Comparative Metabolomic Fingerprinting Analysis of Tomato Fruits from Physalis Species in Mexico’s Balsas Basin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Metabolomic Fingerprinting Analisis using SPME-GC/MS
2.2.1. Sample Preparation and Extraction
2.2.2. Solid-Phase Microextraction (SPME)
2.2.3. Gas Chromatography–Mass Spectrometry (GC/MS)
2.3. Data Analysis
3. Results
3.1. Metabolite Profiling across Physalis Species
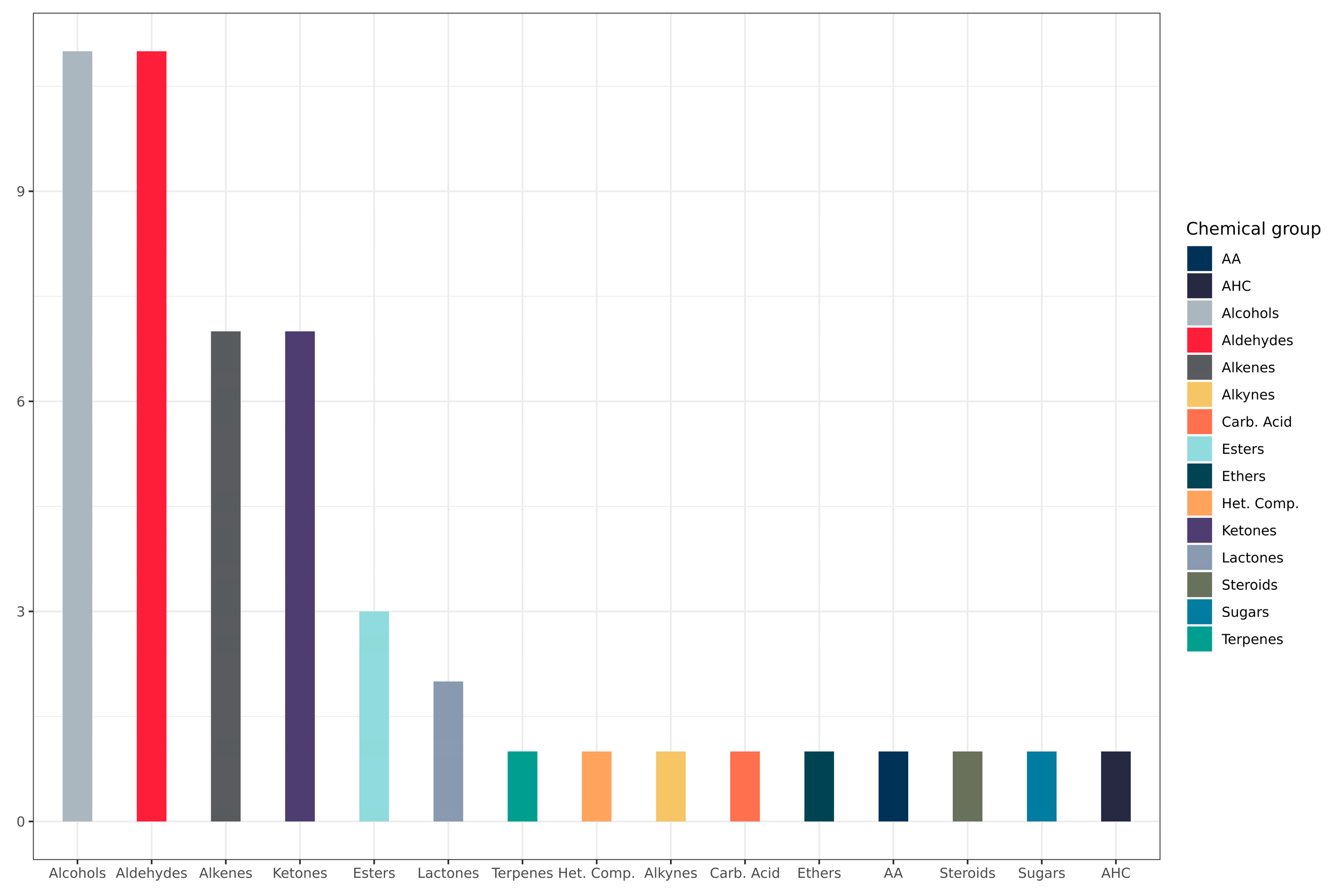
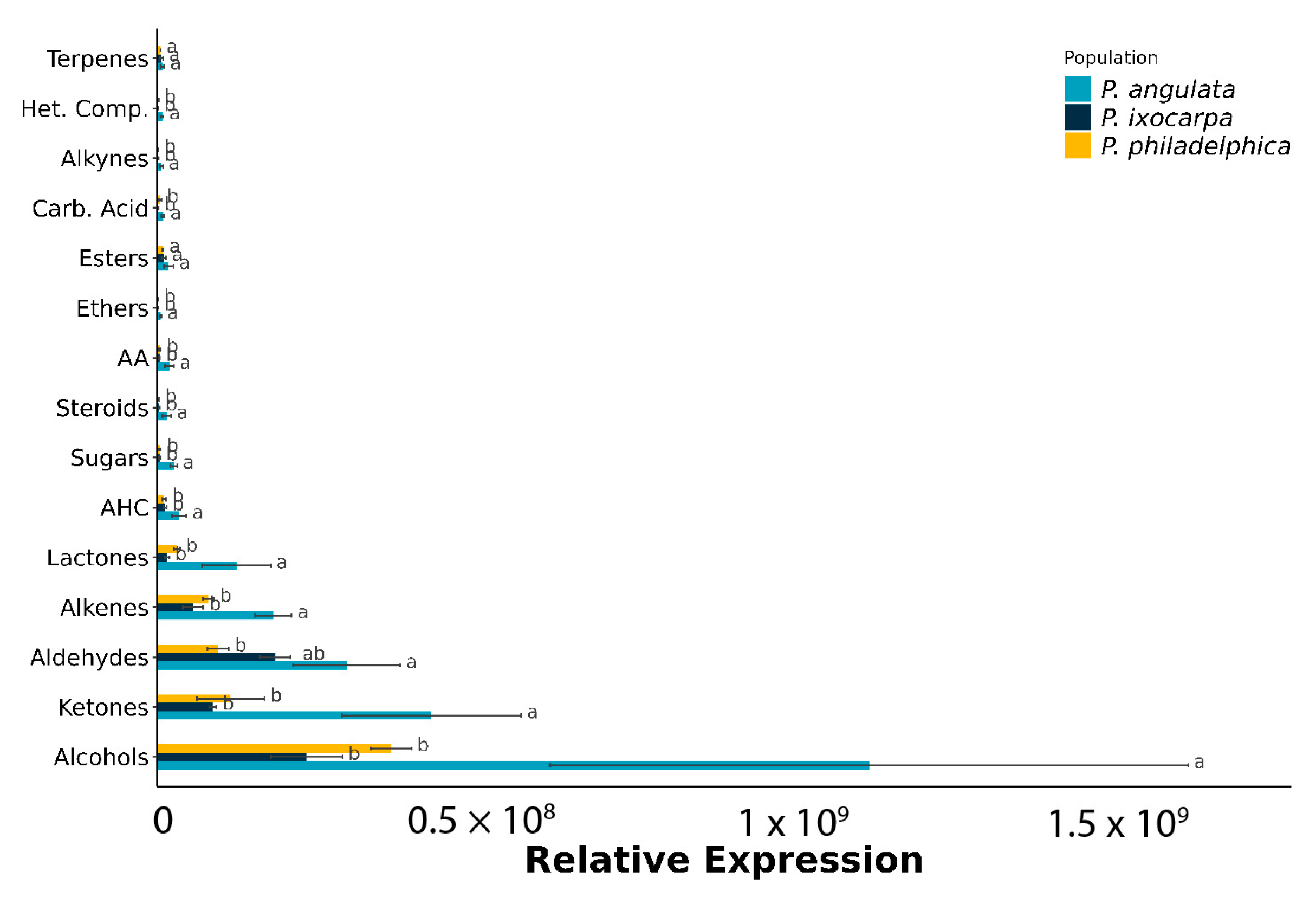
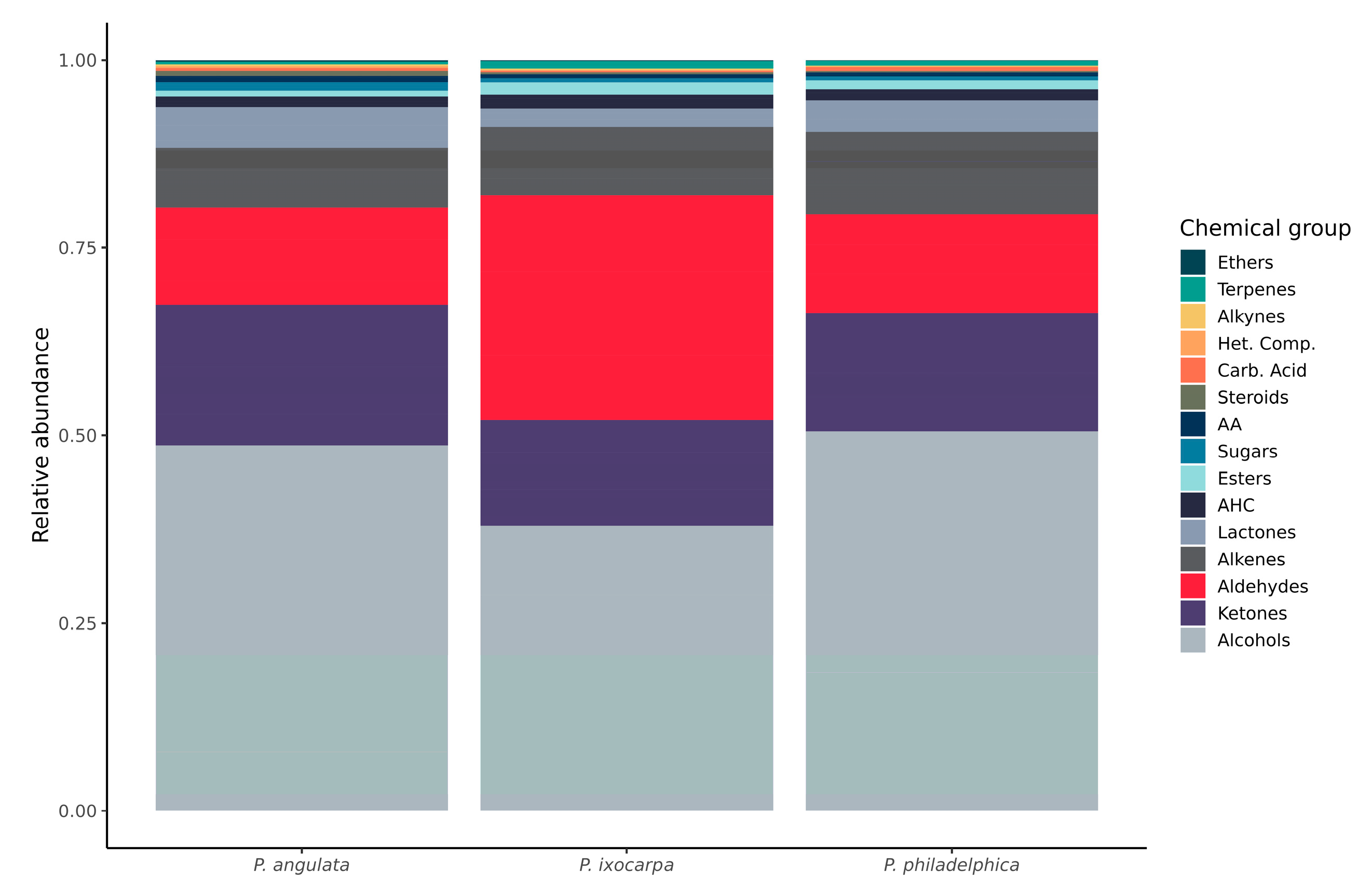
3.2. Metabolite Distribution by Chemical Group
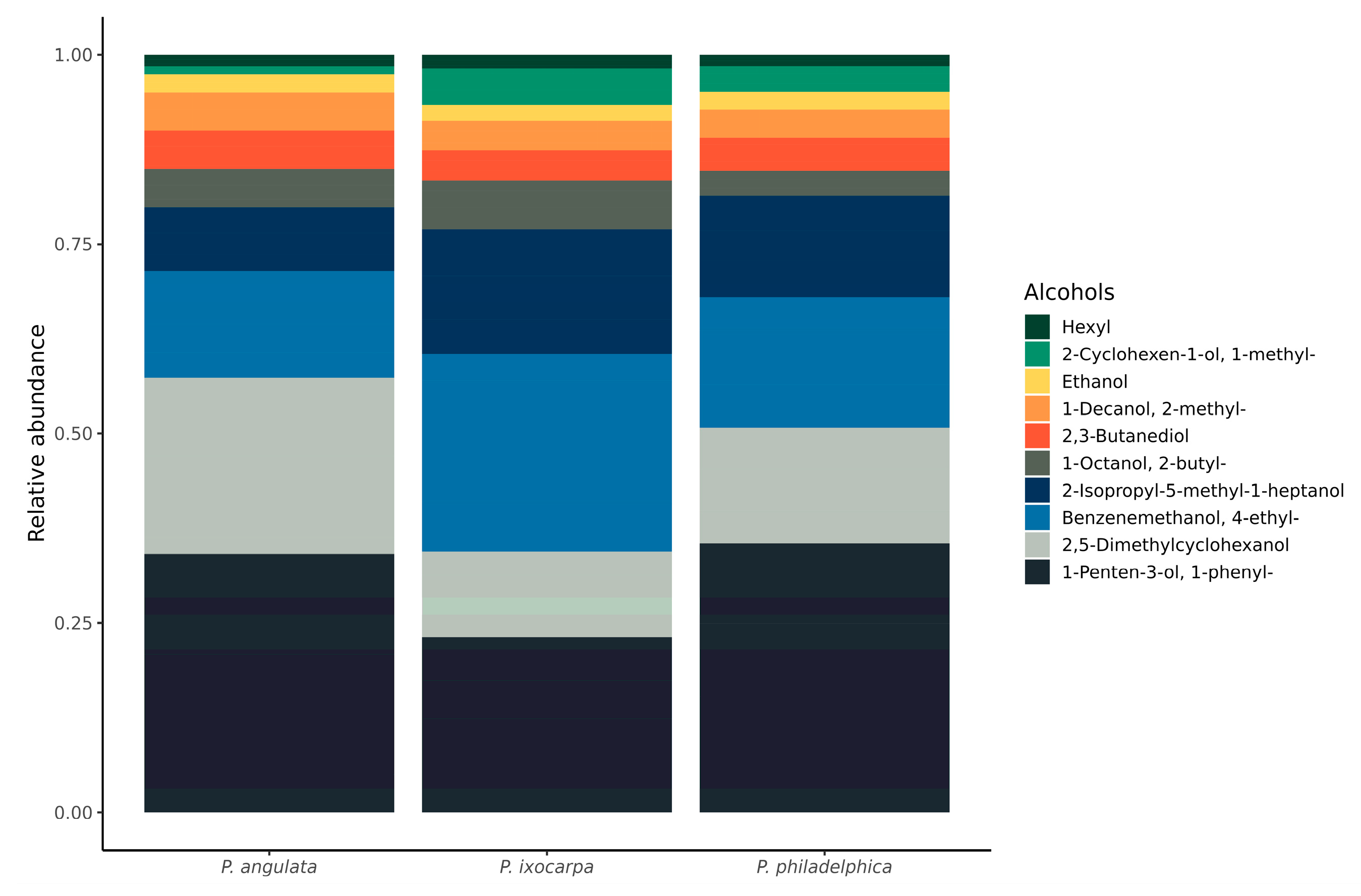
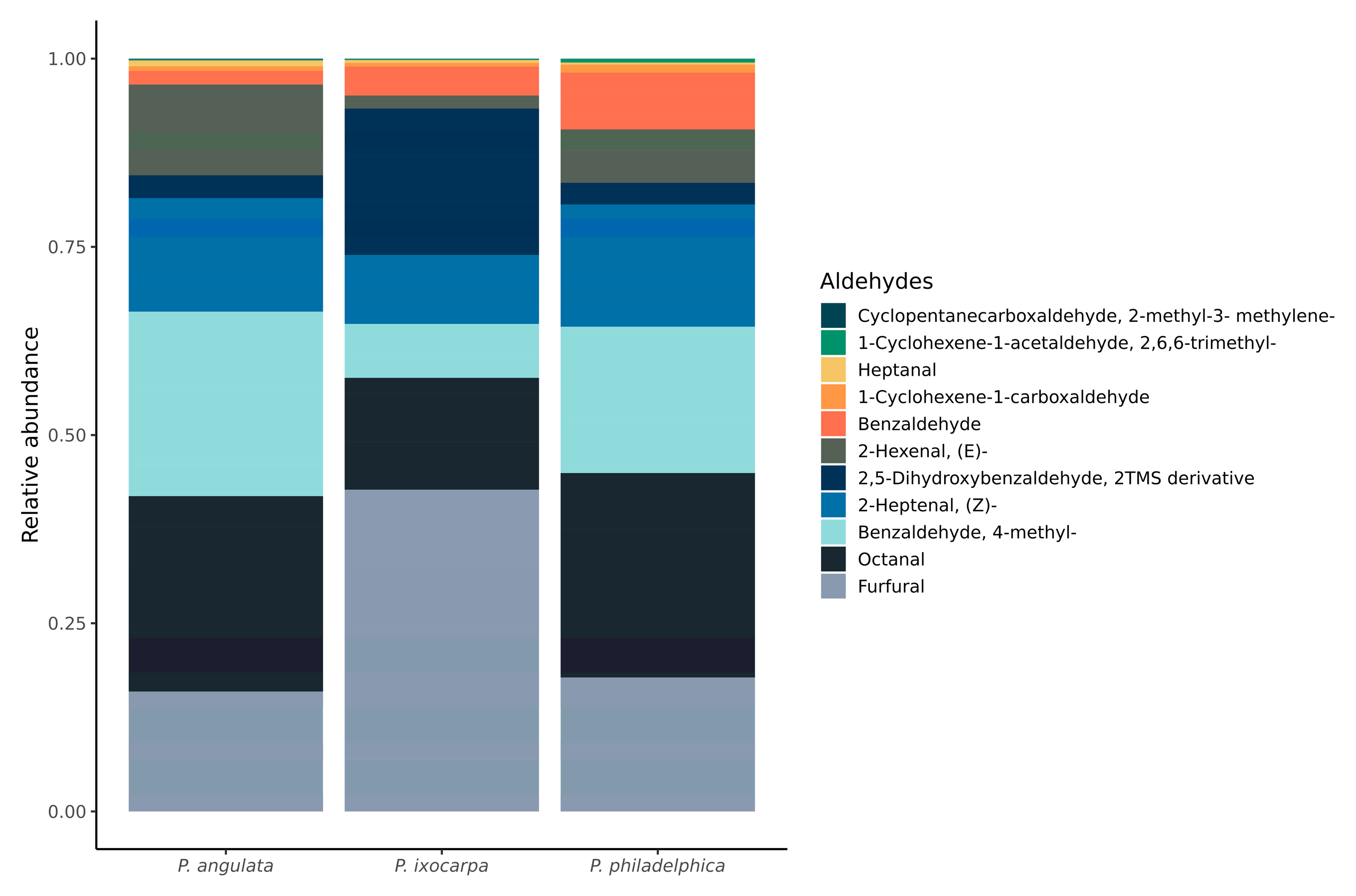
3.3. Alcohol and Aldehyde Groups
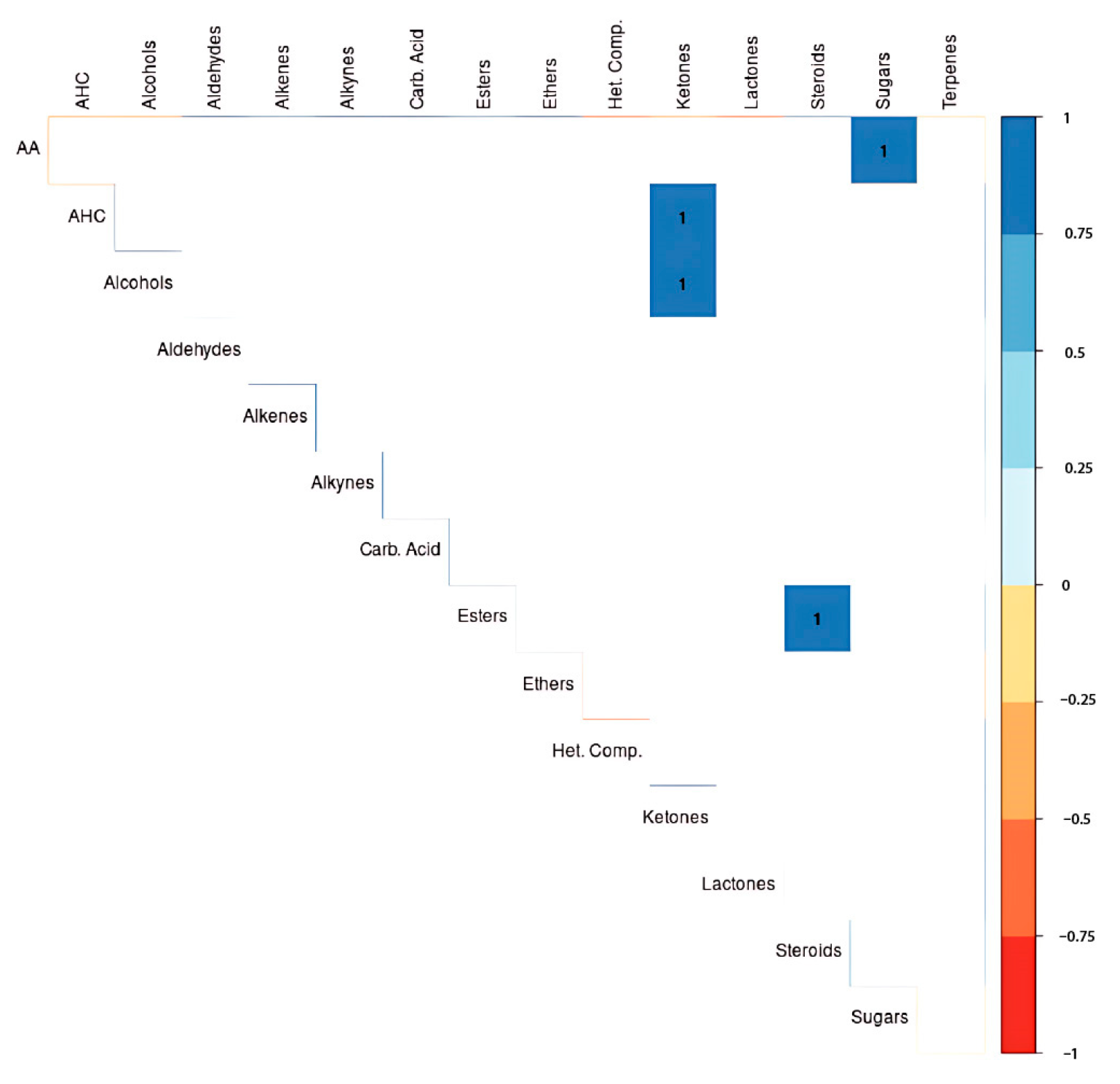
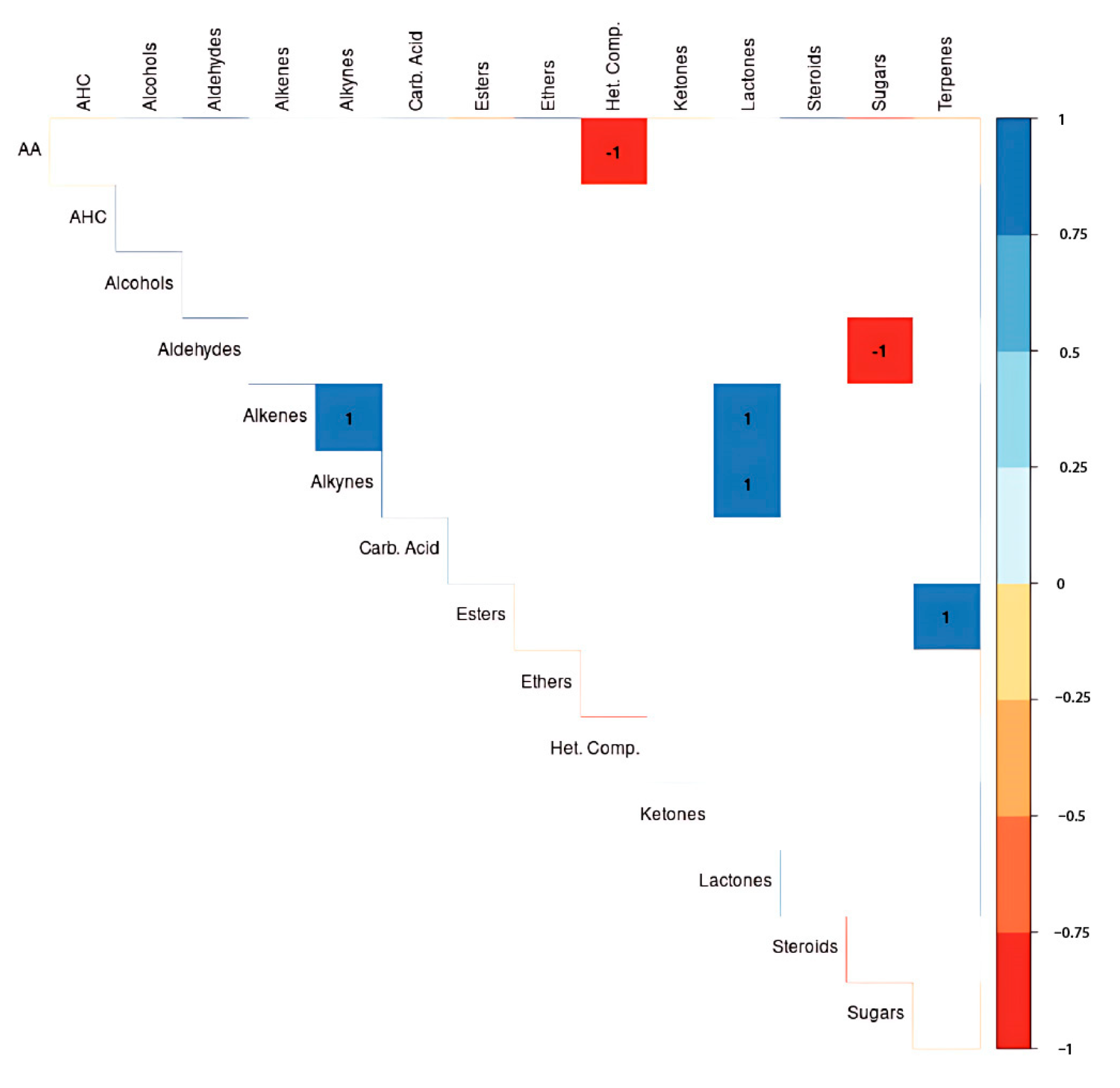
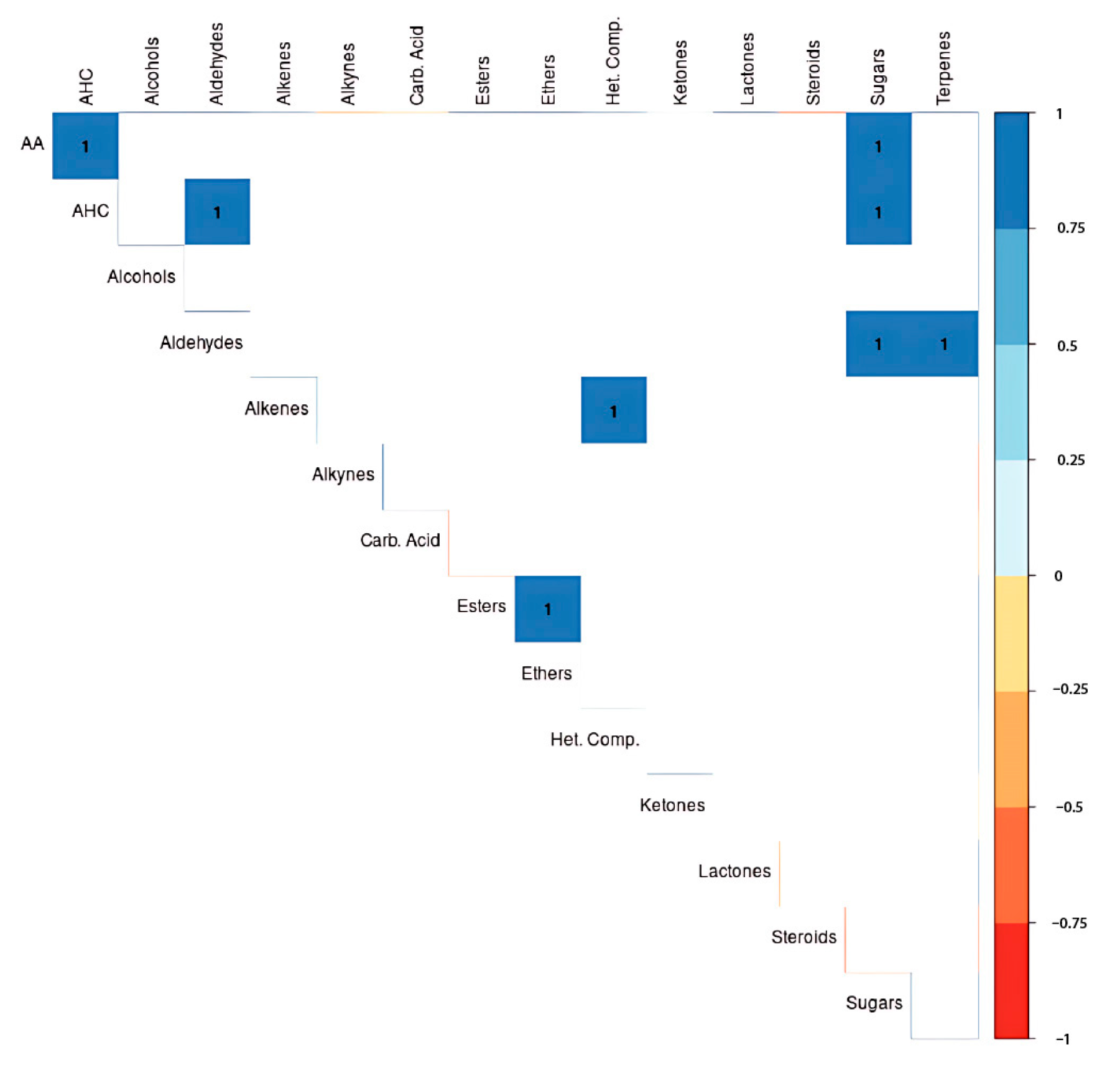
3.4. Metabolite Correlations
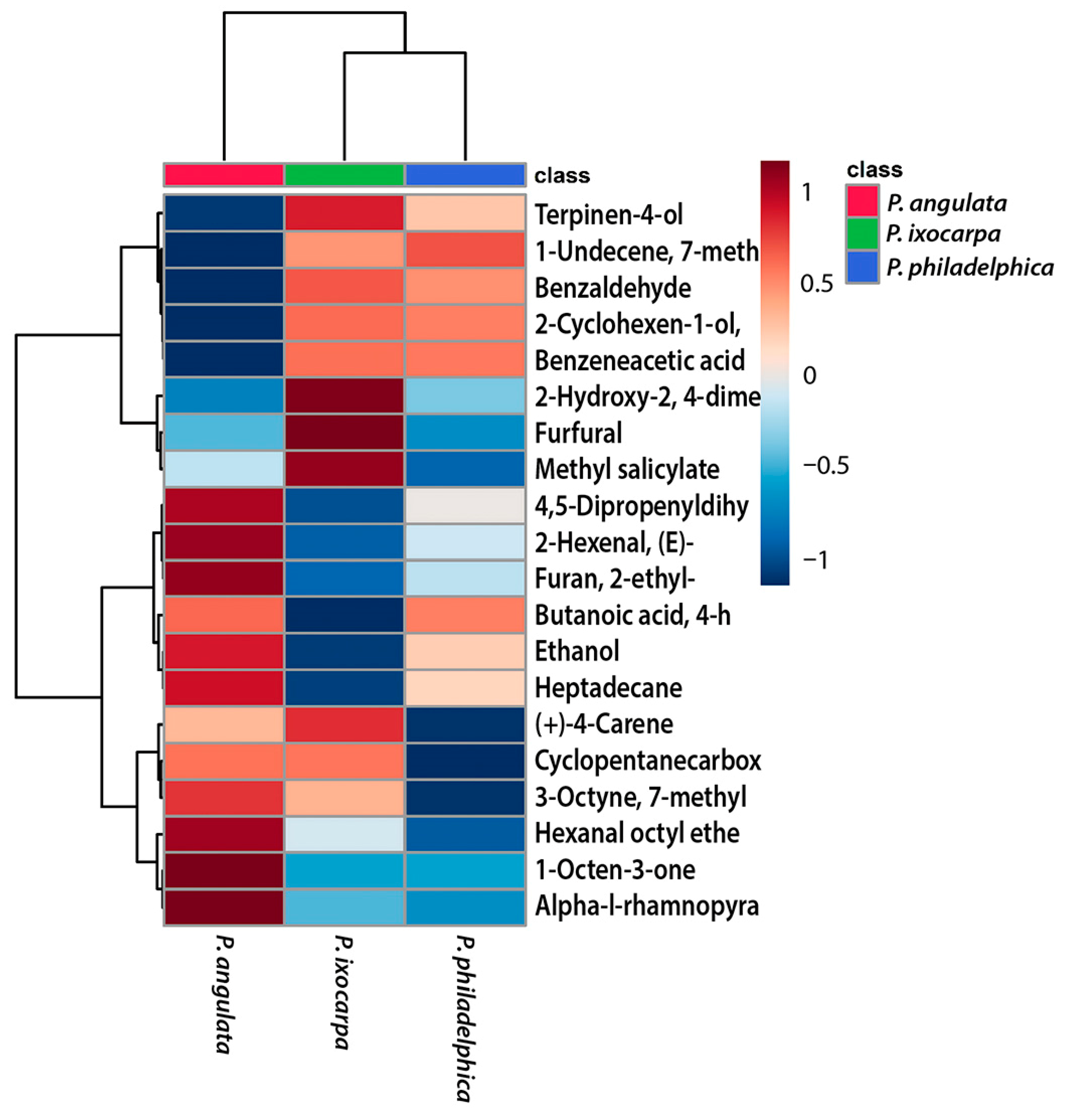
3.5. Heatmap Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Martínez, M.; Vargas-Ponce, O.; Rodríguez, A.; Chiang, F.; Ocegueda, S. Solanaceae family in Mexico. Bot. Sci. 2017, 95, 131–145. [Google Scholar] [CrossRef]
- Martínez, M.; Vargas-Ponce, O.; Zamora-Tavares, P. Taxonomic revision of Physalis in Mexico. Front. Genet. 2023, 14, 1080176. [Google Scholar] [CrossRef]
- Zamora-Tavares, P.; Vargas-Ponce, O.; Sánchez-Martínez, J.; Cabrera-Toledo, D. Diversity and genetic structure of the husk tomato (Physalis philadelphica Lam.) in Western Mexico. Genet. Resour. Crop Evol. 2015, 62, 141–153. [Google Scholar] [CrossRef]
- del Pilar Zamora-Tavares, M.; Martínez, M.; Magallón, S.; Guzmán-Dávalos, L.; Vargas-Ponce, O. Physalis and physaloids: A recent and complex evolutionary history. Mol. Phylogenetics Evol. 2016, 100, 41–50. [Google Scholar] [CrossRef]
- Solís-Montero, L.; Aceves-Chong, L.; Vega-Polanco, M.; Vargas-Ponce, O. Changes in reproductive traits in Physalis philadelphica; An unexpected shift toward self-Incompatibility in a domesticated annual fruit crop. Front. Plant Sci. 2021, 12, 658406. [Google Scholar] [CrossRef]
- Ulrich, D.; Olbricht, K. Fruit organoleptic properties and potential for their genetic improvement. In Breeding for Fruit Quality; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2011; pp. 39–59. [Google Scholar]
- González-Pérez, J.E.; Guerrero-Beltrán, J.Á. Tomatillo or husk tomato (Physalis philadelphica and Physalis ixocarpa): A review. Sci. Hortic. 2021, 288, 110306. [Google Scholar] [CrossRef]
- Sandoval-Padilla, I.; Pérez-Alquicira, J.; Rodríguez, A.; del Pilar Zamora-Tavares, M.; Vargas-Ponce, O. The plastome of the husk tomato (Physalis philadelphica Lam., Solanaceae): A comparative analysis between wild and cultivated pools. Genet. Resour. Crop Evol. 2022, 69, 1391–1405. [Google Scholar] [CrossRef]
- Hernández, J.F.S.; Yáñez, S.B. Aprovechamiento tradicional de las especies de Physalis en México. Rev. Geogr. Agríc. 2009, 43, 81–86. [Google Scholar]
- Valdivia-Mares, L.E.; Zaragoza, F.A.R.; González, J.J.S.; Vargas-Ponce, O. Phenology, agronomic and nutritional potential of three wild husk tomato species (Physalis, Solanaceae) from Mexico. Sci. Hortic. 2016, 200, 83–94. [Google Scholar] [CrossRef]
- Vargas-Ponce, O.; Sánchez Martínez, J.; Zamora Tavares, M.d.P.; Valdivia Mares, L.E. Traditional management of a small-scale crop of Physalis angulata in Western Mexico. Genet. Resour. Crop Evol. 2016, 63, 1383–1395. [Google Scholar] [CrossRef]
- Saavedra, J.d.C.M.; Zaragoza, F.A.R.; Toledo, D.C.; Hernández, C.V.S.; Vargas-Ponce, O. Agromorphological characterization of wild and weedy populations of Physalis angulata in Mexico. Sci. Hortic. 2019, 246, 86–94. [Google Scholar] [CrossRef]
- Martínez-Vega, A.; Oregel-Zamudio, E.; García-Ruíz, I.; Villapando-Arteaga, E.; Torres-García, J. Genetic and metabolomic differentiation of Physalis ixocarpa Brot. ex Hornem. populations in Michoacan State, Mexico. Genet. Resour. Crop Evol. 2022, 69, 1867–1877. [Google Scholar] [CrossRef]
- Forsberg, E.M.; Huan, T.; Rinehart, D.; Benton, H.P.; Warth, B.; Hilmers, B.; Siuzdak, G. Data processing, multi-omic pathway mapping, and metabolite activity analysis using XCMS Online. Nat. Protoc. 2018, 13, 633–651. [Google Scholar] [CrossRef]
- Tautenhahn, R.; Patti, G.J.; Rinehart, D.; Siuzdak, G. XCMS Online: A web-based platform to process untargeted metabolomic data. Anal. Chem. 2012, 84, 5035–5039. [Google Scholar] [CrossRef]
- Pang, Z.; Chong, J.; Li, S.; Xia, J. MetaboAnalystR 3.0: Toward an optimized workflow for global metabolomics. Metabolites 2020, 10, 186. [Google Scholar] [CrossRef]
- Ariza-Mejía, D.; Oyoque-Salcedo, G.; Angóa-Pérez, V.; Mena-Violante, H.G.; Álvarez-Bernal, D.; Torres-García, J.R. Diversity and Potential Function of the Bacterial Rhizobiome Associated to Physalis ixocarpa Broth. in a Milpa System, in Michoacan, Mexico. Agronomy 2022, 12, 1780. [Google Scholar] [CrossRef]
- Zhao, J.; Sauvage, C.; Zhao, J.; Bitton, F.; Bauchet, G.; Liu, D.; Huang, S.; Tieman, D.M.; Klee, H.J.; Causse, M. Meta-analysis of genome-wide association studies provides insights into genetic control of tomato flavor. Nat. Commun. 2019, 10, 1534. [Google Scholar] [CrossRef]
- Colantonio, V.; Ferrão, L.F.V.; Tieman, D.M.; Bliznyuk, N.; Sims, C.; Klee, H.J.; Munoz, P.; Resende, M.F., Jr. Metabolomic selection for enhanced fruit flavor. Proc. Natl. Acad. Sci. USA 2022, 119, e2115865119. [Google Scholar] [CrossRef]
- Bennett, A.B. Taste: Unraveling tomato flavor. Curr. Biol. 2012, 22, R443–R444. [Google Scholar] [CrossRef]
- Klee, H.J.; Tieman, D.M. The genetics of fruit flavour preferences. Nat. Rev. Genet. 2018, 19, 347–356. [Google Scholar] [CrossRef]
- Heng, Z.; Xu, X.; Xu, X.; Li, Y.; Wang, H.; Huang, W.; Yan, S.; Li, T. Integrated transcriptomic and metabolomic analysis of chili pepper fruits provides new insight into the regulation of the branched chain esters and capsaicin biosynthesis. Food Res. Int. 2023, 169, 112856. [Google Scholar] [CrossRef] [PubMed]
- Ferrão, L.F.V.; Sater, H.; Lyrene, P.; Amadeu, R.R.; Sims, C.A.; Tieman, D.M.; Munoz, P.R. Terpene volatiles mediates the chemical basis of blueberry aroma and consumer acceptability. Food Res. Int. 2022, 158, 111468. [Google Scholar] [CrossRef]
- Zhang, C.-R.; Khan, W.; Bakht, J.; Nair, M.G. New antiinflammatory sucrose esters in the natural sticky coating of tomatillo (Physalis philadelphica), an important culinary fruit. Food Chem. 2016, 196, 726–732. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Xie, T.; Xie, J.; Chen, C.; Ai, L.; Tian, H. Aroma perceptual interactions of benzaldehyde, furfural, and vanillin and their effects on the descriptor intensities of Huangjiu. Food Res. Int. 2020, 129, 108808. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arias-Martínez, S.; Oyoque-Salcedo, G.; Gutiérrez-Cárdenas, O.G.; Oregel-Zamudio, E.; Torres-García, J.R. Comparative Metabolomic Fingerprinting Analysis of Tomato Fruits from Physalis Species in Mexico’s Balsas Basin. Horticulturae 2024, 10, 600. https://doi.org/10.3390/horticulturae10060600
Arias-Martínez S, Oyoque-Salcedo G, Gutiérrez-Cárdenas OG, Oregel-Zamudio E, Torres-García JR. Comparative Metabolomic Fingerprinting Analysis of Tomato Fruits from Physalis Species in Mexico’s Balsas Basin. Horticulturae. 2024; 10(6):600. https://doi.org/10.3390/horticulturae10060600
Chicago/Turabian StyleArias-Martínez, Sergio, Guadalupe Oyoque-Salcedo, Oscar Giovanni Gutiérrez-Cárdenas, Ernesto Oregel-Zamudio, and Jesús Rubén Torres-García. 2024. "Comparative Metabolomic Fingerprinting Analysis of Tomato Fruits from Physalis Species in Mexico’s Balsas Basin" Horticulturae 10, no. 6: 600. https://doi.org/10.3390/horticulturae10060600
APA StyleArias-Martínez, S., Oyoque-Salcedo, G., Gutiérrez-Cárdenas, O. G., Oregel-Zamudio, E., & Torres-García, J. R. (2024). Comparative Metabolomic Fingerprinting Analysis of Tomato Fruits from Physalis Species in Mexico’s Balsas Basin. Horticulturae, 10(6), 600. https://doi.org/10.3390/horticulturae10060600










