Abstract
The propagation of Crocus sativus L. relies exclusively on corm multiplication. As underground storage organs, corms are susceptible to a wide range of pathogens, environmental stresses, and diseases, making traditional propagation methods often ineffective with the loss of valuable material. In vitro propagation offers an alternative for the saffron culture under controlled conditions. In particular, the innovative application of the Temporary Immersion System (TIS) represents a technological advancement for enhancing biomass production with a reduction in operational costs. The current study utilized the Plantform™ bioreactor to propagate in vitro saffron corms from the ‘Abruzzo’ region (Italy), integrating machine learning models to assess its performance. The evaluation of saffron explants after 30, 60, and 90 days of culture showed a marked improvement in growth and microcorm production compared to conventional in vitro culture on semisolid medium, supported by the machine learning analysis. Indeed, the Random Forest algorithm revealed a predictive accuracy with an R2 value of 0.81 for microcorm number, showcasing the capability of machine learning models to forecast propagation outcomes effectively. These results confirm that applying TIS in saffron culture could lead to economically viable, large biomass production within a controlled environment, irrespective of seasonality. This study represents the first endeavor to use TIS technology to enhance the in vitro propagation of saffron in conjunction with machine learning, suggesting an innovative approach for cultivating high-value crops like saffron.
1. Introduction
Saffron (Crocus sativus L.) is a perennial plant growing widely in Iran, Morocco, Greece, Türkiye, China, and many other countries [1]. It is an ancient plant; the first mention of its cultivation dates around 2300 B.C. by King Sargon, and it was identified in a fresco painting in the Knossos palace (Minos, Crete in Greek) dated 1700–1600 B.C. [2]. It is a plant with traditional importance, indeed it was used in local cultural, intellectual, physical, and spiritual areas [3]. The commercial part of the saffron plant consists of dry red stigma or red powder, also called ‘red gold’, used as a spice to obtain dye and scents, and as medicinal drugs to human health since ancient times.
The saffron plant is vegetatively propagated through corm multiplication, particularly by forming daughter corms from the mother plant. Corms, which are underground organs, are susceptible to a wide range of pathogens and diseases that can lead to significant losses in valuable production. Additionally, saffron propagation is labor-intensive, as the corms must be manually removed and replanted [4].
Therefore, different propagation pathways, such as in vitro culture, have been investigated. In vitro propagation has already been successfully applied to saffron from different geographical origins, i.e., Iran [5], India [6], Morocco [7], Türkiye [8], China [9], and Spain [10], allowing its culture in sterile, controlled conditions and without season dependency. However, the Temporary Immersion System (TIS) approach can be considered for large-scale in vitro propagation and rapid acclimatization. This system is based on alternating cycles of temporary immersion of the cultured explants into the liquid medium followed by a dry period. The alternating contact between the plants and the liquid medium can encourage the development and adaptation of plants to the next stage of acclimatization [11,12,13]. Moreover, there is the possibility of reducing hand labor and the cost of production markedly. Indeed, careful positioning of explants in the agarized medium is not necessary, and the replacement of the new fresh liquid medium is easily performed. TIS allows us to achieve many other advantages [14], and it was applied with success on several ornamental, fruit, and woody species [15,16,17,18,19,20,21,22,23,24]. TIS application requires the use of plant bioreactors that can be of different types and sometimes are equipped for gas exchange by ventilation phases [14,25]. In this study, the PlantformTM bioreactor was used; it consists of a single container made of polycarbonate transparent and is autoclavable at 120 °C (http://www.plantform.se: accessed on 8 April 2024). Inside, there is a basket containing plant material. Filters and silicon tubes are connected to timers and air pumps to regulate the immersion and ventilation times. The containers can be placed above each other, saving space in the climate chamber. The PlantformTM bioreactor was applied to propagate saffron corms coming from the geographical origin ‘Abruzzo region’ (Italy).
Many researchers frequently find it challenging to use conventional statistical methods to assess big and complex datasets in the context of in vitro micropropagation, a composite biological process impacted by genotypes, culture medium, and environment [26]. Recently, new technology based on artificial intelligence, such as machine learning, is developing quickly in several scientific and industrial domains [27] but its integration within the plant and agricultural sciences remains relatively emergent. Artificial Neural Networks (ANNs), described as a class of nonlinear computational strategies, are employed for various tasks encompassing data clustering, generating forecasts, and classifying complex systems. Machine learning algorithms emerge as potent and predictive tools for decision-making in the sector of in vitro plant propagation, due to their proficiency in clarifying and defining the complexity of processes that involve a multitude of factors. Currently, these models have been applied in various in vitro culture investigations, including micropropagation, regeneration and in vitro organogenesis, stress physiology, and salt stress [28,29,30,31,32,33].
Therefore, this study aimed to evaluate the response of the Italian saffron ecotype from Abruzzo to the culture in the PlantformTM bioreactor in terms of growth and microcorm production compared to the conventional in vitro propagation on semisolid medium. To support the research scope, the artificial neural network (ANN) analysis and machine learning (ML) algorithms (Gaussian process, GP; random forest, RF; support vector machine; SVM) were integrated to model and predict the effects of two culture systems on different growth parameters.
2. Materials and Methods
2.1. Plant Material, Sterilization, and In Vitro Establishment
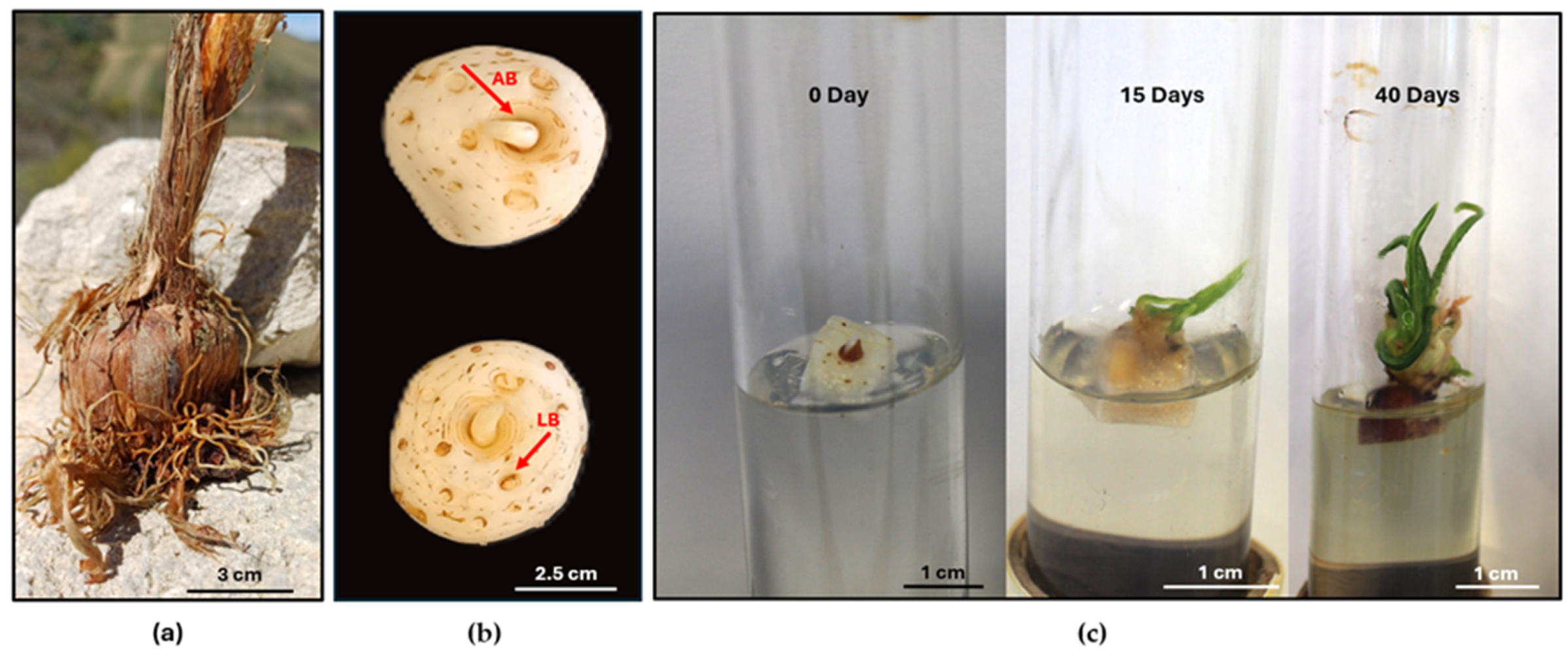
For in vitro culture establishment, healthy saffron corms were collected from Navelli (Protected Designation of Origin) situated in the Abruzzo region (42°14′19″ N 13°43′46″ E). The corms were used as source of explants (Figure 1a). For cleaning and disinfection of corms, the external tunic was removed, and the corms were treated with 0.1% fungicide (Enovit Metil FL; SIPCAM—Soc, Milan, Italy) for 20 min and then sterilized with 80% (v/v) ethanol for 1 min, followed by dipping in 0.2% (w/v) HgCl2 for 20 min. Following that, the corms were rinsed three times with distilled sterile water. The apical and the lateral buds from the corms were excised (in total 250 buds) at the end of October and used for in vitro culture (Figure 1b). The buds were cultured initially in glass tubes (Figure 1c) and then transferred in 500 mL glass jars on Murashige and Skoog (MS, Sigma-Aldrich, St. Louis, MO, USA) medium [34] containing 1 mg L−1 6-benzyladenine; (BAP; Sigma-Aldrich, St. Louis, MO, USA), 1 mg L−1 1-Naphthaleneacetic acid (NAA; Sigma-Aldrich, St. Louis, MO), 100 mg L−1 Ascorbic acid (Carlo Erba, Cornaredo, MI, USA), 30 g L−1 sucrose, and 3 g L−1 Gelrite™ (Sigma-Aldrich, St. Louis, MO, USA), mentioned as MS1. The pH of the media was adjusted to 5.8. The cultures were kept at 24 °C, under a 16 h photoperiod with 60 μmol m−2 s1 of photosynthetically active radiation provided by cool-white fluorescent lamps and subcultured every 30 days. After three subcultures, new, well-developed in vitro corms were used to start the TIS and semisolid cultures.
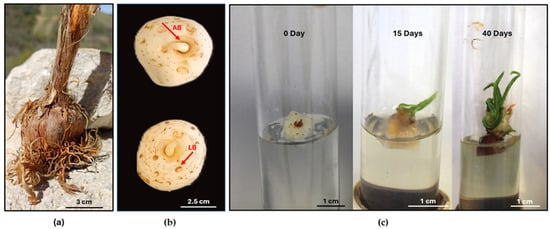
Figure 1.
Corms of saffron from the Abruzzo region (a); corms with apical buds (AB) and lateral buds (LB) used for in vitro culture (b); development of initial explants in in vitro culture (c).
2.2. Preparation of In Vitro Cultures in Temporary Immersion System and Semisolid Medium
In the PlantformTM bioreactor, 500 mL of liquid MS1 medium was used. A total of 6 explants were assessed. The immersion and ventilation periods of the TIS culture were controlled using air pumps and a timer placed in the climate chamber; the immersion frequency was 5 min every 4 h, with 15 min aeration every 4 h. The experiment was repeated twice. In the semisolid culture, each jar was prepared with 100 mL of MS1 medium, and 6 jars were set up. One corm was placed per jar.
The cultures were kept for 30, 60, and 90 days under the same culture conditions as stated above. In the TIS culture, at each evaluation period, only the MS1 was replaced without removing the corms, while in the semisolid medium, the explants were transferred to a fresh MS1 as they stood. The number of new microcorms, number of developed shoots, and root formation were evaluated for each period. Moreover, corms weight was recorded to estimate the Relative Growth Rate (RGR) index of the cultures after 30, 60, and 90 days using the following formula: [ln FW final − ln FW initial] × 100/days of culture (ln = natural logarithm; FW: fresh weight, [22]). RGR index is based on the initial and final fresh weights of the plant material and the time of culture.
2.3. Statistical Analysis
The present study employed the t-test method using R-programming to perform a rigorous statistical analysis to examine the differences between two culture systems concerning several attributes. The t-test is a statistical tool commonly used, that allowed the comparison of the mean values of each feature between the TIS and semisolid medium, offering important information about any differences influenced by the experimental settings. The experiment was repeated twice and consisted of three replicates with two explants used either for TIS or semisolid per each replicate.
2.4. Modeling Procedure
In this study, we used three Machine Learning (ML) algorithms—Gaussian process (GP), Random Forest (RF), and Support Vector Machine (SVM), as well as the well-known ANN-based Multilayer Perceptron (MLP) to model and predict the micropropagation and rooting efficiency of saffron through TIS and the semisolid medium. To thoroughly evaluate the anticipated performances of the MLP and ML models, we partitioned the dataset into training and testing subsets, and we used a 10-fold cross-validation technique.
TIS and semisolid medium are the input variables. On the other hand, the microcorm, shoot, and root numbers were the target (output) variables. R-programming was used with the Caret package (Classification and Regression Training) to implement coding. Several metrics were used to assess and compare the precision and accuracy of the MLP and ML models. These metrics included the root means square error (RMSE), which shows how closely the regression line matches the observed data points. The coefficient of determination (R2) shows the degree of relationship between the model and dependent variable, and the mean absolute error (MAE) computes the average error between the predicted and observed values (Equations (1)–(3)).
where Yi is the actual value; Ŷi is the predicted value; Ỹ is the mean of the actual values; and n is the sample count.
2.4.1. Multilayer Perceptron
With an input layer, an output layer, and one or more hidden layers, the Multilayer Perceptron (MLP) is a popular example of an Artificial Neural Network (ANN). The input and output variables from the training set were used to train the MLP using a supervised training technique. Until the desired value in Equation (4) was attained, the training procedure was repeated [26].
where n is the number of observations; ys is the sth observation variable; and ŷs is the sth of the predicted variable.
2.4.2. Gaussian Process
To better comprehend the spread of random variables, the Gaussian Process (GP) model, which extends the Gaussian probability distribution, is a useful tool for supervised learning. This model works very well for solving regression and classification issues. It is a non-parametric classifier that calculates the likelihood that input samples belong to particular classes, particularly for binary datasets. One of its main features is its capacity to operate effectively with tiny datasets and produce reliable, accurate, and computationally efficient results [35]. Equation (5) presents the mathematical reasoning for each input (x) and matching output (y).
2.4.3. Random Forest
The RF approach is well-known for its effectiveness and simplicity of usage, and it has proven successful in both regression and classification problems. Prior studies have demonstrated that the RF model has several noteworthy qualities, such as its capacity to avoid overfitting, adeptness at managing noise, and effective handling of a high number of data [36]. The trained tree makes the final decision in the RF technique, which uses bagging, also referred to as bootstrap aggregation. The basic idea of the RF model is shown in Equation (6).
2.4.4. Support Vector Machines
Even with relatively small datasets, the SVM approach demonstrates efficiency; however, a significant amount of training data is often required for effective learning. Additionally, the SVM framework lessens issues with other methods, such as overfitting, slow convergence rates, and becoming trapped in local minima. Due to their higher resistance to the challenges sometimes associated with classic artificial intelligence approaches, SVMs are distinguished by this special quality [37]. The SVM algorithm, which helps identify which class has the longest separator plane, is declared in Equation (7).
3. Results and Discussion
3.1. In Vitro Propagation of Saffron
Many papers that have reported on in vitro saffron propagation come from several countries [5,7,38,39,40,41] and some reviews [42,43,44] have collected all the information on this fundamental method for propagating the Crocus spp. [2], especially in C. sativus, to develop callus or new shoot buds and obtain new microcorms formation. The in vitro culture system is a biotechnological tool that enhances saffron propagation by increasing biomass production and plant quality in a sterile and controlled environment, independent of seasonal availability. Indeed, the low multiplication rate and the bulb rot are the major issues in the traditional propagation of saffron, considering that about four or five corms per mother corm are produced in one year, and soil infestation reduces productivity.
Different types of explants were used to obtain embryogenic callus, microcorms, or shoot regeneration, but corms or small parts of corms, including one bud, were generally better explants for the production of shoots and new cormlets [45]. In our research, the initial explants were a small portion of corms with apical or lateral buds, excised in October, from the corms. The best period for introducing saffron buds in vitro culture seems to be September–December [46,47].
Corms collected from fields are contaminated with soil and contaminants, thus their sterilization is a crucial step towards the success of the in vitro culture, and the optimization of the disinfection protocol is essential for ensuring large-scale corm production. Different disinfection processes have been applied to saffron corms [48]. In our study, a sequence of treatments involving ETOH, a fungicide, HgCl2, and sterilized water was identified as the best combination to obtain an 87% survival rate of explants, in agreement with findings by Taheri-Dehkordi et al. [5]. Furthermore, as reported by Yasmin et al. [47], the in vitro development of saffron buds is season-dependent, with the highest success rates observed from September to October (95%) and followed by November to December (85%). Therefore, employing an effective set of sterilization steps combined with choosing the best period for initiating in vitro cultures led to a high survival rate of explants.
Different media were used for the in vitro culture of saffron as reported in the detailed review of Salami et al. [45]. Our preliminary results showed that MS medium containing 1 mg L−1 BA and 1 mg L−1 NAA was the best combination for the in vitro culture of the Italian ecotype and was consequently applied in the current study.
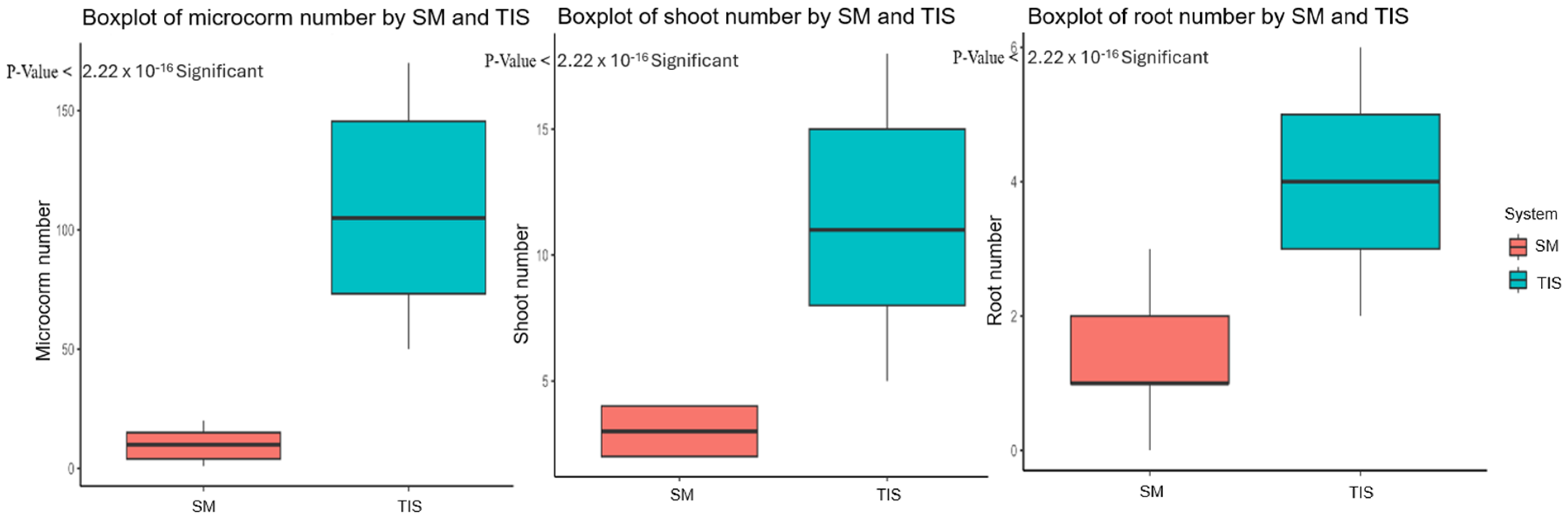
The conventional in vitro propagation using the semisolid medium is proposed as a system that increases the number of corms produced [49,50,51], but it is often a cost-labor method. Consequently, the introduction of semi-automation by plant bioreactors for in vitro propagation has been proposed as a strategy to reduce production costs [52,53,54]. In this study, applying the PlantformTM bioreactor on the Abruzzo saffron ecotype improved in vitro propagation efficiency. Table 1 and Figure 2 present the t-test comparisons between the two distinct groups, TIS and semisolid medium, across different growth parameters (microcorm number, shoot number, and root number). For each parameter, significant differences in mean values between the two culture systems were noted; the p-values indicate statistically significant differences. Notably, TIS outperforms the semisolid medium in the number of microcorms, with a significantly higher mean value of 107.5, which is further supported by a p-value < 0.001. Likewise, a variation in the shoot number was observed, and the TIS yielded a mean value of 11.26, which was greater than the semisolid medium’s mean of 3.07. The statistical significance is confirmed by a corresponding p-value < 0.001, which suggests a significant difference in the number of shoots between the culture systems.

Table 1.
The t-test analysis of growth parameters between TIS and semisolid medium.
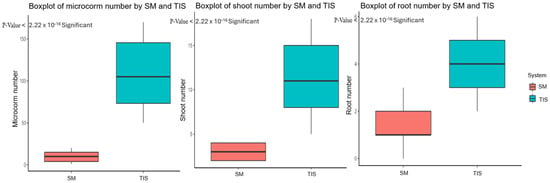
Figure 2.
The t-test boxplot of growth parameters (microcorm, shoot, and root number) in TIS and semisolid medium (SM) cultures.
There is also a significant difference revealed by the root number, where the temporary immersion has a mean value of 4.13, with respect to the mean of 1.38 in semisolid medium; a p-value < 0.001, showing a clear variation in root number between the two systems. Overall, the statistical analysis significantly highlighted the critical role that culture systems play in influencing the investigated parameters (Figure 2).
RGR is an analytical tool used to characterize plant growth. It can be calculated from measurements taken on the same sample at two different times without the loss of vegetal material. This index was also applied in in vitro cultures [22,24,55,56,57]. In the current study, the RGR index demonstrated an improvement in the growth of saffron in TIS than in the semisolid medium since the first evaluation period (30 days). The same trend was confirmed after 60 and 90 days of continuous culture, as indicated in Table 2. These results are in agreement with Gatti et al. [22] on the efficiency of PlantformTM in increasing biomass production of Quercus robur L. over semisolid culture in a microbox. Our findings showed the highest RGR of 1.27 after 90 days of culture in PlantformTM versus 0.87 in the solid medium. A similar RGR of 1.12 and 1.15 was observed at 30 and 60 days, respectively, indicating a considerable increase in biomass obtained during TIS cultures as recorded by Benelli and De Carlo, [24] using the same type of PlantformTM bioreactor for Olea europaea L.

Table 2.
Relative growth rate (RGR index) of C. sativus in PlantformTM and semisolid medium after 30, 60, and 90 days of the beginning of in vitro culture.
In saffron, the application of the TIS was reported in only one study by Blázquez et al. [58], which utilized embryogenic calli as explants to explore and highlight the effects of polyamine metabolism during the somatic embryogenesis process.
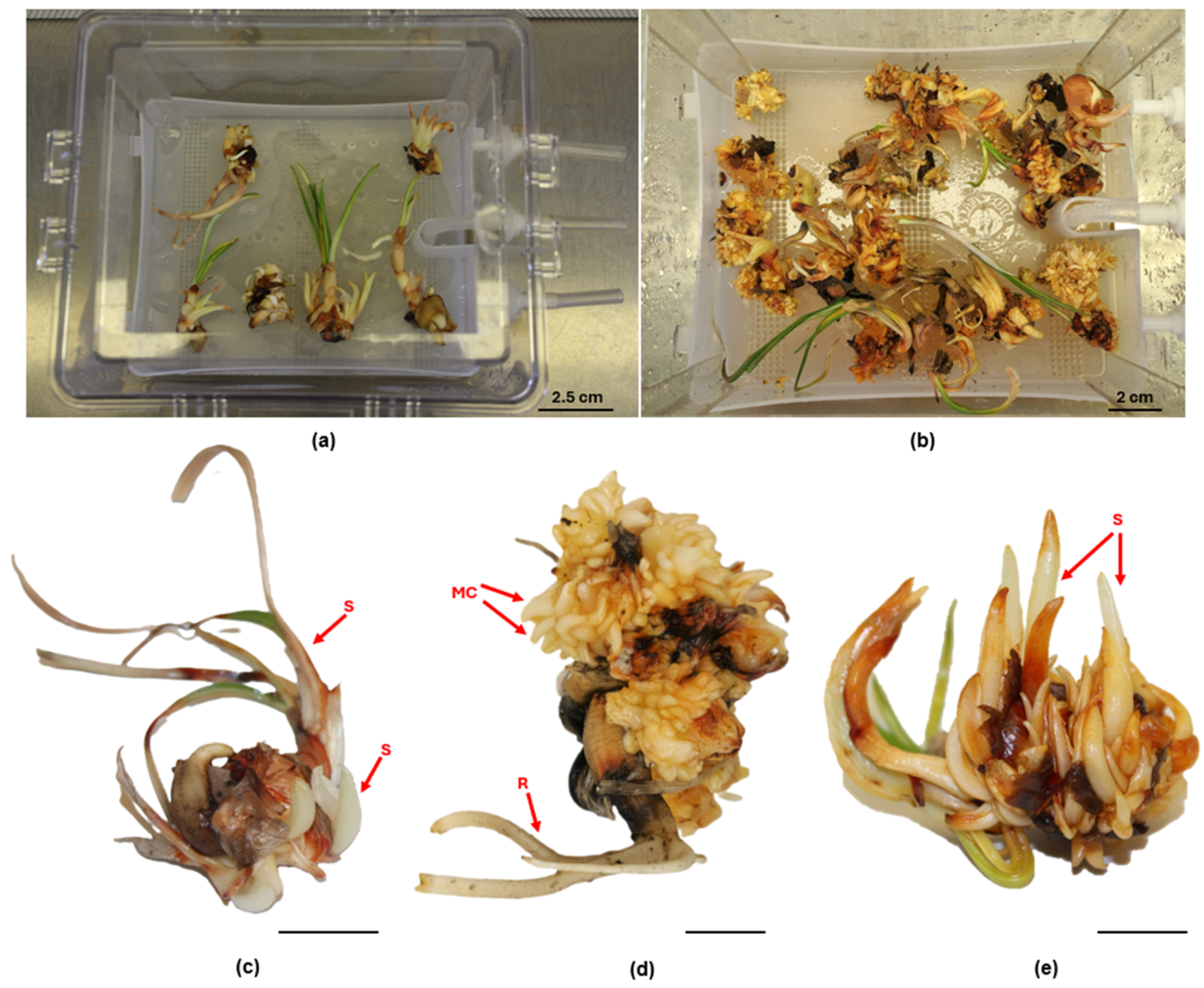
The Rita® bioreactor was used and in this system, the production of embryogenic calli increased four-fold compared to the semisolid medium. This finding is in accordance with our study. Indeed, the explants in PlantformTM yielded a higher number of microcorms (~eleven-fold) with respect to the semisolid medium system (Figure 3b–e). Moreover, it was possible to maintain the corms in vitro culture for 90 days, equivalent to three subcultures, without handling the plant material, requiring only the replacement of the culture medium. The TIS has been applied to several bulbous plants to achieve rapid propagation, particularly ornamental bulbous plants. For instance, in Hippeastrum × chmielii Chm., the use of twin-flasks as a temporary culture bioreactor was effective in obtaining a greater number of new bulbs (6.3–6.5) than the agar-solidified medium (3.9) within 4 weeks [59].

Figure 3.
TIS culture of saffron at the beginning (a) and after 90 days (b) of culture in PlantformTM bioreactor. Explant after 30 days (c), 60 days (d), and 90 days (e); Bars = 1.5 cm. (S: Shoot; MC: Microcorm; R: Root).
Increased bulb induction in Leucojum aestivum L. was achieved using the Rita® bioreactor (VITROPIC, St-Mathieu de Tréviers, France)with an immersion period of 5 min every 2 h. Regardless of the constituents of the culture medium, a bulb rate production of 74.6% was obtained in the TIS system, while in the solid culture media, it was 71.7% [60]. Moreover, the study reported that the bulbs derived from TIS had a higher fresh weight (about 1.6 times) than the bulbs in the solid medium, and the acclimatization step was better in plants obtained from Rita® systems.
Similarly, Ruffoni et al. [61] showed the efficiency of Rita® vessels in producing an increased number of Gladiolus corms, which could be easily transplanted into the field, in contrast to cultures in a semisolid medium. In TIS Gladiolus propagation, an immersion time of 3 min every 3 h was applied for 30, 45, and 90 days of culture.
Several studies have investigated the Lilium spp. propagation in bioreactors [62]. For example, in three oriental lilies [63], bioreactor culture with an immersion time of 5 min every 2 h led to an increase in bulb mass production by 10–13 times. Barberini et al. [64] reported the application of Rita® for multiplying L. bulbiferum, with good results in terms of material quality to be easily transferred in the greenhouse. Lian et al. [65] propagated the Lilium oriental hybrid ‘Casablanca’ in an ebb-and-flow system and obtained a higher number of bulblets (1025) compared with semisolid culture (82), applying 15 min of immersion 4 times per day.
While the TIS culture has shown promising results for many plant species, it does not always yield superior outcomes. For instance, Mirmasoumi and Bakhshaie [66] found that shoot organogenesis from the callus of L. ledebourii was less efficient when callus cultures were grown in the Rita® system compared to a semisolid medium. Similarly, Nesi et al. [67] observed that Lilium hybrids achieved better propagation through a continuous immersion system than the TIS using an ebb and flood system.
For the in vitro production of Solanum tuberosum L., Wongket and Pumisutapon [68] achieved 82–90% of microtubers using TIS (twin-flasks system) with an immersion every 12 h for 2 min than in the solid medium (20%). TIS conditions gave 4.1–4.5 times more microtubers respecting solid medium. The use of Rita® container in potato led to obtaining vigorous seedlings with a higher number of shoots [69], and PlantformTM was allowed to achieve a maximum number of tubers [70] rather than the conventional culture system. A study conducted by Andriani et al. [71] highlighted the significance of using another type of TIS for the micropropagation of S. tuberosum. It was found that explants grown in the SetisTM bioreactor produced a larger number, size, and weight of tubers than the semisolid medium. This improvement was attributed to the direct contact between the explants and the liquid medium during immersion periods, leading to enhanced nutrient uptake and consequently more efficient tuber induction.
The aforementioned findings suggest that the effectiveness of TIS may vary depending on the specific requirements of the plant species. The control and monitoring of different parameters, such as the optimization of the medium culture, immersion, and aeration time are fundamental for customized approaches in plant tissue culture. Particularly, an accurate fitting of the immersion time and dry periods can considerably reduce the hyperhydricity of the tissue, establishing optimal conditions for humidity. In our study, the application of immersion time 5 min every 4 h, which was in line with the reports for the bulbous species.
3.2. Machine Learning Analysis
A comparison of the performance metrics of four different machine learning models used to predict features in TIS is shown in Table 3. The number of microcorms, shoots, and roots was examined with different models: RF, SVM, GP, and MLP. The validity of the model was assessed using MAE, R2, and RMSE metrics. The R2 scores are between 0 and 1, where 1 represents the best possible prediction, and 0 indicates no explanation potential. The RMSE numbers, which typically vary from zero to positive infinity, show the precision of the model. Lower values indicate better performance. Similarly, the MAE scales from zero to positive infinity, with lower values indicating more accuracy; it represents projected accuracy.

Table 3.
Performance matrices of machine learning models for the Temporary Immersion System (TIS).
RF showed the best predictive performance with an R2 value of 0.81 for the microcorm number, and its value can explain about 81% of the variance in microcorms. Moreover, RF produced the lowest results at 0.12 and 0.16 for MAE and RMSE, respectively, indicating the highest predictive accuracy level and the least divergence between the expected and actual values. Compared to the other models, SVM demonstrated comparatively poorer performance metrics, as evidenced by its R-squared value of 0.56, indicating a lower level of predictive potential.
Concerning shoot number prediction, RF once more demonstrated the strongest performance, with an R2 value of 0.75. Among the models, RF had the lowest MAE (0.13) and RMSE (0.17), indicating better overall accuracy than other models, while SVM and MLP models showed the highest RMSE of 0.20.
MLP proved to be the most effective method for estimating root number; its R2 value of 0.78 meant that it could account for almost 78% of the variation in this parameter. Additionally, MLP had the lowest RMSE and MAE of all the models, indicating excellent prediction accuracy and low error in root number prediction. On the other hand, with an R2 value of 0.51, SVM showed the least favorable performance metrics due to a rather poor predictive efficacy in this parameter prediction task. The findings revealed that the RF model outperformed other models in every parameter category, offering the TIS high prediction accuracy and low error rates in forecasting the number of microcorms, shoots, and roots.
However, each model’s performance could vary based on the studied parameters; thus, choosing machine learning methods that are unique to the intended prediction task is important.
Table 4 also provides a detailed examination of RF, SVM, GP, and MLP in semisolid medium culture systems. Regarding microcorm predictions, both RF and SVM showed similar R2 values of 0.75, which can explain almost 75% of the variance in this growth parameter. However, GP and MLP with 0.77 and 0.78, respectively, had higher R2 values, indicating a better capacity to explain variance in microcorms. The MAE ranged from 0.11 to 0.12 for all models and was consistently low, showing a minor average divergence between the predicted and actual values. Similarly, the models were constant regarding the RMSE, which had values between 0.16 and 0.17, showing reliable accuracy in microcorm number prediction.

Table 4.
Performance matrices of machine learning models for a semisolid medium.
GP and MLP led the field in shoot number prediction with the greatest R2 values of 0.69 and 0.70, respectively, indicating their greater ability to explain variance. RF and SVM showed adequate R2 values of 0.65 and 0.68, respectively, remaining slightly behind. Among the models, MLP had the lowest MAE of 0.12, which indicates that it predicts shoot numbers more accurately than the other models. Moreover, MLP had the least overall error with the lowest RMSE of 0.17.
Regarding root number, SVM has the greatest R2 value (0.60), followed by GP (0.59). Both can explain the variance more effectively than other models. The R2 values for RF and MLP were decreased (0.50 and 0.54); this suggests that their prediction power for root numbers is slightly lower. Notably, SVM and GP showed the lowest RMSE and MAE, indicating less error and higher accuracy in root number prediction.
In the case of the semisolid medium, MLP exhibited competitive performance in predicting microcorm and shoot numbers, even though each model displayed a unique performance across many attributes, while SVM seemed to be more successful in predicting the root number. These results highlight the need to choose suitable machine learning algorithms for predictive modeling in semisolid medium by considering particular attributes and corresponding model performances.
When evaluating the performance of the machine learning models, the RF algorithm consistently demonstrated superior accuracy in predicting parameters, particularly within the TIS culture system. Both the GP and the MLP models also performed effectively, showing good results with only slight adjustments in context.
SVM performed competitively in the semisolid medium, especially in root number prediction, while RF performed better in TIS; MLP continuously demonstrated higher accuracy in both system cultures, particularly regarding microcorm and shoot number prediction. Although RF was reliable, model selection should consider the features and culture conditions to maximize prediction efficacy.
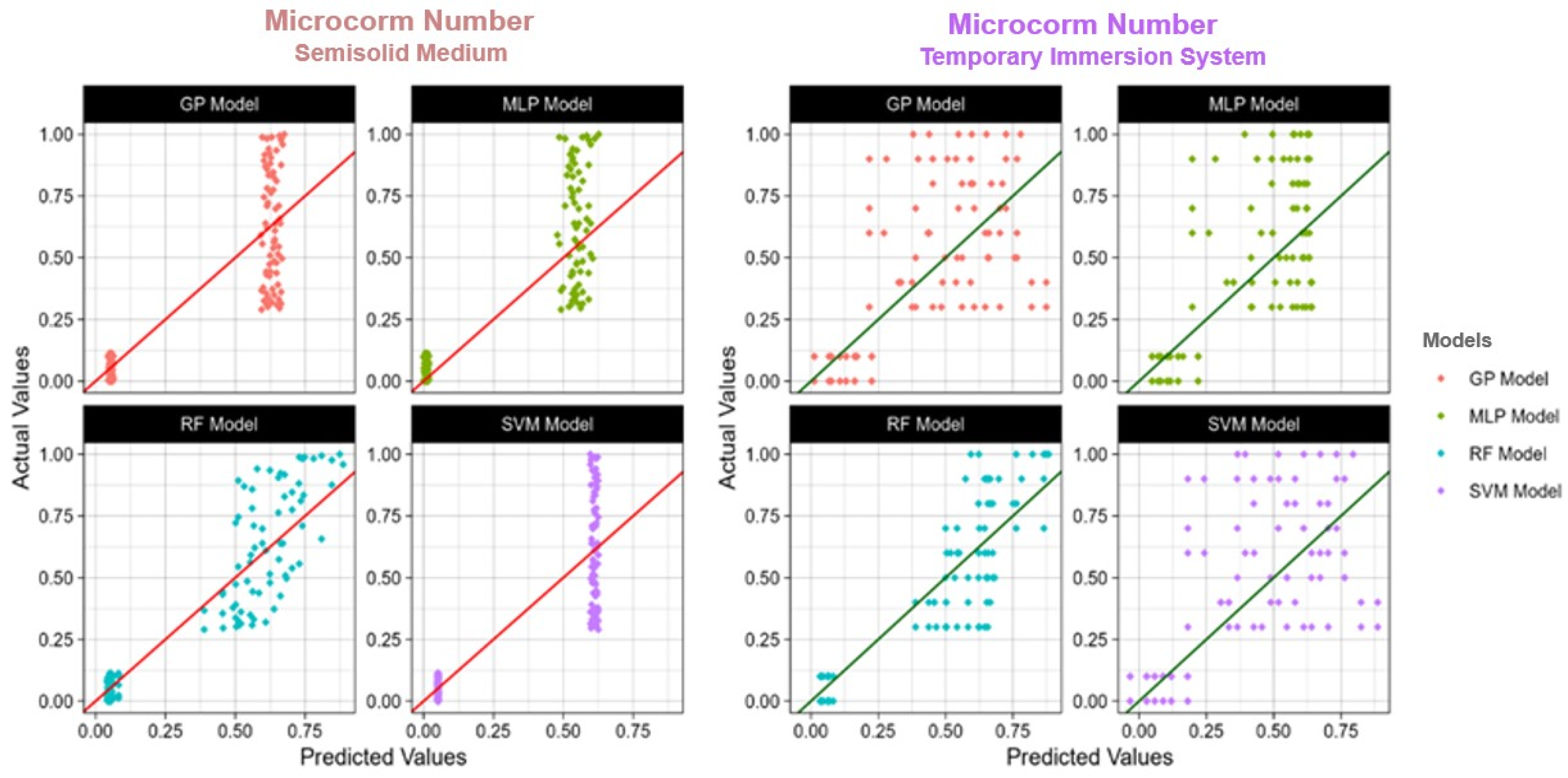
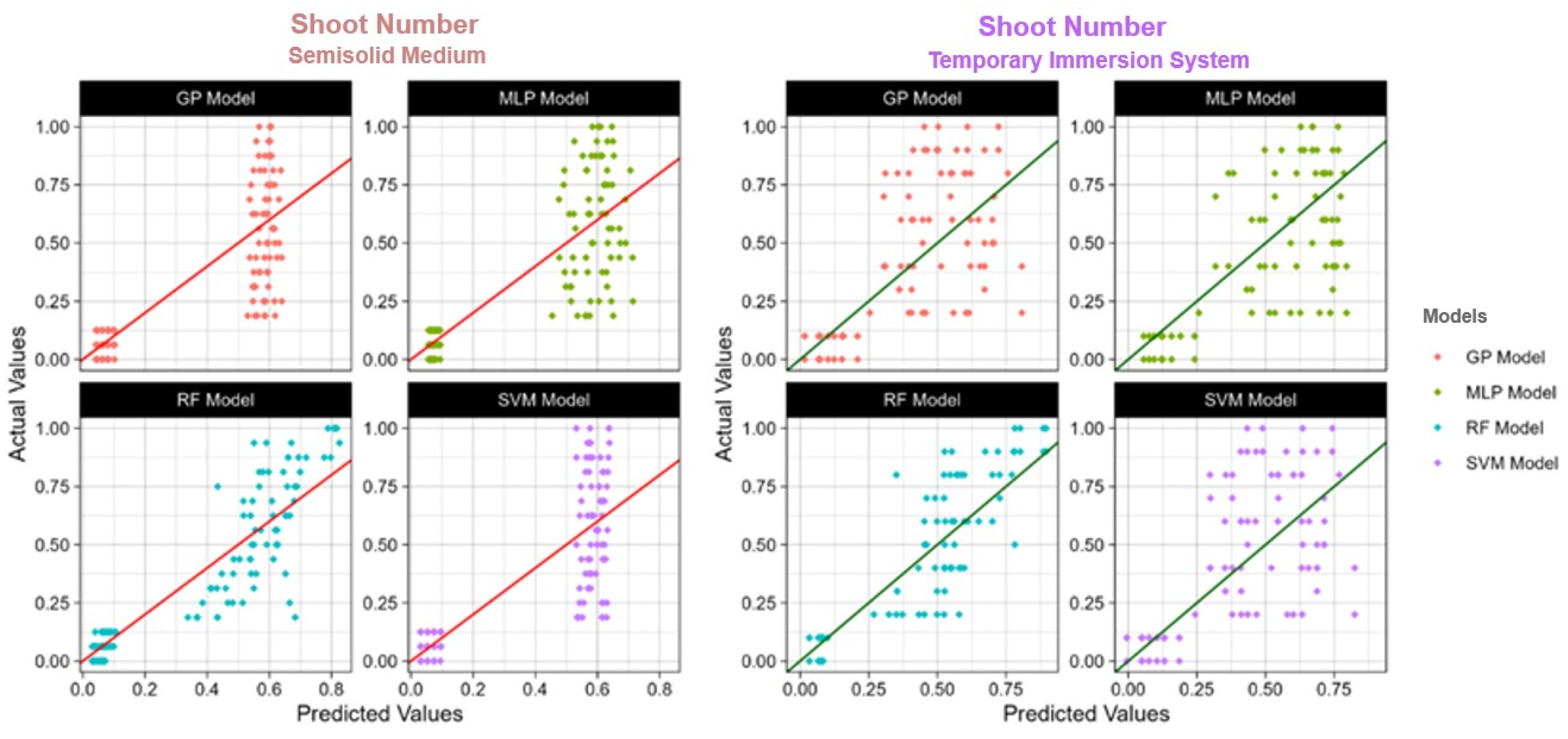
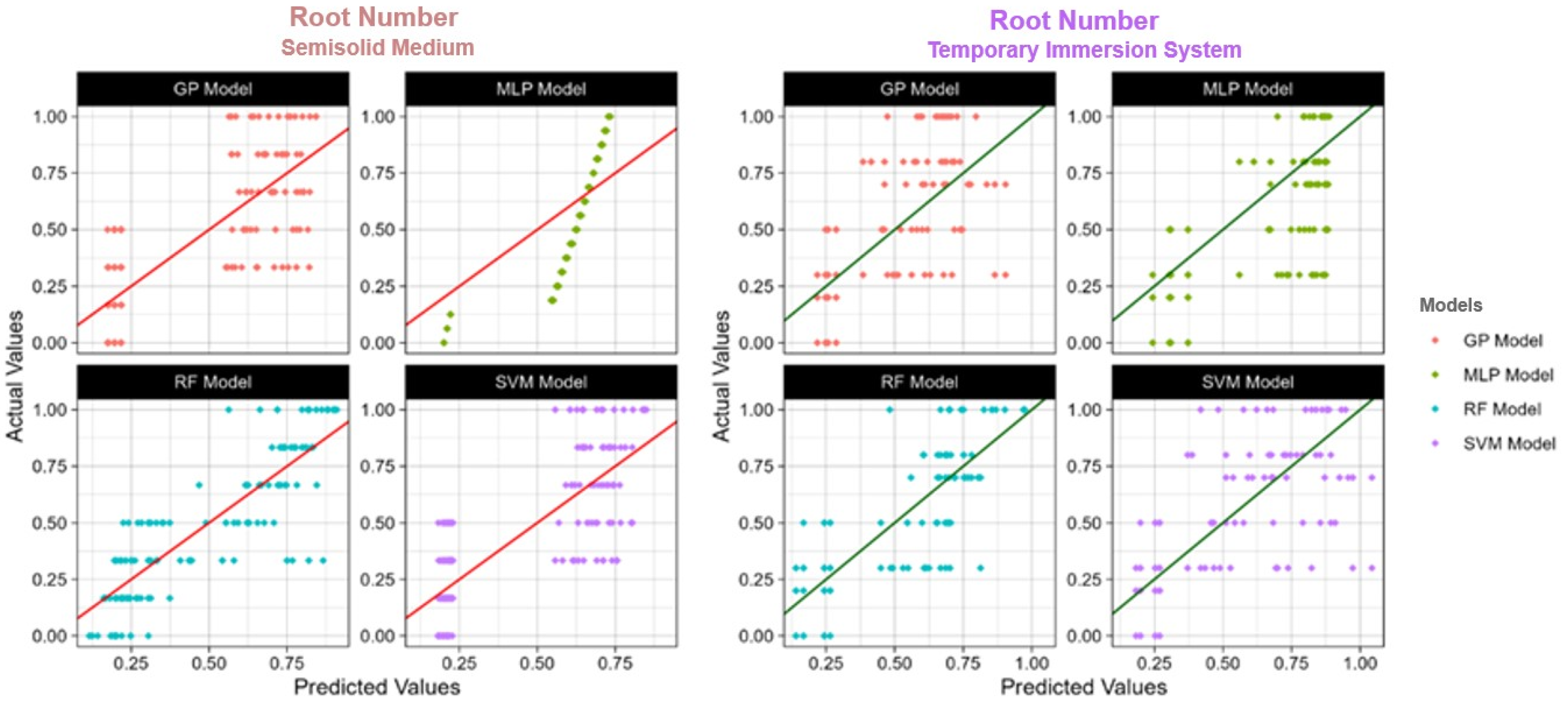
Figure 4, Figure 5 and Figure 6 show the actual and predicted values, making the comparison of observed data points with model forecasts easy. The model performance may be evaluated in the graphic representations, which highlight how well it predicts actual results.
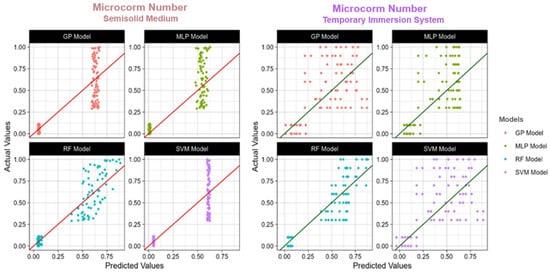
Figure 4.
The actual and predicted value of TIS and semisolid medium microcorm number using four models: RF: Random Forest; SVM: Support Vector Machines; GP: Gaussian Process; MLP: Multilayer Perceptron; R2: Coefficient of determination.
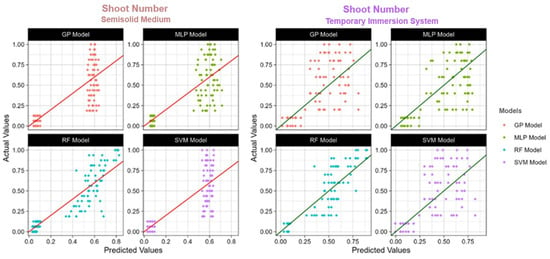
Figure 5.
The actual and predicted value of TIS and semisolid medium shoot number using four models: RF: Random Forest; SVM: Support Vector Machines; GP: Gaussian Process; MLP: Multilayer Perceptron; R2: Coefficient of determination.
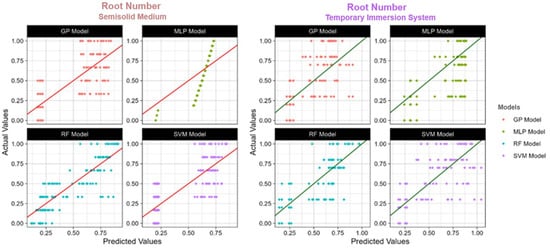
Figure 6.
The actual and predicted value of TIS and semisolid medium root number using four models: RF: Random Forest; SVM: Support Vector Machines; GP: Gaussian Process; MLP: Multilayer Perceptron; R2: Coefficient of determination.
In the general view of machine learning applications within the agricultural domain, particularly focusing on plant tissue culture, our study delves into the comparative effectiveness of the four models used in two distinct culture systems, namely the TIS and semisolid medium. This analysis is in line with other studies [35,37,72,73,74], which have explored various dimensions of machine learning applicability in predicting plant growth parameters under different experimental conditions. Our research underscores the superior predictive performance of RF in the TIS culture, particularly in forecasting the number of microcorms, shoots, and roots. This finding is consistent with the literature, such as Şimşek [35], which highlighted the robustness of RF in detecting water stress effects in strawberries, and Aasim et al. [37], which identified the efficacy of RF in predicting germination and morphological traits in hemp seedlings. The ability of RF to manage complex datasets with high accuracy highlights its value in agricultural predictive modeling, providing a basis for optimizing plant tissue culture conditions. The machine learning analysis section of our study reveals nuanced performances across different models and culture systems. For instance, in the semisolid medium, GP and MLP showed heightened effectiveness in predicting microcorms, aligning with observations of Aasim et al. [72] on the promising applications of quantum computing-enhanced machine learning models in plant tissue culture. This suggests that while RF generally offers a robust performance, the intricate nature of biological data might sometimes favor other models or necessitate a hybrid approach for enhanced prediction accuracy. Moreover, as explored by Aasim et al. [72], the integration of quantum computing techniques in machine learning introduces a groundbreaking perspective on future agricultural research. In our study, the quantum-enhanced algorithms were not applied, but the reported success in other research indicates a potential way for elevating the predictive modeling capabilities in plant science, thereby enhancing the efficiency of plant tissue culture protocols. The lesser predictive potential of SVM which is noted in TIS system is in agreement with Pepe et al. [74], who reported the limitations of SVM in complex plant growth modeling. This reinforces the necessity for a customized selection of models based on the specific dataset characteristics and predictive needs, highlighting the importance of exploring advanced machine learning techniques and potentially quantum computing methods to overcome the current modeling limitations. In essence, our study, alongside the referenced literature, emphasizes the dynamic nature of machine learning applications in agriculture and illustrates the need for constant methodological progress and adaptability to specific research contexts. The evolving complexity of biological data and the emergence of quantum computing herald a new era of possibilities that necessitate ongoing exploration and adaptation of machine learning methodologies in plant tissue culture and wider agricultural sciences.
4. Conclusions
Although in vitro propagation presents a significant opportunity to multiply disease-free saffron microcorms, achieving large-scale production remains a challenge. The Temporary Immersion System (TIS) offers a promising approach to enhance production and support the acclimatization phase for this species. This study represents the first effort to propagate saffron corms using the TIS technique with the PlantformTM bioreactor. Indeed, the microcorm propagation was optimized in the TIS over the conventional in vitro culture on semisolids, with no abnormalities or hyperhydricity in corms production during the culture period. Therefore, the adoption of this innovative system could be a significant step toward producing large quantities of disease-free corms at reduced costs, enhancing the ease of operation and handling of plant material through a semiautomated system. Additionally, TIS might play a crucial role in acclimatization phase since the explants, during periods without immersion in the culture medium, begin to adapt to ex vitro conditions. In this sense, further attention and investigation are needed in future research to improve the acclimatization of saffron. Furthermore, integrating machine learning to predict outcomes in this field opens up novel approaches to solving complex biological questions. These findings provide important insights into the biological mechanisms and contextual factors that influence growth parameters across different experimental conditions.
Author Contributions
Conceptualization, C.B. and W.T.; methodology W.T., T.İ. and C.B.; software, Ö.Ş.; data curation, W.T., Ö.Ş. and T.İ.; writing—original draft preparation, W.T., T.İ. and C.B.; writing—review and editing, W.T., T.İ., N.C., Ö.Ş. and C.B.; visualization, T.İ.; supervision, C.B.; funding acquisition, C.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data are contained within the article.
Acknowledgments
The authors acknowledge the National Council Research of Italy (Bilateral Project 2022–2023: ‘Improving cultivation, quality, and conservation of saffron accessions from Morocco and Italy’).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Cardone, L.; Castronuovo, D.; Perniola, M.; Cicco, N.; Candido, V. Saffron (Crocus sativus L.), the king of spices: An overview. Sci. Hortic. 2020, 272, 109560. [Google Scholar] [CrossRef]
- Khawar, M.K.; Yildirim, M.U.; Sarihan, E. Ex vitro macropropagation of saffron (Crocus sativus L.) corms. In Saffron: The Age-Old Panacea in a New Light; Sarwat, M., Sumaiya, S., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 45–56. [Google Scholar]
- Yildirim, M.U.; Sarihan, E.O.; Khawar, K.M. Ethnomedicinal and traditional usage of saffron (Crocus sativus L.) in Turkey. In Saffron: The Age-Old Panacea in a New Light; Sarwat, M., Sumaiya, S., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 21–31. [Google Scholar]
- Menia, M.; Sadaf Iqbal, I.; Zahida, I.R.; Tahir, I.S.; Kanth, I.R.; Aashq Hussian, I.; Iqbal, S.; Hussian, A. Production technology of saffron for enhancing productivity. J. Pharmacogn. Phytochem. 2018, 7, 1033–1039. [Google Scholar]
- Taheri-Dehkordi, A.; Naderi, R.; Martinelli, F.; Salami, S.A. A robust workflow for indirect somatic embryogenesis and cormlet production in saffron (Crocus sativus L.) and its wild allies; C. caspius and C. speciosus. Heliyon 2020, 6, e05841. [Google Scholar] [CrossRef]
- Chib, S.; Thangaraj, A.; Kaul, S.; Dhar, M.K.; Kaul, T. Development of a system for efficient callus production, somatic embryogenesis and gene editing using CRISPR/Cas9 in Saffron (Crocus sativus L.). Plant Methods 2020, 16, 47. [Google Scholar] [CrossRef]
- Lagram, K.; Ben, M.; Caid, E.; El Aaouam, S.; Lachheb, M.; El, A.; Serghini, M.A. In vitro shoot regeneration and development of microcorms of moroccan saffron (Crocus sativus L.). Atlas J. Plant Biol. 2016, 50–55. [Google Scholar] [CrossRef]
- Sevindik, B.; Mendi, Y.Y. Somatic embryogenesis in Crocus sativus L. In Methods in Molecular Biology; Humana Press Inc.: Totowa, NJ, USA, 2016; Volume 1359, pp. 351–357. [Google Scholar]
- Yang, B.M.; Huang, Y.L.; Xu, W.J.; Bao, L.X. Explant selection and cluster buds induction in vitro of saffron (Crocus sativus L.). Agric. Sci. Technol. Commun 2015, 2, 106–108. [Google Scholar]
- Renau-Morata, B.; Moyá, L.; Nebauer, S.G.; Seguí-Simarro, J.M.; Parra-Vega, V.; Gómez, M.D.; Molina, R.V. The use of corms produced under storage at low temperatures as a source of explants for the in vitro propagation of saffron reduces contamination levels and increases multiplication rates. Ind. Crops Prod. 2013, 46, 97–104. [Google Scholar] [CrossRef]
- Berthouly, M.; Etienne, H. Temporary immersion system: A new concept for use liquid medium in mass propagation. In Liquid Culture Systems for In Vitro Plant Propagation; Hvoslef-Eide, A.K., Preil, W., Eds.; Springer: Dordrecht, The Netherlands, 2005; pp. 165–195. [Google Scholar]
- Yan, H.; Yang, L.; Li, Y. Improved growth and quality of Dioscorea fordii Prain et Burk and Dioscorea alata plantlets using a temporary immersion system. Afr. J. Biotechnol. 2011, 10, 19444–19448. [Google Scholar]
- Yang, S.-H.; Yeh, D.-M. In vitro leaf anatomy, ex vitro photosynthetic behaviors and growth of Calathea orbifolia (Linden) Kennedy plants obtained from semi-solid medium and temporary immersion systems. Plant Cell Tissue Organ Cult. 2008, 93, 201–207. [Google Scholar] [CrossRef]
- De Carlo, A.; Tarraf, W.; Lambardi, M.; Benelli, C. Temporary immersion system for production of biomass and bioactive compounds from medicinal plants. Agronomy 2021, 11, 2414. [Google Scholar] [CrossRef]
- Pérez-Alonso, N.; Jiménez, E.; de Feria, M.; Capote, A.; Barbón, R.; Quiala, E.; Chávez, M. Effect of inoculum density and immersion time on the production of potato microtubers in temporary immersion systems and field studies. Biotecnol. Veg. 2007, 7, 149–154. [Google Scholar]
- Businge, E.; Trifonova, A.; Schneider, C.; Rödel, P.; Egertsdotter, U. Evaluation of a new temporary immersion bioreactor system for micropropagation of cultivars of Eucalyptus, birch and fir. Forests 2017, 8, 196. [Google Scholar] [CrossRef]
- Ruffoni, B.; Savona, M. The temporary immersion system (T.I.S.) for the improvement of micropropagation of ornamental plants. Acta Hortic. 2005, 683, 445–454. [Google Scholar] [CrossRef]
- Elazab, D.; Capuana, M.; Ozudogru, E.A.; Anichini, M.; Lambardi, M. Use of liquid culture with the electis bioreactor for faster recovery of blackberry (Rubus fruticosus L.) shoots from conservation at 4 °C. Horticulturae 2023, 9, 680. [Google Scholar] [CrossRef]
- Vives, K.; Andújar, I.; Lorenzo, J.C.; Concepción, O.; Hernández, M.; Escalona, M. Comparison of different in vitro micropropagation methods of Stevia rebaudiana B. including temporary immersion bioreactor (BIT®). Plant Cell Tissue Organ Cult. 2017, 131, 195–199. [Google Scholar] [CrossRef]
- Ramírez-Mosqueda, M.A.; Iglesias-Andreu, L.G. Evaluation of different temporary immersion systems (BIT®, BIG, and RITA®) in the micropropagation of Vanilla planifolia Jacks. Vitr. Cell. Dev. Biol. Plant 2016, 52, 154–160. [Google Scholar] [CrossRef]
- Nongdam, P.; Beleski, D.G.; Tikendra, L.; Dey, A.; Varte, V.; EL Merzougui, S.; Pereira, V.M.; Barros, P.R.; Vendrame, W.A. Orchid micropropagation using conventional semi-solid and temporary immersion systems: A review. Plants 2023, 12, 1136. [Google Scholar] [CrossRef] [PubMed]
- Gatti, E.; Sgarbi, E.; Ozudogru, E.A.; Lambardi, M. The effect of PlantformTM bioreactor on micropropagation of Quercus robur in comparison to a conventional in vitro culture system on gelled medium, and assessment of the microenvironment influence on leaf structure. Plant Biosyst. 2017, 151, 1129–1136. [Google Scholar] [CrossRef]
- Vidal, N.; Blanco, B.; Cuenca, B. A temporary immersion system for micropropagation of axillary shoots of hybrid chestnut. Plant Cell Tissue Organ Cult. 2015, 123, 229–243. [Google Scholar] [CrossRef]
- Benelli, C.; De Carlo, A. In vitro multiplication and growth improvement of Olea europaea L. cv Canino with temporary immersion system (PlantformTM). 3 Biotech 2018, 8, 317. [Google Scholar] [CrossRef]
- Georgiev, V.; Schumann, A.; Pavlov, A.; Bley, T. Temporary immersion systems in plant biotechnology. Eng. Life Sci. 2014, 14, 607–621. [Google Scholar] [CrossRef]
- Şimşek, Ö.; Sekerci, A.D.; Isak, M.A.; Bulut, F.; Izgü, T.; Tütüncü, M.; Dönmez, D. Optimizing micropropagation and rooting protocols for diverse lavender genotypes: A synergistic approach integrating machine learning techniques. Horticulturae 2024, 10, 52. [Google Scholar] [CrossRef]
- Jan, Z.; Ahamed, F.; Mayer, W.; Patel, N.; Grossmann, G.; Stumptner, M.; Kuusk, A. Artificial intelligence for industry 4.0: Systematic review of applications, challenges, and opportunities. Expert Syst. Appl. 2023, 216, 119456. [Google Scholar] [CrossRef]
- Demirel, F.; Uğur, R.; Popescu, G.C.; Demirel, S.; Popescu, M. Usage of machine learning algorithms for establishing an effective protocol for the in vitro micropropagation ability of black chokeberry (Aronia melanocarpa (Michx.) Elliott). Horticulturae 2023, 9, 1112. [Google Scholar] [CrossRef]
- Sadat-Hosseini, M.; Arab, M.M.; Soltani, M.; Eftekhari, M.; Soleimani, A.; Vahdati, K. Predictive modeling of Persian walnut (Juglans regia L.) in vitro proliferation media using machine learning approaches: A comparative study of ANN, KNN and GEP models. Plant Methods 2022, 18, 48. [Google Scholar] [CrossRef]
- Lozano-Milo, E.; Landin, M.; Gallego, P.P.; García-Pérez, P. Machine learning deciphers genotype and ammonium as key factors for the micropropagation of Bryophyllum sp. medicinal plants. Horticulturae 2022, 8, 987. [Google Scholar] [CrossRef]
- Özcan, E.; Atar, H.H.; Ali, S.A.; Aasim, M. Artificial neural network and decision tree–based models for prediction and validation of in vitro organogenesis of two hydrophytes—Hemianthus callitrichoides and Riccia fluitans. Vitr. Cell. Dev. Biol. Plant 2023, 59, 547–562. [Google Scholar] [CrossRef]
- Hesami, M.; Jones, A.M.P. Application of artificial intelligence models and optimization algorithms in plant cell and tissue culture. Appl. Microbiol. Biotechnol. 2020, 104, 9449–9485. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, H.; Mirzaie-Asl, A.; Abdollahi, M.R.; Tohidfar, M. Enhancing petunia tissue culture efficiency with machine learning: A pathway to improved callogenesis. PLoS ONE 2023, 18, e0293754. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Şimşek, Ö. Machine learning offers insights into the impact of in vitro drought stress on strawberry cultivars. Agriculture 2024, 14, 294. [Google Scholar] [CrossRef]
- Hu, J.; Sun, Y.; Li, G.; Jiang, G.; Tao, B. Probability analysis for grasp planning facing the field of medical robotics. Meas. J. Int. Meas. Confed. 2019, 141, 227–234. [Google Scholar] [CrossRef]
- Aasim, M.; Katırcı, R.; Akgur, O.; Yildirim, B.; Mustafa, Z.; Nadeem, M.A.; Baloch, F.S.; Karakoy, T.; Yılmaz, G. Machine learning (ML) algorithms and artificial neural network for optimizing in vitro germination and growth indices of industrial hemp (Cannabis sativa L.). Ind. Crops Prod. 2022, 181, 114801. [Google Scholar] [CrossRef]
- Sharma, K.D.; Rathour, R.; Sharma, R.; Goel, S.; Sharma, T.R.; Singh, B.M. In vitro cormlet development in Crocus sativus. Biol. Plant. 2008, 52, 709–712. [Google Scholar] [CrossRef]
- Zeybek, E.; Önde, S.; Kaya, Z. Improved in vitro micropropagation method with adventitious corms and roots for endangered saffron. Cent. Eur. J. Biol. 2012, 7, 138–145. [Google Scholar] [CrossRef]
- Mahmoud, K.B.; Rebaii, J.; Jemai, N.; Jedidi, E. In vitro micropropagation attempts of the high value spice saffron (Crocus sativus L.). Rev. Des. Régions Arid. 2020, 727–734. Available online: https://www.researchgate.net/publication/352056079_In_vitro_micropropagation_attempts_of_the_high_value_spice_saffron_Crocus_sativus_L (accessed on 29 April 2024).
- Mereu, A.; Dorsaf, K.; Scarpa, G. In vitro culture of saffron: Hormones influence on the development of new shoots and callus. Plant Cell Biotechnol. Mol. Biol. 2019, 20, 511–520. [Google Scholar]
- Ahmad, M.; Zaffar, G.; Habib, M.; Arshid, A.; Dar, N.A.; Dar, Z.A. Saffron (Crocus sativus L.) in the light of biotechnological approaches: A review. Sci. Res. Essays 2014, 9, 13–18. [Google Scholar]
- Gantait, S.; Vahedi, M. In vitro regeneration of high value spice Crocus sativus L.: A concise appraisal. J. Appl. Res. Med. Aromat. Plants 2015, 2, 124–133. [Google Scholar] [CrossRef]
- Tahiri, A.; Mazri, M.A.; Karra, Y.; Ait Aabd, N.; Bouharroud, R.; Mimouni, A. Propagation of saffron (Crocus sativus L.) through tissue culture: A review. J. Hortic. Sci. Biotechnol. 2023, 98, 10–30. [Google Scholar] [CrossRef]
- Salami, S.A. Tissue culture techniques for saffron improvement. In The Saffron Genome. Compendium of Plant Genomes; Vakhlu, J., Ambardar, S., Salami, S.A., Kole, C., Eds.; Springer: Cham, Switzerland, 2022; pp. 195–220. [Google Scholar]
- Devi, K.; Sharma, M.; Singh, M.; Singh Ahuja, P. In vitro cormlet production and growth evaluation under greenhouse conditions in saffron (Crocus sativus L.)—commercially important crop. Eng. Life Sci. 2011, 11, 189–194. [Google Scholar] [CrossRef]
- Yasmin, S.; Nehvi, F.A.; Wani, S.A. Tissue culture as an alternative for commercial corm production in saffron: A heritage crop of Kashmir. Afr. J. Biotechnol. 2013, 12, 3940–3946. [Google Scholar]
- Teixeira da Silva, J.A. Disinfection of explants for saffron (Crocus sativus) tissue culture. Environ. Exp. Biol. 2016, 14, 183–198. [Google Scholar] [CrossRef]
- Raja, W.; Zaffer, G.; Wani, S.A. In vitro microcorm formation in saffron (Crocus sativus L.). Acta Hortic. 2007, 739, 291–296. [Google Scholar] [CrossRef]
- Soukrat, S.; Benlhabib, O.; Alfaiz, C.; Lage, M. Boosting saffron (Crocus sativus L.) micro-propagation through in vitro corm production. Acta Hortic. 2017, 1184, 87–95. [Google Scholar] [CrossRef]
- Lagram, K.; El Merzougui, S.; Boudadi, I.; Ben El Caid, M.; El Boullani, R.; El Mousadik, A.; Serghini, M.A. In vitro shoot formation and enrooted mini-corm production by direct organogenesis in saffron (Crocus sativus L.). Vegetos 2023. Available online: https://link.springer.com/article/10.1007/s42535-023-00639-9 (accessed on 29 April 2024). [CrossRef]
- Datta, S.K.; Chakrabarty, D.; Janakiram, T. Low cost tissue culture: An overview. J. Plant Sci. Res. 2017, 33, 181–199. [Google Scholar]
- Naik, R.; Bhushan, A.; Gupta, R.K.; Walia, A.; Gaur, A. Low cost tissue culture technologies in vegetables: A review. Int. J. Biochem. Res. Rev. 2020, 29, 66–78. [Google Scholar] [CrossRef]
- Sota, V.; Benelli, C.; Çuko, B.; Papakosta, E.; Depaoli, C.; Lambardi, M.; Kongjika, E. Evaluation of ElecTIS bioreactor for the micropropagation of Malus sylvestris (L.) Mill., an important autochthonous species of Albania. Hortic. Sci. 2021, 48, 12–21. [Google Scholar] [CrossRef]
- Lambardi, M.; Roncasaglia, R.; Previati, A.; De Carlo, A.; Dradi, G.; Da Re, F.; Calamai, L. In vitro slow growth storage of fruit rootstocks inside gas-tight or gas-permeable containers. Acta Hortic. 2006, 725, 483–488. [Google Scholar] [CrossRef]
- Ozudogru, E.A.; Benelli, C.; Dradi, G.; Lambardi, M. Effect of culture container and carbohydrate content on in vitro slow growth storage of the cherry rootstock ‘Gisela®5’. Acta Physiol. Plant. 2017, 39, 2–9. [Google Scholar] [CrossRef]
- Gianguzzi, V.; Sottile, F. Temporary immersion system as an innovative approach for in vitro propagation of Sorbus domestica L. Horticulturae 2024, 10, 164. [Google Scholar] [CrossRef]
- Blázquez, S.; Piqueras, A.; Sema, M.D.; Casas, J.L.; Fernández, J.A. Somatic embryogenesis in saffron: Optimisation through temporary immersion and polyamine metabolism. Acta Hortic. 2004, 650, 269–276. [Google Scholar] [CrossRef]
- Ilczuk, A.; Winkelmann, T.; Richartz, S.; Witomska, M.; Serek, M. In vitro propagation of Hippeastrum × chmielii Chm. Influence of flurprimidol and the culture in solid or liquid medium and in temporary immersion systems. Plant Cell Tissue Organ Cult. 2005, 83, 339–346. [Google Scholar] [CrossRef]
- Ptak, A. Leucojum aestivum L. in vitro bulbs induction and acclimatization. Cent. Eur. J. Biol. 2014, 9, 1011–1021. [Google Scholar] [CrossRef]
- Ruffoni, B.; Savona, M.; Barberini, S. Biotechnological support for the development of new Gladiolus hybrids. Floric. Ornam. Biotechnol. 2012, 6, 45–52. [Google Scholar]
- Lian, M.L.; Chakrabarty, D.; Paek, K.Y. Bulblet formation from bulbscale segments of Lilium using bioreactor system. Biol. Plant. 2003, 46, 199–203. [Google Scholar] [CrossRef]
- Seon, J.H.; Kim, Y.S.; Son, S.H.; Paek, K.Y. The fed-batch culture system using bioreactor for the bulblets production of oriental lilies. Acta Hortic. 2000, 520, 53–59. [Google Scholar] [CrossRef]
- Barberini, S.; Savona, M.; Ruffoni, B. Temporary immersion culture of Lilium bulbiferum. Acta Hortic. 2011, 900, 377–384. [Google Scholar] [CrossRef]
- Lian, M.; Chakrabarty, D.; Paek, K.Y. Growth and uptake of sucrose and mineral ions by bulblets of Lilium oriental hybrid “Casablanca” during bioreactor culture. J. Hortic. Sci. Biotechnol. 2002, 77, 253–257. [Google Scholar] [CrossRef]
- Mirmasoumi, M.; Bakhshaie, M. Effects of liquid, temporary immersion bioreactor and solid culture systems on micropropagation of Lilium ledebourii via bulblet microscales—An endangered valuable plant with ornamental potential. Prog. Biol. Sci. 2015, 5, 169–180. [Google Scholar]
- Nesi, B.; Lazzereschi, S.; Pecchioli, S.; Grassotti, A.; Burchi, G.; Cardarelli, M.; Cardona Suarez, C.M.; Colla, G. Development, selection and propagation of interspecific hybrids of Lilium. Acta Hortic. 2014, 1027, 155–164. [Google Scholar] [CrossRef]
- Wongket, A.; Pumisutapon, P. Effect of feeding frequency and period in temporary immersion system on microtuberization of potato. In Proceedings of the 24th Tri-University International Joint Seminar and Symposium 2017 Mie University, Tsu, Japan, 23–27 October 2017; pp. 123–125. [Google Scholar]
- Peña-Rojas, G.; Sanchez, H.; Barahona, I.R.; Ayme, V.A.; Segura-Turkowsky, M.; Jimenez, R.E. Alternative inputs for micropropagation of Solanum tuberosum, Ullucus tuberosus and Oxalis tuberosa in semisolid and liquid medium and temporary immersion system. Trop. Subtrop. Agroecosyst. 2020, 23, 1–15. [Google Scholar] [CrossRef]
- Al-Shareefi, M.J.H.; Abbass, J.A.; Abdulhussein, M.A.A. Effect of light sources and culture systems on microtubers production of potato (Solanum Tuberosum L.) in vitro. Int. J. Agric. Stat. Sci. 2020, 16, 679–686. [Google Scholar]
- Andriani, S.; Siregar, L.A.M.; Safni, I. Microtubers production by using Temporary Immersion System (TIS) bioreactor to potato varieties. IOP Conf. Ser. Earth Environ. Sci. 2021, 886, 012005. [Google Scholar] [CrossRef]
- Aasim, M.; Katırcı, R.; Acar, A.Ş.; Ali, S.A. A comparative and practical approach using quantum machine learning (QML) and support vector classifier (SVC) for light emitting diodes mediated in vitro micropropagation of black mulberry (Morus nigra L.). Ind. Crops Prod. 2024, 213, 118397. [Google Scholar] [CrossRef]
- Kirtis, A.; Aasim, M.; Katırcı, R. Application of artificial neural network and machine learning algorithms for modeling the in vitro regeneration of chickpea (Cicer arietinum L.). Plant Cell Tissue Organ Cult. 2022, 150, 141–152. [Google Scholar] [CrossRef]
- Pepe, M.; Hesami, M.; Small, F.; Jones, A.M.P. Comparative analysis of machine learning and evolutionary optimization algorithms for precision micropropagation of Cannabis sativa: Prediction and validation of in vitro shoot growth and development based on the optimization of light and carbohydrate sou. Front. Plant Sci. 2021, 12, 757869. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).