Overexpression of CBL-Interacting Protein Kinases 23 Improves Tolerance to Low-Nitrogen Stress in Potato Plants
Abstract
1. Introduction
2. Materials and Method
2.1. Potato Transformation and Growth Conditions
2.2. Hydroponics of Potato Seedlings and Nitrogen Content Measurement
2.3. Bioinformatics Analysis
2.4. Quantitative Real-Time PCR
2.5. Subcellular Localization Analysis
2.6. Yeast Two-Hybrid Analysis
2.7. Luciferase Complementary Imaging Assay
2.8. Statistical Assay
3. Results
3.1. StCIPK23 Phylogenetic Relationships
3.2. Subcellular Localization of StCIPK23
3.3. StCIPK23 Overexpression Improves Tolerance to Low-Nitrogen Stress
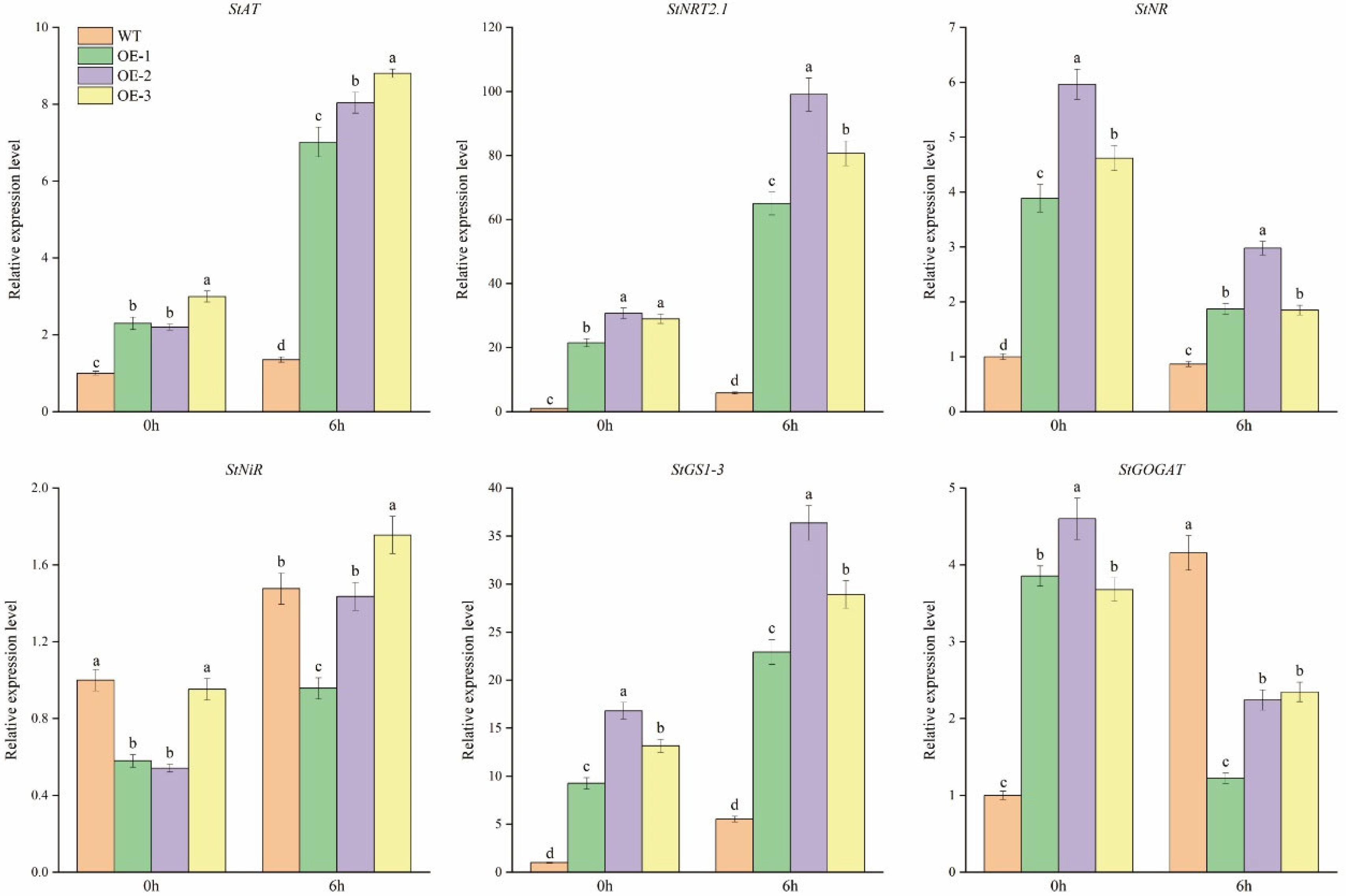
3.4. StCIPK23 Adjusted the Expression Level of Genes in Nitrogen Metabolism
3.5. Interaction between StCIPK23 and StCBL3
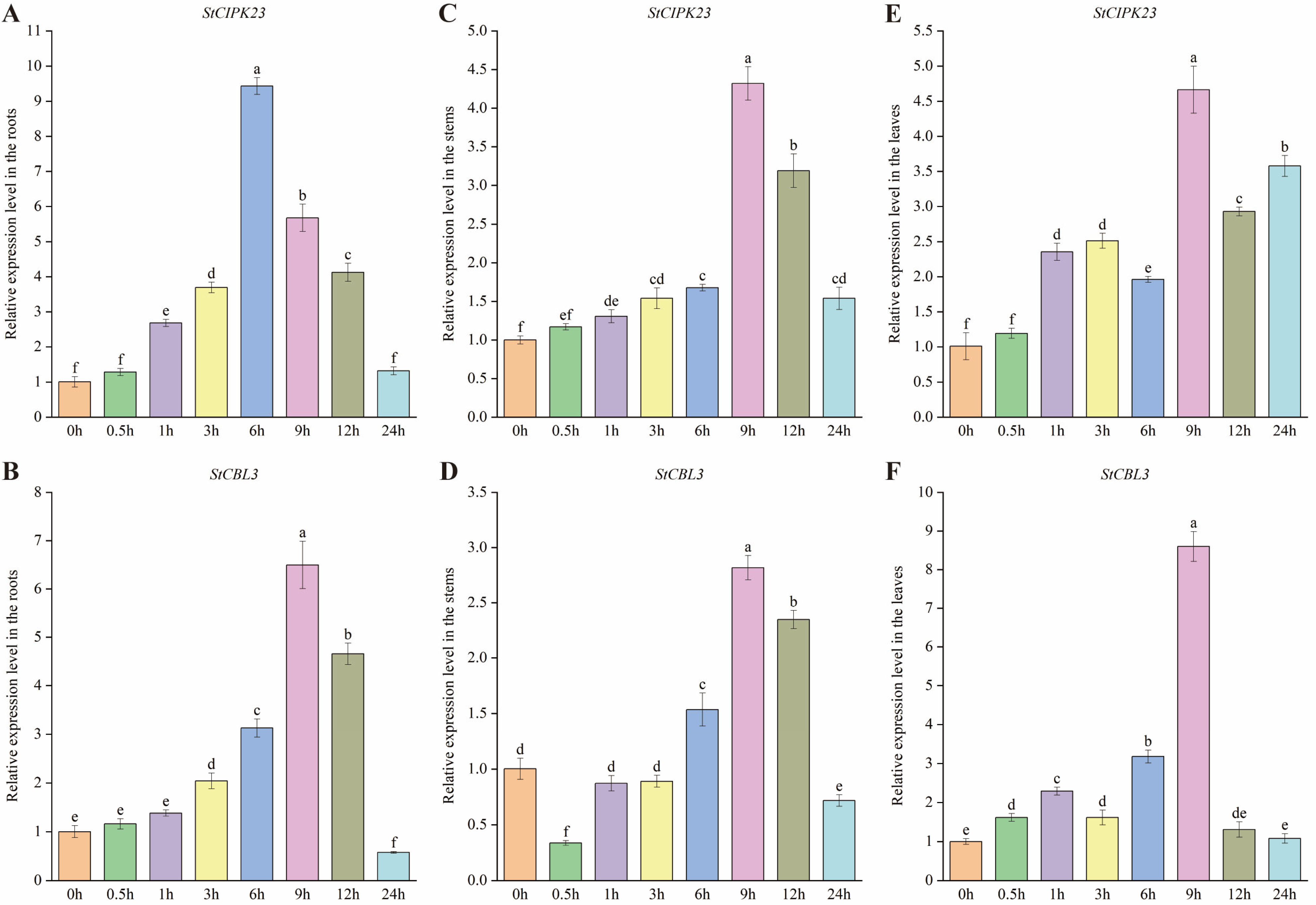
3.6. StCIPK23 and StCBL3 Share the Same Low-Nitrogen Response Pattern
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jansky, S.H.; Jin, L.P.; Xie, K.Y.; Xie, C.H.; Spooner, D.M. Potato production and breeding in China. Potato Res. 2009, 52, 57–65. [Google Scholar] [CrossRef]
- Kikuchi, A.; Huynh, H.D.; Endo, T.; Watanabe, K. Review of recent transgenic studies on abiotic stress tolerance and futuremolecular breeding in potato. Breed Sci. 2015, 65, 85–102. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, J.K.; Plett, D.; Garnett, T.; Chakrabarti, S.K.; Singh, R.K. Integrated genomics, physiology and breeding approaches for improving nitrogen use efficiency in potato: Translating knowledge from other crops. Funct. Plant Biol. 2018, 45, 587–605. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wang, R.; Gao, Y.; Wang, C.; Zhao, L.; Xu, N.; Chen, K.E.; Qi, S.; Zhang, M.; Tsay, Y.F.; et al. The Arabidopsis CPSF30-L gene plays an essential role in nitrate signaling and regulates the nitrate transceptor gene NRT1.1. New Phytol. 2017, 216, 1205–1222. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, H.; Jiang, Z.; Wang, W.; Xu, R.; Wang, Q.; Zhang, Z.; Li, A.; Liang, Y.; Ou, S.; et al. Genomic basis of geographical adaptation to soil nitrogen in rice. Nature 2021, 590, 600–605. [Google Scholar] [CrossRef]
- Tiwari, J.K.; Buckseth, T.; Devi, S.; Varshney, S.; Sahu, S.; Patil, V.U.; Zinta, R.; Ali, N.; Moudgil, V.; Singh, R.K.; et al. Physiological and genome-wide RNA-sequencing analyses identify candidate genes in a nitrogen-use efficient potato cv. Kufri Gaurav. Plant Physiol. Biochem. 2020, 154, 171–183. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Hsu, P.K.; Tsay, Y.F. Uptake, allocation and signaling of nitrate. Trends Plant Sci. 2012, 17, 458–467. [Google Scholar] [CrossRef]
- Wei, Q.R.; Yin, Y.B.; Deng, B.; Song, X.W.; Gong, Z.P.; Shi, Y. Transcriptome analysis of nitrogen-deficiency-responsive genes in two potato cultivars. Agronomy 2023, 13, 2164. [Google Scholar] [CrossRef]
- Sakuraba, Y.; Chaganzhana; Mabuchi, A.; Iba, K.; Yanagisawa, S. Enhanced NRT1.1/NPF6.3 expression in shoots improves growth under nitrogen deficiency stress in Arabidopsis. Commun. Biol. 2021, 4, 256. [Google Scholar] [CrossRef]
- Sanyal, S.K.; Mahiwal, S.; Nambiar, D.M.; Pandey, G.K. CBL-CIPK module-mediated phosphoregulation: Facts and hypothesis. Biochem. J. 2020, 477, 853–871. [Google Scholar] [CrossRef]
- Tang, R.J.; Wang, C.; Li, K.L.; Luan, S. The CBL-CIPK calcium signaling network: Unified paradigm from 20 years of discoveries. Trends Plant Sci. 2020, 25, 604–617. [Google Scholar] [CrossRef]
- Xiang, Y.; Huang, Y.; Xiong, L. Characterization of stress-responsive CIPK genes in rice for stress tolerance improvement. Plant Physiol. 2007, 144, 1416–1428. [Google Scholar] [CrossRef]
- Ma, X.; Li, Q.H.; Yu, Y.N.; Qiao, Y.M.; Haq, S.U.; Gong, Z.H. The CBL-CIPK pathway in plant response to stress signals. Int. J. Mol. Sci. 2020, 21, 5668. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Guo, X.; Xu, Y.; Li, H.; Ma, L.; Yao, X.; Weng, Y.; Guo, Y.; Liu, C.M.; Chong, K. OsCIPK7 point-mutation leads to conformation and kinase-activity change for sensing cold response. J. Integr. Plant Biol. 2019, 61, 1194–1200. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, T.; John, S.J.; Chen, M.; Chang, J.; Yang, G.; He, G. A CBL-interacting protein kinase TaCIPK27 confers drought tolerance and exogenous ABA sensitivity in transgenic Arabidopsis. Plant Physiol. Biochem. 2018, 123, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.Q.; Zheng, Z.Z.; Pan, W.H.; Pan, J.W. Functions and action mechanisms of CBL-CIPK signaling system in plants. Plant Physiol. J. 2014, 50, 641–650. [Google Scholar]
- Li, R.; Zhang, J.; Wei, J.; Wang, H.; Wang, Y.; Ma, R. Functions and mechanisms of the CBL-CIPK signaling system in plant response to abiotic stress. Prog. Nat. Sci. 2009, 19, 667–676. [Google Scholar] [CrossRef]
- Cui, X.Y.; Du, Y.T.; Fu, J.D.; Yu, T.F.; Wang, C.T.; Chen, M.; Chen, J.; Ma, Y.Z.; Xu, Z.S. Wheat CBL-interacting protein kinase 23 positively regulates drought stress and ABA responses. BMC Plant Biol. 2018, 18, 93. [Google Scholar] [CrossRef]
- Ma, X.; Gai, W.X.; Li, Y.; Yu, Y.N.; Ali, M.; Gong, Z.H. The CBL-interacting protein kinase CaCIPK13 positively regulates defence mechanisms against cold stress in pepper. J. Exp. Bot. 2022, 73, 1655–1667. [Google Scholar] [CrossRef]
- Ma, R.; Liu, W.; Li, S.; Zhu, X.; Yang, J.; Zhang, N.; Si, H. Genome-wide identification, characterization and expression analysis of the CIPK gene family in potato (Solanum tuberosum L.) and the role of StCIPK10 in response to drought and osmotic stress. Int. J. Mol. Sci. 2021, 22, 13535. [Google Scholar] [CrossRef]
- Zhu, X.; Wang, F.F.; Li, S.; Feng, Y.; Yang, J.W.; Zhang, N.; Si, H.J. Calcium-dependent protein kinase 28 maintains potato photosynthesis and its tolerance under water deficiency and osmotic stress. Int. J. Mol. Sci. 2022, 23, 8795. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zhang, N.; Wang, K.T.; Zheng, Z.Y.; Wei, J.J.; Si, H.J. CBL-Interacting Protein Kinases 18 (CIPK18) gene positively regulates drought resistance in potato. Int. J. Mol. Sci. 2023, 24, 3613. [Google Scholar] [CrossRef] [PubMed]
- Deng, K.; Wang, W.; Feng, L.; Yin, H.; Xiong, F.J.; Ren, M.Z. Target of rapamycin regulates potassium uptake in Arabidopsis and potato. Plant Physiol. Biochem. 2020, 155, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Y.; Li, J.; Zou, X.; Lu, L.M.; Li, L.Q.; Ni, S.; Liu, F. Ectopic expression of AtCIPK23 enhances tolerance against low-K+ stress in transgenic potato. Am. J. Potato Res. 2010, 88, 153–159. [Google Scholar] [CrossRef]
- Liu, J.R.; Song, J.; Zhuang, X.Y.; Lu, Y.F.; Wang, Q.; Yang, S.M.; Lu, L.M.; Wang, X.Y.; Li, L.Q. Overexpression of cytosolic glyceraldehyde-3-phosphate dehydrogenase 1 gene improves nitrogen absorption and utilization in potato. Horticulturae 2023, 9, 1105. [Google Scholar] [CrossRef]
- Yuan, T.T.; Zhu, C.L.; Li, G.Z.; Liu, Y.; Yang, K.B.; Li, Z.; Song, X.Z.; Gao, Z.M. An integrated regulatory network of mRNAs, micro RNAs, and IncRNAs involved in nitrogen metabolism of moso bamboo. Front. Genet. 2022, 13, 854346. [Google Scholar] [CrossRef] [PubMed]
- Zebarth, B.J.; Rosen, C.J. Research perspective on nitrogen bmp development for potato. Am. J. Potato Res. 2007, 84, 3–18. [Google Scholar] [CrossRef]
- O’Brien, J.A.A.; Vega, A.; Bouguyon, E.; Krouk, G.; Gojon, A.; Coruzzi, G.; Gutiérrez, R.A.A. Nitrate transport, sensing, and responses in plants. Mol. Plant. 2016, 9, 837–856. [Google Scholar] [CrossRef]
- Kuwata, M.; Izawa, K.; Takeba, G. Comparison between induction of flowering by nitrogen deficiency and that by short days or continuous irradiation with blue or low-intensity white light in lemna paucicostata. Plant Cell Physiol. 1989, 30, 1139–1144. [Google Scholar]
- Footitt, S.; Huang, Z.Y.; Clay, H.A.; Mead, A.; Finch-Savage, W.E. Temperature, light and nitrate sensing coordinate Arabidopsis seed dormancy cycling, resulting in winter and summer annual phenotypes. Plant J. 2013, 74, 1003–1015. [Google Scholar] [CrossRef]
- McAinsh, M.R.; Pittman, J.K. Shaping the calcium signature. New Phytol. 2009, 181, 275–294. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.Y.; Wang, Y.P.; Yao, J.; Yang, X.; Wu, K.Y.; Teng, G.X.; Gong, B.C.; Xu, Y. Genome–wide Investigation of the CBL–CIPK gene family in oil persimmon: Evolution, function and expression analysis during development and stress. Horticulturae 2023, 9, 30. [Google Scholar] [CrossRef]
- Tang, R.J.; Zhao, F.G.; Garcia, V.J.; Kleist, T.J.; Yang, L.; Zhang, H.X.; Luan, S. Tonoplast CBL-CIPK calcium signaling network regulates magnesium homeostasis in Arabidopsis. Proc. Natl. Acad. Sci. USA 2015, 112, 3134–3139. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.W.; Fan, H.M.; Sun, C.H.; Yuan, M.Y.; Geng, X.; Ding, X.; Ma, R.; Yan, N.; Sun, X.; Zheng, C.S. New insights into the role of chrysanthemum calcineurin B-like interacting protein kinase CmCIPK23 in nitrate signaling in Arabidopsis roots. Sci. Rep. 2022, 12, 1018. [Google Scholar] [CrossRef]
- Li, P.; Tangchun, Z.; Li, L.; Zhuo, X.; Jiang, L.; Wang, J.; Cheng, T.; Zhang, Q. Identification and comparative analysis of the CIPK gene family and characterization of the cold stress response in the woody plant Prunus mume. Peer J. 2019, 7, e6847. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Gai, W.X.; Qiao, Y.M.; Ali, M.; Wei, A.M.; Luo, D.X.; Li, Q.H.; Gong, Z.H. Identification of CBL and CIPK gene families and functional characterization of CaCIPK1 under Phytophthora capsici in pepper (Capsicum annuum L.). BMC Genom. 2019, 20, 775. [Google Scholar] [CrossRef]
- Kim, B.G.; Waadt, R.; Cheong, Y.H.; Pandey, G.K.; Dominguez-Solis, J.R.; Schültke, S.; Lee, S.C.; Kudla, J.; Luan, S. The calcium sensor CBL10 mediates salt tolerance by regulating ion homeostasis in Arabidopsis. Plant J. 2007, 52, 473–484. [Google Scholar] [CrossRef]
- D’Angelo, C.; Weinl, S.; Batistic, O.; Pandey, G.K.; Cheong, Y.H.; Schültke, S.; Albrecht, V.; Ehlert, B.; Schulz, B.; Harter, K.; et al. Alternative complex formation of the Ca2+-regulated protein kinase CIPK1 controls abscisic acid-dependent and independent stress responses in Arabidopsis. Plant J. 2006, 48, 857–872. [Google Scholar] [CrossRef]
- Batistič, O.; Sorek, N.; Schültke, S.; Yalovsky, S.; Kudla, J. Dual fatty acyl modification determines the localization and plasma membrane targeting of CBL/CIPK Ca2+ signaling complexes in Arabidopsis. Plant Cell 2008, 20, 1346–1362. [Google Scholar] [CrossRef]
- Xu, J.; Li, H.D.; Chen, L.Q.; Wang, Y.; Liu, L.L.; He, L.; Wu, W.H. A protein kinase, interacting with two calcineurin B-like proteins, regulates K+ transporter AKT1 in Arabidopsis. Cell 2006, 125, 1347–1360. [Google Scholar] [CrossRef]
- Gao, S.W.; Yang, Y.Y.; Guo, J.L.; Zhang, X.; Feng, M.X.; Su, Y.C.; Que, Y.X.; Xu, L.P. Ectopic expression of sugarcane ScAMT1.1 has the potential to improve ammonium assimilation and grain yield in transgenic rice under low nitrogen stress. Int. J. Mol. Sci. 2023, 24, 1595. [Google Scholar] [CrossRef] [PubMed]
- Mhamdi, M.; Abid, G.; Chikh, R.H.; Razgallah, N.; Hassen, A. Effect of genotype and growing season on nitrate accumulation and expression patterns of nitrate transporter genes in potato (Solanum tuberosum L. ) Arch. Agron. Soil Sci. 2016, 11, 1508–1520. [Google Scholar] [CrossRef]
- Ródenas, R.; Vert, G. Regulation of root nutrient transporters by CIPK23: ‘One Kinase to Rule Them All’. Plant Cell Physiol. 2021, 62, 553–563. [Google Scholar] [CrossRef]
- Hu, H.C.; Wang, Y.Y.; Tsay, Y.F. AtCIPK8, a CBL-interacting protein kinase, regulates the low-affinity phase of the primary nitrate response. Plant J. 2009, 57, 264–278. [Google Scholar] [CrossRef]
- Ma, Q.J.; Zhao, C.Y.; Hu, S.; Zuo, K.J. The calcium sensor CBL7 odulates plant responses to low nitrate in Arabidopsis. Biochem. Biophys. Res.Commun. 2015, 468, 59–65. [Google Scholar] [CrossRef]
- Ma, Q.J.; Zhao, C.Y.; Hu, S.; Zuo, K.J. Arabidopsis calcium-dependent protein kinase CPK6 regulates drought tolerance under high nitrogen by the phosphorylation of NRT1.1. J. Exp. Bot. 2023, 74, 5682–5693. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.U.; Li, P.H.; Amjad, H.; Khan, A.Q.; Arafat, Y.; Waqas, M.; Li, Z.; Noman, A.; Islam, W.; Wu, L.K.; et al. Exploring the potential of overexpressed OsCIPK2 rice as a nitrogen utilization efficient crop and analysis of its associated rhizo-compartmental microbial communities. Int. J. Mol. Sci. 2019, 20, 3636. [Google Scholar] [CrossRef]
- Wang, W.; Hu, B.; Yuan, D.; Liu, Y.; Che, R.; Hu, Y.; Ou, S.; Liu, Y.; Zhang, Z.; Wang, H. Expression of the nitrate transporter gene osnrt1. 1a/osnpf6. 3 confers high yield and early maturation in rice. Plant Cell 2018, 30, 638–651. [Google Scholar] [CrossRef]
- Fan, X.; Tang, Z.; Tan, Y.; Zhang, Y.; Luo, B.; Yang, M.; Lian, X.; Shen, Q.; Miller, A.J.; Xu, G. Overexpression of a ph-sensitive nitrate transporter in rice increases crop yields. Proc. Natl. Acad. Sci. USA 2016, 113, 7118–7123. [Google Scholar] [CrossRef]
- Straub, T.; Ludewig, U.; Neuhäuser, B. The kinase CIPK23 inhibits ammonium transport in Arabidopsis thaliana. Plant Cell 2017, 29, 409–422. [Google Scholar] [CrossRef]
- Ho, C.H.; Lin, S.H.; Hu, H.C.; Tsay, Y.F. CHL1 functions as a nitrate sensor in plants. Cell 2009, 138, 1184–1194. [Google Scholar] [CrossRef]
- Verma, P.; Sanyal, S.K.; Pandey, G.K. Ca2+-CBL-CIPK: A modulator system for efficient nutrient acquisition. Plant Cell Rep. 2021, 40, 2111–2122. [Google Scholar] [CrossRef]
- Liu, K.H.; Liu, M.H.; Lin, Z.W.; Wang, Z.F.; Chen, B.Q.; Liu, C.; Guo, A.P.; Konishi, M.; Yanagisawa, S.C.; Wagner, G.; et al. NIN-like protein 7 transcription factor is a plant nitrate sensor. Science 2022, 377, 1419–1425. [Google Scholar] [CrossRef] [PubMed]
- Fredes, I.; Moreno, S.; Díaz, F.P.; Gutiérrez, R.A. Nitrate signaling and the control of Arabidopsis growth and development. Curr. Opin. Plant Biol. 2019, 47, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Ju, C.; Zhang, Z.; Deng, J.; Miao, C.; Wang, Z.; Wallrad, L.; Javed, L.; Fu, D.; Zhang, T.; Kudla, J.; et al. Ca2+-dependent successive phosphorylation of vacuolar transporter MTP8 by CBL2/3-CIPK3/9/26 and CPK5 is critical for manganese homeostasis in Arabidopsis. Mol. Plant 2022, 15, 419–437. [Google Scholar] [CrossRef]
- Yin, X.; Xia, Y.; Xie, Q.; Cao, Y.; Wang, Z.; Hao, G.; Song, J.; Zhou, Y.; Jiang, X. The protein kinase complex CBL10-CIPK8-SOS1 functions in Arabidopsis to regulate salt tolerance. J. Exp. Bot. 2020, 71, 1801–1814. [Google Scholar] [CrossRef]
- Kudla, J.; Xu, Q.; Harter, K.; Gruissem, W.; Luan, S. Genes for calcineurin B-like proteins in Arabidopsis are differentially regulated by stress signals. Proc. Natl. Acad. Sci. USA 1999, 96, 4718–4723. [Google Scholar] [CrossRef]
- Maierhofer, T.; Diekmann, M.; Offenborn, J.N.; Lind, C.; Bauer, H.; Hashimoto, K.; Al-Rasheid, K.A.S.; Luan, S.; Kudla, J.; Geiger, D.; et al. Site- and kinase-specific phosphorylation-mediated activation of SLAC1, a guard cell anion channel stimulated by abscisic acid. Plant Biol. 2014, 7, ra86. [Google Scholar] [CrossRef] [PubMed]
- Chu, L.C.; Offenborn, J.N.; Steinhorst, L.; Wu, X.N.; Xi, L.; Li, Z.; Jacquot, A.; Lejay, L.; Kudla, J.; Schulze, W.X. Plasma membrane calcineurin B-like calcium-ion sensor proteins function in regulating primary root growth and nitrate uptake by affecting global phosphorylation patterns and microdomain protein distribution. New Phytol. 2020, 229, 2223–2237. [Google Scholar] [CrossRef]
- Du, X.Q.; Wang, F.L.; Li, H.; Jing, S.; Yu, M.; Li, J.G.; Wu, W.H.; Kudla, J.; Wang, Y. The transcription factor MYB59 regulates K+/NO3- translocation in the Arabidopsis response to low K+ stress. Plant Cell 2019, 31, 699–714. [Google Scholar] [CrossRef]
- Wang, Z.F.; Mi, T.W.; Gao, Y.Q.; Feng, H.Q.; Wu, W.H.; Wang, Y. STOP1 Regulates LKS1 Transcription and coordinates K+/NH4+ balance in Arabidopsis response to low-K+ stress. Int. J. Mol. Sci. 2022, 23, 383. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, F.; Lu, Y.; Li, Z.; Zhang, L.; Xie, M.; Ren, B.; Lu, L.; Li, L.; Yang, C. Overexpression of CBL-Interacting Protein Kinases 23 Improves Tolerance to Low-Nitrogen Stress in Potato Plants. Horticulturae 2024, 10, 526. https://doi.org/10.3390/horticulturae10050526
Huang F, Lu Y, Li Z, Zhang L, Xie M, Ren B, Lu L, Li L, Yang C. Overexpression of CBL-Interacting Protein Kinases 23 Improves Tolerance to Low-Nitrogen Stress in Potato Plants. Horticulturae. 2024; 10(5):526. https://doi.org/10.3390/horticulturae10050526
Chicago/Turabian StyleHuang, Feiyun, Yifei Lu, Zi Li, Lang Zhang, Minqiu Xie, Bi Ren, Liming Lu, Liqin Li, and Cuiqin Yang. 2024. "Overexpression of CBL-Interacting Protein Kinases 23 Improves Tolerance to Low-Nitrogen Stress in Potato Plants" Horticulturae 10, no. 5: 526. https://doi.org/10.3390/horticulturae10050526
APA StyleHuang, F., Lu, Y., Li, Z., Zhang, L., Xie, M., Ren, B., Lu, L., Li, L., & Yang, C. (2024). Overexpression of CBL-Interacting Protein Kinases 23 Improves Tolerance to Low-Nitrogen Stress in Potato Plants. Horticulturae, 10(5), 526. https://doi.org/10.3390/horticulturae10050526



