Comparative Growth and Bacoside Production in Diploid and Tetraploid Bacopa monnieri (L.) Wettst. Cultivated Indoors via Hydroponic and Soil Culture Systems
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cultivation of B. monnieri
2.2. Growth and Biomass Measurements of B. monnieri
2.3. Analysis of Chlorophyll and Carotenoid Content in Leave of B. monnieri
2.4. Extraction of B. monnieri Phytochemicals
2.5. Quantification of Total Phenolic Content in B. monnieri
2.6. Quantification of Total Flavonoid Content in B. monnieri
2.7. Quantification of Total Triterpenoid Content in B. monnieri
2.8. Quantification of Bacoside Content in B. monnieri
2.9. Quantification of DPPH Radical Scavenging Activity of B. monnieri
2.10. Analysis of Data
3. Results
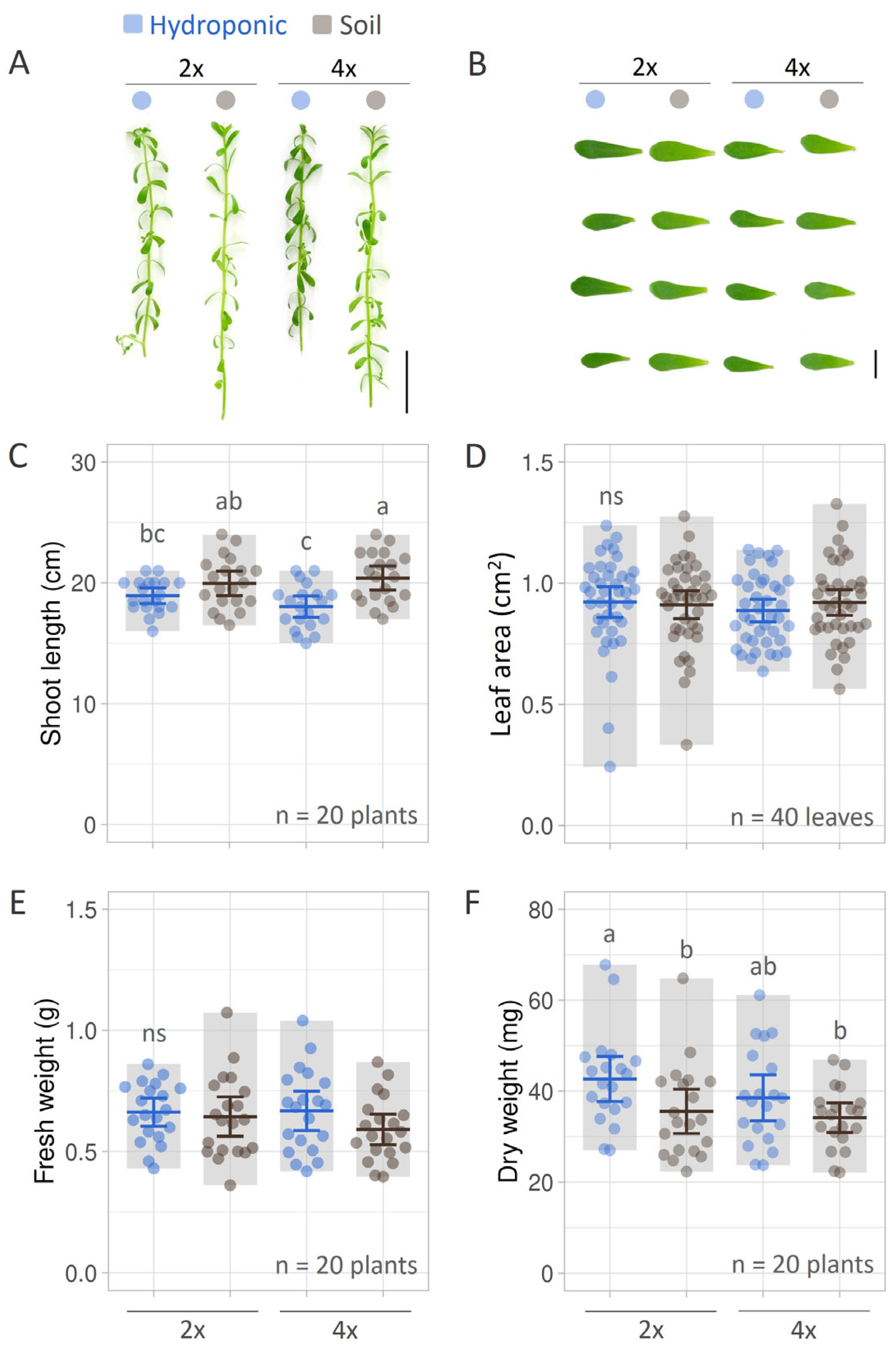
3.1. Growth and Biomass of B. monnieri
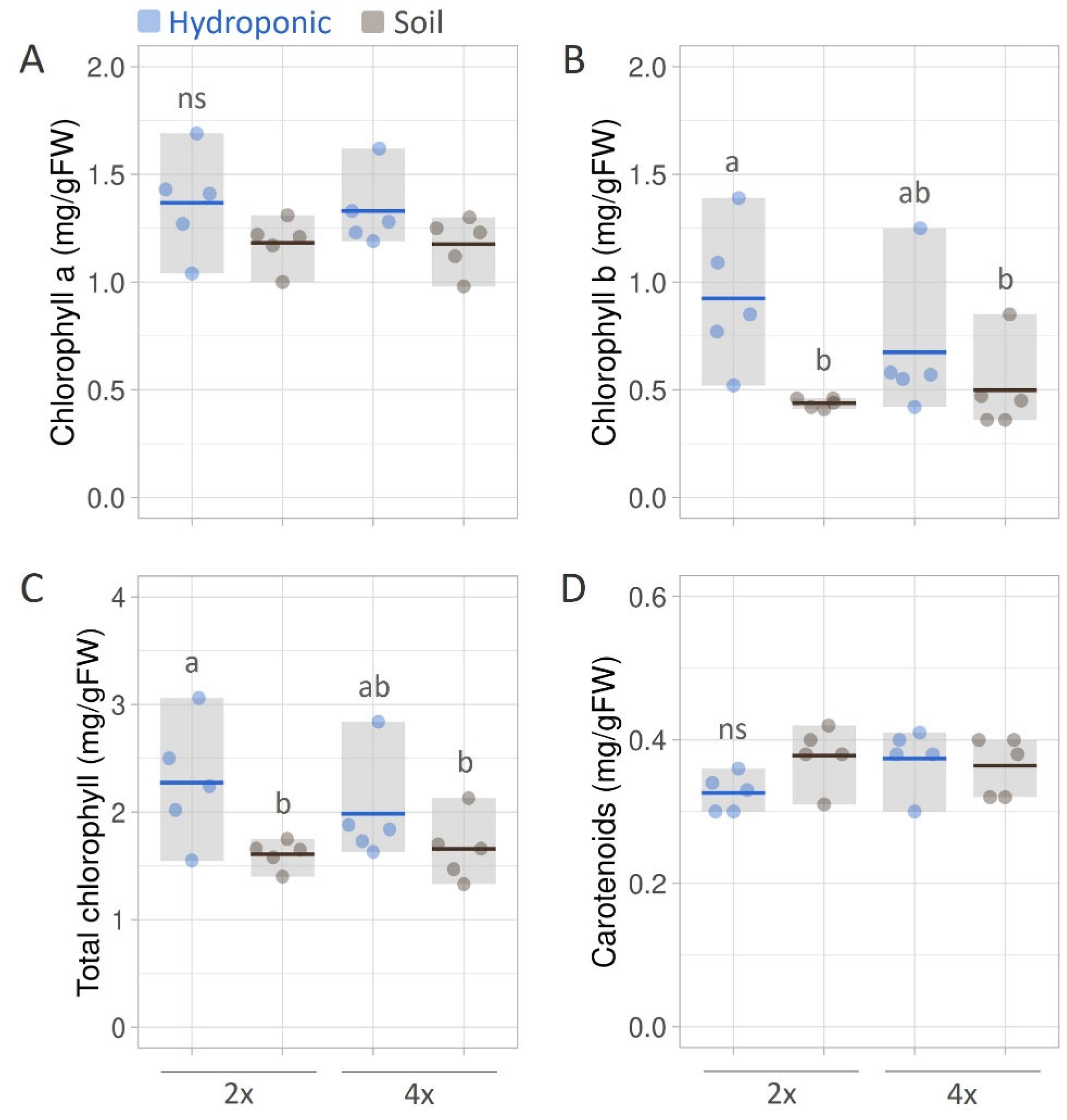
3.2. Chlorophyll and Carotenoid Contents in Leaves of B. monnieri
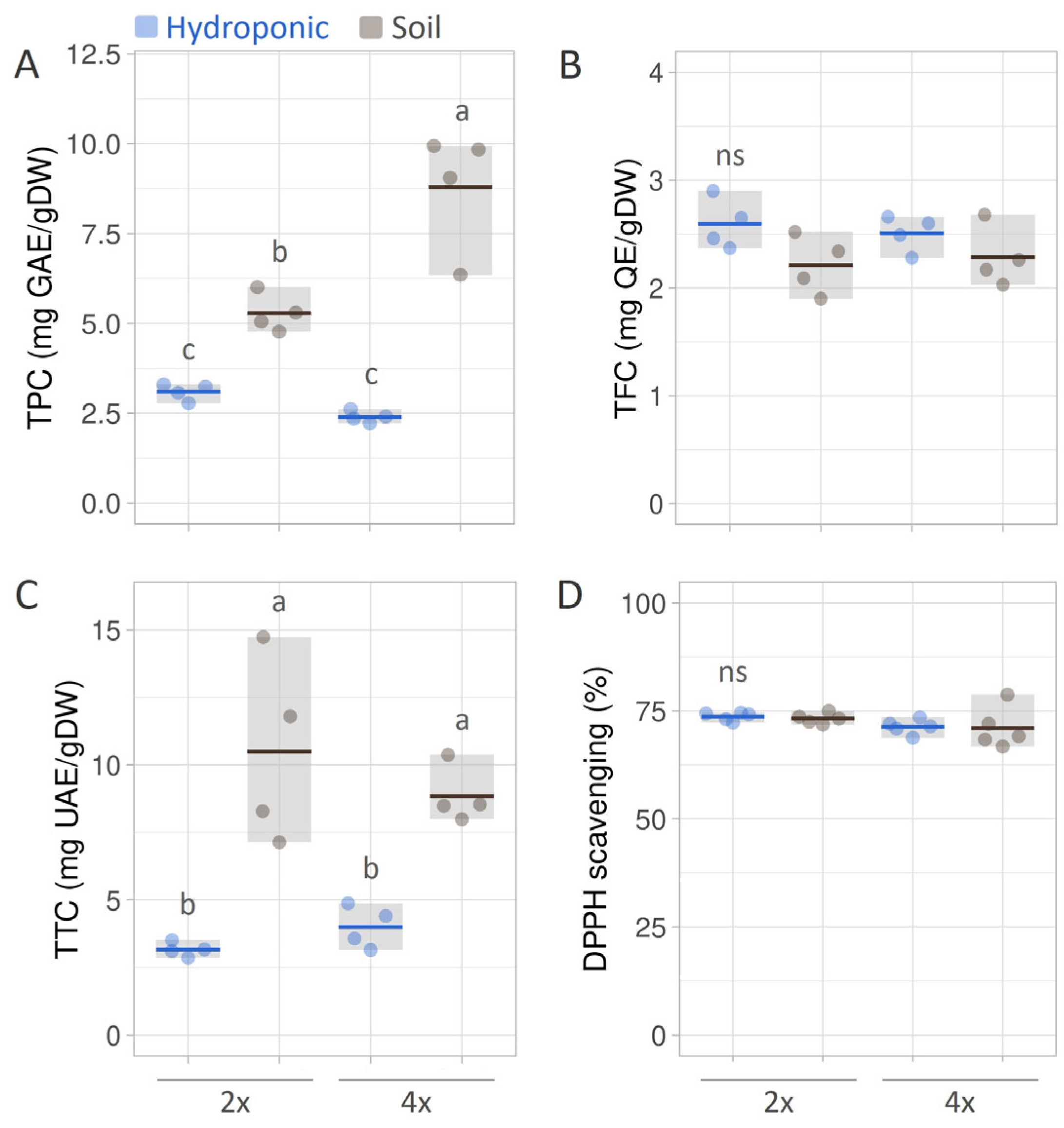
3.3. Total Phenolic, Total Flavonoid, and Total Triterpenoid Contents in B. monnieri
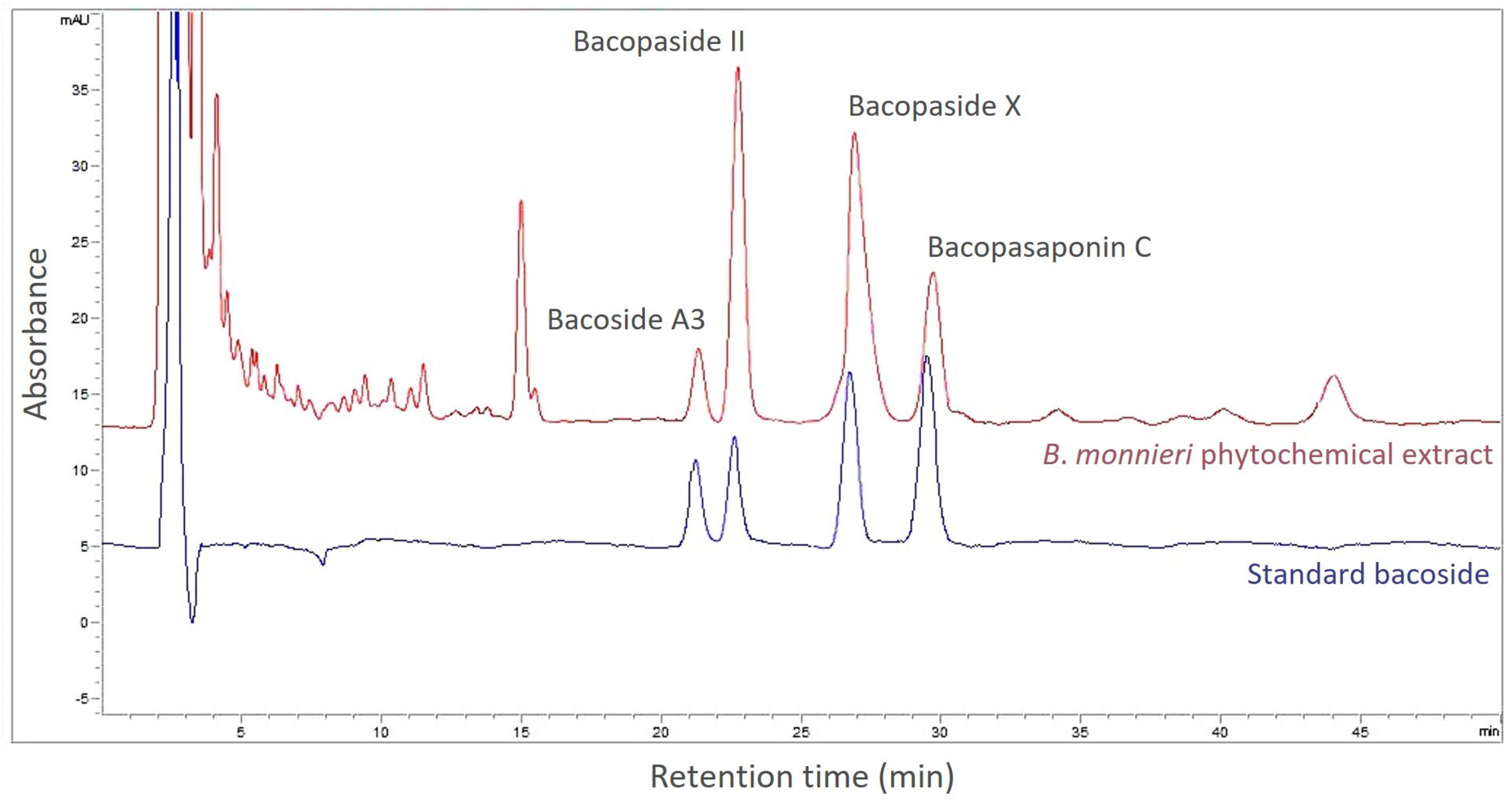
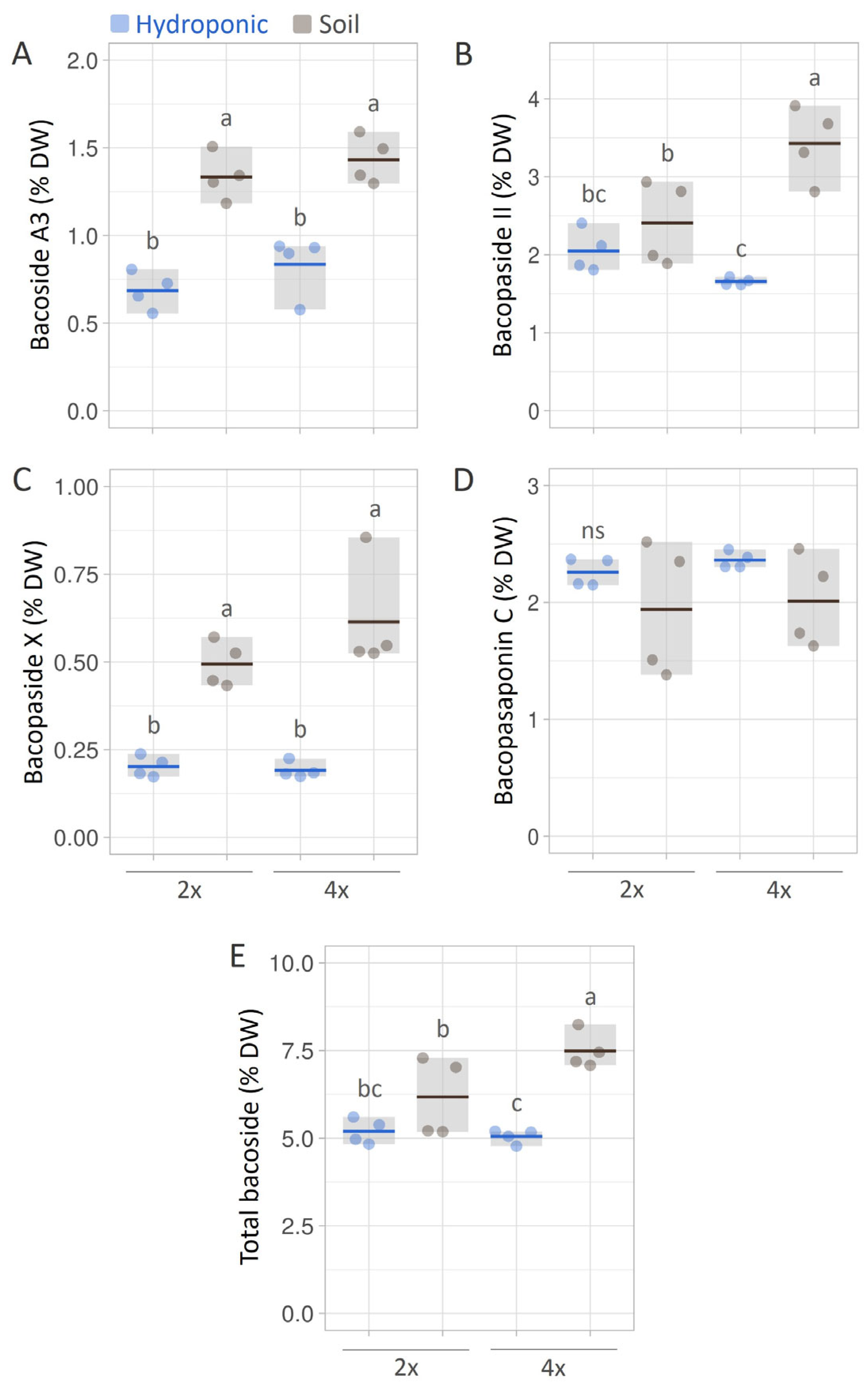
3.4. Bacoside Contents in B. monnieri
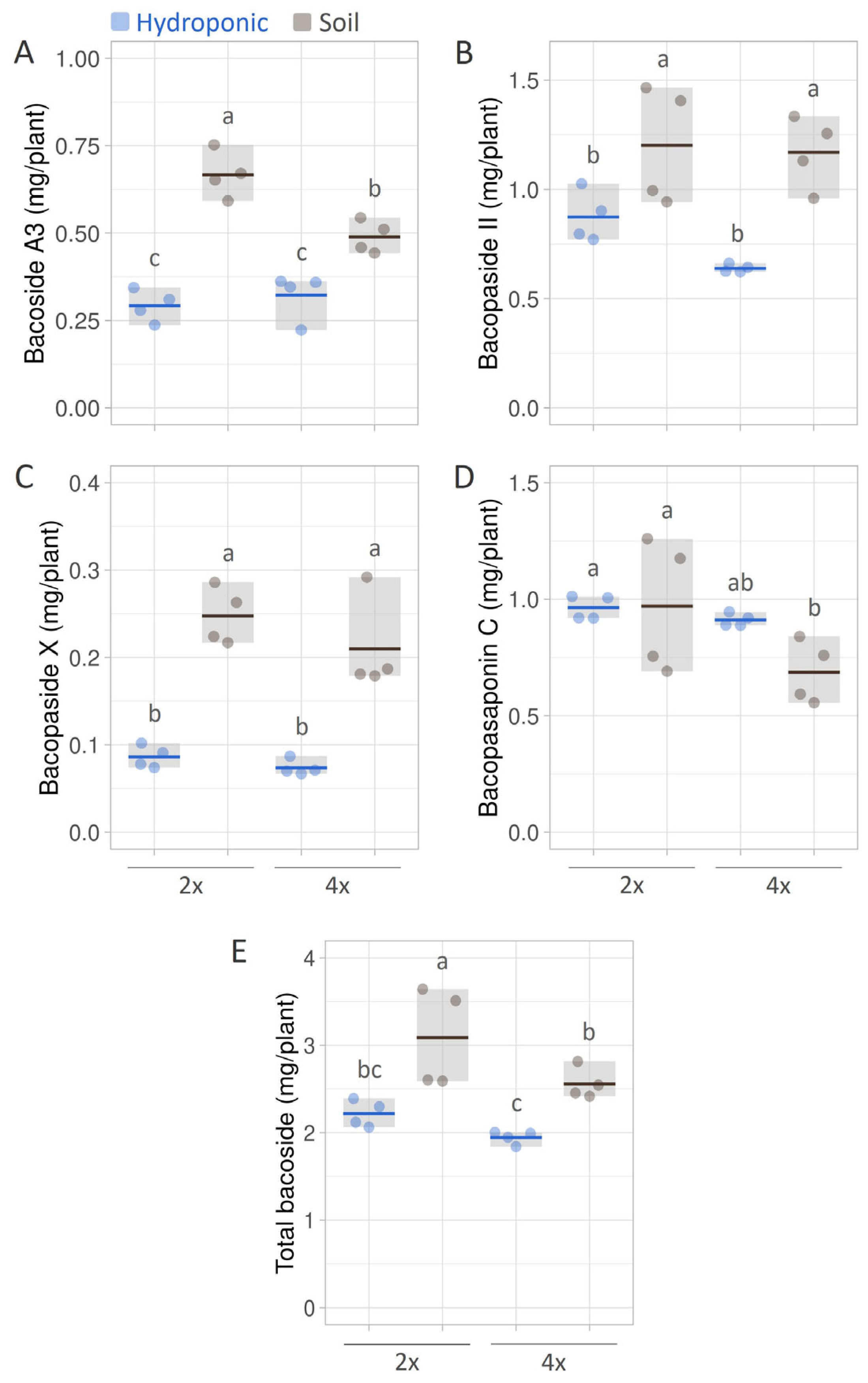
3.5. Bacoside Yields in B. monnieri
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mondal, S.; Bhar, K.; Mondal, P.; Panigrahi, N.; Sahoo, S.K.; Swetha, P.; Chakraborty, S.; Teja, N.Y.; Parveen, N. In quest of the mysterious holistic Vedic herb Bacopa monnieri (L.) Pennell. Pharmacogn. Res. 2023, 15, 410–454. [Google Scholar] [CrossRef]
- Limpeanchob, N.; Jaipan, S.; Rattanakaruna, S.; Phrompittayarat, W.; Ingkaninan, K. Neuroprotective effect of Bacopa monnieri on beta-amyloid-induced cell death in primary cortical culture. J. Ethnopharmacol. 2008, 120, 112–117. [Google Scholar] [CrossRef]
- Sathyanarayanan, V.; Thomas, T.; Einöther, S.J.; Dobriyal, R.; Joshi, M.K.; Krishnamachari, S. Brahmi for the better? New findings challenging cognition and anti-anxiety effects of Brahmi (Bacopa monniera) in healthy adults. Psychopharmacology 2013, 227, 299–306. [Google Scholar] [CrossRef]
- Jansen, R.L.M.; Brogan, B.; Whitworth, A.J.; Okello, E.J. Effects of five Ayurvedic herbs on locomotor behaviour in a Drosophila melanogaster Parkinson’s disease model. Phytother. Res. 2014, 28, 1789–1795. [Google Scholar] [CrossRef]
- Micheli, L.; Spitoni, S.; Di Cesare Mannelli, L.; Bilia, A.R.; Ghelardini, C.; Pallanti, S. Bacopa monnieri as augmentation therapy in the treatment of anhedonia, preclinical and clinical evaluation. Phytother. Res. 2020, 34, 2331–2340. [Google Scholar] [CrossRef]
- Ramasamy, S.; Chin, S.P.; Sukumaran, S.D.; Buckle, M.J.C.; Kiew, L.V.; Chung, L.Y. In silico and in vitro analysis of bacoside A aglycones and its derivatives as the constituents responsible for the cognitive effects of Bacopa monnieri. PLoS ONE 2015, 10, e0126565. [Google Scholar] [CrossRef]
- Jeyasri, R.; Muthuramalingam, P.; Suba, V.; Ramesh, M.; Chen, J.T. Bacopa monnieri and their bioactive compounds inferred multi-target treatment strategy for neurological diseases: A cheminformatics and system pharmacology approach. Biomolecules 2020, 10, 536. [Google Scholar] [CrossRef]
- Nopparat, J.; Sujipuli, K.; Ratanasut, K.; Weerawatanakorn, M.; Prasarnpun, S.; Thongbai, B.; Laothaworn, W.; Inthima, P. Exploring the excellence of commercial Brahmi products from Thai online markets: Unraveling phytochemical contents, antioxidant properties and DNA damage protection. Heliyon 2024, 10, e24509. [Google Scholar] [CrossRef]
- Sanyal, R.; Nandi, S.; Pandey, S.; Chatterjee, U.; Mishra, T.; Datta, S.; Prasanth, D.A.; Anand, U.; Mane, A.B.; Kant, N.; et al. Biotechnology for propagation and secondary metabolite production in Bacopa monnieri. Appl. Microbiol. Biotechnol. 2022, 106, 1837–1854. [Google Scholar] [CrossRef]
- Phrompittayarat, W.; Jetiyanon, K.; Wittaya-Areekul, S.; Putalun, W.; Tanaka, H.; Khan, I.; Ingkaninan, K. Influence of seasons, different plant parts, and plant growth stages on saponin quantity and distribution in Bacopa monnieri. Songklanakarin J. Sci. Technol. 2011, 33, 193–199. [Google Scholar]
- Bansal, M.; Reddy, M.S.; Kumar, A. Seasonal variations in harvest index and bacoside A contents amongst accessions of Bacopa monnieri (L.) Wettst. collected from wild populations. Physiol. Mol. Biol. Plants 2016, 22, 407–413. [Google Scholar] [CrossRef]
- Luo, L.; Wang, B.; Jiang, J.; Fitzgerald, M.; Huang, Q.; Wei, J.; Yang, C.; Zhang, H.; Dong, L.; Chen, S. Heavy metal contaminations in herbal medicines: Determination, comprehensive risk assessments, and solutions. Front. Pharmacol. 2021, 11, 595335. [Google Scholar] [CrossRef]
- Sinha, S. Accumulation of Cu, Cd, Cr, Mn and Pb from artificially contaminated soil by Bacopa monnieri. Environ. Monit. Assess. 1999, 57, 253–264. [Google Scholar] [CrossRef]
- Shukla, O.P.; Dubey, S.; Rai, U.N. Preferential accumulation of cadmium and chromium: Toxicity in Bacopa monnieri L. under mixed metal treatments. Bull. Environ. Contam. Toxicol. 2007, 78, 252–257. [Google Scholar] [CrossRef]
- Dineshkumar, M.; Sivalingam, A.; Thirumarimurugan, M. Phytoremediation potential of Bacopa monnieri in the removal of heavy metals. J. Environ. Biol. 2019, 40, 753–757. [Google Scholar] [CrossRef]
- Bisht, V.K.; Uniyal, R.C.; Sharma, S.M. Assessment of heavy metal content in herbal raw materials traded in India. S. Afr. J. Bot. 2022, 148, 154–161. [Google Scholar] [CrossRef]
- Wei, X.; Zhao, X.; Long, S.; Xiao, Q.; Guo, Y.; Qiu, C.; Qiu, H.; Wang, Y. Wavelengths of LED light affect the growth and cannabidiol content in Cannabis sativa L. Ind. Crops Prod. 2021, 165, 113433. [Google Scholar] [CrossRef]
- Bok, G.; Hahm, S.; Shin, J.; Park, J. Optimizing indoor hemp cultivation efficiency through differential day–night temperature treatment. Agronomy 2023, 13, 2636. [Google Scholar] [CrossRef]
- Velazquez-Gonzalez, R.S.; Garcia-Garcia, A.L.; Ventura-Zapata, E.; Barceinas-Sanchez, J.D.O.; Sosa-Savedra, J.C. A review on hydroponics and the technologies associated for medium-and small-scale operations. Agriculture 2022, 12, 646. [Google Scholar] [CrossRef]
- Csambalik, L.; Divéky-Ertsey, A.; Gál, I.; Madaras, K.; Sipos, L.; Székely, G.; Pusztai, P. Sustainability perspectives of organic farming and plant factory systems—From divergences towards synergies. Horticulturae 2023, 9, 895. [Google Scholar] [CrossRef]
- Pennisi, G.; Pistillo, A.; Orsini, F.; Cellini, A.; Spinelli, F.; Nicola, S.; Fernandez, J.A.; Crepaldi, A.; Gianquinto, G.; Marcelis, L.F. Optimal light intensity for sustainable water and energy use in indoor cultivation of lettuce and basil under red and blue LEDs. Sci. Hortic. 2020, 272, 109508. [Google Scholar] [CrossRef]
- Nguyen, T.K.L.; Lee, J.H.; Lee, G.O.; Cho, K.M.; Cho, D.Y.; Son, K.H. Optimization of cultivation type and temperature for the production of Balloon flower (Platycodon grandiflorum A. DC) sprouts in a plant factory with artificial lighting. Horticulturae 2022, 8, 315. [Google Scholar] [CrossRef]
- Selma, M.V.; Luna, M.C.; Martínez-Sánchez, A.; Tudela, J.A.; Beltrán, D.; Baixauli, C.; Gil, M.I. Sensory quality, bioactive constituents and microbiological quality of green and red fresh-cut lettuces (Lactuca sativa L.) are influenced by soil and soilless agricultural production systems. Postharvest Biol. Technol. 2012, 63, 16–24. [Google Scholar] [CrossRef]
- Phantong, P.; Machikowa, T.; Saensouk, P.; Muangsan, N. Comparing growth and physiological responses of Globba schomburgkii Hook. f. and Globba marantina L. under hydroponic and soil conditions. Emir. J. Food Agric. 2018, 30, 157–164. [Google Scholar] [CrossRef]
- Maurer, D.; Sadeh, A.; Chalupowicz, D.; Barel, S.; Shimshoni, J.A.; Kenigsbuch, D. Hydroponic versus soil-based cultivation of sweet basil: Impact on plants’ susceptibility to downy mildew and heat stress, storability and total antioxidant capacity. J. Sci. Food Agric. 2023, 103, 7809–7815. [Google Scholar] [CrossRef]
- Inthima, P.; Sujipuli, K. Improvement of growth and bacoside production in Bacopa monnieri through induced autotetraploidy with colchicine. PeerJ 2019, 7, e7966. [Google Scholar] [CrossRef]
- Hoagland, D.R.; Arnon, D.I. The water-culture method for growing plants without soil. Calif. Agric. Exp. Sta. Circ. 1950, 347, 1–32. [Google Scholar]
- Abràmoff, M.D.; Magalhães, P.J.; Ram, S.J. Image processing with ImageJ. Biophotonics Int. 2004, 11, 36–42. [Google Scholar]
- Ramírez-Mosqueda, M.A.; Iglesias-Andreu, L.G. Evaluation of different temporary immersion systems (BIT®®, BIG, and RITA®®) in the micropropagation of Vanilla planifolia Jacks. In Vitro Cell. Dev. Biol. Plant 2016, 52, 154–160. [Google Scholar] [CrossRef]
- Postma, M.; Goedhart, J. PlotsOfData—A web app for visualizing data together with their summaries. PLoS Biol. 2019, 17, e3000202. [Google Scholar] [CrossRef]
- Dhami, N.; Mishra, A.D. Phytochemical variation: How to resolve the quality controversies of herbal medicinal products? J. Herb. Med. 2015, 5, 118–127. [Google Scholar] [CrossRef]
- Kulhari, A.; Sheorayan, A.; Bajar, S.; Sarkar, S.; Chaudhury, A.; Kalia, R.K. Investigation of heavy metals in frequently utilized medicinal plants collected from environmentally diverse locations of north western India. SpringerPlus 2013, 2, 676. [Google Scholar] [CrossRef]
- Mishra, A.; Mishra, A.K.; Tiwari, O.P.; Jha, S. Studies on metals and pesticide content in some Ayurvedic formulations containing Bacopa monnieri L. J. Integr. Med. 2016, 14, 44–50. [Google Scholar] [CrossRef]
- Surendran, U.; Chandran, C.; Joseph, E.J. Hydroponic cultivation of Mentha spicata and comparison of biochemical and antioxidant activities with soil-grown plants. Acta Physiol. Plant. 2017, 39, 26. [Google Scholar] [CrossRef]
- Abu-Shahba, M.S.; Mansour, M.M.; Mohamed, H.I.; Sofy, M.R. Comparative cultivation and biochemical analysis of iceberg lettuce grown in sand soil and hydroponics with or without microbubbles and macrobubbles. J. Soil Sci. Plant Nutr. 2021, 21, 389–403. [Google Scholar] [CrossRef]
- Majid, M.; Khan, J.N.; Shah, Q.M.A.; Masoodi, K.Z.; Afroza, B.; Parvaze, S. Evaluation of hydroponic systems for the cultivation of Lettuce (Lactuca sativa L., var. Longifolia) and comparison with protected soil-based cultivation. Agric. Water Manag. 2021, 245, 106572. [Google Scholar] [CrossRef]
- Wimmerova, L.; Keken, Z.; Solcova, O.; Bartos, L.; Spacilova, M. A comparative LCA of aeroponic, hydroponic, and soil cultivations of bioactive substance producing plants. Sustainability 2022, 14, 2421. [Google Scholar] [CrossRef]
- Ucar, E.; Ozyigit, Y.; Demirbas, A.; Yasin Guven, D.; Turgut, K. Effect of different nitrogen doses on dry matter ratio, chlorophyll and macro/micro nutrient content in sweet herb (Stevia rebaudiana Bertoni). Commun. Soil Sci. Plant Anal. 2017, 48, 1231–1239. [Google Scholar] [CrossRef]
- Muhammad, I.; Yang, L.; Ahmad, S.; Farooq, S.; Al-Ghamdi, A.A.; Khan, A.; Zeeshan, M.; Elshikh, M.S.; Abbasi, A.M.; Zhou, X.B. Nitrogen fertilizer modulates plant growth, chlorophyll pigments and enzymatic activities under different irrigation regimes. Agronomy 2022, 12, 845. [Google Scholar] [CrossRef]
- Banchio, E.; Bogino, P.C.; Santoro, M.; Torres, L.; Zygadlo, J.; Giordano, W. Systemic induction of monoterpene biosynthesis in Origanum× majoricum by soil bacteria. J. Agric. Food Chem. 2010, 58, 650–654. [Google Scholar] [CrossRef]
- Eshaghi Gorgi, O.; Fallah, H.; Niknejad, Y.; Barari Tari, D. Effect of Plant growth promoting rhizobacteria (PGPR) and mycorrhizal fungi inoculations on essential oil in Melissa officinalis L. under drought stress. Biologia 2022, 77, 11–20. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, Z.; Chen, Z.; Kowalchuk, G.A.; Fu, X.; Kuramae, E.E. Microbial inoculants modulate growth traits, nutrients acquisition and bioactive compounds accumulation of Cyclocarya paliurus (Batal.) Iljinskaja under degraded field condition. For. Ecol. Manag. 2021, 482, 118897. [Google Scholar] [CrossRef]
- Bharti, N.; Yadav, D.; Barnawal, D.; Maji, D.; Kalra, A. Exiguobacterium oxidotolerans, a halotolerant plant growth promoting rhizobacteria, improves yield and content of secondary metabolites in Bacopa monnieri (L.) Pennell under primary and secondary salt stress. World J. Microbiol. Biotechnol. 2013, 29, 379–387. [Google Scholar] [CrossRef]
- Jagtap, R.R.; Mali, G.V.; Waghmare, S.R.; Nadaf, N.H.; Nimbalkar, M.S.; Sonawane, K.D. Impact of plant growth promoting rhizobacteria Serratia nematodiphila RGK and Pseudomonas plecoglossicida RGK on secondary metabolites of turmeric rhizome. Biocatal. Agric. Biotechnol. 2023, 47, 102622. [Google Scholar] [CrossRef]
- Sabzehzari, M.; Hoveidamanesh, S.; Modarresi, M.; Mohammadi, V. Morphological, anatomical, physiological, and cytological studies in diploid and tetraploid plants of Plantago psyllium. Plant Cell Tissue Organ Cult. 2019, 139, 131–137. [Google Scholar] [CrossRef]
- Corneillie, S.; De Storme, N.; Van Acker, R.; Fangel, J.U.; De Bruyne, M.; De Rycke, R.; Geelen, D.; Willats, W.G.; Vanholme, B.; Boerjan, W. Polyploidy affects plant growth and alters cell wall composition. Plant Physiol. 2019, 179, 74–87. [Google Scholar] [CrossRef]
- Tang, Z.Q.; Chen, D.L.; Song, Z.J.; He, Y.C.; Cai, D.T. In Vitro induction and identification of tetraploid plants of Paulownia tomentosa. Plant Cell Tissue Organ Cult. 2010, 102, 213–220. [Google Scholar] [CrossRef]
- Xu, C.; Zhang, Y.; Han, Q.; Kang, X. Molecular mechanism of slow vegetative growth in Populus tetraploid. Genes 2020, 11, 1417. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, J.; Qiu, B.; Ma, Z.; Lu, T.; Kang, X.; Yang, J. Induction and characterization of tetraploid through zygotic chromosome doubling in Eucalyptus urophylla. Front. Plant Sci. 2022, 13, 870698. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Inthima, P.; Supaibulwatana, K. Comparative Growth and Bacoside Production in Diploid and Tetraploid Bacopa monnieri (L.) Wettst. Cultivated Indoors via Hydroponic and Soil Culture Systems. Horticulturae 2024, 10, 574. https://doi.org/10.3390/horticulturae10060574
Inthima P, Supaibulwatana K. Comparative Growth and Bacoside Production in Diploid and Tetraploid Bacopa monnieri (L.) Wettst. Cultivated Indoors via Hydroponic and Soil Culture Systems. Horticulturae. 2024; 10(6):574. https://doi.org/10.3390/horticulturae10060574
Chicago/Turabian StyleInthima, Phithak, and Kanyaratt Supaibulwatana. 2024. "Comparative Growth and Bacoside Production in Diploid and Tetraploid Bacopa monnieri (L.) Wettst. Cultivated Indoors via Hydroponic and Soil Culture Systems" Horticulturae 10, no. 6: 574. https://doi.org/10.3390/horticulturae10060574
APA StyleInthima, P., & Supaibulwatana, K. (2024). Comparative Growth and Bacoside Production in Diploid and Tetraploid Bacopa monnieri (L.) Wettst. Cultivated Indoors via Hydroponic and Soil Culture Systems. Horticulturae, 10(6), 574. https://doi.org/10.3390/horticulturae10060574





