Do Climate Conditions Affect the Quality of the Apiaceae Fruits’ Essential Oils?
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
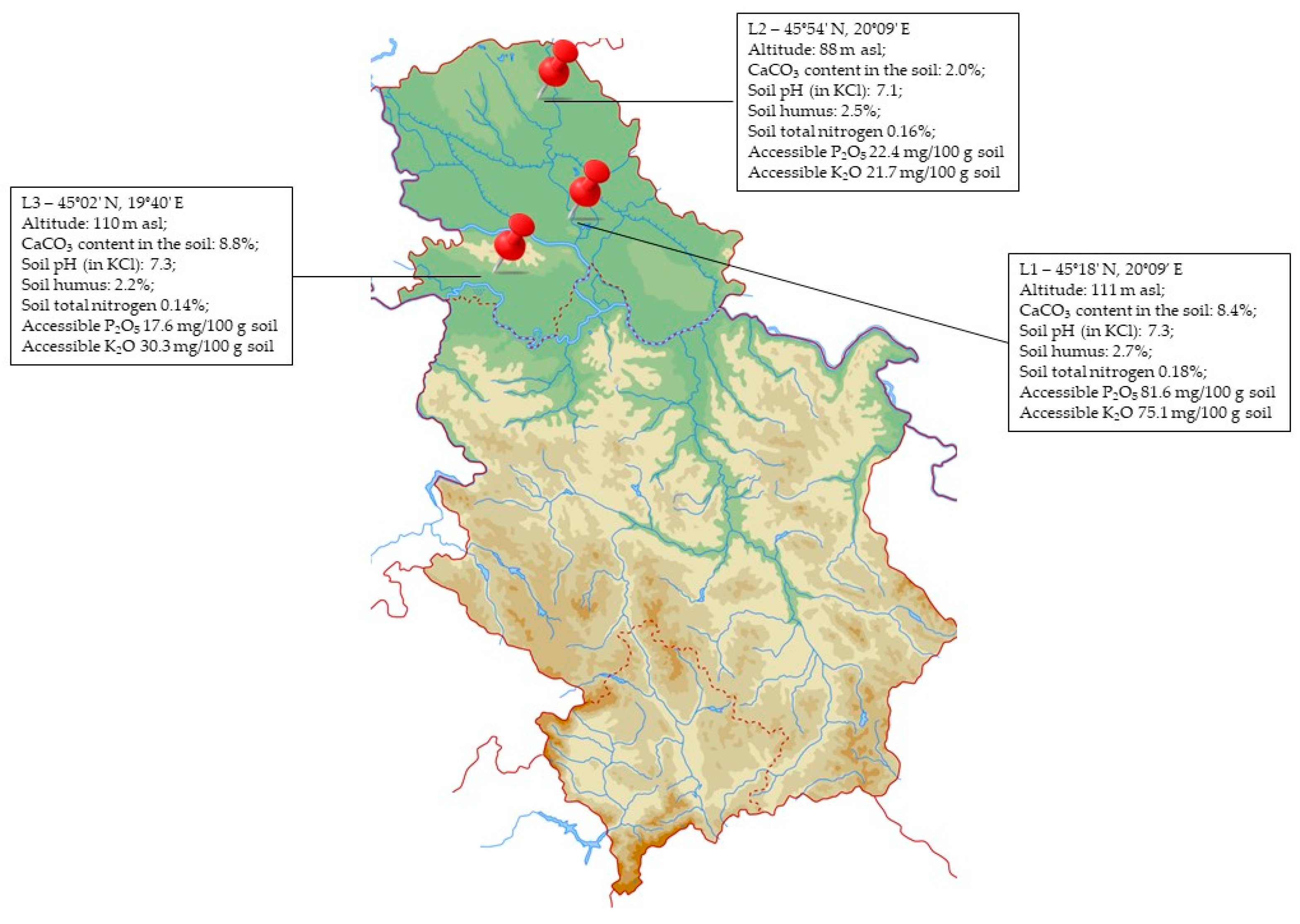
2.2. Experiment Locations and Main Environmental Characteristics
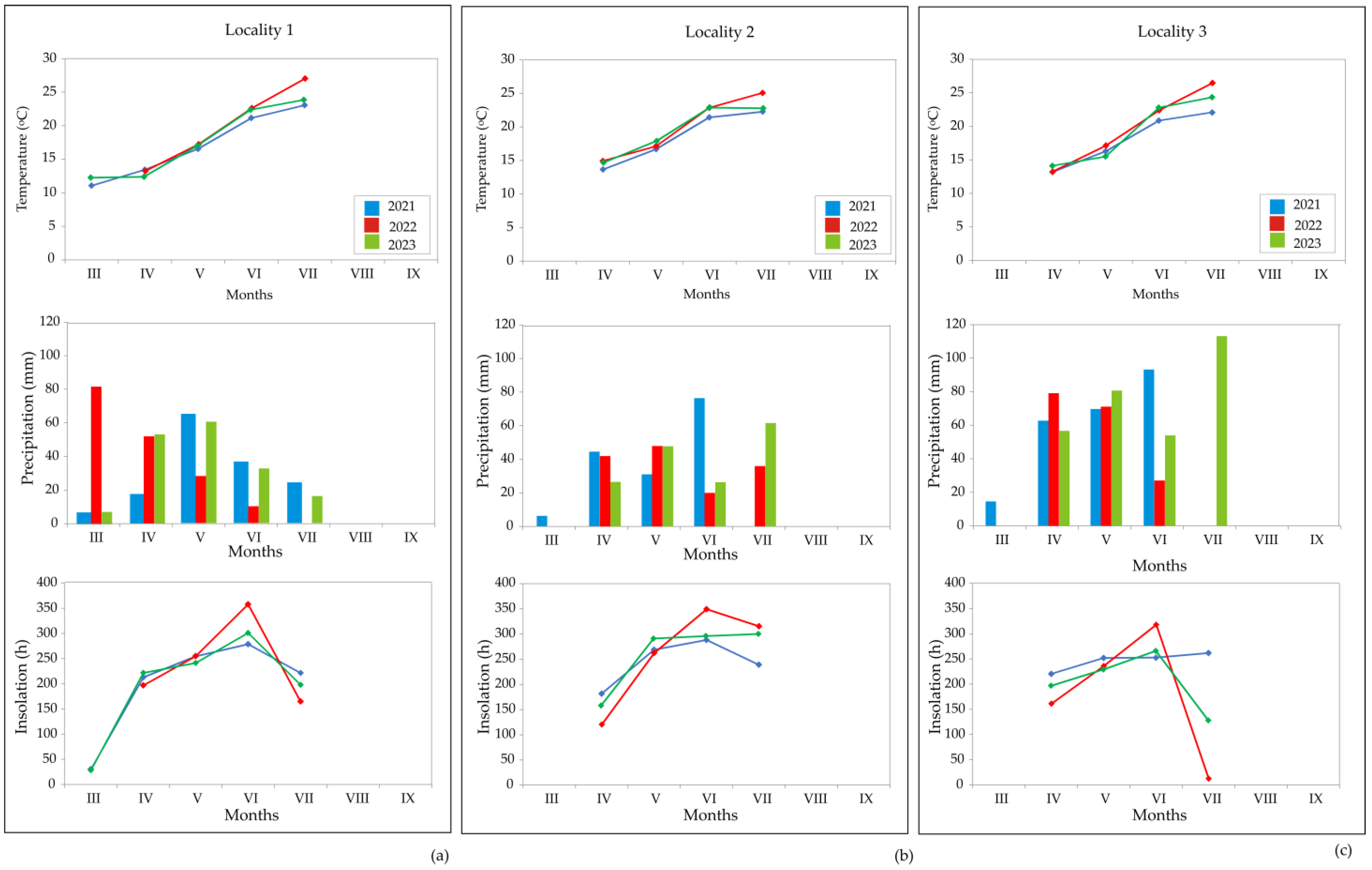
2.2.1. Weather Conditions during the Coriander Vegetation Period
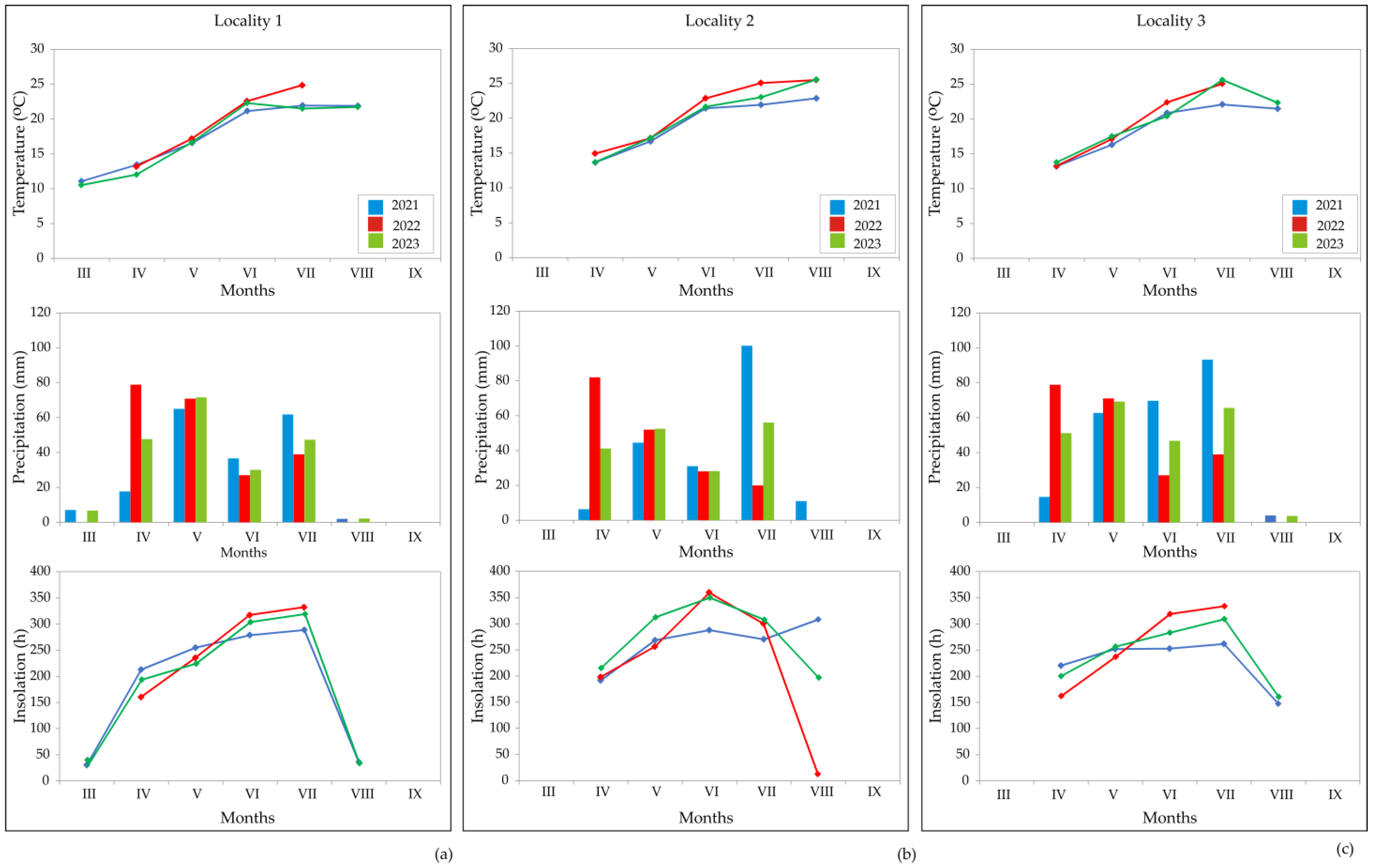
2.2.2. Weather Conditions during the Aniseed Vegetation Period
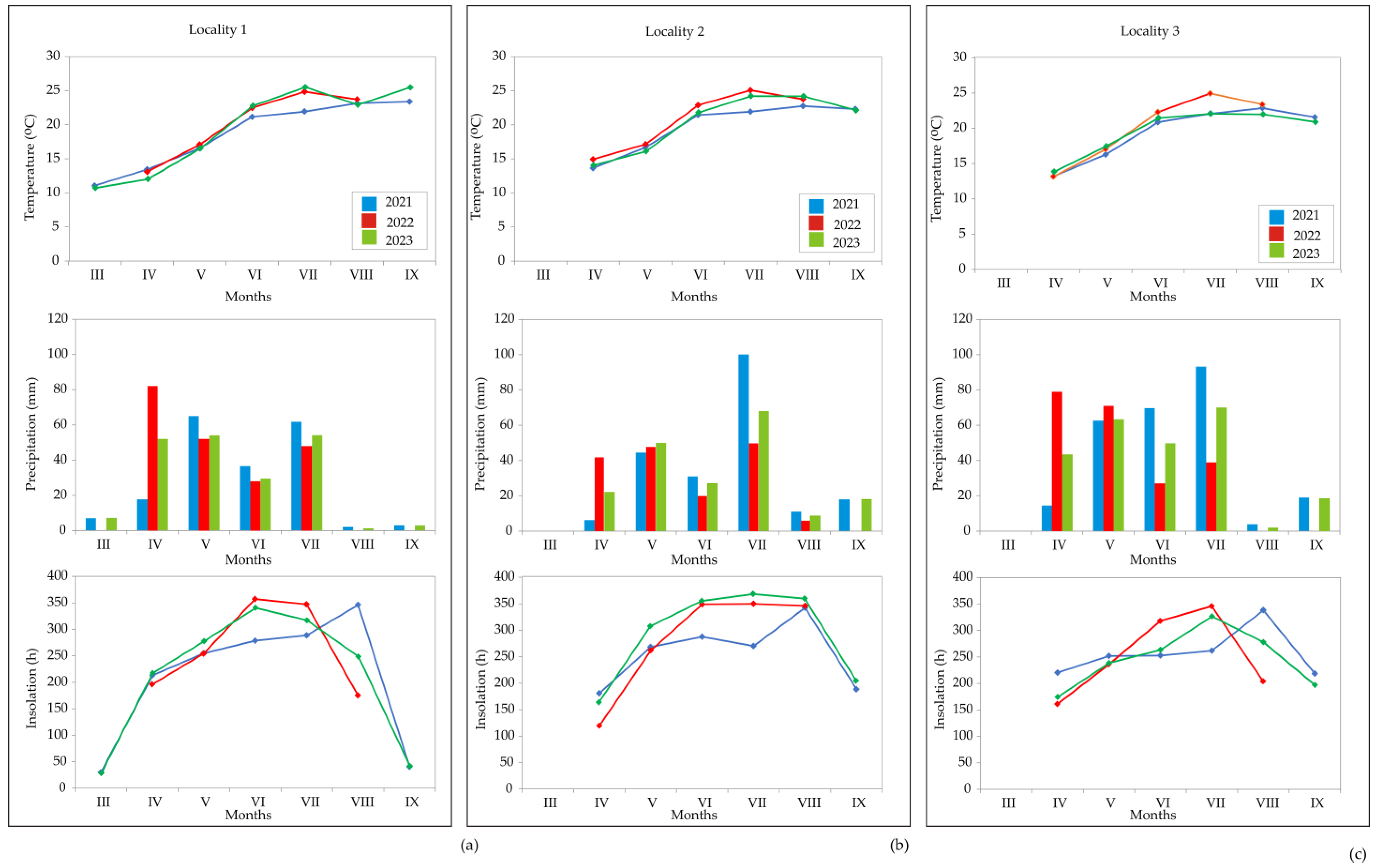
2.2.3. Weather Conditions during the Annual Caraway Vegetation Period
2.3. Essential Oil Extraction
2.4. Gas Chromatographic–Mass Spectrometric Analysis
2.5. Statistical Analysis
3. Results
3.1. Essential Oil Quality
3.2. Color Correlation Analysis
3.3. Cluster Analysis
3.4. Principal Component Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Aćimović, M.G. Nutraceutical potential of Apiaceae. In Bioactive Molecules in Food. Reference Series in Phytochemistry; Mérillon, J.M., Ramawat, K., Eds.; Springer: Cham, Switzerland, 2019; pp. 1311–1341. [Google Scholar] [CrossRef]
- Nouioura, G.; Lyoussi, B.; Derwich, E. Rekindling desire: Unveiling the Aphrodisiac potential of Apiaceae Elixirs. Phytomed. Plus 2024, 4, 100530. [Google Scholar] [CrossRef]
- Sharma, N.; Tan, M.A.; An, S.S.A. Mechanistic aspects of Apiaceae family spices in ameliorating Alzheimer’s disease. Antioxidants 2021, 10, 1571. [Google Scholar] [CrossRef]
- Aćimović, M.; Kostadinović, L.M.; Popović, S.; Dojčinović, N. Apiaceae seeds as functional food. J. Agric. Sci. 2015, 60, 237–246. [Google Scholar] [CrossRef]
- Sayed-Ahmad, B.; Talou, T.; Saad, Z.; Hijazi, A.; Merah, O. The Apiaceae: Ethnomedicinal family as source for industrial uses. Ind. Crops Prod. 2017, 109, 661–671. [Google Scholar] [CrossRef]
- Li, X.M.; Tian, S.L.; Pang, Z.C.; Shi, J.Y.; Feng, Z.S.; Zhang, Y.M. Extraction of Cuminum cyminum essential oil by combination technology of organic solvent with low boiling point and steam distillation. Food Chem. 2009, 115, 1114–1119. [Google Scholar] [CrossRef]
- Boudraa, H.; Kadri, N.; Mouni, L.; Madani, K. Microwave-assisted hydrodistillation of essential oil from fennel seeds: Optimization using Plackett–Burman design and response surface methodology. J. Appl. Res. Med. Aromat. Plants 2021, 23, 100307. [Google Scholar] [CrossRef]
- Jayakodi, Y.; Thiviya, P.; Gamage, A.; Evon, P.; Madhujith, T.; Merah, O. Antioxidant activity of essential oils extracted from Apiaceae family plants. Agrochemicals 2024, 3, 57–69. [Google Scholar] [CrossRef]
- Gajić, I.; Stanojević, L.; Dinić, A.; Stanojević, J.; Nikolić, L.; Nikolić, V.; Savić, V. The chemical composition of the essential oil and volatile compounds from caraway fruit (Carum carvi L.) extracted by headspace-solid phase microextraction and the antioxidant activity. Adv. Technol. 2020, 9, 37–43. [Google Scholar] [CrossRef]
- Gladikostić, N.; Ikonić, B.; Teslić, N.; Zeković, Z.; Božović, D.; Putnik, P.; Bursać Kovačević, D.; Pavlić, B. Essential oils from Apiaceae, Asteraceae, Cupressaceae and Lamiaceae families grown in Serbia: Comparative chemical profiling with in vitro antioxidant activity. Plants 2023, 12, 745. [Google Scholar] [CrossRef]
- Mahendra, P.; Bisht, S. Coriandrum sativum: A daily use spice with great medicinal effect. Pharmacogn. J. 2011, 3, 84–88. [Google Scholar] [CrossRef]
- Mohammed, F.S.; Uysal, I.; Sevindik, E.; Dogan, M.; Sevindik, M. Pimpinella Species (Anise): Traditional use, mineral, nutrient and chemical contents, biological activities. Int. J. Tradit. Complement. Med. Res. 2023, 4, 97–105. [Google Scholar] [CrossRef]
- Naquibuddin, M.; Hamiduddin, S.M.; Kowser, T. Zeera siyah (Carum carvi Linn.): A culinary spice and an important drug of unani medicine. Int. J. Herb. Med. 2021, 9, 101–105. [Google Scholar]
- Al-Khayri, J.M.; Banadka, A.; Nandhini, M.; Nagella, P.; Al-Mssallem, M.Q.; Alessa, F.M. Essential oil from Coriandrum sativum: A review on its phytochemistry and biological activity. Molecules 2023, 28, 696. [Google Scholar] [CrossRef] [PubMed]
- Sobhani, Z.; Mohtashami, L.; Amiri, M.S.; Ramezani, M.; Emami, S.A.; Simal-Gandara, J. Ethnobotanical and phytochemical aspects of the edible herb Coriandrum sativum L. J. Food Sci. 2022, 87, 1386–1422. [Google Scholar] [CrossRef]
- Jeya, K.R.; Veerapagu, M.; Sangeetha, V. Antimicrobial and antioxidant properties of Coriandrum sativum L. seed essential oil. Am. J. Essent. Oils Nat. Prod. 2019, 7, 06–10. [Google Scholar]
- Sriti, J.; Bettaieb, I.; Bachrouch, O.; Talou, T.; Marzouk, B. Chemical composition and antioxidant activity of the coriander cake obtained by extrusion. Arab. J. Chem. 2019, 12, 1765–1773. [Google Scholar] [CrossRef]
- Hajlaoui, H.; Arraouadi, S.; Noumi, E.; Aouadi, K.; Adnan, M.; Khan, M.A.; Kadri, A.; Snoussi, M. Antimicrobial, antioxidant, anti-acetylcholinesterase, antidiabetic, and pharmacokinetic properties of Carum carvi L. and Coriandrum sativum L. essential oils alone and in combination. Molecules 2021, 26, 3625. [Google Scholar] [CrossRef] [PubMed]
- Aouini, J.; Bachrouch, O.; Msaada, K.; Fares, N.; Jallouli, S.; Médiouni Ben Jemâa, J.; Soliman, T.M.A.; Sriti, J. Screening of antimicrobial and insecticidal properties of essential oils extracted from three Tunisian aromatic and medicinal plants. Int. J. Environ. Health Res. 2024, 34, 923–933. [Google Scholar] [CrossRef]
- Kajal, A.; Singh, R. Coriandrum sativum seeds extract mitigate progression of diabetic nephropathy in experimental rats via AGEs inhibition. PLoS ONE 2019, 14, e0213147. [Google Scholar] [CrossRef]
- Samojlik, I.; Lakić, N.; Mimica-Dukić, N.; Đaković-Švajcer, K.; Božin, B. Antioxidant and hepatoprotective potential of essential oils of coriander (Coriandrum sativum L.) and caraway (Carum carvi L.) (Apiaceae). J. Agric. Food Chem. 2010, 58, 8848–8853. [Google Scholar] [CrossRef]
- Özbek, H.; Kırmızı, N.İ.; Cengiz, N.; Erdoğan, E. Hepatoprotective effects of Coriandrum sativum essential oil against acute hepatotoxicity induced by carbon tetrachloride on rats. Acta Pharm. Sci. 2016, 54, 35–40. [Google Scholar] [CrossRef]
- Mandal, S.; Mandal, M. Coriander (Coriandrum sativum L.) essential oil: Chemistry and biological activity. Asian Pac. J. Trop. Biomed. 2015, 5, 421–428. [Google Scholar] [CrossRef]
- Acimovic, M.; Tesevic, V.; Todosijevic, M.; Djisalov, J.; Oljaca, S. Compositional characteristics of the essential oil of Pimpinella anisum and Foeniculum vulgare grown in Serbia. Bot. Serb. 2015, 39, 4–19. [Google Scholar]
- Shojaii, A.; Fard, M.A. Review of pharmacological properties and chemical constituents of Pimpinella anisum. Int. Sch. Res. Netw. 2012, 2012, 510795. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Shahrajabian, M.H.; Cheng, Q. Anise (Pimpinella anisum L.), a dominant spice and traditional medicinal herb for both food and medicinal purposes. Cogent Biol. 2019, 5, 1673688. [Google Scholar] [CrossRef]
- Bettaieb Rebey, I.; Aidi Wannes, W.; Ben Kaab, S.; Bourgou, S.; Saidani Tounsi, M.; Ksouri, R.; Laure Fauconnier, M. Bioactive compounds and antioxidant activity of Pimpinella anisum L. accessions at different ripening stages. Sci. Hortic. 2019, 246, 453–461. [Google Scholar] [CrossRef]
- El-Sayed, S.M.; Ahmed, N.; Selim, S.; Al-Khalaf, A.A.; El Nahhas, N.; Abdel-Hafez, S.H.; Sayed, S.; Emam, H.M.; Ibrahim, M.A.R. Acaricidal and antioxidant activities of anise oil (Pimpinella anisum) and the oil’s effect on protease and acetylcholinesterase in the two-spotted spider mite (Tetranychus urticae Koch). Agriculture 2022, 12, 224. [Google Scholar] [CrossRef]
- Bakhshi, M.; Kamalinejad, M.; Shokri, M.; Forouzani, G.; Heidari, F.; Tofangchiha, M. In vitro antibacterial effect of Pimpinella anisum essential oil on Enterococcus faecalis, Lactobacillus casei, Actinomyces naeslundii, and Aggregatibacter actinomycetemcomitans. Folia Medica 2022, 64, 799–806. [Google Scholar] [CrossRef] [PubMed]
- Dumitrescu, E.; Muselin, F.; Tîrziu, E.; Folescu, M.; Dumitrescu, C.S.; Orboi, D.M.; Cristina, R.T. Pimpinella anisum L. essential oil a valuable antibacterial and antifungal alternative. Plants 2023, 12, 2428. [Google Scholar] [CrossRef]
- Iannarelli, R.; Marinelli, O.; Morelli, M.B.; Santoni, G.; Amantini, C.; Nabissi, M.; Maggi, F. Aniseed (Pimpinella anisum L.) essential oil reduces pro-inflammatory cytokines and stimulates mucus secretion in primary airway bronchial and tracheal epithelial cell lines. Ind. Crops Prod. 2018, 114, 81–86. [Google Scholar] [CrossRef]
- Aćimović, M.; Milić, N. Perspectives of the Apiaceae hepatoprotective effects—A review. Nat. Prod. Commun. 2017, 12, 309–317. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. Assessment report on Pimpinella anisum L., fructus and Pimpinella anisum L., aetheroleum. Committee on Herbal Medicinal Products (HMPC), EMEA/HMPC/321181/2012. Available online: https://www.ema.europa.eu/en/documents/herbal-report/final-assessment-report-pimpinella-anisum-l-fructus-and-pimpinella-anisum-l-aetheroleum_en.pdf (accessed on 24 March 2024).
- Assami, K.; Chemat, S.; Meklati, B.Y.; Chemat, F. Ultrasound-assisted aromatisation with condiments as an enabling technique for olive oil flavouring and shelf life enhancement. Food Anal. Methods 2016, 9, 982–990. [Google Scholar] [CrossRef]
- Tomović, V.; Šojić, B.; Savanović, J.; Kocić-Tanackov, S.; Pavlić, B.; Jokanović, M.; Đorđević, V.; Parunović, N.; Martinović, A.; Vujadinović, D. Caraway (Carum carvi L.) essential oil improves quality of dry-fermented sausages produced with different levels of sodium nitrite. J. Food Process. Preserv. 2021, 46, e15786. [Google Scholar] [CrossRef]
- Ahmad, B.S.; Talou, T.; Straumite, E.; Sabovics, M.; Kruma, Z.; Saad, Z.; Hijazi, A.; Merah, O. Protein bread fortification with cumin and caraway seeds and by-product flour. Foods 2018, 7, 28. [Google Scholar] [CrossRef] [PubMed]
- Kostova, I.; Vlaseva, R.; Ivanova, M.; Damyanova, S.; Ivanova, N.; Stoyanova, A. Studying the possibilities of using of essential oils in dairy products. 2. Caraway (Carum carvi L.). Indian J. Appl. Res. 2015, 5, 83–85. [Google Scholar]
- Trifan, A.; Aprotosoaie, A.C.; Cioancă, O.; Hăncianu, M.; Jităreanu, A.; Gille, E.; Miron, A. Antioxidant activity of essential oil from Carum carvi L. cultivated in north-eastern Romania. Rev. Med. Chir. Soc. Med. Nat. 2016, 120, 732–736. [Google Scholar]
- Seidler-Łożykowska, K.; Kędzia, B.; Karpińska, E.; Bocianowski, J. Microbiological activity of caraway (Carum carvi L.) essential oil obtained from different origin. Acta Sci. Agron. 2013, 35, 495–500. [Google Scholar] [CrossRef]
- Kamaleeswari, M.; Nalini, N. Dose–response efficacy of caraway (Carum carvi L.) on tissue lipid peroxidation and antioxidant profile in rat colon carcinogenesis. J. Pharm. Pharmacol. 2006, 58, 1121–1130. [Google Scholar] [CrossRef] [PubMed]
- Alasmari, E.A.; Mehanna, A.S. Cytotoxic effects of S-(+)-carvone on selected human cancer cell lines. J. Anal. Pharm. Res. 2019, 8, 149–158. [Google Scholar]
- Eidi, A.; Eidi, M.; Haeri Rohani, A.; Basati, F. Hypoglycemic effect of ethanolic extract of Carum carvi L. seeds in normal and streptozotocin-induced diabetic rats. J. Med. Plants 2010, 9, 106–113. [Google Scholar]
- Haidari, F.; Seyed-Sadjadi, N.; Taha-Jalali, M.; Mohammed-Shahi, M. The effect of oral administration of Carum carvi on weight, serum glucose, and lipid profile in streptozotocininduced diabetic rats. Saudi Med. J. 2011, 32, 695–700. [Google Scholar] [PubMed]
- European Medicines Agency. Herbal Medicine: Summary for the Public—Caraway Fruit Carum carvi L., Fructus. Available online: https://www.ema.europa.eu/en/documents/herbal-summary/caraway-fruit-summary-public_en.pdf (accessed on 23 March 2024).
- Karalija, E.; Dahija, S.; Tarkowski, P.; Zeljković, S.Ć. Influence of climate-related environmental stresses on economically important essential oils of Mediterranean Salvia sp. Front. Plant Sci. 2022, 13, 864807. [Google Scholar] [CrossRef] [PubMed]
- Grulova, D.; De Martino, L.; Mancini, E.; Salamon, I.; De Feo, V. Seasonal variability of the main components in essential oil of Mentha× piperita L. J. Sci. Food Agr. 2015, 95, 621–627. [Google Scholar] [CrossRef] [PubMed]
- Yavari, A.; Nazeri, V.; Sefidkon, F.; Hassani, M.E. Influence of some environmental factors on the essential oil variability of Thymus migricus. Nat. Prod. Commun. 2010, 5, 943–948. [Google Scholar] [CrossRef] [PubMed]
- Aćimović, M.; Lončar, B.; Stanković Jeremić, J.; Cvetković, M.; Pezo, L.; Pezo, M.; Todosijević, M.; Tešević, V. Weather conditions influence on lavandin essential oil and hydrolate quality. Horticulturae 2022, 8, 281. [Google Scholar] [CrossRef]
- Seidler-Lozykowska, K. Effect of the selected weather conditions on essential oil, α-bisabolol and chamazulene content in flower heads of chamomile [Chamomilla recutita (L.) Rausch.]. J. Essent. Oil Res. 2010, 22, 45–48. [Google Scholar] [CrossRef]
- Mohamadi, N.; Rajaei, P. Effects of different ecological condition on the quality and quantity of essential oils of Artemisia persica Boiss. Populations from Kerman, Iran. J. Essent. Oil Bear. Plants 2016, 19, 200–207. [Google Scholar] [CrossRef]
- Council of Europe and European Pharmacopeia Commission. European Pharmacopoeia; Version 7.0; Council of Europe: Strasbourg, France, 2010. [Google Scholar]
- ISO 3516:1997; Oil of Coriander Fruits (Coriandrum sativum L.), 2nd ed. International Standards: Geneva, Switzerland, 1997.
- ISO 3475:2020; Essential Oil of Aniseed (Pimpinella anisum L.), 3rd ed. International Standards: Geneva, Switzerland, 2020.
- ISO 8896:2016; Essential Oil of Caraway (Carum carvi L.), 2nd ed. International Standards: Geneva, Switzerland, 2016.
- ISO 10693; Soil Quality—Determination of Carbonate Content—Volumetric Method. International Standards: Geneva, Switzerland, 1995.
- ISO 10390; Soil Quality—Determination of pH. International Standards: Geneva, Switzerland, 1994.
- ISO 14235; Soil Quality—Determination of Organic Carbon by Sulfochromic Oxidation. International Standards: Geneva, Switzerland, 1998.
- Bremner, J.M. Determination of nitrogen in soil by Kjeldahl method. J. Agric. Sci. 1960, 55, 11–33. [Google Scholar] [CrossRef]
- Egner, H.; Riehm, H.; Domingo, W.R. Untersuchungen über die chemische Bodenanalyse als Grundlage für die Beurteilung des Nahrstoffzustandes der Boden, II: Chemische Extractionsmetoden zu Phosphor-und Kaliumbestimmung. K. Lantbrukshügskolans Ann. 1960, 26, 199–215. [Google Scholar]
- Aćimović, M.; Oljača, S.; Tešević, V.; Todosijević, M.; Djisalov, J. Evaluation of caraway essential oil from different production areas of Serbia. Hort. Sci. 2014, 41, 122–130. [Google Scholar] [CrossRef]
- Saxena, S.N.; Swarup Meena, R.; Vishal, M.K.; John, S.; Kumar Sharma, L.; Mishra, B.K.; Agarwal, D. Variation in essential oil constituents of coriander (Coriandrum sativum L.) germplasm across coriander growing regions in India. J. Essent. Oil Res. 2022, 34, 173–180. [Google Scholar] [CrossRef]
- Shams, M.; Esfahan, S.Z.; Esfahan, E.Z.; Dashtaki, H.N.; Dursun, A.; Yildirim, E. Effects of climatic factors on the quantity of essential oil and dry matter yield of coriander (Coriandrum sativum L.). Indian J. Sci. Technol. 2016, 9, 1–4. [Google Scholar] [CrossRef]
- Wang, F.; Gao, Q.; Ji, G.; Wang, J.; Ding, Y.; Wang, S. Effects of light intensity and photoperiod on morphological development and photosynthetic characteristics of coriander. Horticulturae 2024, 10, 215. [Google Scholar] [CrossRef]
- Aćimović, M.; Oljača, S.; Jaćimović, G.; Dražić, S.; Tasić, S. Benefits of environmental conditions for growing coriander in Banat Region, Serbia. Nat. Prod. Commun. 2011, 6, 1465–1468. [Google Scholar] [PubMed]
- Chandrakala, R.; Venkatesan, K.; Senthamizh Selvi, B.; Senthil, N.; Karthikeyan, G.; Vellai Kumar, S. Variation in seed yield and essential oil composition of coriander (Coriandrum sativum L.). J. Essent. Oil Res. 2024, 36, 222–233. [Google Scholar] [CrossRef]
- Sawargaonkar, S.L.; Singh, A.K.; Tiwari, J.; Singh, K.P.; Sao, A.; Paraye, P.M.; Painkra, S.K.; Rathia, G.R.; Sahu, S.; Rao, S.S.; et al. Genotype × environment interaction and stability of indigenous coriander (Coriandrum sativum L.) genotypes for seed yield in different agro-climatic zones of Chhattisgarh. J. Spices Aromat. Crops 2019, 28, 122–130. [Google Scholar] [CrossRef]
- Talebi, S.M.; Naser, A.; Ghorbanpour, M. Chemical composition and antimicrobial activity of the essential oils in different populations of Coriandrum sativum L. (coriander) from Iran and Iraq. Food Sci. Nutr. 2024, 1–11. [Google Scholar] [CrossRef]
- Freires, I.D.A.; Murata, R.M.; Furletti, V.F.; Sartoratto, A.; Alencar, S.M.D.; Figueira, G.M.; de Oliveira Rodrigues, J.A.; Duarte, M.C.T.; Rosalen, P.L. Coriandrum sativum L. (coriander) essential oil: Antifungal activity and mode of action on Candida spp., and molecular targets affected in human whole-genome expression. PLoS ONE 2014, 9, e99086. [Google Scholar] [CrossRef] [PubMed]
- Shojaiefar, S.; Sabzalian, M.R.; Mirlohi, A.; Mirjalili, M.H. Seed yield stability with modified essential oil content and composition in self-compatible progenies of bitter fennel (Foeniculum vulgare Mill.). Ind. Crops Prod. 2022, 182, 114821. [Google Scholar] [CrossRef]
- Mehravi, S.; Hanifei, M.; Gholizadeh, A.; Khodadadi, M. Water deficit stress changes in physiological, biochemical and antioxidant characteristics of anise (Pimpinella anisum L.). Plant Physiol. Biochem. 2023, 201, 107806. [Google Scholar] [CrossRef]
- Aćimović, M.; Korać, J.; Jacimović, G.; Oljača, S.; Djukanović, L.; Vuga-Janjatov, V. Influence of ecological conditions on seeds traits and essential oil contents in anise (Pimpinella anisum L.). Not. Bot. Horti. Agrobot. 2014, 42, 232–238. [Google Scholar]
- Iannarelli, R.; Caprioli, G.; Sut, S.; Dall’Acqua, S.; Fiorini, D.; Vittori, S.; Maggi, F. Valorizing overlooked local crops in the era of globalization: The case of aniseed (Pimpinella anisum L.) from Castignano (central Italy). Ind. Crops Prod. 2017, 104, 99–110. [Google Scholar] [CrossRef]
- Bettaieb Rebey, I.; Bourgou, S.; Aidi Wannes, W.; Hamrouni Selami, I.; Saidani Tounsi, M.; Marzouk, B.; Fauconnier, M.L.; Ksouri, R. Comparative assessment of phytochemical profiles and antioxidant properties of Tunisian and Egyptian anise (Pimpinella anisum L.) seeds. Plant Biosyst. Int. J. Deal. Asp. Plant Biol. 2018, 152, 971–978. [Google Scholar] [CrossRef]
- Sedláková, J.; Kocourková, B.; Lojková, L.; Kubá?, V. The essential oil content in caraway species (Carum carvi L.). Hort. Sci. 2003, 30, 73–79. [Google Scholar] [CrossRef]
- Fang, R.; Jiang, C.H.; Wang, X.Y.; Zhang, H.M.; Liu, Z.L.; Zhou, L.; Du, S.S.; Deng, Z.W. Insecticidal activity of essential oil of Carum Carvi fruits from China and its main components against two grain storage insects. Molecules 2010, 15, 9391–9402. [Google Scholar] [CrossRef] [PubMed]
- Kallio, H.; Kerrola, K.; Alhonmaki, P. Carvone and limonene in caraway fruits (Carum carvi L.) analyzed by supercritical carbon dioxide extraction-gas chromatography. J. Agric. Food Chem. 1994, 42, 2478–2485. [Google Scholar] [CrossRef]
- Clark, G. A profile: An aroma chemical—Carvone. Perfum. Flavorist 1989, 14, 35–40. [Google Scholar]
- Erasto, P.; Viljoen, A.M. Limonene—A review: Biosynthetic, ecological and pharmacological relevance. Nat. Prod. Commun. 2008, 3, 1193–1202. [Google Scholar] [CrossRef]
- Bouwmeester, H.J.; Gershenzon, J.; Konings, M.C.; Croteau, R. Biosynthesis of the monoterpenes limonene and carvone in the fruit of caraway. I. Demonstration of enzyme activities and their changes with development. Plant Physiol. 1998, 117, 901–912. [Google Scholar] [CrossRef]
- Chizzola, R. Composition of the essential oil of wild grown caraway in meadows of the Vienna region (Austria). Nat. Prod. Commun. 2014, 9, 581–582. [Google Scholar] [CrossRef]
- von Maydell, D.; Beleites, C.; Stache, A.M.; Riewe, D.; Krahmerc, A.; Marthe, F. Genetic variation of annual and biennial caraway (Carum carvi) germplasm offers diverse opportunities for breeding. Ind. Crops Prod. 2024, 208, 117798. [Google Scholar] [CrossRef]
- Ibrahim, M.F.; Ali, M.M.; Lamlom, S.; Kalaji, H.M.; Yousef, A.F. Climate change necessitates a change in the cultivation date of caraway (Carum carvi L.). J. Water Land Dev. 2022, 54, 39–49. [Google Scholar] [CrossRef]
- Solberg, S.O.; Göransson, M.; Petersen, M.A.; Yndgaard, F.; Jeppson, S. Caraway essential oil composition and morphology: The role of location and genotype. Biochem. Syst. Ecol. 2016, 66, 351–357. [Google Scholar] [CrossRef]
- Bosko, R.; Vagnerova, L.; Pluhackova, H.; Sofrova, J.; Smirous, P. The variability of caraway (Carum carvi L.) essential oils. MendelNet 2016, 23, 30–34. [Google Scholar]
- Seidler-Łożykowska, K.; Baranska, M.; Baranski, R.; Krol, D. Raman analysis of caraway (Carum carvi L.) single fruits. Evaluation of essential oil content and its composition. J. Agric. Food Chem. 2010, 58, 5271–5275. [Google Scholar] [CrossRef]
- Dobhal, P.; Purohit, V.K.; Chandra, S.; Rawat, S.; Prasad, P.; Bhandari, U.; Trivedi, V.L.; Nautiyal, M.C. Climate-induced changes in essential oil production and terpene composition in Alpine aromatic plants. Plant Stress 2024, 12, 100445. [Google Scholar] [CrossRef]
- Hälvä, S.; Craker, L.E.; Simon, J.E.; Charles, D.J. Growth and essential oil in dill, Anethum graveolens L., in response to temperature and photoperiod. J. Herbs Spices Med. Plants 1993, 1, 47–56. [Google Scholar] [CrossRef]
- Mirmohammadmakki, F.; Gharachorloo, M.; Ghavami, M.; Abdossi, V.; Azizinezhad, R. Quantitative changes in essential oils contents of parsley (Petroselinum crispum) harvested in three consecutive months of spring. J. Food Bioprocess Eng. 2023, 6, 48–55. [Google Scholar]
- Pirmohammadi, M.; Abai, M.R.; Shayeghi, M.; Vatandoost, H.; Rahimi, S.; Pirmohammadi, M. Influence of agro-climatic conditions on chemical compositions and repellency effect of Mentha longifolia plant against malaria vector, Anopheles stephensi. Toxin Rev. 2023, 42, 115–121. [Google Scholar] [CrossRef]
- Aćimović, M.; Zeremski, T.; Šovljanski, O.; Lončar, B.; Pezo, L.; Zheljazkov, V.D.; Pezo, M.; Šuput, D.; Kurunci, Z. Seasonal variations in essential oil composition of immortelle cultivated in Serbia. Horticulturae 2022, 8, 1183. [Google Scholar] [CrossRef]
- Kurpis, J.; Serrato-Cruz, M.A.; Arroyo, T.P.F. Modeling the effects of climate change on the distribution of Tagetes lucida Cav. (Asteraceae). Glob. Ecol. Conserv. 2019, 20, e00747. [Google Scholar] [CrossRef]
- Aćimović, M.; Ljujić, J.; Vulić, J.; Zheljazkov, V.D.; Pezo, L.; Varga, A.; Tumbas Šaponjac, V. Helichrysum italicum (Roth) G. Don essential oil from Serbia: Chemical composition, classification and biological activity—May it be a suitable new crop for Serbia? Agronomy 2021, 11, 1282. [Google Scholar] [CrossRef]
- Kiprovski, B.; Zeremski, T.; Varga, A.; Cabarkapa, I.; Filipović, J.; Lončar, B.; Aćimović, M. Essential oil quality of lavender grown outside its native distribution range: A study from Serbia. Horticulturae 2023, 9, 816. [Google Scholar] [CrossRef]
- Aćimović, M.; Filipović, V.; Stanković, J.; Cvetković, M.; Đukanović, L. The influence of environmental conditions on Carum carvi L. var. annum seed quality. Ratar. Povrt. 2015, 52, 91–96. [Google Scholar] [CrossRef]
- Bouwmeester, H.J.; Smid, H.G. Seed yield in caraway (Carum carvi). 1. Role of pollination. J. Agric. Sci. 1995, 124, 235–244. [Google Scholar] [CrossRef]
- Malhotra, S.K. Caraway. In Handbook of Herbs and Spicesm, 2nd ed.; Peter, K.V., Ed.; Woodhead Publishing Series in Food Science, Technology and Nutrition; Woodhead Publishing: Sawston, UK, 2012; pp. 225–248. [Google Scholar]
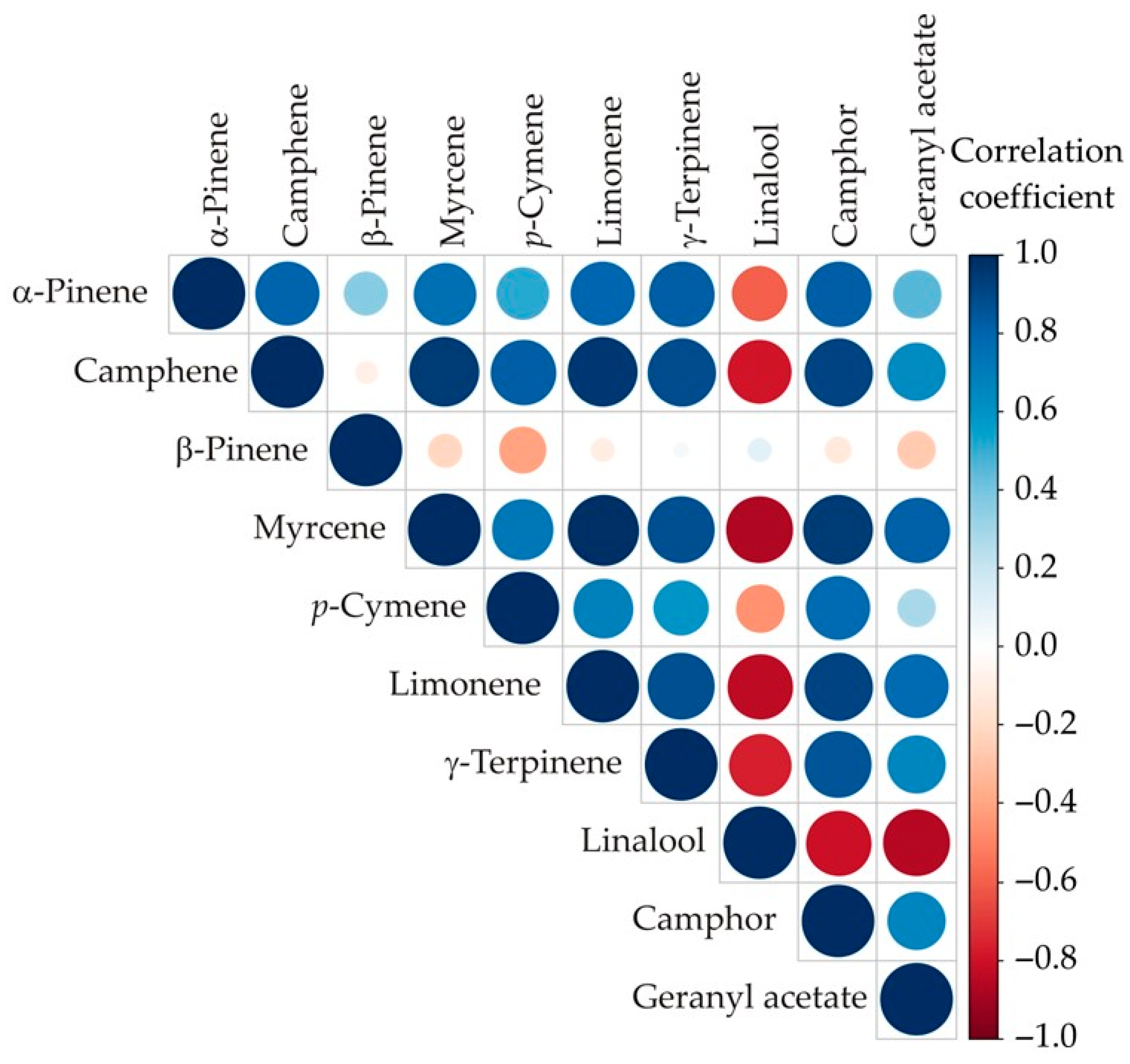
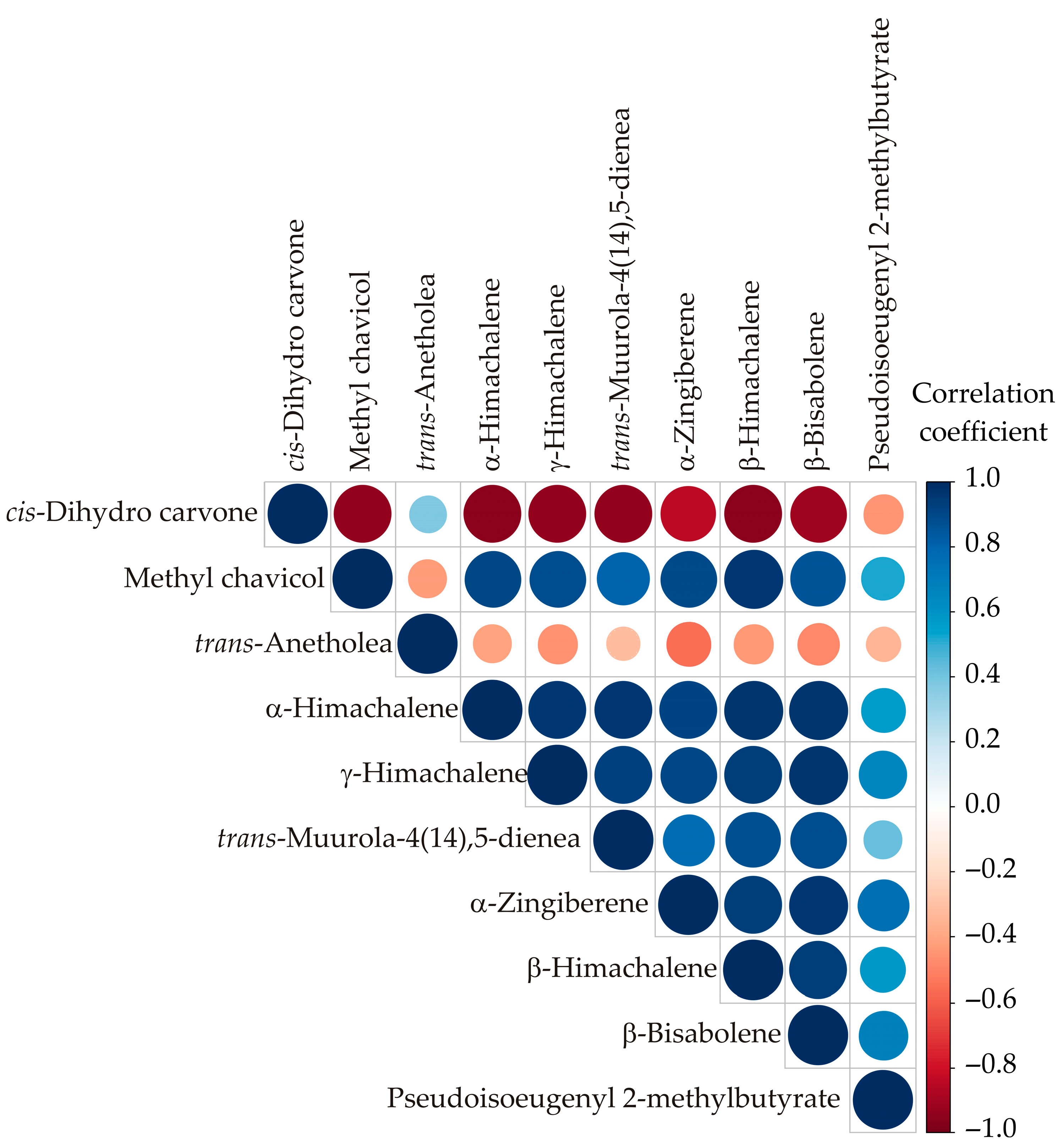

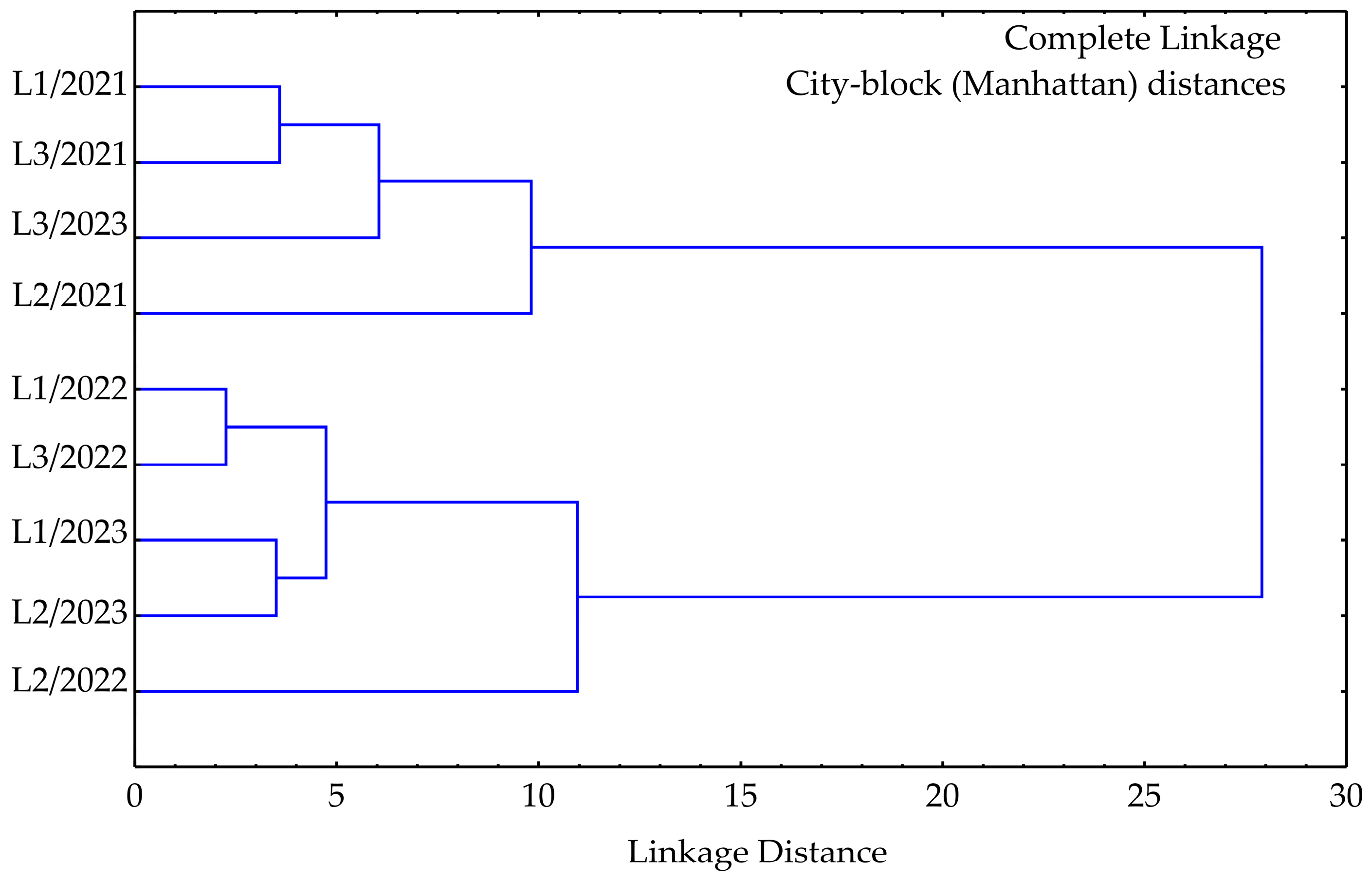

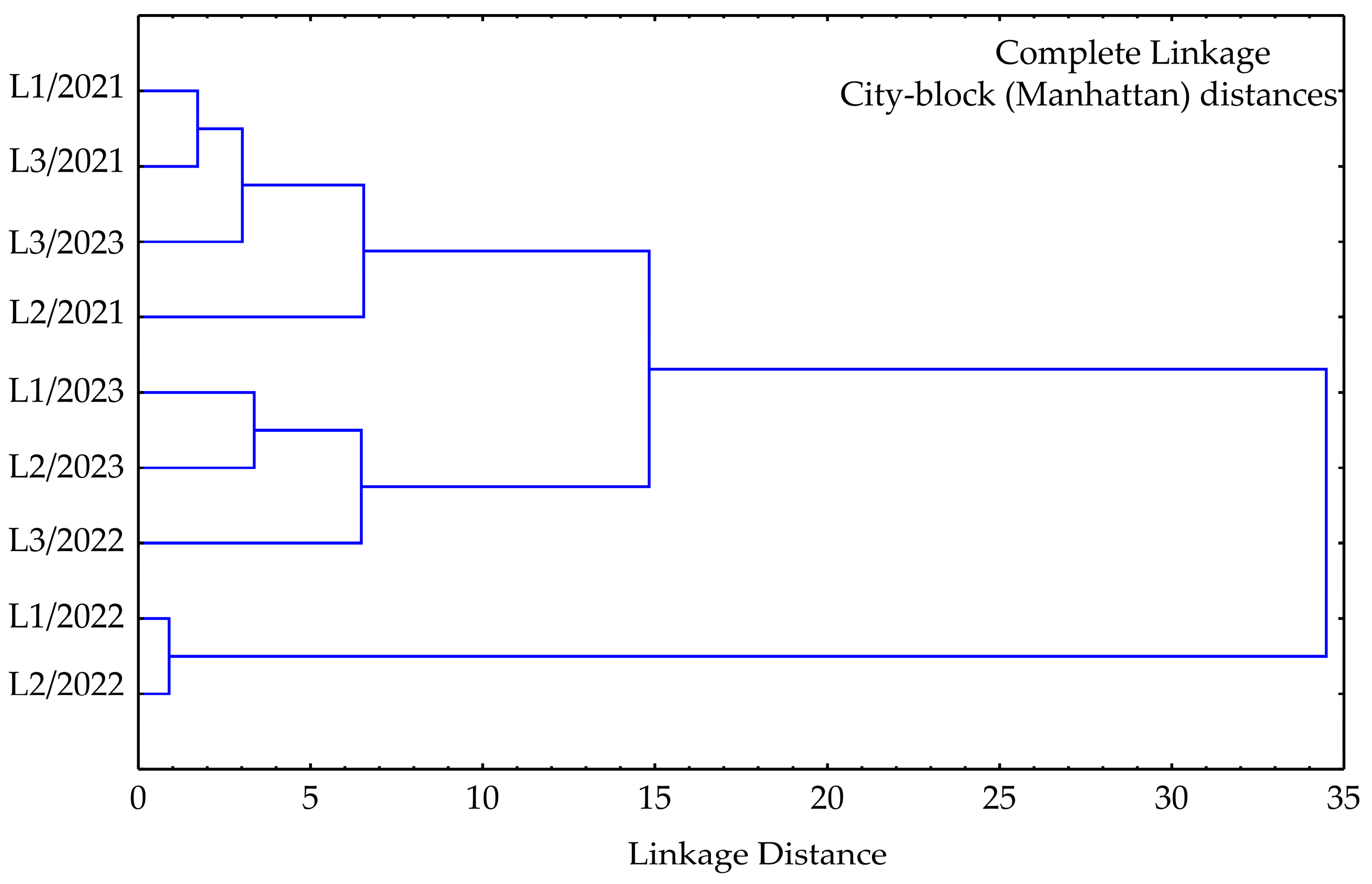
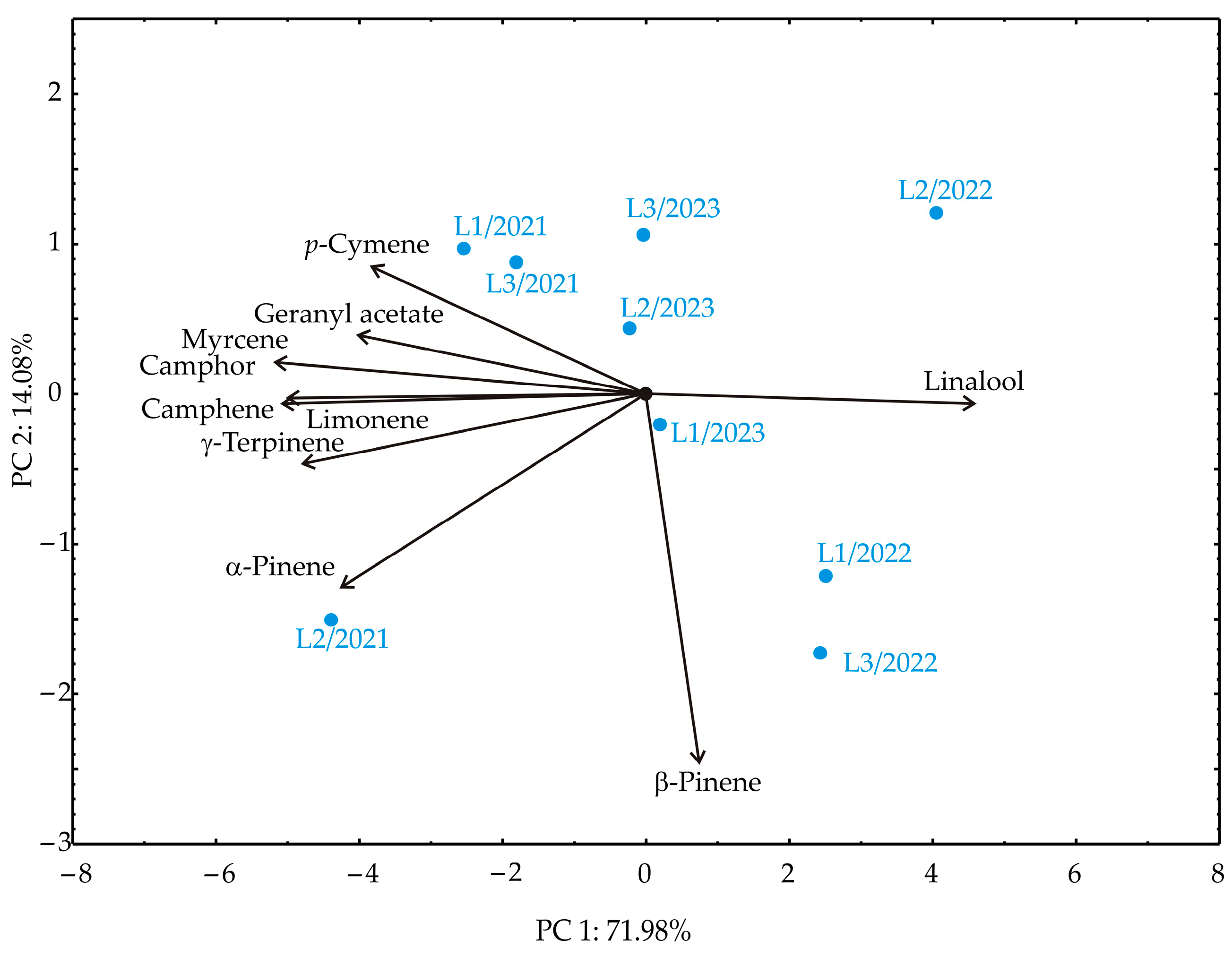
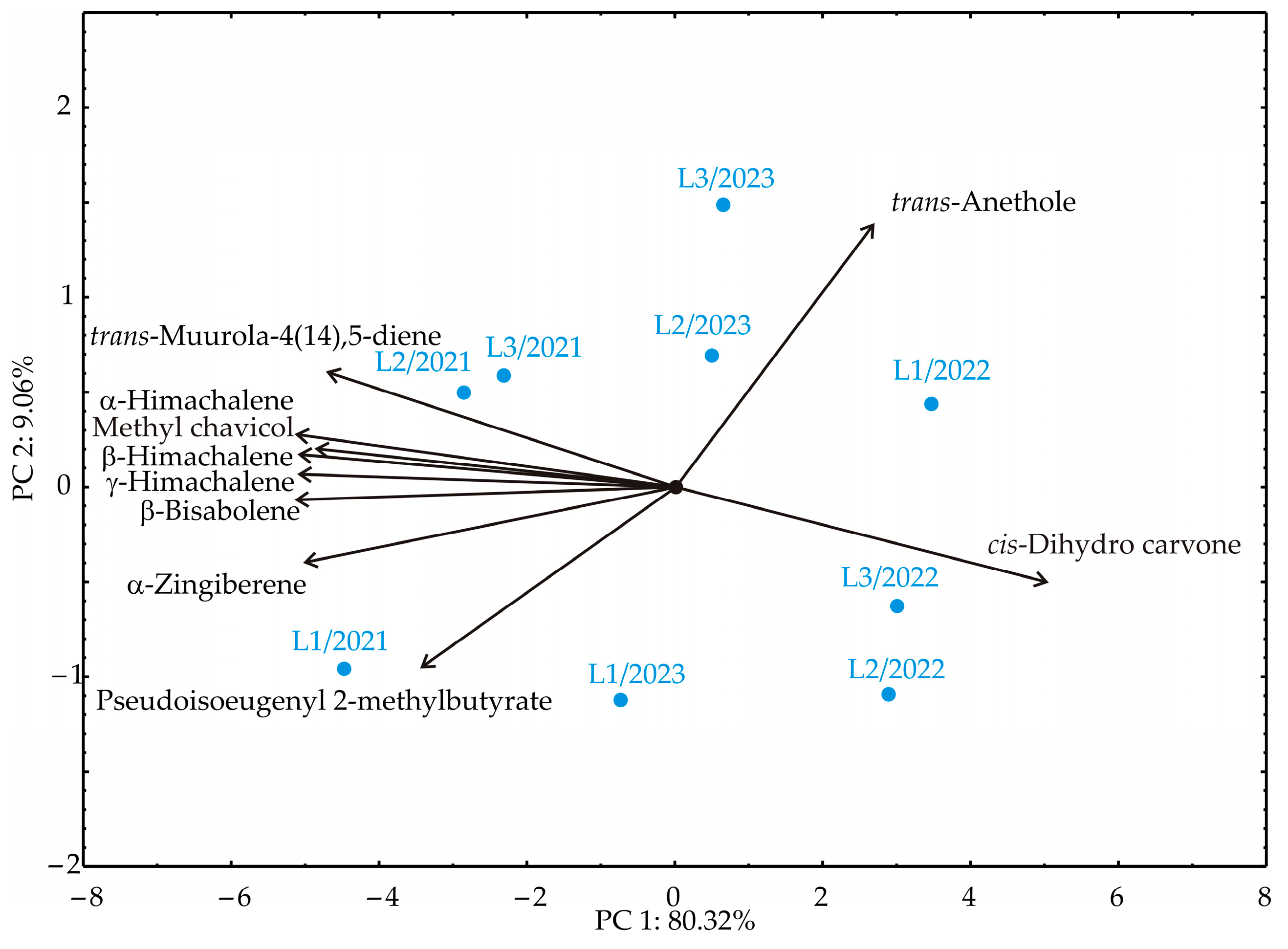

| No | Compound | RT | Mean | SD | Range (%) | L1 | L2 | L3 | ISO 3516 [52] | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Min | Max | KT | Kp | Ki | KT | Kp | Ki | KT | Kp | Ki | ||||||
| 1 | α-pinene | 58.270 | 8.5 | 1.0 | 6.6 | 10.5 | −0.39 | −3.26 | −0.79 | −2.40 | −2.30 | −3.85 | 0.04 | −0.16 | −0.02 | 3.0–7.0 |
| 2 | camphene | 62.286 | 0.9 | 0.2 | 0.6 | 1.2 | −0.12 | −0.70 | −0.21 | −0.39 | −0.26 | −0.57 | −0.16 | −0.37 | −0.26 | - |
| 3 | β-pinene | 70.277 | 0.8 | 0.0 | 0.7 | 0.8 | 0.03 | 0.23 | 0.06 | −0.05 | −0.09 | −0.10 | 0.07 | 0.13 | 0.10 | - |
| 4 | myrcene | 74.054 | 0.6 | 0.3 | 0.1 | 1.1 | −0.19 | −1.24 | −0.34 | −0.62 | −0.58 | −0.99 | −0.45 | −0.99 | −0.69 | 0.5–1.5 |
| 5 | p-cymene | 85.589 | 0.8 | 0.2 | 0.5 | 1.1 | −0.20 | −1.22 | −0.34 | −0.17 | −0.08 | −0.24 | −0.17 | −0.37 | −0.26 | - |
| 6 | limonene | 87.005 | 1.8 | 0.4 | 1.2 | 2.5 | −0.17 | −1.13 | −0.31 | −0.80 | −0.62 | −1.22 | −0.48 | −1.07 | −0.74 | 2.0–5.0 |
| 7 | γ-terpinene | 98.079 | 7.6 | 0.8 | 6.1 | 8.5 | −0.31 | −1.71 | −0.51 | −1.61 | −0.69 | −2.22 | −0.43 | −1.03 | −0.70 | 2.0–7.0 |
| 8 | linalool | 115.735 | 72.6 | 5.0 | 65.3 | 80.5 | 2.01 | 8.43 | 2.94 | 8.92 | 11.94 | 15.76 | 3.96 | 6.98 | 5.51 | 65–78 |
| 9 | camphor | 132.597 | 3.1 | 0.5 | 2.5 | 3.9 | −0.33 | −2.24 | −0.60 | −0.82 | −1.08 | −1.44 | −0.42 | −0.91 | −0.64 | 4.0–6.0 |
| 10 | geranyl acetate | 238.200 | 1.6 | 0.8 | 0.6 | 3.0 | −0.31 | −2.09 | −0.56 | −1.10 | −1.06 | −1.76 | −1.05 | −2.30 | −1.62 | 1.0–3.5 |
| No | Compound | RT | Mean | SD | Range | L1 | L2 | L3 | ISO 3475 [53] | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Min | Max | KT | Kp | Ki | KT | Kp | Ki | KT | Kp | Ki | ||||||
| 1 | cis-dihydro carvone | 1192 | 0.1 | 0.1 | 0.0 | 0.3 | 0.13 | 0.23 | 0.15 | 0.17 | −0.75 | 0.35 | 0.06 | 0.13 | 0.58 | - |
| 2 | methyl chavicol | 1200 | 0.5 | 0.3 | 0.2 | 0.9 | −0.34 | −0.60 | −0.39 | −0.21 | 0.96 | −0.44 | −0.19 | −0.37 | −1.61 | 0.5–3.0 |
| 3 | trans-anethole | 1294 | 94.9 | 4.3 | 92.4 | 96.8 | 3.06 | 6.63 | 3.26 | 0.48 | −29.17 | 3.70 | 5.76 | 0.86 | −0.30 | 87–94 |
| 4 | α-himachalene | 1452 | 0.2 | 0.1 | 0.1 | 0.3 | −0.10 | −0.18 | −0.12 | −0.10 | 0.38 | −0.20 | −0.04 | −0.10 | −0.43 | - |
| 5 | γ-himachalene | 1482 | 2.5 | 0.5 | 1.8 | 3.4 | −0.69 | −1.12 | −0.81 | −0.58 | 3.61 | −1.30 | −0.32 | −0.48 | −2.05 | 1.0–5.0 |
| 6 | trans-muurola−4(14),5-diene | 1485 | 0.3 | 0.2 | 0.1 | 0.6 | −0.15 | −0.26 | −0.17 | −0.25 | 1.15 | −0.52 | −0.09 | −0.19 | −0.84 | - |
| 7 | α-zingiberene | 1499 | 0.2 | 0.1 | 0.1 | 0.5 | −0.21 | −0.37 | −0.24 | −0.09 | 0.33 | −0.18 | −0.06 | −0.11 | −0.50 | - |
| 8 | β-himachalene | 1504 | 0.2 | 0.0 | 0.1 | 0.2 | −0.05 | −0.09 | −0.06 | −0.03 | 0.20 | −0.08 | −0.01 | −0.04 | −0.19 | - |
| 9 | β-bisabolene | 1512 | 0.1 | 0.1 | 0.0 | 0.3 | −0.13 | −0.22 | −0.15 | −0.11 | 0.44 | −0.22 | −0.03 | −0.07 | −0.32 | - |
| 10 | pseudo isoeugenyl 2-methylbutyrate | 1848 | 0.8 | 0.2 | 0.3 | 1.3 | −0.48 | −0.84 | −0.55 | 0.03 | 0.02 | 0.04 | −0.01 | 0.02 | 0.10 | 0.3–2.0 |
| No | Compound | RT | Mean | SD | Range | L1 | L2 | L3 | ISO 8896 [54] | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Min | Max | KT | Kp | Ki | KT | Kp | Ki | KT | Kp | Ki | ||||||
| 1 | myrcene | 7.447 | 0.2 | 0.1 | 0.1 | 0.3 | −0.08 | −0.05 | −0.10 | −0.07 | −0.12 | −0.09 | −0.22 | −0.08 | −0.21 | 0.2–0.7 |
| 2 | p-cymene | 8.581 | 0.1 | 0.1 | 0.0 | 0.3 | 0.15 | 0.10 | 0.20 | 0.28 | 0.83 | 0.33 | 0.02 | 0.01 | 0.02 | - |
| 3 | limonene | 8.780 | 61.0 | 6.4 | 54.0 | 70.3 | 8.26 | 6.47 | 11.40 | 12.06 | 12.25 | 14.01 | 7.83 | 2.82 | 5.61 | 33–45 |
| 4 | cis-limonene oxide | 12.780 | 0.1 | 0.1 | 0.0 | 0.3 | −0.12 | −0.08 | −0.15 | −0.05 | −0.10 | −0.06 | −0.20 | −0.07 | −0.18 | - |
| 5 | trans-limonene oxide | 12.970 | 0.2 | 0.1 | 0.0 | 0.3 | −0.03 | −0.02 | −0.04 | 0.09 | 0.14 | 0.10 | −0.20 | −0.07 | −0.18 | - |
| 6 | trans-dihydro carvone | 15.869 | 0.2 | 0.1 | 0.1 | 0.3 | 0.02 | 0.02 | 0.03 | 0.05 | 0.11 | 0.06 | −0.29 | −0.10 | −0.27 | - |
| 7 | trans-carveol | 16.553 | 0.2 | 0.1 | 0.1 | 0.3 | 0.01 | 0.01 | 0.02 | 0.15 | 0.23 | 0.17 | 0.12 | 0.04 | 0.10 | - |
| 8 | neo iso-dihydro carveol | 17.038 | 0.1 | 0.1 | 0.0 | 0.3 | 0.14 | 0.10 | 0.18 | 0.26 | 0.51 | 0.30 | 0.23 | 0.08 | 0.21 | - |
| 9 | carvone | 17.738 | 36.8 | 6.1 | 27.4 | 44.5 | −8.34 | −6.05 | −11.10 | −15.10 | −29.91 | −17.56 | −6.95 | −2.53 | −3.95 | 50–63 |
| 10 | trans-caryophyllene | 25.399 | 0.2 | 0.0 | 0.1 | 0.2 | 0.06 | 0.04 | 0.08 | 0.06 | 0.09 | 0.06 | 0.00 | 0.00 | −0.02 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lončar, B.; Pezo, L.; Pezo, M.; Jovanović, A.; Šuput, D.; Radosavljević, M.; Aćimović, M. Do Climate Conditions Affect the Quality of the Apiaceae Fruits’ Essential Oils? Horticulturae 2024, 10, 577. https://doi.org/10.3390/horticulturae10060577
Lončar B, Pezo L, Pezo M, Jovanović A, Šuput D, Radosavljević M, Aćimović M. Do Climate Conditions Affect the Quality of the Apiaceae Fruits’ Essential Oils? Horticulturae. 2024; 10(6):577. https://doi.org/10.3390/horticulturae10060577
Chicago/Turabian StyleLončar, Biljana, Lato Pezo, Milada Pezo, Aca Jovanović, Danijela Šuput, Miloš Radosavljević, and Milica Aćimović. 2024. "Do Climate Conditions Affect the Quality of the Apiaceae Fruits’ Essential Oils?" Horticulturae 10, no. 6: 577. https://doi.org/10.3390/horticulturae10060577
APA StyleLončar, B., Pezo, L., Pezo, M., Jovanović, A., Šuput, D., Radosavljević, M., & Aćimović, M. (2024). Do Climate Conditions Affect the Quality of the Apiaceae Fruits’ Essential Oils? Horticulturae, 10(6), 577. https://doi.org/10.3390/horticulturae10060577










