growth of harmful micro-organisms. In addition, organic acids are involved in energy metabolism and may play a role in supporting metabolic processes in the human body. The accumulation of organic acids, including alpha-hydroxyisobutyric acid and 2-hydroxybutanoic acid, can improve the overall acidity and nutritional profile of dried peels. In addition, organic acids such as sodium valproate, which is commonly used as an anticonvulsant, may have potential therapeutic effects. The increased presence of these organic acids may contribute to the health-promoting aspects of dried orange peel, making it a valuable source of bioactive compounds.
Androsin (hym05321, Figure 5C) is among the most up-accumulated DAMs in CR5 as compared to CR1. It is an active compound isolated from Picrorhiza kurroa Royle ex Benth, with anti-asthmatic effects [34].
A total of 19 DAMs were detected as saccharides and alcohols in CR5 (Supplementary Table S2). Among these metabolites, only one (D(+)-melezitose-o-rhamnoside) was down-accumulated, whereas 18 (including D-mannitol, xylitol, D-sorbitol, D-galacitol, L-arabitol) were up-accumulated (Supplementary Table S2).
KEGG enrichment analysis identified the most enriched pathways as tropare, piperdine and pyridine alkaloid biosynthesis, pentose and glucuronate interconversions, fructose and mannose metabolism, ether lipid metabolism, D-arginine and D-ornithine metabolism, phenylalanine metabolism and phenylpropanoid biosynthesis pathways. Interestingly, flavonoid biosynthesis was not detected among the most enriched pathways (Figure 5D).
It can be concluded that after five years of storage, the metabolism of major metabolites, such as amino acids and polysaccharides, is more pronounced compared to the biosynthesis of secondary metabolites, such as flavonoids.
3.3.3. Metabolome Changes between CR5 and CR10
Comparison of DAMs between CR5 and CR10 identified 190 up-accumulated and 237 down-accumulated metabolites (Figure 4B, Supplementary Table S3). The comparative analysis revealed several exclusive DAMs in CR5 or CR10 (Supplementary Table S3). Notably, a significant up-regulation was observed in CR10 for several compounds belonging to different metabolite classes (Figure 5E). Luteolin-7-O-glucoside, a flavonoid, showed a remarkable 14.96 increase and LysoPE 18:1, a lipid, showed a 14.01 upregulation. Other upregulated metabolites included pinocembrin (flavonoid), pyrogallic acid (phenolic acid), 6-hydroxy-4-methylcoumarin and 4-hydroxy-7-methoxycoumarin-β-rhamnoside (coumarins). These results suggest that prolonged storage of citrus peels resulted in a significant increase in the concentration of certain metabolites, which may affect the overall chemical composition and bioactive properties of the dried peels. Conversely, several metabolites showed significant down-regulation in CR10 compared to CR5, including dihydrocaffeoylspermine and tricin 4′-O-glucoside (flavonoids), nicotinic acid hexoside (vitamins) and various alkaloids, suggesting a complex and dynamic response to prolonged storage.
Of the 88 differentially accumulated phenolic acids, 31 were up-accumulated. Pyrogallic acid and 4-O-caffeoylquinic acid (criptochlorogenic acid) were among the top 10 most up-accumulated metabolites. However, the majority of phenolic compounds showed down-accumulation in CR10 compared to CR5 (Supplementary Table S3).
Out of 110 flavonoid DAMs, 71 were up-accumulated. The flavonoids that showed a notable increase in concentration between 5 and 10 years of storage in dried citrus peel included a wide range of compounds. Notable flavonoids in this accumulation include luteolin 7-O-glucoside (cynaroside), pinocembrin (dihydrochrysin), chrysin 5-O-glucoside (toringin), kaempferol 3-O-galactoside (Trifolin), hesperetin, homoeriodictyol, isosakuranetin (4′-methylnaringenin), luteolin, apigenin 7-O-glucoside (cosmosiin) and others. The substantial fold changes observed in these flavonoids suggest a significant increase in their abundance during the prolonged storage period, implying potential alterations in the health-promoting properties of dried citrus peels (Supplementary Table S3).
Of the 25 differentially accumulated organic acids, 19 were up-accumulated (Supplementary Table S3). Among these, certain organic acids exhibited substantial fold changes, reflecting a notable effect of storage time. Interestingly, 2-isopropylmalate showed the highest log2fold change of 4.18, indicating a significant increase in its concentration. In contrast, some organic acids showed a decrease in concentration in CR10 compared to CR5. Guanidinoethyl sulphonate, 5-hydroxyhexanoic acid, 2-aminoethanesulphonic acid, creatine, 3-hydroxypropanoic acid and sodium valproate experienced decreases, introducing a complex dynamic to the metabolomic changes associated with prolonged storage.
KEGG enrichment analysis identified the most enriched pathways as glycolysis/gluconeogenesis, starch and sucrose metabolism, fructose and mannose metabolism, tropare, piperdine and pyridine alkaloid biosynthesis, the sulphur relay system, indole alkaloid biosynthesis and flavonoid biosynthesis (Figure 5F).
4. Discussion
The known health benefits of chenpi intrigue consumers who seek out the most authentic and aged material. This significantly influences its market value [14]. In order to find out if aging time has any differences in the metabolic composition, analysis was performed using chenpi stored for 1, 5 and 10 years. First, the total flavonoid and total phenolic content were determined, followed by the study of global metabolomic profiles by UPLC-MS/MS.
Earlier studies have highlighted that long-term storage of chenpi shows an increased levels of total flavonoids [19] as well as total phenolics [35]. In the case of total phenolics, the C. reticulata peels aged for 5 and 13 years showed a significant increase compared to those freshly harvested or the ones aged for one year [35]. Our results that the total phenolics increased significantly in both the CR5 and CR10 compared to CR1 are consistent with this report. However, there were non-significant differences in CR5 and CR10 for total phenolics in Chuan chenpi derived from Dahongpao red oranges. This reduction has also been observed in the C. reticulata from the Xinhui region of Guangdong province, China [36]. Some studies have explained that the reduced total phenolics could be due to a decrease in both soluble and insoluble phenolic acid ester fraction with time [19]. Similarly, the total flavonoid content increased significantly from CR1 to CR5. However, CR10 showed a significant decrease compared to CR5. A somewhat similar observation has been reported in Guang chenpi samples from the Xinhui region of Guangdong province, China, particularly for polymethoxyflavones [10]. These results highlight that Chuan chenpi aged for different time periods are useful from their composition point of view.
Using UPLC-MS/MS, a relatively higher number of compounds (781) were identified from Chuan chenpi compared to the earlier reports in Guang chenpi (53) [36] and Chachi chenpi (47) [12]. The dominant class of compounds was flavonoids and phenolic acids (~48%), which is consistent with the earlier reports [37,38,39,40]. However, the detection of the other compound classes highlights that Chuan chenpi contains a diverse range of metabolites. As for the changes in the metabolome composition, aging for 5 years seems to be most suitable for Chuan chenpi because of the higher content of saccharides and alcohols, glycerol ester and phenolic acids in CR5 compared to CR10. This is also consistent with our physiochemical analysis-based results for total phenols (Figure 1), whereas lipids, free fatty acids and others largely remained unchanged between the CR5 and CR10 aging time, though the composition of each group of compounds differed within years. Thus, a higher molecular heterogeneity exists between different aging periods in Chuan chenpi [36]. Coumarins [41], lignans [42], nucleotides and derivatives [43] and alkaloids [44] are important compounds with pharmacological effects including antioxidant and anti-cancer activities. Their decreasing trends in CR5 and CR10 suggest that careful consideration should be given to aged Chuan chenpi for specific purposes.
The five-year-long aging of Chuan chenpi resulted in the increased accumulation of α-linolenic acid and γ-linolenic, Lysine butyrate, 1-feruloyl-sn-glycerol, 3,5-dicaffeoylquinic acid, caffeic acid, protocatechuic acid, and 23 flavonoids, 17 organic acids, androsin, and 18 saccharides (Supplementary Table S2; Figure 5). These are important results considering the usage of Chuan chenpi in traditional Chinese medicine. Of these, α-linolenic acid has a prospective cardioprotective effect when consumed via the diet [45]. γ-linolenic plays a role as a substrate for eicosanoids synthesis, cholesterol transport and oxidation and is a component of lipid membranes [46]. Similarly, the other compounds offer a range of beneficial health and disease-protective effects. Therefore, their increased accumulation makes CR5 a suitable material for the health industry. The increased presence of many compounds classified as amino acids and fatty acids together with the flavonoids and organic acids in CR10 compared to CR1 and CR5 indicate aging for a longer time is suitable for Chuan chenpi like other cultivars of C. reticulata from other Chinese citrus growing regions [20].
Although there was a slight decrease in total flavonoids (based on cumulate metabolite intensity of all compounds), the increased content of 23/71 flavonoids is consistent with the earlier reports [47]. However, considering the individual flavonoid’s contents, CR10 is better, as 63/116 compounds in this class were u- accumulated compared to CR1 and 71/110 compared to CR5. Similarly, the increase in organic acids’ content with aging time allows us to conclude that the longer the aging time, the better the health properties Chuan chenpi would have [10,21]. Moreover, the organic acids play important roles in metabolic processes [48], and therefore on energy metabolism [48] and possibly as antioxidant defendants [49]. However, the downregulation of creatine in CR10 suggests changes in energy metabolism [50], while the decrease in γ-aminobutyric acid may influence neurotransmitter regulation [51]. This intricate balance of organic acids underscores the dynamic nature of the biochemical composition in stored citrus peels, revealing potential implications for their utility in various applications, from functional foods to pharmaceuticals. The changes in the organic acids from CR5 to CR10 signify the intricate response of organic acids to prolonged storage, implying potential implications for the overall chemical composition and properties of the dried citrus peels [52]. However, the changes in the overall metabolome with aging time are complex, indicating both short- and long-term storage have their own influence on the content of specific classes of compounds. From this, it can be understood that CR5 and CR10 are useful if the required product should contain saccharides, flavonoids, organic acids, phenolics or other classes of compounds.
5. Conclusions
Based on the current data, it is concluded that the different periods of peel aging affect the composition of the Chuan chenpi metabolome. The Chuan chenpi metabolome comprises 48% flavonoids and phenolic acids, with the remaining portion consisting of amino acids, alkaloids, organic acids, saccharides and alcohols, nucleotides, free fatty acids, lipids, vitamins, coumarins, lignans and other compounds. Our results show that prolonged aging of Chuan chenpi can significantly and positively affect the phenolic and flavonoid content. Finally, both CR5 and CR10 offer a range of health-beneficial compounds and should be used for specific purposes based on their metabolome composition.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/horticulturae10040421/s1, Supplementary Table S1: List of differentially accumulated metabolites between CR1 and CR10, Supplementary Table S2: List of differentially accumulated metabolites between CR1 and CR5, Supplementary Table S3: List of differentially accumulated metabolites between CR5 and CR10.
Author Contributions
Conceptualization, W.Z. and Q.N.; methodology, W.Z., X.F., Y.Z., X.C., T.F., C.X. and Q.N.; software, X.F., Y.Z. and X.C.; validation, W.Z. and X.F.; formal analysis, W.Z., X.F., Y.Z., X.C., T.F., C.X. and Q.N.; investigation, W.Z., X.F., Y.Z., X.C., T.F., C.X. and Q.N.; resources, W.Z.; data curation, W.Z.; writing—original draft preparation, W.Z.; writing—review and editing, Q.N.; visualization, X.F.; supervision, Q.N.; project administration, Q.N.; funding acquisition, Q.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Chongqing Technical Innovation and Application Development Special Project (SCTB2022TIAD-ZXX0043) and Chongqing University Research and Innovation Group Project (CXQTP19037).
Data Availability Statement
All datasets produced as a result of this work have been given in the main manuscript or as supplementary datasets.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Yu, X.; Sun, S.; Guo, Y.; Liu, Y.; Yang, D.; Li, G.; Lü, S. Citri reticulatae Pericarpium (Chenpi): Botany, ethnopharmacology, phytochemistry, and pharmacology of a frequently used traditional Chinese medicine. J. Ethnopharmacol. 2018, 220, 265–282. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Tao, H.; Cao, Y.; Ho, C.-T.; Jin, S.; Huang, Q. Prevention of obesity and type 2 diabetes with aged citrus peel (Chenpi) extract. J. Agric. Food Chem. 2016, 64, 2053–2061. [Google Scholar] [CrossRef]
- Zhang, M.; Zhu, J.; Zhang, X.; Zhao, D.-g.; Ma, Y.-y.; Li, D.; Ho, C.-T.; Huang, Q. Aged citrus peel (Chenpi) extract causes dynamic alteration of colonic microbiota in high-fat diet induced obese mice. Food Funct. 2020, 11, 2667–2678. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Cao, Y.; Ho, C.-T.; Jin, S.; Huang, Q. Aged citrus peel (Chenpi) extract reduces lipogenesis in differentiating 3T3-L1 adipocytes. J. Funct. Foods 2017, 34, 297–303. [Google Scholar] [CrossRef]
- Zhou, L.; Gu, W.; Kui, F.; Gao, F.; Niu, Y.; Li, W.; Zhang, Y.; Guo, L.; Wang, J.; Guo, Z. The mechanism and candidate compounds of aged citrus peel (Chenpi) preventing chronic obstructive pulmonary disease and its progression to lung cancer. Food Nutr. Res. 2021, 65, 7526. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.-H.; Chan, Y.-F.; Pan, M.-H.; Tung, Y.-C.; Su, Z.-Y. Aged citrus peel (Chenpi) prevents acetaminophen-induced hepatotoxicity by epigenetically regulating Nrf2 pathway. Am. J. Chin. Med. 2019, 47, 1833–1851. [Google Scholar] [CrossRef] [PubMed]
- Falduto, M.; Smedile, F.; Zhang, M.; Zheng, T.; Zhu, J.; Huang, Q.; Weeks, R.; Ermakov, A.M.; Chikindas, M.L. Anti-obesity effects of Chenpi: An artificial gastrointestinal system study. Microb. Biotechnol. 2022, 15, 874–885. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Gao, Z.; Wang, C.; Ma, J.; Li, G.; Fu, F.; Guo, J.; Shan, Y. Effects of different treatment methods of dried citrus peel (Chenpi) on intestinal microflora and short-chain fatty acids in healthy mice. Front. Nutr. 2021, 8, 702559. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Kou, X.; Wang, L.; Ji, R.; Ma, C.; Wang, H. Effective hydroxylation of tangeretin from Citrus Peel (Chenpi) by edible acids and its improvement in antioxidant and anti-lipase activities. LWT 2019, 116, 108469. [Google Scholar] [CrossRef]
- Sun, X.; Deng, H.; Shan, B.; Shan, Y.; Huang, J.; Feng, X.; Tang, X.; Ge, Y.; Liao, P.; Yang, Q. Flavonoids contribute most to discriminating aged Guang Chenpi (Citrus reticulata ‘Chachi’) by spectrum-effect relationship analysis between LC-Q-Orbitrap/MS fingerprint and ameliorating spleen deficiency activity. Food Sci. Nutr. 2023, 11, 7039–7060. [Google Scholar] [CrossRef]
- Daduo, L.; Chao, C.; Rongwei, L. Protective effect of flavonoids from pericarpium Citri reticulatae (Chenpi) against oxidative stress induced by exhaustive exercise. Afr. J. Microbiol. Res. 2011, 5, 50–56. [Google Scholar]
- Yang, M.; Jiang, Z.; Wen, M.; Wu, Z.; Zha, M.; Xu, W.; Zhang, L. Chemical variation of Chenpi (Citrus peels) and corresponding correlated bioactive compounds by LC-MS metabolomics and multibioassay analysis. Front. Nutr. 2022, 9, 825381. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Gao, Z.; Yang, Y.; Pan, S.; Yin, J.; Yu, X. Rapid identification of the storage age of dried tangerine peel using a hand-held near infrared spectrometer and machine learning. J. Near Infrared Spectrosc. 2022, 30, 31–39. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, W.; Qian, M.; Wen, Z.; Bai, W.; Zeng, X.; Wang, H.; Xian, Y.; Dong, H. Recent advances in the authentication (geographical origins, varieties and aging time) of tangerine peel (Citri reticulatae pericarpium): A review. Food Chem. 2024, 442, 138531. [Google Scholar] [CrossRef] [PubMed]
- Dai, G.; Wu, L.; Zhao, J.; Guan, Q.; Zeng, H.; Zong, M.; Fu, M.; Du, C. Classification of Pericarpium Citri reticulatae (Chenpi) age using surface-enhanced Raman spectroscopy. Food Chem. 2023, 408, 135210. [Google Scholar] [CrossRef] [PubMed]
- Li, A. Tangerine peel treasures for a healthy body and tasty treats. Shine: Beyond a Single Story, 25 January 2022. [Google Scholar]
- Li, J.; Qiu, G.; Tang, R.; Zhang, J. Clinical study on treatment of functional dyspepsia with Pericarpium Citri reticulatae from Xinhui preserved for twenty years. J. New Chin. Med. 2011, 43, 7–10. [Google Scholar]
- Qiu, G.; Li, J.; Tang, R.; Zhang, J. Study of treatment effect used five year Xinhui Chenpi to functional dyspepsia. Chin. Arch. Tradit. Chin. Med. 2011, 29, 346–348. [Google Scholar]
- Choi, M.-Y.; Chai, C.; Park, J.H.; Lim, J.; Lee, J.; Kwon, S.W. Effects of storage period and heat treatment on phenolic compound composition in dried Citrus peels (Chenpi) and discrimination of Chenpi with different storage periods through targeted metabolomic study using HPLC-DAD analysis. J. Pharm. Biomed. Anal. 2011, 54, 638–645. [Google Scholar] [CrossRef]
- Li, F.; Lu, Y.; Li, C.; Huang, R.; Tian, E.; Tan, E.; Yang, Z.; Li, H.; Chao, Z. trnL-trnF copy number is inversely correlated with storage time of Guang Chenpi, the aged sun-dried peels of Citrus reticulata ‘Chachi’. J. Stored Prod. Res. 2022, 97, 101982. [Google Scholar] [CrossRef]
- Yue, F.; Zhang, F.; Qu, Q.; Wang, C.; Qin, Y.; Ma, L.; Jia, Y.; Ismael, M.; Jiang, Y.; Sun, T. Effects of ageing time on the properties of polysaccharide in tangerine peel and its bacterial community. Food Chem. 2023, 417, 135812. [Google Scholar] [CrossRef]
- Wang, F.; Hu, Y.; Chen, H.; Chen, L.; Liu, Y. Exploring the roles of microorganisms and metabolites in the 30-year aging process of the dried pericarps of Citrus reticulata ‘Chachi’ based on high-throughput sequencing and comparative metabolomics. Food Res. Int. 2023, 172, 113117. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wen, H.; Kong, J.; Hu, Z.; Hu, Y.; Zeng, J.; Chen, X.; Zhang, H.; Chen, J.; Xu, J. Flavor characterization of Citri reticulatae Pericarpium (Citrus reticulata ‘Chachiensis’) with different aging years via sensory and metabolomic approaches. Food Chem. 2024, 443, 138616. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Mao, X.; Guo, L.; Zhou, Z. Comparative Analysis of the Impact of Three Drying Methods on the Properties of Citrus reticulata Blanco cv. Dahongpao Powder and Solid Drinks. Foods 2023, 12, 2514. [Google Scholar] [CrossRef] [PubMed]
- Luan, A.; Zhang, W.; Yang, M.; Zhong, Z.; Wu, J.; He, Y.; He, J. Unveiling the molecular mechanism involving anthocyanins in pineapple peel discoloration during fruit maturation. Food Chem. 2023, 412, 135482. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.; Bi, X.; Fu, X.; Li, Y.; Li, G.; Li, Y.; Liu, D.; Yang, Y.; Shi, R.; Dong, W. Comparative Metabolome Profiles and Antioxidant Potential of Four Coffea arabica L. Varieties Differing in Fruit Color. Diversity 2023, 15, 724. [Google Scholar] [CrossRef]
- De Winter, J.C. Using the Student’s t-test with extremely small sample sizes. Pract. Assess. Res. Eval. 2019, 18, 10. [Google Scholar]
- Bouyahya, A.; Taha, D.; Benali, T.; Zengin, G.; El Omari, N.; El Hachlafi, N.; Khalid, A.; Abdalla, A.N.; Ardianto, C.; Tan, C.S. Natural sources, biological effects, and pharmacological properties of cynaroside. Biomed. Pharmacother. 2023, 161, 114337. [Google Scholar] [CrossRef] [PubMed]
- M Calderon-Montano, J.; Burgos-Morón, E.; Pérez-Guerrero, C.; López-Lázaro, M. A review on the dietary flavonoid kaempferol. Mini Rev. Med. Chem. 2011, 11, 298–344. [Google Scholar] [CrossRef] [PubMed]
- Al-Bzour, M.H.; Bsieso, Y.; Gammoh, O.; Alqudah, M.; Qnais, E.Y.; Wedyan, M.; Alqudah, A. Exploring the antinociceptive potential of homoeriodictyol in nociception models. J. Pharm. Pharmacogn. Res. 2024, 12, 615–623. [Google Scholar] [CrossRef]
- Choi, H.J.; Song, H.-H.; Lee, J.-S.; Ko, H.-J.; Song, J.-H. Inhibitory effects of norwogonin, oroxylin A, and mosloflavone on enterovirus 71. Biomol. Ther. 2016, 24, 552. [Google Scholar] [CrossRef]
- Qiu, M.; Wei, W.; Zhang, J.; Wang, H.; Bai, Y.; Guo, D.-A. A Scientometric Study to a Critical Review on Promising Anticancer and Neuroprotective Compounds: Citrus Flavonoids. Antioxidants 2023, 12, 669. [Google Scholar] [CrossRef]
- Mokdad-Bzeouich, I.; Mustapha, N.; Sassi, A.; Bedoui, A.; Ghoul, M.; Ghedira, K.; Chekir-Ghedira, L. Investigation of immunomodulatory and anti-inflammatory effects of eriodictyol through its cellular anti-oxidant activity. Cell Stress Chaperones 2016, 21, 773–781. [Google Scholar] [CrossRef] [PubMed]
- Dorsch, W.; Müller, A.; Christoffel, V.; Stuppner, H.; Antus, S.; Gottsegen, A. Antiasthmatic acetophenones—An in vivo study on structure activity relationship. Phytomedicine 1994, 1, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Chen, G.; Fu, X.; Liu, R.-H. Effects of aging on the phytochemical profile and antioxidative activity of Pericarpium Citri reticulatae ‘Chachiensis’. RSC Adv. 2016, 6, 105272–105281. [Google Scholar] [CrossRef]
- Tian, C.; Xu, H.; Li, J.; Han, Z. Characteristics and intestinal immunomodulating activities of water-soluble pectic polysaccharides from Chenpi with different storage periods. J. Sci. Food Agric. 2018, 98, 3752–3757. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Zeng, W.; Huang, K.-E.; Li, D.-X.; Chen, W.; Yu, X.-Q.; Ke, X.-H. Discrimination of Citrus reticulata Blanco and Citrus reticulata ‘Chachi’ as well as the Citrus reticulata ‘Chachi’ within different storage years using ultra high performance liquid chromatography quadrupole/time-of-flight mass spectrometry based metabolomics approach. J. Pharm. Biomed. Anal. 2019, 171, 218–231. [Google Scholar] [PubMed]
- Qin, K.; Zheng, L.; Cai, H.; Cao, G.; Lou, Y.; Lu, T.; Shu, Y.; Zhou, W.; Cai, B. Characterization of chemical composition of Pericarpium Citri reticulatae volatile oil by comprehensive two-dimensional gas chromatography with high-resolution time-of-flight mass spectrometry. Evid.-Based Complement. Altern. Med. 2013, 2013, 237541. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Yuan, W.; Wen, B.; Miao, Y.; Li, Y.; Li, J.; Fan, C.; Liu, M.; Wang, J.; Chen, J. Comparisons of Metabolite Composition and Antioxidant Activities in Citrus Reticulata ‘Chachi’ Extracts Using Different Solvents with Uplc-Qtof/Ms Based on a Metabolomics Approach. Ms Based on a Metabolomics Approach 2023. [Google Scholar]
- Zhang, J.; Wu, X.; Qiu, J.; Zhang, L.; Zhang, Y.; Qiu, X.; Huang, Z.; Xu, W. Comprehensive comparison on the chemical profile of Guang Chen Pi at different ripeness stages using untargeted and pseudotargeted metabolomics. J. Agric. Food Chem. 2020, 68, 8483–8495. [Google Scholar] [CrossRef]
- J Matos, M.; Vazquez-Rodriguez, S.; Fonseca, A.; Uriarte, E.; Santana, L.; Borges, F. Heterocyclic antioxidants in nature: Coumarins. Curr. Org. Chem. 2017, 21, 311–324. [Google Scholar] [CrossRef]
- Teodor, E.D.; Moroeanu, V.; Radu, G.L. Lignans from medicinal plants and their anticancer effect. Mini Rev. Med. Chem. 2020, 20, 1083–1090. [Google Scholar] [CrossRef] [PubMed]
- Sridharan, K.; Gogtay, N.J. Therapeutic nucleic acids: Current clinical status. Br. J. Clin. Pharmacol. 2016, 82, 659–672. [Google Scholar] [CrossRef] [PubMed]
- Adamski, Z.; Blythe, L.L.; Milella, L.; Bufo, S.A. Biological activities of alkaloids: From toxicology to pharmacology. Toxins 2020, 12, 210. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-B.; Nam, Y.A.; Kim, H.S.; Hayes, A.W.; Lee, B.-M. α-Linolenic acid: Nutraceutical, pharmacological and toxicological evaluation. Food Chem. Toxicol. 2014, 70, 163–178. [Google Scholar] [CrossRef] [PubMed]
- Białek, M.; Rutkowska, J. The importance of γ-linolenic acid in the prevention and treatment. Adv. Hyg. Exp. Med. 2015, 69, 892–904. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Chen, S.; Gu, G.; Qiu, J.; Chen, Y.; Jia, Z.; Tang, H. Comparison of the content of flavonoids, total phenols, and carotenoids and antioxidant activity in guang Citri reticulatae pericarpium during the aging time. Pharmacogn. Mag. 2020, 16, 375–381. [Google Scholar]
- Sauer, S.W.; Okun, J.G.; Hoffmann, G.F.; Koelker, S.; Morath, M.A. Impact of short-and medium-chain organic acids, acylcarnitines, and acyl-CoAs onmitochondrial energy metabolism. Biochim. Biophys. Acta (BBA)-Bioenerg. 2008, 1777, 1276–1282. [Google Scholar] [CrossRef] [PubMed]
- Pereira, D.M.; Valentão, P.; Andrade, P.B. Organic acids of plants and mushrooms: Are they antioxidants. Funct. Plant Sci. Biotechnol. 2009, 3, 103–113. [Google Scholar]
- Oudman, I.; Clark, J.F.; Brewster, L.M. The effect of the creatine analogue beta-guanidinopropionic acid on energy metabolism: A systematic review. PLoS ONE 2013, 8, e52879. [Google Scholar] [CrossRef]
- Li, K.; Xu, E. The role and the mechanism of γ-aminobutyric acid during central nervous system development. Neurosci. Bull. 2008, 24, 195–200. [Google Scholar] [CrossRef]
- Xie, J.; Deng, B.; Wang, W.; Zhang, H. Changes in sugar, organic acid and free amino acid levels and the expression of genes involved in the primary metabolism of oleocellosis in citrus peels. J. Plant Physiol. 2023, 280, 153877. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).