Antioxidative Response of Duckweed (Lemna minor L.) to Rhizosphere-Associated Pseudomonas Strains and Exogenous Indole-3-Acetic Acid
Abstract
1. Introduction
2. Materials and Methods
2.1. Duckweed (L. minor L.) Culture Conditions and Co-Cultivation with Pseudomonas Strains
2.2. Photosynthetic Pigment Content
2.3. Oxidative Stress Assessment
2.3.1. Localization of Superoxide Anion (O2•−) Production and Hydrogen Peroxide (H2O2) Accumulation
2.3.2. Lipid Peroxidation and Hydrogen Peroxide (H2O2) Content
2.3.3. Analysis of Antioxidant Enzyme Activity
2.4. Statistical Analysis
3. Results and Discussion
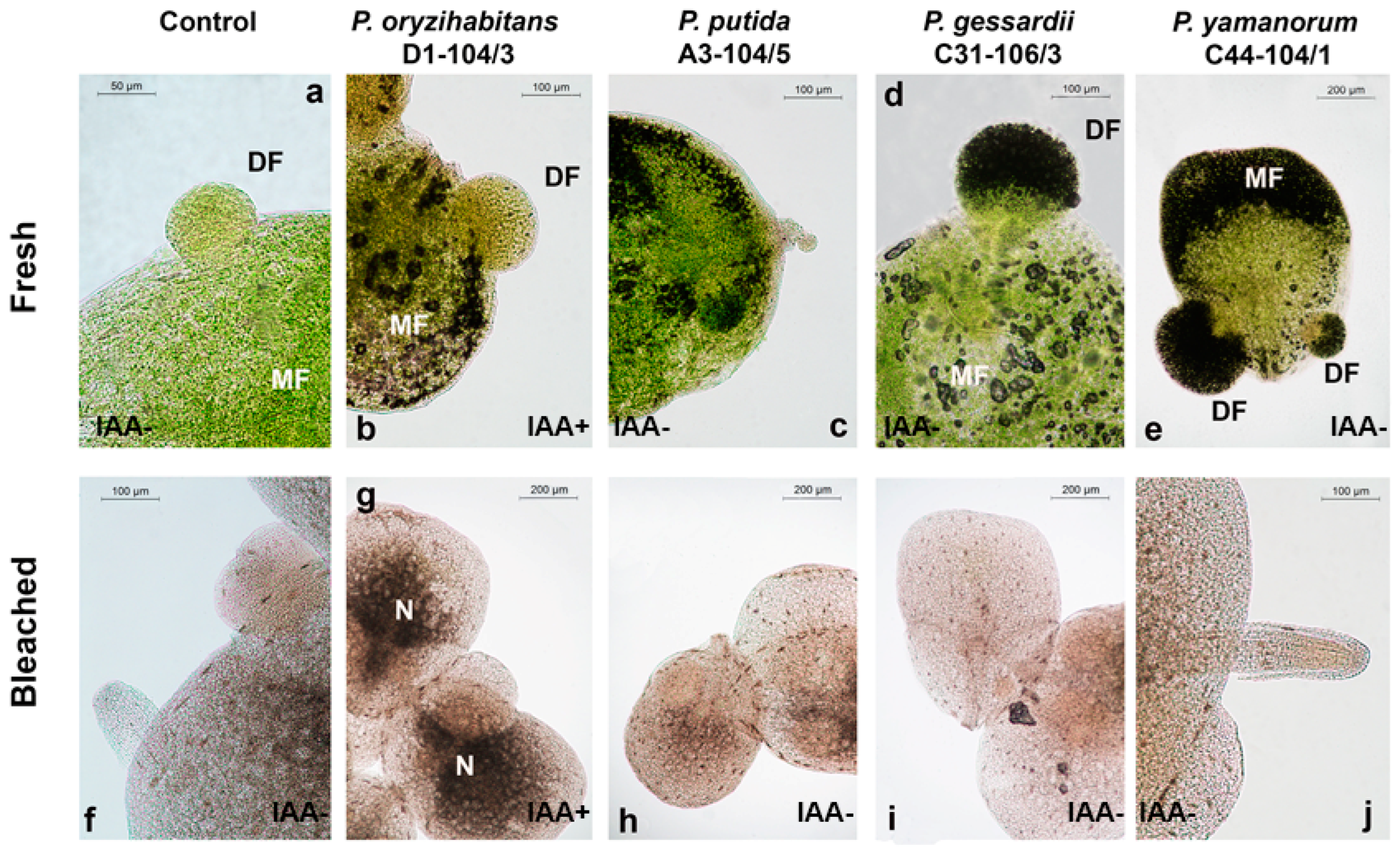
3.1. Growth Parameters of Duckweeds and Surface Density of Pseudomonas Bacteria
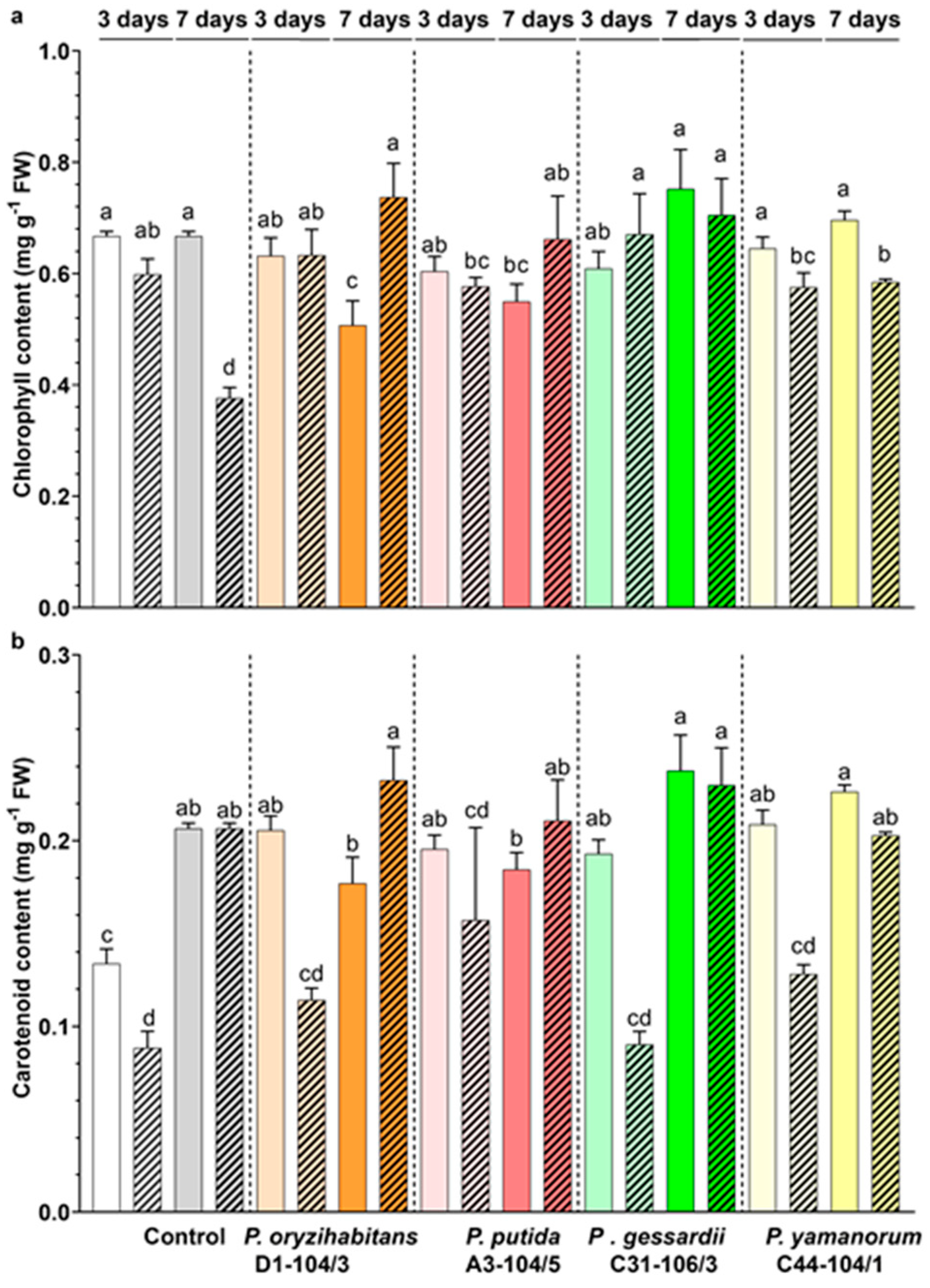
3.2. Effects of Pseudomonas Strains and IAA on the Photosynthetic Pigment Content in Duckweeds
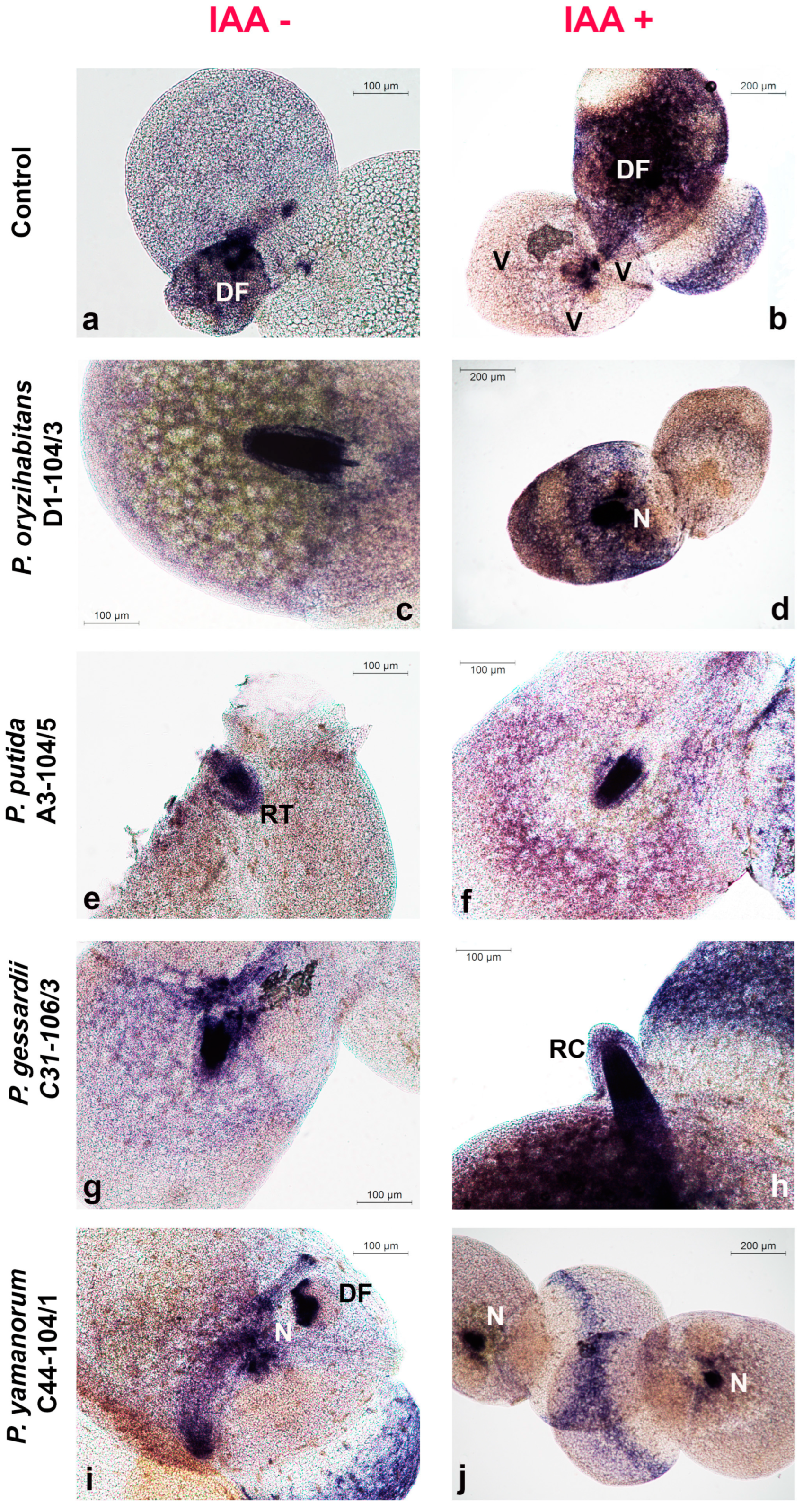
3.3. Histochemical Assessment of O2•− Production and H2O2 Accumulation
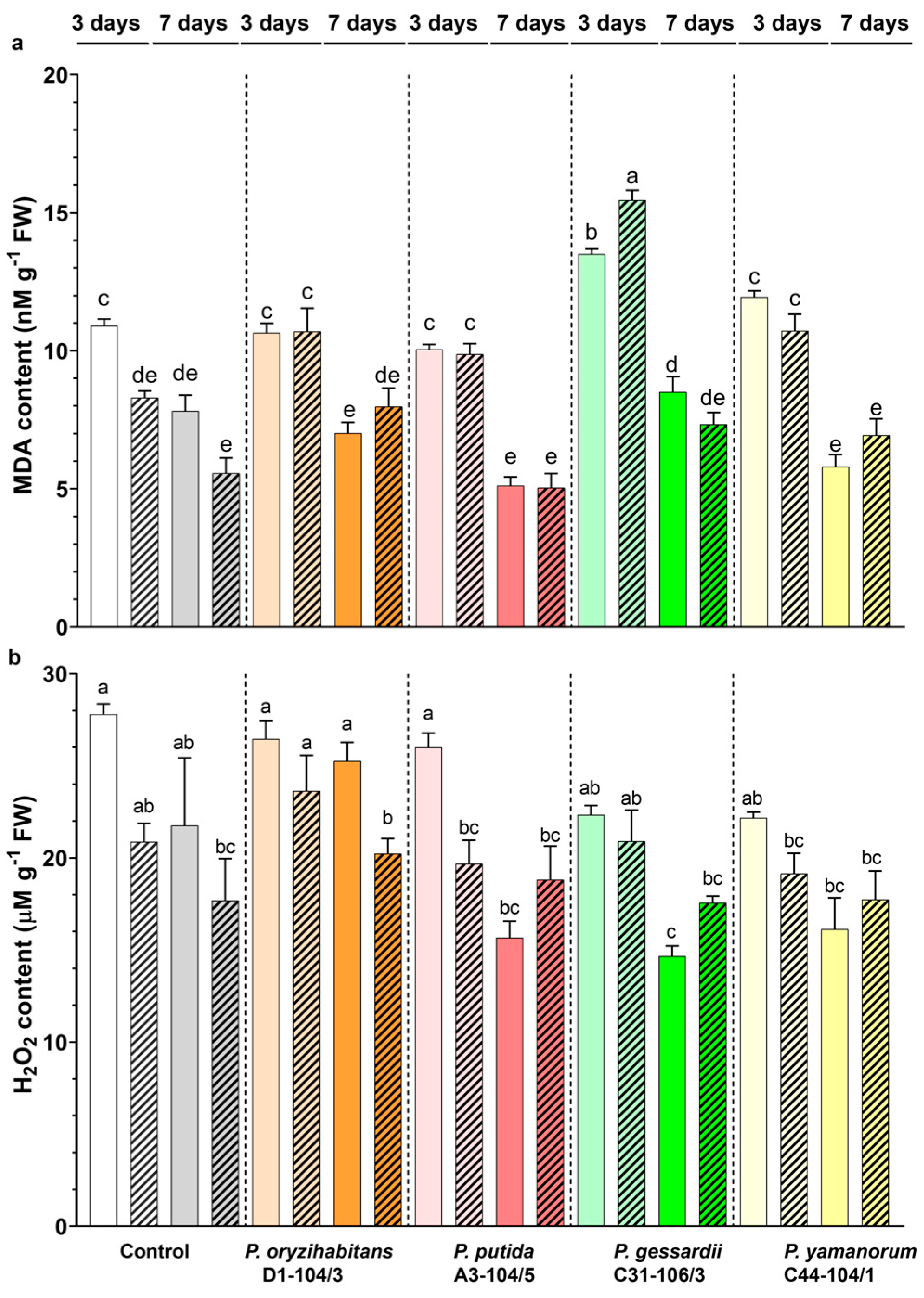
3.4. Effects of Pseudomonas Strains and IAA on the MDA and H2O2 Content in Duckweeds
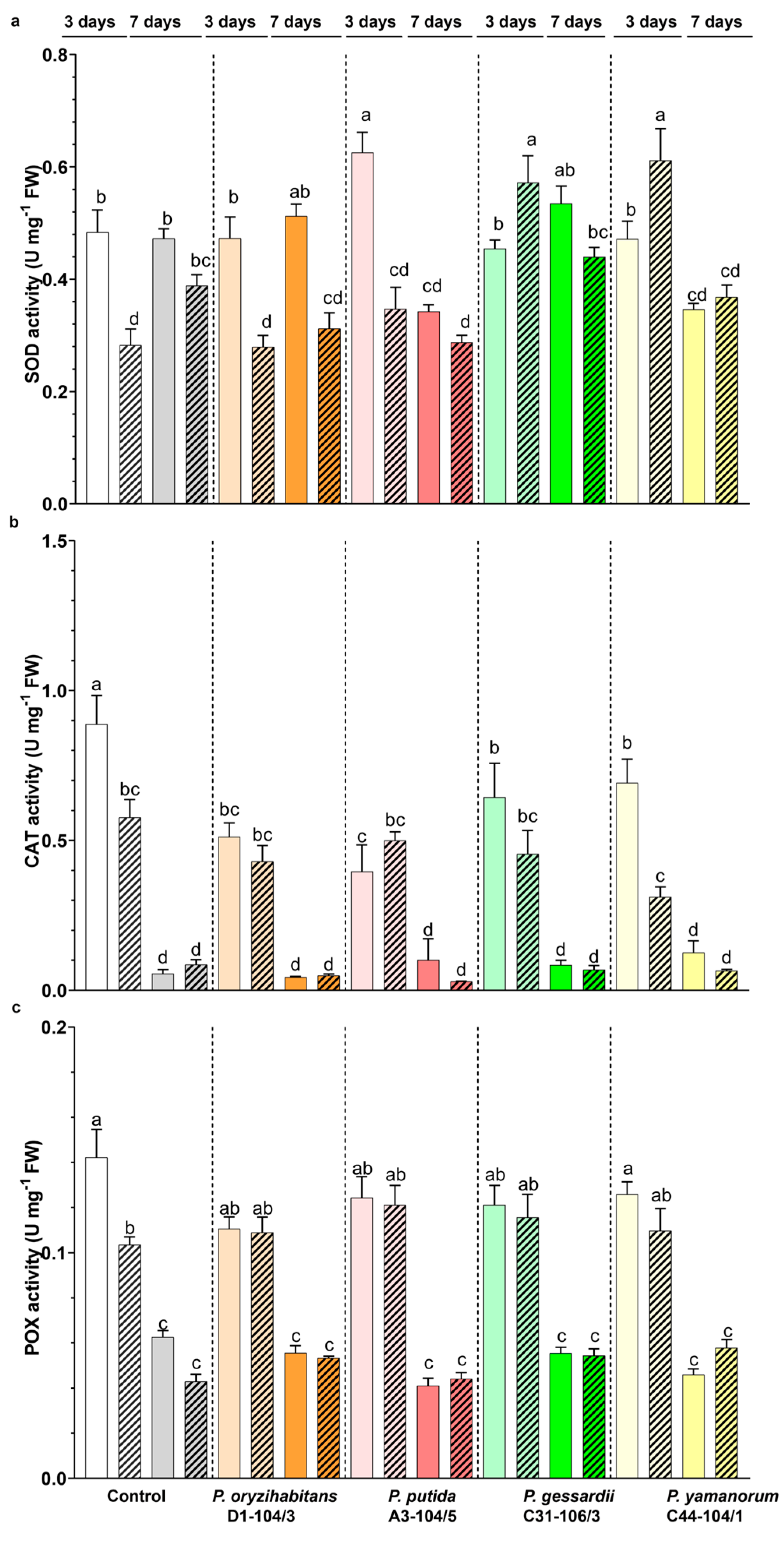
3.5. Effects of Pseudomonas Strains and IAA on the Antioxidant Enzyme Activity of Duckweeds
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PGPB | plant-growth-promoting bacteria |
| ACC | aminocyclopropane-1-carboxylic acid |
| IAA | indole-3-acetic acid |
| GA | gibberellic acid |
| CAT | catalase |
| POX | peroxidase |
| SOD | superoxide dismutase |
| MDA | malondialdehyde |
| H2O2 | hydrogen peroxide |
| O2•− | superoxide anion |
| ROS | reactive oxygen species |
| TCA | trichloroacetic acid |
| TBA | tiobarbiturate |
| MS medium | Murashige–Skoog medium |
| PVPP | polyvynilpyrrolidone phosphate |
| DTT | dithiothreitol |
| PMSF | phenylmethylsulfonyl fluoride |
| NBT | nitroblue tetrazolium |
| DAB | diaminobenzidine-3,3’-tetrachloride |
References
- Ziegler, P.; Appenroth, K.J.; Sree, K.S. Survival Strategies of Duckweeds, the World’s Smallest Angiosperms. Plants 2023, 12, 2215. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, P.; Sree, K.S.; Appenroth, K.J. Duckweeds for water remediation and toxicity testing. Toxicol. Environ. Chem. 2016, 98, 1127–1154. [Google Scholar] [CrossRef]
- Escobar, C.M.; Escobar, A.C. Duckweed: A tiny aquatic plant with enormous potential for bioregenerative life support systems. In Proceedings of the International Conference on Environmental Systems, Charleston, South Carolina, 16–20 July 2017; pp. 1–9. Available online: http://www.fao.org/ag/againfo/resources/documents/DW/Dw2.htm (accessed on 16 April 2024).
- Xu, J.; Shen, Y.; Zheng, Y.; Smith, G.; Sun, X.S.; Wang, D.; Zhao, Y.; Zhang, W.; Li, Y. Duckweed (Lemnaceae) for potentially nutritious human food: A review. Food Rev. Int. 2023, 39, 3620–3634. [Google Scholar] [CrossRef]
- Acosta, K.; Appenroth, K.J.; Borisjuk, L.; Edelman, M.; Heinig, U.; Jansen, M.A.; Oyama, T.; Pasaribu, B.; Schubert, I.; Sorrels, S.; et al. Return of the Lemnaceae: Duckweed as a model plant system in the genomics and postgenomics era. Plant Cell 2021, 33, 3207–3234. [Google Scholar] [CrossRef] [PubMed]
- Ishizawa, H.; Kuroda, M.; Morikawa, M.; Ike, M. Differential oxidative and antioxidative response of duckweed Lemna minor toward plant growth promoting/inhibiting bacteria. Plant Physiol. Biochem. 2017, 118, 667–673. [Google Scholar] [CrossRef] [PubMed]
- Varga, M.; Horvatić, J.; Čelić, A. Short term exposure of Lemna minor and Lemna gibba to mercury, cadmium and chromium. Cent. Eur. J. Biol. 2013, 8, 1083–1093. [Google Scholar] [CrossRef]
- Ishizawa, H.; Tada, M.; Kuroda, M.; Inoue, D.; Ike, M. Performance of plant growth-promoting bacterium of duckweed under different kinds of abiotic stress factors. Biocatal. Agric. Biotechnol. 2019, 19, 101146. [Google Scholar] [CrossRef]
- Basiglini, E.; Pintore, M.; Forni, C. Effects of treated industrial wastewaters and temperatures on growth and enzymatic activities of duckweed (Lemna minor L.). Ecotoxicol. Environ. Saf. 2018, 153, 54–59. [Google Scholar] [CrossRef]
- Vandenkoornhuyse, P.; Quaiser, A.; Duhamel, M.; Le Van, A.; Dufresne, A. The importance of the microbiome of the plant holobiont. New Phytol. 2015, 206, 1196–1206. [Google Scholar] [CrossRef]
- Ishizawa, H.; Kuroda, M.; Morikawa, M.; Ike, M. Evaluation of environmental bacterial communities as a factor affecting the growth of duckweed Lemna minor. Biotechnol. Biofuels 2017, 10, 62. [Google Scholar] [CrossRef]
- Gómez-Godínez, L.J.; Aguirre-Noyola, J.L.; Martínez-Romero, E.; Arteaga-Garibay, R.I.; Ireta-Moreno, J.; Ruvalcaba-Gómez, J.M. A Look at Plant-Growth-Promoting Bacteria. Plants 2023, 12, 1668. [Google Scholar] [CrossRef] [PubMed]
- Rouphael, Y.; Colla, G. Editorial: Biostimulants in Agriculture. Front. Plant Sci. 2020, 11, 40. [Google Scholar] [CrossRef] [PubMed]
- Nordstedt, N.P.; Jones, M.L. Isolation of Rhizosphere Bacteria That Improve Quality and Water Stress Tolerance in Greenhouse Ornamentals. Front. Plant Sci. 2020, 11, 826. [Google Scholar] [CrossRef] [PubMed]
- Ware, A.; Jones, D.H.; Flis, P.; Chrysanthou, E.; Smith, K.E.; Kümpers, B.M.; Yant, L.; Atkinson, J.A.; Wells, D.M.; Bhosale, R.; et al. Loss of ancestral function in duckweed roots is accompanied by progressive anatomical reduction and a re-distribution of nutrient transporters. Curr. Biol. 2023, 33, 1795–1802.e4. [Google Scholar] [CrossRef] [PubMed]
- Utami, D.; Kawahata, A.; Sugawara, M.; Jog, R.N.; Miwa, K.; Morikawa, M. Effect of exogenous general plant growth regulators on the growth of the Duckweed Lemna minor. Front. Chem. 2018, 6, 251. [Google Scholar] [CrossRef] [PubMed]
- Popržen, T.; Nikoli, I. Characterization of the IAA-Producing and -Degrading Pseudomonas Strains Regulating Growth of the Common Duckweed (Lemna minor L.). Int. J. Mol. Sci. 2023, 24, 17207. [Google Scholar] [CrossRef]
- Bakker, P.A.H.M.; Berendsen, R.L.; Van Pelt, J.A.; Vismans, G.; Yu, K.; Li, E.; Van Bentum, S.; Poppeliers, S.W.M.; Gil, J.J.S.; Zhang, H.; et al. The Soil-Borne Identity and Microbiome-Assisted Agriculture: Looking Back to the Future. Mol. Plant 2020, 13, 1394–1401. [Google Scholar] [CrossRef] [PubMed]
- Albright, M.B.N.; Louca, S.; Winkler, D.E.; Feeser, K.L.; Haig, S.-J.; Whiteson, K.L.; Emerson, J.B.; Dunbar, J. Solutions in microbiome engineering: Prioritizing barriers to organism establishment. ISME J. 2022, 16, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Đurić, M.; Subotić, A.; Prokić, L.; Trifunović-Momčilov, M.; Milošević, S. Alterations in Physiological, Biochemical, and Molecular Responses of Impatiens walleriana to Drought by Methyl Jasmonate Foliar Application. Genes 2023, 14, 1072. [Google Scholar] [CrossRef]
- Lipid Peroxidation Protocol (Health & Packer, 1968). Available online: https://plant-stress.weebly.com/uploads/7/6/3/3/7633398/lipid_peroxidation_protocol.pdf (accessed on 16 April 2024).
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants protective role of exogenous polyamines. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Radulović, O.; Stanković, S.; Stanojević, O.; Vujčić, Z.; Dojnov, B.; Trifunović-Momčilov, M.; Marković, M. Antioxidative responses of duckweed (Lemna minor L.) to phenol and rhizosphere-associated bacterial strain hafnia paralvei c32-106/3. Antioxidants 2021, 10, 1719. [Google Scholar] [CrossRef] [PubMed]
- Milošević, S.; Simonović, A.; Cingel, A.; Jevremović, S.; Todorović, S.; Filipović, B.; Subotić, A. Response of antioxidative enzymes to long-term Tomato spotted wilt virus infection and virus elimination by meristem-tip culture in two Impatiens species. Physiol. Mol. Plant Pathol. 2012, 79, 79–88. [Google Scholar] [CrossRef]
- Beyer, W.F.; Fridovich, I. Assaying for superoxide dismutase activity: Some large consequences of minor changes in conditions. Anal. Biochem. 1987, 161, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Antonić, D.; Milošević, S.; Cingel, A.; Lojić, M.; Trifunović-Momčilov, M.; Petrić, M.; Subotić, A.; Simonović, A. Effects of exogenous salicylic acid on Impatiens walleriana L. grown in vitro under polyethylene glycol-imposed drought. S. Afr. J. Bot. 2016, 105, 226–233. [Google Scholar] [CrossRef]
- Rariz, G.; Ferrando, L.; Echegoyen, N.; Scavino, A.F. Antagonism between Azospirillum brasilense Az39 and Pseudomonas oryzihabitans, a seed-borne endophyte, in growing rice plants. Rev. Agronómica Noroeste Argent. 2017, 37, 45–56. [Google Scholar]
- Hardoim, P.R.; Hardoim, C.C.P.; van Overbeek, L.S.; van Elsas, J.D. Dynamics of seed-borne rice endophytes on early plant growth stages. PLoS ONE 2012, 7, e30438. [Google Scholar] [CrossRef] [PubMed]
- Pandit, A.; Adholeya, A.; Cahill, D.; Brau, L.; Kochar, M. Microbial biofilms in nature: Unlocking their potential for agricultural applications. J. Appl. Microbiol. 2020, 129, 199–211. [Google Scholar] [CrossRef] [PubMed]
- Kraft, M.; Kuglitsch, R.; Kwiatkowski, J.; Frank, M.; Grossmann, K. Indole-3-acetic acid and auxin herbicides up-regulate 9-cis-epoxycarotenoid dioxygenase gene expression and abscisic acid accumulation in cleavers (Galium aparine): Interaction with ethylene. J. Exp. Bot. 2007, 58, 1497–1503. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Alonso, M.-M.; Ortiz-García, P.; Moya-Cuevas, J.; Lehmann, T.; Sánchez-Parra, B.; Björk, R.G.; Karim, S.; Amirjani, M.R.; Aronsson, H.; Wilkinson, M.D.; et al. Endogenous indole-3-acetamide levels contribute to the crosstalk between auxin and abscisic acid, and trigger plant stress responses in Arabidopsis. J. Exp. Bot. 2021, 72, 459–475. [Google Scholar] [CrossRef]
- Gamalero, E.; Glick, B.R. Recent Advances in Bacterial Amelioration of Plant Drought and Salt Stress. Biology 2022, 11, 437. [Google Scholar] [CrossRef]
- Berrios, L.; Rentsch, J.D. Linking Reactive Oxygen Species (ROS) to Abiotic and Biotic Feedbacks in Plant Microbiomes: The Dose Makes the Poison. Int. J. Mol. Sci. 2022, 23, 4402. [Google Scholar] [CrossRef]
- Tzipilevich, E.; Russ, D.; Dangl, J.L.; Benfey, P.N. Plant immune system activation is necessary for efficient root colonization by auxin-secreting beneficial bacteria. Cell Host Microbe 2021, 29, 1507–1520.e4. [Google Scholar] [CrossRef] [PubMed]
- López-Farfán, D.; Reyes-Darias, J.A.; Matilla, M.A.; Krell, T. Concentration dependent effect of plant root exudates on the chemosensory systems of Pseudomonas putida KT2440. Front. Microbiol. 2019, 10, 432640. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xu, Z.; Chen, L.; Xun, W.; Shu, X.; Chen, Y.; Sun, X.; Wang, Z.; Ren, Y.; Shen, Q.; et al. Root colonization by beneficial rhizobacteria. FEMS Microbiol. Rev. 2024, 48, fuad066. [Google Scholar] [CrossRef] [PubMed]
- Schlechter, R.O.; Miebach, M.; Remus-Emsermann, M.N.P. Driving factors of epiphytic bacterial communities: A review. J. Adv. Res. 2019, 19, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Karpinska, B.; Foyer, C.H. Superoxide signalling and antioxidant processing in the plant nucleus. J. Exp. Bot. 2024, erae090. [Google Scholar] [CrossRef] [PubMed]
- Radulović, O.; Stanković, S.; Uzelac, B.; Tadić, V.; Trifunović-Momčilov, M.; Lozo, J.; Marković, M. Phenol removal capacity of the common duckweed (Lemna minor L.) and six phenol-resistant bacterial strains from its rhizosphere: In vitro evaluation at high phenol concentrations. Plants 2020, 9, 599. [Google Scholar] [CrossRef]
- Lemon, G.D.; Posluszny, U. Comparative shoot development and evolution in the Lemnaceae. Int. J. Plant Sci. 2000, 161, 733–748. [Google Scholar] [CrossRef]
- Huang, H.; Ullah, F.; Zhou, D.X.; Yi, M.; Zhao, Y. Mechanisms of ROS regulation of plant development and stress responses. Front. Plant Sci. 2019, 10, 800. [Google Scholar] [CrossRef]
- Zandalinas, S.I.; Fichman, Y.; Mittler, R. Vascular bundles mediate systemic reactive oxygen signaling during light stress. Plant Cell 2020, 32, 3425–3435. [Google Scholar] [CrossRef]
- Takahashi, H.; Yamauchi, T.; Colmer, T.D.; Nakazono, M. Aerenchyma formation in plants. Plant Cell Monogr. 2014, 21, 247–265. [Google Scholar] [CrossRef] [PubMed]
- Ishizawa, H.; Kuroda, M.; Inoue, D.; Morikawa, M.; Ike, M. Community dynamics of duckweed-associated bacteria upon inoculation of plant growth-promoting bacteria. FEMS Microbiol. Ecol. 2020, 96, fiaa101. [Google Scholar] [CrossRef] [PubMed]
- De Vleesschauwer, D.; Djavaheri, M.; Bakker, P.A.H.M.; Höfte, M. Pseudomonas fluorescens WCS374r-induced systemic resistance in rice against Magnaporthe oryzae is based on pseudobactin-mediated priming for a salicylic acid-repressible multifaceted defense response. Plant Physiol. 2008, 148, 1996–2012. [Google Scholar] [CrossRef] [PubMed]
- Xing, Z.; Wu, X.; Zhao, J.; Zhao, X.; Zhu, X.; Wang, Y.; Fan, H.; Chen, L.; Liu, X.; Duan, Y. Isolation and identification of induced systemic resistance determinants from Bacillus simplex Sneb545 against Heterodera glycines. Sci. Rep. 2020, 10, 11586. [Google Scholar] [CrossRef] [PubMed]
- Bakker, P.A.H.M.; Pieterse, C.M.J.; Van Loon, L.C. Induced systemic resistance by fluorescent Pseudomonas spp. Phytopathology 2007, 97, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Alquéres, S.; Meneses, C.; Rouws, L.; Rothballer, M.; Baldani, I.; Schmid, M.; Hartmann, A. The bacterial superoxide dismutase and glutathione reductase are crucial for endophytic colonization of rice roots by Gluconacetobacter diazotrophicus PAL5. Mol. Plant-Microbe Interact. 2013, 26, 937–945. [Google Scholar] [CrossRef] [PubMed]
- Tarkowski, Ł.P.; Signorelli, S.; Considine, M.J.; Montrichard, F. Integration of reactive oxygen species and nutrient signalling to shape root system architecture. Plant Cell Environ. 2023, 46, 379–390. [Google Scholar] [CrossRef]
- Santos, A.D.; Silveira, J.A.; Bonifacio, A.; Rodrigues, A.C.; Figueiredo, M.D. Antioxidant response of cowpea co-inoculated with plant growth-promoting bacteria under salt stress. Braz. J. Microbiol. 2018, 49, 513–521. [Google Scholar] [CrossRef] [PubMed]
- Santos, C.D.; Franco, O.L. Pathogenesis-Related Proteins (PRs) with Enzyme Activity Activating Plant Defense Responses. Plants 2023, 12, 2226. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Peng, Y.; Guo, L.; Li, C. Root colonization of encapsulated klebsiella oxytoca rs-5 on cotton plants and its promoting growth performance under salinity stress. Eur. J. Soil Biol. 2014, 60, 81–87. [Google Scholar] [CrossRef]
- Kang, S.M.; Shahzad, R.; Khan, M.A.; Hasnain, Z.; Lee, K.E.; Park, H.S.; Kim, L.R.; Lee, I.J. Ameliorative effect of indole-3-acetic acid- and siderophore-producing Leclercia adecarboxylata MO1 on cucumber plants under zinc stress. J. Plant Interact. 2021, 16, 30–41. [Google Scholar] [CrossRef]
- Fatima, H.; Ahmed, A. Indole-3-acetic acid synthesizing chromium-resistant bacteria can mitigate chromium toxicity in Helianthus annuus L. Plant Soil Environ. 2020, 66, 216–221. [Google Scholar] [CrossRef]
- Song, R.; Xia, Y.; Zhao, Z.; Yang, X.; Zhang, N. Effects of plant growth regulators on the contents of rutin, hyperoside and quercetin in Hypericum attenuatum Choisy. PLoS ONE 2023, 18, e0285134. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popržen, T.; Jevremović, S.; Milošević, S.; Đurić, M.; Uzelac, B.; Stanković, S.; Radulović, O. Antioxidative Response of Duckweed (Lemna minor L.) to Rhizosphere-Associated Pseudomonas Strains and Exogenous Indole-3-Acetic Acid. Horticulturae 2024, 10, 562. https://doi.org/10.3390/horticulturae10060562
Popržen T, Jevremović S, Milošević S, Đurić M, Uzelac B, Stanković S, Radulović O. Antioxidative Response of Duckweed (Lemna minor L.) to Rhizosphere-Associated Pseudomonas Strains and Exogenous Indole-3-Acetic Acid. Horticulturae. 2024; 10(6):562. https://doi.org/10.3390/horticulturae10060562
Chicago/Turabian StylePopržen, Tatjana, Slađana Jevremović, Snežana Milošević, Marija Đurić, Branka Uzelac, Slaviša Stanković, and Olga Radulović. 2024. "Antioxidative Response of Duckweed (Lemna minor L.) to Rhizosphere-Associated Pseudomonas Strains and Exogenous Indole-3-Acetic Acid" Horticulturae 10, no. 6: 562. https://doi.org/10.3390/horticulturae10060562
APA StylePopržen, T., Jevremović, S., Milošević, S., Đurić, M., Uzelac, B., Stanković, S., & Radulović, O. (2024). Antioxidative Response of Duckweed (Lemna minor L.) to Rhizosphere-Associated Pseudomonas Strains and Exogenous Indole-3-Acetic Acid. Horticulturae, 10(6), 562. https://doi.org/10.3390/horticulturae10060562










