Characterization of Mixtures of Rugulopteryx okamurae Compost and Plant Residues to Determine the Most Effective Composition as a Substrate and Source of Nutrients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experiment Location and Period of Study
2.2. Origin of the Materials and Characterization
- -
- Experiment 1 (2020–2021): The R. okamurae seaweed was collected from the coast near the town of Tarifa (Cadiz, Spain) in August 2020. It weighed 1730 kg and filled 5 m3, with a density of 0.346 kg L−1 and a moisture content of 75%. The seaweed drifts were subjected to a water treatment with a conductivity of 0.6 dS·m−1, which resulted in a reduction of their high electrical conductivity (EC) from 84 dS m−1 to levels of 13 dS·m−1 (Figure 2). The ratio used to wash the biomass algae was 1:1.4 (volume/volume), and each batch had an average volume of 941 L (295 kg). As the algae sample to be composted was 1730 kg, it was divided into 6 batches for washing. The salt washing time for each batch was 48 h, which was determined via EC measurements of the wash water and biomass at 24 and 48 h. After 48 h, the water was removed, and the biomass was transferred to drying tables, where it remained for 3 to 10 days, depending on the weather. After drying, eight subsamples were taken from each batch to determine the percentage of moisture, dry matter, and EC of the saturated paste extract. The mean EC of the six batches was 22 dS m−1, where batch 1 went from 85.7 dS m−1 to 9.9 dS m−1, batch 2 from 72.9 dS m−1 to 16.2 dS m−1, batch 3 from 75.5 dS m−1 to 31.6 dS m−1, batch 4 from 84.2 dS m−1 to 28.0 dS m−1, batch 5 from 87.1 dS m−1 to 35.0 dS m−1, and batch 6 from 99.8 dS m−1 to 27.1 dS m−1. Given the lower EC of the first two batches, it was deemed necessary to conduct a second washing, combining batches 3 + 4 and 5 + 6, obtaining an average final EC of 12.4 dS m−1 for all batches. The same procedure was performed with a biomass/water ratio (1:1.4). The water consumption was approximately 12 m3 for the algae used in experiment 1, with a final biomass/water ratio of 1:2.17.
- -
- Experiment 2 (2021–2022): In this experiment, 5 m3 of R. okamurae algae was collected, which is the same amount as in the previous experiment. The source of the algae was the Guadalmesí beach in Tarifa (Cadiz, Spain), a coastal area with mostly pebbles. The use of pebbles was intended to reduce the amount of sand in the algae collection, which could reduce the quality of the compost. The above material was received in August 2021 and stored at the “Servicios Medioambientales Las Chozas” industrial composting plant in El Ejido, Almeria (Spain). It was left to dry out in the open during the same month. One solution to avoid having to wash the algae before composting is to mix it with other materials [55]. Some authors, such as Illera et al. [56], demonstrated that it is possible to lower the EC for a few hours in an organic substrate as compost for soilless culture with water (15 h) or using a nutritive solution for a long period of time of cultive (60 days).
2.3. Treatment Composition
2.4. Parameter Evaluation
2.4.1. Compost Control Parameter Monitoring
2.4.2. Quality of Compost
Physical–Chemical Parameters of Compost
Germination Bioassays
2.5. Statistical Analysis
3. Results and Discussion
3.1. Assessment of the Composting Process
3.1.1. Temperature
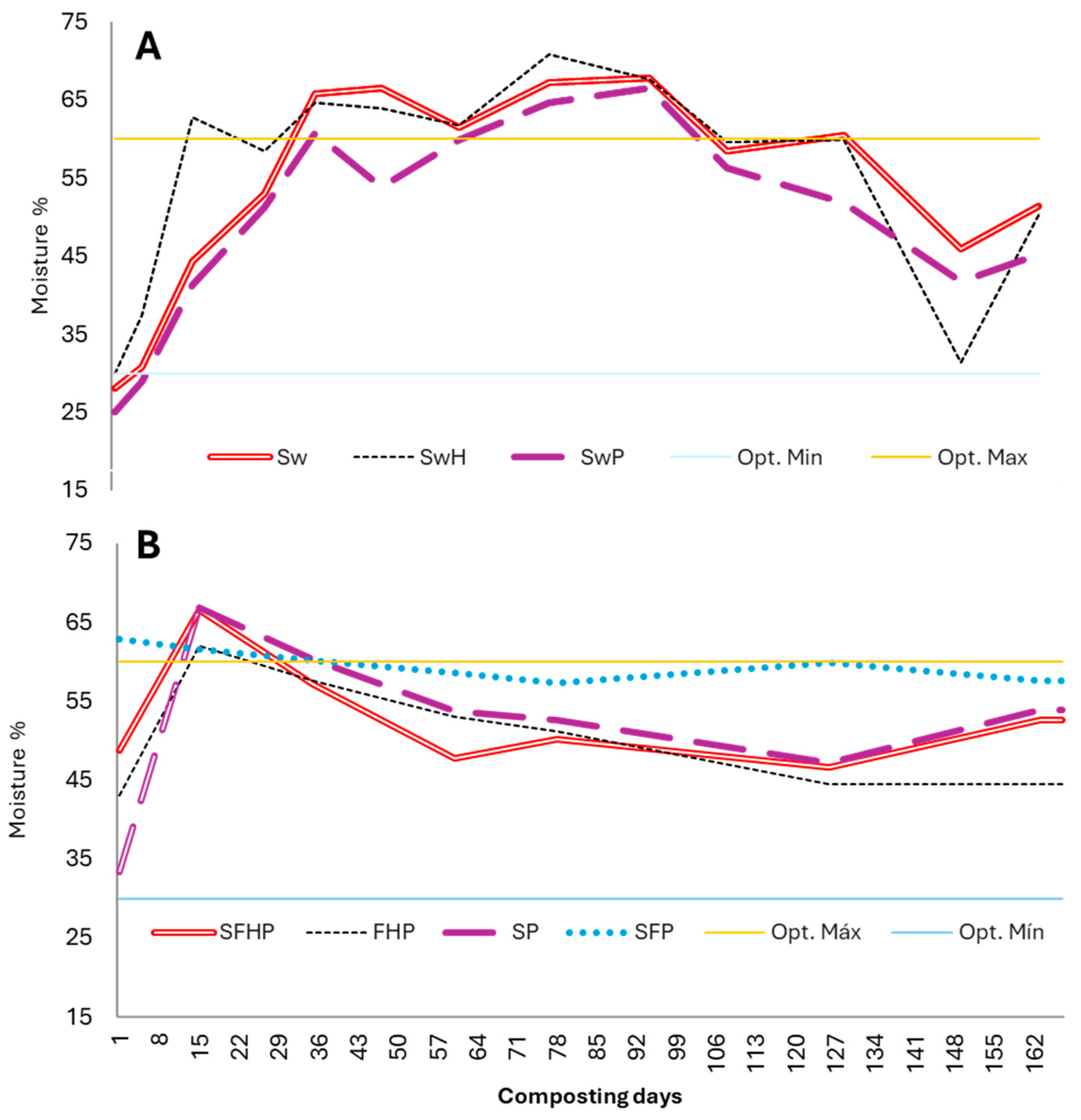
3.1.2. Moisture
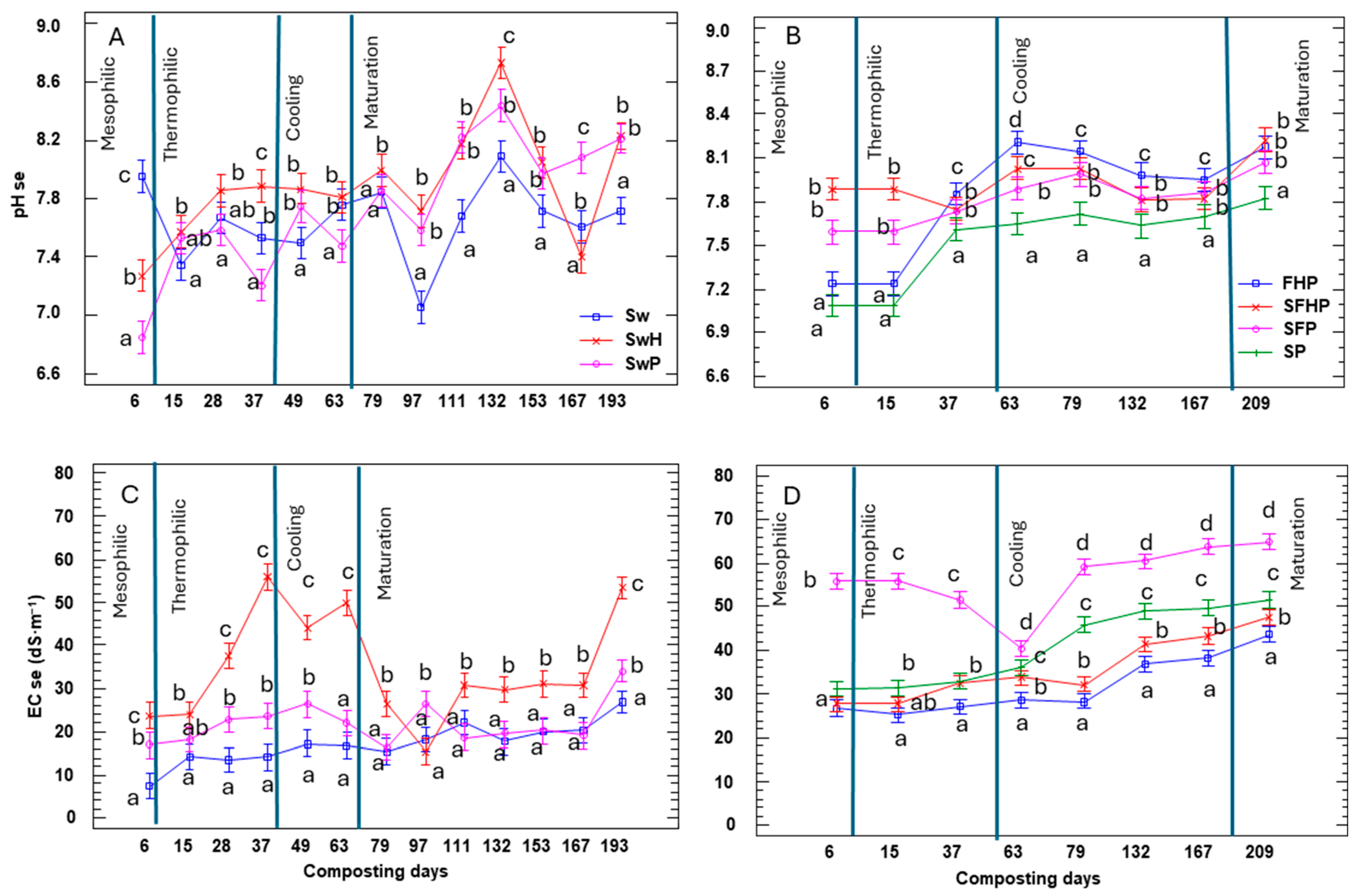
3.1.3. Parameters Monitored: Organic Matter, Total Nitrogen, C/N Ratio, and pH and EC
3.2. Quality of Compost
3.2.1. Physical–Chemical Parameters of Compost
| Experiment 1 | Experiment 2 | Ideal Substrate [76] | Manure [81] | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Parameters | Units | Sw | SwH | SwP | SFHP | FHP | SP | SFP | ||
| pH se | 7.7 a | 8.2 c | 8.2 bc | 8.2 c | 8.2 bc | 7.8 a | 8.1 b | 5.0–7.0 | 8.9 | |
| EC se | dS m−1 | 26.9 a | 53.4 b | 34.0 b | 47.6 d | 43.7 c | 51.6 e | 64.9 f | <3.5 | 29.8 |
| Moisture | % | 39.4 bc | 44.8 d | 43.5 d | 41.0 c | 39.0 b | 37.3 a | 48.2 e | 43.7 | |
| Real density | g cm−3 | 1.8 a | 1.8 a | 1.9 b | 1.8 a | 1.9 b | 1.8 a | 1.8 a | ||
| Bulk density | g cm−3 | 0.2 a | 0.2 a | 0.3 b | 0.4 c | 0.5 d | 0.3 b | 0.4 c | <0.75 | |
| Porosity | % | 90.4 c | 86.9 bc | 83.9 b | 78.7a | 74.7 a | 83.0 b | 79.44 a | >85 | |
| Saturation | % | 330.0 d | 200.0 c | 180.0 b | 174.7 b | 155.7 a | 211.8 c | 183.0 b | ||
| Dry matter | % | 60.6 cd | 55.2 b | 56.5 b | 59.0 c | 61.0 d | 62.7 e | 51.8 a | ||
| Experiment 1 | Experiment 2 | Compost [48] | Manure [81] | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Parameters | Units | Sw | SwH | SwP | SFHP | FHP | SP | SFP | ||
| Organic matter | % | 54.8 b | 57.1 bc | 47.1 a | 54.25 b | 44.65 a | 59.25 c | 59.15 c | - | 56 |
| N | % | 4.4 e | 3.8 d | 3.1 c | 2.6 b | 1.8 a | 2.3 b | 2.5 b | 3 | 1.8 |
| Organic carbon | % | 36.0 c | 32.6 bc | 28.8 a | 31.2 b | 25.98 a | 34.4 c | 34.38 c | 63.7 | 32.2 |
| C/N | 8.2 a | 8.7 ab | 9.4 b | 12.0 c | 14.4 d | 14.9 d | 14.1 d | 21.2 | 17.9 | |
| P | % | 0.46 a | 3.0 c | 0.9 ab | 1.4 bc | 1.1 ab | 0.7 ab | 1.4 bc | 0.2 * | 1.1 |
| K | % | 0.24 a | 1.68 c | 0.6 b | 2.5 d | 3.6 f | 2.9 e | 2.5 d | 0.4 * | 2.4 |
| Ca | % | 6.5 a | 12.45 bc | 14.3 c | 13.71 c | 14.1 c | 11.5 b | 12.4 bc | 13.6 * | 11.2 |
| Mg | % | 1.1 a | 1.8 a | 1.3 a | 2.8 b | 3.0 b | 2.7 b | 2.9 b | 0.6 * | 1.6 |
| Na | % | 1.5 c | 1.2 b | 1.1 a | 2.2 d | 2.7 e | 4.1 g | 3.5 f | 0.4 * | |
| S | % | 0.1 a | 0.3 c | 0.2 b | 0.2 b | 0.2 b | 0.4 d | 0.4 d | 0.1 * | |
| ∑cations (CEC) | meq·(100 g−1) | 125.4 c | 164.9 e | 111.7 b | 125.3 c | 99.1 a | 145.7 d | 159.1 e | ||
| Heavy Metals | Experiment 1 | Experiment 2 | Spanish Legislation [76,84] | Compost [48] | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| (mg kg−1) | Sw | SwH | SwP | SFHP | FHP | SP | SFP | Cat. A | Cat. B | SwP |
| Cd | 0.25 c | 0.15 a | 0.33 d | 0.21 b | 0.23 c | 0.16 a | 0.23 c | <0.7 | <2 | 0.04 |
| Cu | 11.4 a | 22.45 b | 157.9 d | 33.5 c | 36.45 c | 25.00 b | 32.95 c | <70 | <300 | 12.1 |
| Cr | 6.9 a | 10.6 ab | 14.8 bc | 22.5 d | 49.5 e | 16.2 bcd | 18.2 cd | <70 | <250 | 11.4 |
| Hg | <0.050 a | <0.050 a | <0.050 a | <0.050 a | 0.13 c | 0.102 bc | 0.057 ab | <0.4 | <1.5 | - |
| Ni | 9.2 ab | 8.1 a | 8.25 a | 9.0 ab | 15.15 c | 9.15 ab | 11.05 b | <25 | <90 | 6.13 |
| Pb | 4.2 a | 8.0 cd | 4.25 a | 5.75 b | 7.4 c | 5.25 ab | 8.85 d | <45 | <150 | 7.04 |
| Zn | 91.1 b | 85.5 ab | 159.4 d | 62.9 a | 124.05 c | 79.15 ab | 66.5 a | <200 | <500 | 28.1 |
| Fe | 3309.3 a | 4423.7 b | 3122.9 a | 4223.8 b | 5839.4 d | 4640.5 bc | 4975.3 c | 4400 | ||
| Mn | 42.3 a | 91. b8 | 180.8 e | 91.0 b | 119.6 d | 96.6 bc | 102.2 c | 166 | ||
3.2.2. Phytotoxicity Test of Compost
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Davidson, A.D.; Campbell, M.L.; Hewitt, C.L.; Schaffelke, B. Assessing the impacts of non indigenous marine macrogalgae: An update of current knowledge. Bot. Mar. 2015, 58, 55–79. [Google Scholar] [CrossRef]
- El Aamri, F.; Idhalla, M.; Tamsouri, M.N. Ocurrence of the invasive brown seaweed Rugulopteryx okamurae (EY Dawson) IK Wang, WJ Lee and HS Kim (Dictyotales, Phaeophyta) in Morocco (Mediterranean Sea). MedFAR 2018, 1, 92–96. [Google Scholar]
- García-Gómez, J.C.; González, A.R.; Maestre, M.J.; Espinosa, F. Detect coasta disturbances and climate change effects in coralligenous community through sentinel stations. PLoS ONE 2020, 15, e0231641. [Google Scholar] [CrossRef]
- Ruitton, S.; Blanfuné, A.; Boudouresque, C.F.; Guillemain, D.; Michotey, V.; Roblet, S.; Thibault, D.; Thibaut, T.; Verlaque, M. Rapid Spread of the Invasive Brown Alga Rugulopteryx okamurae in a National Park in Provence (France, Mediterranean Sea). Water 2021, 13, 2306. [Google Scholar] [CrossRef]
- Faria, J.; Prestes, A.C.L.; Moreu, I.; Cacabelos, E.; Martins, G.M. Dramatic changes in the structure of shallow-water marine benthic communities following the invasion by Rugulopteryx okamurae (Dictyotales, Ochrophyta) in Azores (NE Atlantic). Mar. Pollut. Bull. 2022, 175, 113358. [Google Scholar] [CrossRef] [PubMed]
- DeClerck, L.; Leliaert, F.; Verbruggen, H.; Lane, C.E.; DePaula, J.C.; Payo, D.I.; Coppejans, E. A revised classification of the dictyoteae (Dictyotales, Phaeophyceae) based on rbcL and 26S ribosomal DNA sequence data analyses. J. Phycol. 2006, 42, 1271–1288. [Google Scholar] [CrossRef]
- Altamirano-Jeschke, M.; De la Rosa Álamos, J.; Martínez Medina, F.J. Arribazones de la especia exótica Rugulopteryx okamurae (EY Dawson) en el Estrecho de Gibraltar. 2016. Repositorio Universidad de Málaga. Available online: http://hdl.handle.net/10630/12433 (accessed on 5 October 2023).
- García-Gómez, J.C.; Florido, M.; Olaya-Ponzone, L.; Rey Díaz de Rada, J.; Donázar-Aramendía, I.; Chacón, M.; Quintero, J.J.; Magariño, S.; Megina, C. Monitoring Extreme Impacts of Rugulopteryx okamurae (Dictyotales, Ochrophyta) in El Estrecho Natural Park (Biosphere Reserve). Showing Radical Changes in the Underwater Seascape. Front. Ecol. Evol. 2021, 9, 639161. [Google Scholar] [CrossRef]
- García-Gómez, J.C.; Florido, M.; Olaya-Ponzone, L.; Sempere-Valverde, J.; Megina, C. The invasive macroalga Rugulopteryx okamurae: Substrata plasticity and spatial colonization pressure on resident macroalgae. Front. Ecol. Evol. 2021, 9, 631754. [Google Scholar] [CrossRef]
- Altamirano, M.; De la Rosa, J.D.; Carmona, R.; Zanolla, M.; Muñoz, A.R. Macroalgas invasoras en las costas andaluzas. Algas 2019, 55e, 10–13. [Google Scholar]
- CAGPyDS Resultados de Los Trabajos con Rugulopteryx Okamurae en la ZEC y PN del Estrecho en el Marco del Convenio Suscrito Entre Amaya, Agapa, y Ocean Cleaner Technology S.L.; Consejería de Agricultura, Ganadería, Pesca y Desarrollo Sostenible de la Junta de Andalucía; Agencia de Medio Ambiente y Agua de Andalucía: Seville, Spain, 2020.
- Figueroa, F.L.; Vega, J.; Gómez-Valderrama, M.; Korbee, N.; Mercado, J.M.; Bañares, E. Invasión de la especie exótica Rugulopteryx okamurae en Andalucía I: Estudios preliminares de la actividad fotosintética. Algas 2020, 56, 35. [Google Scholar]
- Morand, P.; Briand, X. Excessive growth of macroalgae: A symptom of environmental disturbance. Bot. Mar. 1996, 39, 491–516. [Google Scholar] [CrossRef]
- Illera-Vives, M.; Labandeira, S.S.; Lopez-Mosquera, M.E. Production of compost from marine waste: Evaluation of the product for use in ecological agriculture. J. Appl. Phycol. 2013, 25, 1395–1403. [Google Scholar] [CrossRef]
- Ocaña, O.; Afonso-Carrillo J y Ballesteros, E. Proliferación masiva de una especie dictiotálica (Phaeophyceae, Ochrophyta) por el Estrecho de Gibraltar (Nota de investigación). Rev. Acad. Cañar. Ciencia. 2016, 28, 165–170. [Google Scholar]
- El Aamri, F.; Idhalla M y Tamsouri, M.N. Presencia del alga parda invasora Rugulopteryx okamurae (EY Dawson) IK Hwang, WJ Lee and HS Kim (Dictyotales, Phaeophyta) en Marruecos (Mar Mediterráneo). MedFAR 2018, 1, 92–96. [Google Scholar]
- MITECO. 2021–2022—Estrategia de Control de Rugulopteryx okamurae. Enlace. Available online: https://www.miteco.gob.es/content/dam/miteco/es/biodiversidad/publicaciones/estrategias/estrategia_rokamurae_cs_28072022_tcm30-543560.pdf (accessed on 24 January 2023).
- Castaldi, P.; Melis, P. Growth and yield characteristics and heavy metal content on tomatoes grown in different growing media. Commun. Soil Sci. Plant Anal. 2004, 35, 85–98. [Google Scholar] [CrossRef]
- Abdool-Ghany, A.A.; Pollier, C.G.; Oehlert, A.M.; Swart, P.K.; Blare, T.; Moore, K.; Solo-Gabriele, H.M. Assessing quality and beneficial uses of Sargassum compost. Waste Manag. 2023, 171, 545–556. [Google Scholar] [CrossRef] [PubMed]
- Guillén, J.; MartínezVidal, J.; Triviño, A.; Soler, G.; Fages, E.; Torre, L. Guía de Buenas Prácticas Para la Gestión, Recogida y Tratamiento de Los Arribazones de Algas y Plantas Marinas en Las Costas; Proyecto Seamatter LIFE11 ENV/ES/000600; Instituto de Ecología Litoral: El Campello, Spain, 2014; 24p. [Google Scholar]
- Ruiz, J.L.; Salas Sanjuan, M.D.C. The use of plant growth promoting bacteria for biofertigation; effects on concentrations of nutrients in inoculated aqueous vermicompost extract and on the yield and quality of tomatoes. Biol. Agric. Hortic. 2022, 38, 145–161. [Google Scholar] [CrossRef]
- Carricondo-Martínez, I.; Falcone, D.; Berti, F.; Orsini, F.; Salas-Sanjuan MD, C. Use of Agro-Waste as a Source of Crop Nutrients in Intensive Horticulture System. Agronomy 2022, 12, 447. [Google Scholar] [CrossRef]
- Berti, F.; Salas-Sanjuán MD, C.; Hernández-López, F.; Correa-Bustos, A.; Segura-Pérez, M.L. Use of Compost Based on Invasive Algae Rugulopteryx okamurae as a Peat Alternative in Nursery Growing Media. Agronomy 2023, 13, 948. [Google Scholar] [CrossRef]
- Hussain, A.; Iqbal, K.; Aziem, S.; Mahato, P.; Negi, A.K. A review on the science of growing crops without soil (soilless culture)—A novel alternative for growing crops. Int. J. Agric. Crop Sci. 2014, 7, 833. [Google Scholar]
- Savvas, D.; Gianquinto, G.; Tuzel, Y.; Gruda, N. Soilless Culture. Good Agricultural Practices for Greenhouse Vegetable Crops, Principles for Mediterranean Climate Areas. 217. FAO Plant Production and Protection Paper. 2013; pp. 303–354. Available online: https://www.fao.org/3/a-i3284e.pdf (accessed on 10 January 2024).
- Barrett, G.E.; Alexander, P.D.; Robinson, J.S.; Bragg, N.C. Achieving environmentally sustainable growing media for soilless plant cultivation systems–A review. Sci. Hortic. 2016, 212, 220–234. [Google Scholar] [CrossRef]
- Abad, M.; Noguera, N.P.; y Carrión, C. Los sustratos en los cultivos sin suelo. In Tratado de Cultivo Sin Suelo; Urrestarazu, M., Ed.; Mundi-Prensa: Madrid, Spain, 2004; pp. 113–158. Available online: https://www.researchgate.net/publication/259286675_Tratado_de_cultivo_sin_suelo (accessed on 30 March 2024).
- Shang, X.C.; Zhang, M.; Zhang, Y.; Hou, X.; Yang, L. Waste seaweed compost and rhizosphere bacteria Pseudomonas koreensis promote tomato seedlings growth by benefiting properties, enzyme activities and rhizosphere bacterial community in coastal saline soil of Yellow River Delta, China. Waste Manag. 2023, 172, 33–42. [Google Scholar] [CrossRef]
- Cooper, J.; Greenberg, I.; Ludwig, B.; Hippich, L.; Fischer, D.; Glaser, B.; Kaiser, M. Effect of biochar and compost on soil properties and organic matter in aggregate size fractions under field conditions. Agric. Ecosyst. Environ. 2020, 295, 106882. [Google Scholar] [CrossRef]
- Wei, Y.; Wang, N.; Lin, Y.; Zhan, Y.; Ding, X.; Liu, Y.; Zhang, A.; Ding, G.; Xu, T.; Li, J. Recycling of nutrients from organic waste by advanced compost technology—A case study. Bioresour. Technol. 2021, 337, 125411. [Google Scholar] [CrossRef]
- Zemke-White, W.L.; Ohno, M. World seaweed utilisation: An end-of-century summary. J. Appl. Phycol. 1999, 11, 369–376. [Google Scholar] [CrossRef]
- Blunden, G. Agricultural uses of seaweed and seaweed products. In European Seaweed Resources: Uses and Potential; Guiry, M.D., Blunden, G., Eds.; Wiley: Chichester, England, 1991; pp. 65–81. [Google Scholar]
- Khan, W.; Rayirath, U.P.; Subramanian, S.; Jithesh, M.N.; Rayorath, P.; Hodges, D.M.; Critchley, A.T.; Craigie, J.S.; Norrie, J.; Prithiviraj, B. Seaweed extracts as biostimulants of plant growth and development. J. Plant Growth Regul. 2009, 27, 270–279. [Google Scholar] [CrossRef]
- Gibilisco, P.E.; Negrin, V.L.; Idaszkin, Y.L. Assessing the use of two halophytes species and seaweed composting in Cu-pollution remediation strategies. Mar. Pollut. Bull. 2022, 176, 113413. [Google Scholar] [CrossRef] [PubMed]
- Heo, S.J.; Park, E.J.; Lee, K.W.; Jeon, Y.J. Antioxidant activities of enzymatic extracts from brown seaweeds. Bioresour. Technol. 2005, 96, 1613–1623. [Google Scholar] [CrossRef]
- Wang, M.P.; Chen, L.; Li, Y.T.; Chen, L.; Liu, Z.Y.; Wang, X.J.; Yan, P.S.; Qin, S. Responses of soil microbial communities to a short-term application of seaweed fertilizer revealed by deep amplicon sequencing. Appl. Soil Ecol. 2018, 125, 288–296. [Google Scholar] [CrossRef]
- Casal-Porras, I.; Zubía, E.; Brun, F.G. Dilkamural: A novel chemical weapon involved in the invasive capacity of the alga Rugulopteryx okamurae in the Strait of Gibraltar. Estuar. Coast. Shelf Sci. 2021, 257, 107398. [Google Scholar] [CrossRef]
- Barcellos, L.; Pham, C.K.; Menezes, G.; Bettencourt, R.; Rocha, N.; Carvalho, M.; Felgueiras, H.P. A concise review on the potential applications of Rugulopteryx okamurae macroalgae. Mar. Drugs 2023, 21, 40. [Google Scholar] [CrossRef] [PubMed]
- Patón, D.; García-Gómez, J.C.; Loring, J.; Torres, A. Composting the invasive toxic seaweed Rugulopteryx okamurae using five invertebrate species, and a mini-review on composting Macroalgae. Waste Biomass Valorization 2023, 14, 167–184. [Google Scholar] [CrossRef]
- Cole, A.J.; Roberts, D.A.; Garside, A.L.; de Nys, R.; Paul, N.A. Seaweed compost for agricultural crop production. J. Appl. Phycol. 2016, 28, 629–642. [Google Scholar] [CrossRef]
- Winberg, P.; de Mestre, C.; Willis, S. Evaluating Microdictyon umbilicatum bloom biomass as a compost conditioner for Australian, native coastal plants, Rhagodia candoleana and Banksia integrifolia. Compost. Sci. Util. 2013, 21, 64–74. [Google Scholar]
- Crouch, I.J.; Van Staden, J. Evidence for the Presence of Plant Growth Regulators in Commercial Seaweed Products. Plant Growth Regul. 1993, 13, 21–29. [Google Scholar] [CrossRef]
- Maze, J.; Morand, P.; Potoky, P. Stabilization of Green Tides Ulva by a Method of Composting with a View to Pollution Limitation. J. Appl. Phycol. 1993, 5, 183–190. [Google Scholar] [CrossRef]
- González Henríquez, M.N.; Jaizme-Vega MCAlcoverro Pedrola, T.R.; Haroun Tabraue, J.; Socorro Monzón, A.R. Aprovechamiento de Arribazones Naturales y Residuos Vegetales de Jardinería Como Fuente de Materia Orgánica Para la Elaboración del compost. 2011. Available online: https://www.icia.es/icia/index.php?option=com_content&view=article&id=1188:aprovechamiento-de-arribazones-naturales-y-residuos-vegetales-de-jardineria-como-fuente-de-materia-o&catid=71&Itemid=100060 (accessed on 3 March 2024).
- Geider, R.; La Roche, J. Redfeld revisited: Variability of C:N: P in marine microalgae and its biochemical basis. Eur. J. Phycol. 2002, 37, 1–17. [Google Scholar] [CrossRef]
- De la Lama-Calvente, D.; Fernandez-Rodriguez, M.; Llanos, J.; Mancilla-Leyton, J.; Borja, R. Enhancing methane production from the invasive macroalga Rugulopteryx okamurae through anaerobic co-digestion with olive mil solid waste: Process performance and kinetic analysis. J. Appl. Phycol. 2021, 33, 4113–4124. [Google Scholar] [CrossRef]
- Han, W.; Clarke, W.; Pratt, S. Composting of waste algae: A review. Waste Manag. 2014, 34, 1148–1155. [Google Scholar] [CrossRef]
- Madejón, E.; Panettieri, M.; Madejón, P.; Perez-de-Mora, A. Composting as sustainable managing option for seaweed blooms on recreational beaches. Waste Biomass Valorization 2022, 13, 863–875. [Google Scholar] [CrossRef]
- Reglamento (UE) 2019/1009 del Parlamento Europeo y del Consejo, de 5 de junio de 2019, Por el Que se Establecen Disposiciones Relativas a la Puesta a Disposición en el Mercado de los Productos Fertilizantes UE y se Modifican los Reglamentos (CE) n.o 1069/2009 y (CE) n.o 1107/2009 y se Deroga el Reglamento (CE) n.o 2003/2003 (Texto Pertinente a Efectos del EEE). Available online: http://data.europa.eu/eli/reg/2019/1009/oj (accessed on 29 February 2024).
- Carricondo-Martínez, I.; Berti., F.; Salas-Sanjuán., M.d.C. Different Organic Fertilisation Systems Modify Tomato Quality: An Opportunity for Circular Fertilisation in Intensive Horticulture. Agronomy 2022, 12, 174. [Google Scholar] [CrossRef]
- Real Decreto 999/2017, de 24 de Noviembre, Sobre Productos Fertilizantes. BOE-A-2017-14332. Available online: https://www.boe.es/eli/es/rd/2017/11/24/999 (accessed on 23 January 2024).
- Murray, M.; Skene, K.; Haynes, K. The Circular Economy: An Interdisciplinary Exploration of the Concept and Application in a Global Context. J. Bus. Ethics 2015, 140, 369–380. [Google Scholar] [CrossRef]
- Ley 3/2023, de 30 de marzo, de Economía Circular de Andalucía. Available online: https://www.boe.es/eli/es-an/l/2023/03/30/3 (accessed on 23 January 2024).
- Arnfield, A.J. “Köppen Climate Classification”. Encyclopedia Britannica. 16 October 2023. Available online: https://www.britannica.com/science/Koppen-climate-classification (accessed on 17 January 2024).
- Chong, C. Experiences with wastes and composts in nursery substrates. HortRechnology 2005, 15, 739–747. [Google Scholar] [CrossRef]
- Vives, M.I.; Mosquera ME, L.; Fabal, A.L.; del Carmen Salas-Sanjuan, M. Acondicionamiento de un compost salino para su uso como sustrato de cultivo. Recur. Rurais 2012, 18, 13–19. [Google Scholar]
- Hesse, P.R. A Textbook of Soil Chemical Analysis; John Murray: London, UK, 1971. [Google Scholar]
- Guo, R.; Li, G.; Jiang, T.; Schuchardt, F.; Chen, T.; Zhao, Y.; Shen, Y. Effect of aeration rate, C/N ratio and moisture content on the stability and maturity of compost. Bioresour. Technol. 2012, 112, 171–178. [Google Scholar] [CrossRef]
- de Boodt, M.; Verdonck, O. The physical properties of the substrates in horticulture. Acta Hort. 1972, 26, 37–44. [Google Scholar] [CrossRef]
- Raviv, M.; Wallach, R.; Silber, A.; Bar-Tal, A. Substrates and their analysis. In Hydroponic Production of Vegetables and Ornamentals; Embryo Publications: Athens, Greece, 2002; Chapter 2; pp. 25–102. ISBN 9789608002128. [Google Scholar]
- Ansorena Miner, J. Sustratos. In Propiedades y Caracterización; Mundi-Prensa: Madrid, Spain, 1994; 172p. [Google Scholar]
- TMECC, 2001. Test Methods for the Examination of Composting and Compost. US Composting Council. 2001. Available online: https://www.compostingcouncil.org/page/tmecc (accessed on 29 February 2024).
- Zucconi, F.M.; Monaco, A.; Forte, M. Phytotoxins during the stabilization of organic matter. In Composting of Agricultural and Other Wastes; Gasser, J.K.R., Ed.; Elsevier Applied Science Publishers: New York, NY, USA, 1985; pp. 73–86. [Google Scholar]
- Zucconi, F.M.; Pera, A.; Forte, M.; De Bertoldi, M. Evaluating toxicity of immature compost. BioCycle 1981, 22, 5457. [Google Scholar]
- Zucconi, F.; De Bertoldi, M. Production and characterization of compost. BioCycle 1987, 28, 56–61. [Google Scholar]
- Dang, B.T.; Ramaraj, R.; Le, M.V.; Tomoaki, I.; Pham, T.T.; Le Na, P.T.; Tran, D.P. Current application of seaweed waste for composting and biochar: A review. Bioresour. Technol. 2023, 375, 128830. [Google Scholar] [CrossRef]
- Moreno-Casco, J.; Mormeneo-Bernat. Microbiología y bioquímica del proceso de compostaje. In Compostaje; Moreno, J., Moral, R., Eds.; Ediciones Mundi-Prensa: Madrid, Spain, 2005. [Google Scholar]
- Kim, E.; Lee, D.H.; Won, S.; Ahn, H. Evaluation of optimum moisture content for composting of beef manure and bedding material mixtures using oxygen uptake measurement. Asian-Australas. J. Anim. Sci. 2016, 29, 753–758. [Google Scholar] [CrossRef]
- Sembera, J.A.; Meier, E.J.; Waliczek, T.M. Composting as an alternative management strategy for sargassum drifts on coastlines. Horttechnology 2018, 28, 80–84. [Google Scholar] [CrossRef]
- Yang, Q.; Zhang, S.; Li, X.; Rong, K.; Li, J.; Jiang, L. Effects of microbial inoculant and additives on pile composting of cow manure. Front. Microbiol. 2023, 13, 1084171. [Google Scholar] [CrossRef] [PubMed]
- Ceylan, F. Effects of composts obtained from hazelnut wastes on the cultivation of pepper (Capsicum annuum) seedlings. Sci. Rep. 2024, 14, 3019. [Google Scholar] [CrossRef] [PubMed]
- Amacher, J.K.; Koenig, R.; Kitchen, B. Salinity and plant tolerance. AG-SO 2000, 3, 1–8. [Google Scholar]
- Al-Dulaimi, O.; Rateb, M.E.; Hursthouse, A.S.; Thomson, G.; Yaseen, M. The brown seaweeds of Scotland, their importance and applications. Environments 2021, 8, 59. [Google Scholar] [CrossRef]
- Michalak, I.; Chojnacka, K. Algal compost–toward sustainable fertilization. Rev. Inorg. Chem. 2013, 33, 161–172. [Google Scholar] [CrossRef]
- Michalak, I.; Tuhy, L.; Chojnacka, K. Co-Composting of Algae and Efect of the Compost on Germination and Growth of Lepidium sativum. Pol. J. Environ. Stud. 2013, 25, 1107–1115. [Google Scholar] [CrossRef] [PubMed]
- Real Decreto 865/2010, de 2 de Julio, Sobre Sustratos de Cultivo. BOE-A-2010-11153. Available online: https://www.boe.es/eli/es/rd/2010/07/02/865 (accessed on 29 February 2024).
- Mapama—Ministerio De Medio Ambiente Y Medio Ruraly Marino Gobierno, D. E. Obtain of Manual de Compostaje. 2008. Available online: https://www.miteco.gob.es/content/dam/miteco/es/calidad-y-evaluacion-ambiental/temas/prevencion-y-gestion-residuos/Manual%20de%20compostaje%202011%20PAGINAS%201-24_tcm30-185556.pdf (accessed on 15 January 2024).
- Abad, M.; Noguera, P.; Noguera, V.; y Segura, M.L. Los sustratos para el semillero hortícola. Planteles. Compend. Hortic. 1999, 13, 59–68. [Google Scholar]
- MARM, 2010—Ministerio de Medio Ambiente y Medio Rural y Marino, Madrid (España). Guía Práctica de la Fertilización Racional de los Cultivos en España. Available online: https://www.mapa.gob.es/es/agricultura/publicaciones/Publicaciones-fertilizantes.aspx (accessed on 9 February 2024).
- Cabrera, R.I. Propiedades, uso y manejo de sustratos de cultivo para la producción de plantas en maceta. Rev. Chapingo Ser. Hortic. 1999, 5, 5–11. [Google Scholar] [CrossRef]
- Segura, M.L. Fertirrigación de Cultivos Hortícolas en Condiciones Salinas con Sistema Enarenado y Sustratos Alternativos. Ph.D. Thesis, Universidad Autónoma de Madrid, Madrid, Spain, 1995. [Google Scholar]
- Illera, M. Reducción de la salinidad en un compost: ¿Lavado o mezcla? In Libro de Resúmenes de las III Jornadas de Compostaje de la REC; 2012; pp. 127–130. ISBN 978-84-8408-788-5. Available online: https://www.researchgate.net/publication/272089824_REDUCCION_DE_LA_SALINIDAD_EN_UN_COMPOST_LAVADO_O_MEZCLA (accessed on 15 January 2024).
- Lopez, M.J.; Masaguer, A.; Paredes, C.; Perez, L.; Muñoz, M.; Salas, M.C.; Hernandez, R. De Resíduos a Recursos: El Camino hacia la Sostenibilidad; Mundi-Prensa: Madrid, Spain, 2015; pp. 91–121. ISBN 9781512938319. [Google Scholar]
- Real Decreto 506/2013, de 28 de Junio, Sobre Productos Fertilizantes. Available online: https://www.boe.es/eli/es/rd/2013/06/28/506/con (accessed on 29 February 2024).
- EU. Heavy Metals and Organic Compounds from Wastes Used as Organic Fertilisers. Available online: https://ec.europa.eu/environment/pdf/waste/compost/hm_finalreport.pdf (accessed on 4 April 2024).
- Tiquia, S.M.; Tam NF, Y.; Hodgkiss, I.J. Effects of composting on phytotoxicity of spent pig-manure sawdust litter. Environ. Pollut. 1996, 93, 249–256. [Google Scholar] [CrossRef]







| Experiment 1 | Experiment 2 | ||||
|---|---|---|---|---|---|
| Month | Tm | Pt | Month | Tm | Pt |
| November 2020 | 17.9 °C | 8 mm | September 2021 | 26 °C | 0.0 mm |
| December 2020 | 13.9 °C | 0 mm | October 2021 | 21.5 °C | 24.1 mm |
| January 2021 | 12.24 °C | 67.9 mm | November 2021 | 15.8 °C | 5.8 mm |
| February 2021 | 14.8 °C | 1.6 mm | December 2021 | 14.6 °C | 0.0 mm |
| March 2021 | 15.4 °C | 51.7 mm | January 2022 | 12.7 °C | 4.3 mm |
| April 2021 | 17.38 °C | 11.1 mm | February 2022 | 14.1 °C | 4.6 mm |
| May 2021 | 15.4 °C | 16.3 mm | March 2022 | 15.5 °C | 78.7 mm |
| April 2022 | 16.1 °C | 38.0 mm |
| Experiment 1 | Experiment 2 | |||||||
|---|---|---|---|---|---|---|---|---|
| Units | Sw | H | P | S | F | H | P | |
| pH (1:25) | 7.8 | 7.2 | 7.7 | 8.4 | 5.8 | 7.1 | 7.0 | |
| EC (1:25) | dS m−1 | 3.7 | 6.4 | 3.6 | 15.3 | 4.0 | 4.8 | 4.0 |
| EC (se) | dS m−1 | 7.4 | - | - | 113.4 | - | 23.9 | 37.0 |
| pH (se) | 7.9 | - | - | 7.7 | - | 7.0 | 7.0 | |
| Dry matter | % | 94.9 | 97.3 | 97.0 | 36.9 | 7.0 | 91.2 | 75.9 |
| Moisture | % | 5.1 | 2.7 | 3.0 | 63.1 | 93.0 | 8.8 | 24.1 |
| Organic matter | % | 83.0 | 80.4 | 77.7 | 60.7 | 88.0 | 73.0 | 92.4 |
| Ash | % | 17.0 | 19.6 | 22.3 | 39.3 | 12.0 | 27.0 | 7.6 |
| N | % | 4.7 | 1.1 | 1.5 | 3.5 | 3.3 | 2.3 | 1.2 |
| C | % | 48.3 | 46.7 | 45.2 | 35.3 | 51.2 | 42,4 | 53.7 |
| C/N | % | 10.2 | 41.7 | 30.7 | 9.9 | 15.4 | 18.4 | 45.1 |
| P | % | 0.5 | 0.9 | 0.5 | 0.7 | 2.5 | 1.4 | 0.7 |
| K | % | 0.2 | 5.5 | 1.8 | 1.6 | 4.8 | 3.2 | 1.2 |
| Ca | % | 5.0 | 6.3 | 8.8 | 9.1 | 0.6 | 7.0 | 7.6 |
| Mg | % | 1.7 | 2.0 | 1.2 | 2.7 | 0.5 | 1.9 | 1.5 |
| Na | % | 3.4 | 0.8 | 1.1 | 10.8 | 0.1 | 0.4 | 1.9 |
| S | % | 0.2 | 0.4 | 0.1 | 0.4 | 0.2 | 0.4 | 0.4 |
| Cl | % | 2.2 | 2.9 | 1.8 | 13.0 | 0.5 | 1.2 | 1.7 |
| Experiment | Treatment | C/N | Ref. |
|---|---|---|---|
| 1 | 100% washed seaweed | 10.5 | Sw |
| 33% washed seaweed + 67% horticultural waste | 21.3 | SwH | |
| 33% washed seaweed + 67% gardening pruning | 20.4 | SwP | |
| 2 | 15% seaweed + 15% fruit + 30% horticultural waste + 40% gardening pruning | 27.8 | SFHP |
| 20% fruit + 40% horticultural waste + 40% gardening pruning | 28.6 | FHP | |
| 35% seaweed + 65% gardening pruning | 32.7 | SP | |
| 30% seaweed + 20% fruit + 50% gardening pruning | 29.1 | SFP |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Correa-Bustos, A.; Berti, F.; Salas-Sanjuán, M.d.C.; Segura-Pérez, M.L. Characterization of Mixtures of Rugulopteryx okamurae Compost and Plant Residues to Determine the Most Effective Composition as a Substrate and Source of Nutrients. Horticulturae 2024, 10, 567. https://doi.org/10.3390/horticulturae10060567
Correa-Bustos A, Berti F, Salas-Sanjuán MdC, Segura-Pérez ML. Characterization of Mixtures of Rugulopteryx okamurae Compost and Plant Residues to Determine the Most Effective Composition as a Substrate and Source of Nutrients. Horticulturae. 2024; 10(6):567. https://doi.org/10.3390/horticulturae10060567
Chicago/Turabian StyleCorrea-Bustos, Amelia, Francesca Berti, María del Carmen Salas-Sanjuán, and María Luz Segura-Pérez. 2024. "Characterization of Mixtures of Rugulopteryx okamurae Compost and Plant Residues to Determine the Most Effective Composition as a Substrate and Source of Nutrients" Horticulturae 10, no. 6: 567. https://doi.org/10.3390/horticulturae10060567







