Structure and Function of Blueberry Fruit and Flowers: Stomata, Transpiration and Photoassimilation
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Low-Temperature (LT) Cryo SEM
2.2. Fruit Porometry and Chlorophyll
3. Results
3.1. Perianth and Sepal Development
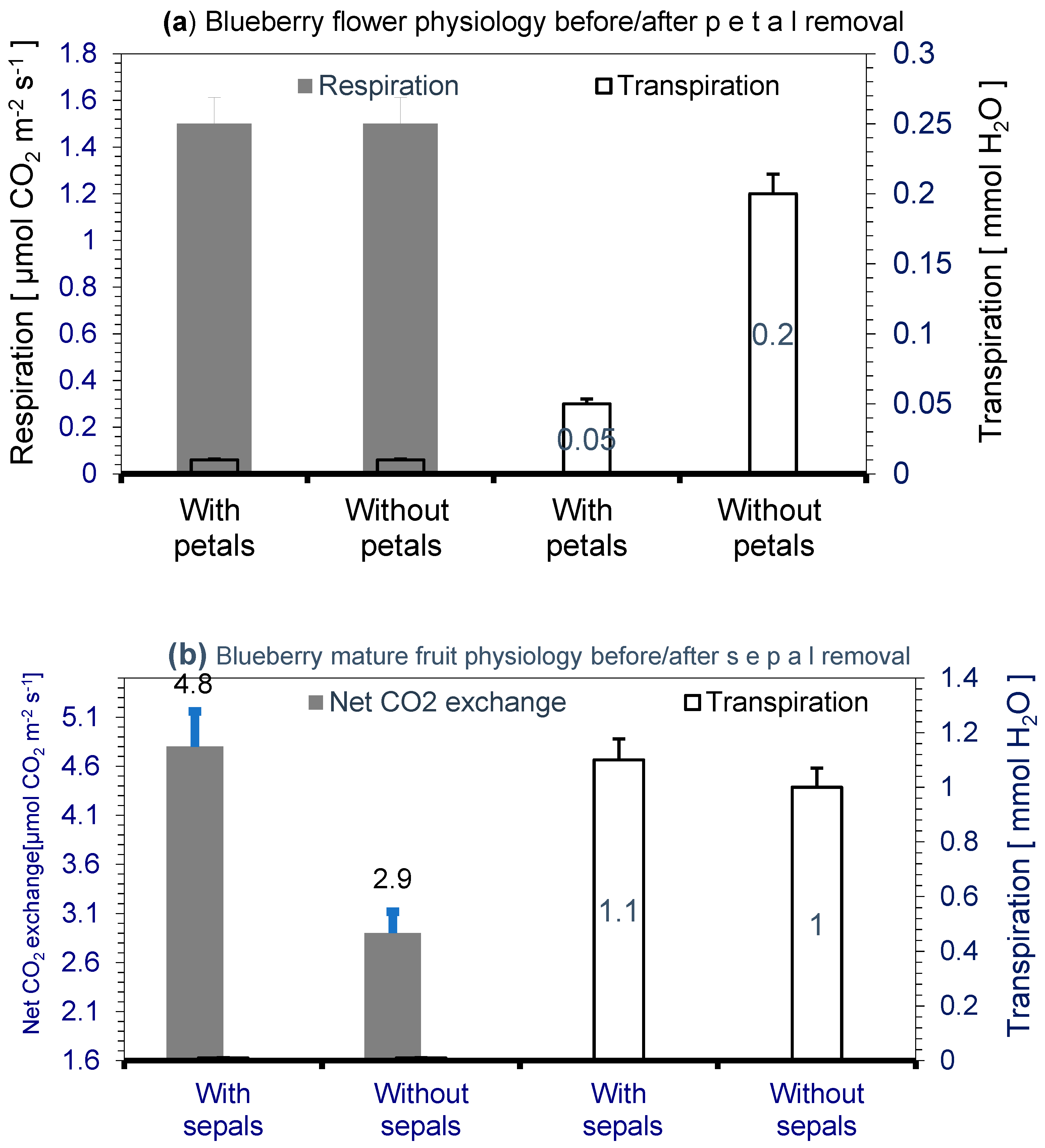
3.2. Respiration and Transpiration of the Flower
3.3. The Blueberry Ovary
3.4. Transpiration and CO2 Uptake of the Berry
3.5. Chlorophyll
4. Discussion
4.1. Stomatal Frequency
4.2. CO2 Exchange
4.3. Transpiration
5. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Seehuber, C.; Damerow, L.; Blanke, M. Regulation of source: Sink relationship, fruit set, fruit growth and fruit quality in European plum (Prunus domestica L.)-using thinning for crop load management. Plant Growth Regul. 2011, 65, 335–341. [Google Scholar] [CrossRef]
- Blanke, M. Regulatory mechanisms in source: Sink relationships—Invited review. Acta Hortic. 2009, 835, 13–20. [Google Scholar] [CrossRef]
- Blanke, M.M.; Lenz, F. Fruit photosynthesis—A review. Plant Cell Environ. 1989, 12, 31–46. [Google Scholar] [CrossRef]
- Fukano, Y.; Tachiki, Y. Evolutionary ecology of climacteric and nonclimacteric fruits. Biol. Lett. 2021, 17, 20210352. [Google Scholar] [CrossRef]
- Duan, Y.; Tarafdar, A.; Chaurasia, D.; Singh, A.; Bhargava, P.C.; Yang, J.; Li, Z.; Ni, X.; Tian, Y.; Li, H.; et al. Blueberry fruit valorization and valuable constituents: A review. Int. J. Food Microbiol. 2022, 381, 109890. [Google Scholar] [CrossRef]
- Freeman, B.; Albrigo, L.G.; Biggs, R.H. Cuticular waxes of developing leaves and fruit of blueberry, Vaccinium ashei Reade cv. Bluegem. J. Amer. Soc. Hort. Sci. 1979, 104, 398–403. [Google Scholar] [CrossRef]
- Roth, I. Handbuch der Pflanzenanatomie, Fruits of Angiosperms; Band X, Part 1; Borntraeger: Berlin, Germany, 1977; p. 431. [Google Scholar]
- Pantastico, E.B. Structure of fruit and vegetables. In Postharvest Physiology, Handling and Utilization of Tropical and Subtropical Fruit and Vegetables; Pantastico, E.B., Ed.; AVI Westport: Westport, CT, USA, 1975; pp. 1–24. [Google Scholar]
- Farag, K.M.; Patta, J.P. Ultrastructure and surface morphology of Vaccinium macrocarpon Arit with reference to Ethrel penetration. Acta Hortic. 1989, 241, 378–383. [Google Scholar]
- Bergmann, H.F. Changes in the rate of respiration of the fruits of the cultivated blueberry during ripening. Science 1959, 70, 15. [Google Scholar] [CrossRef]
- Hall, L.V.; Forsyth, F.R. Respiration rates of the fruits of the lowbush blueberry. Can. J. Plant Sci. 1967, 47, 157–159. [Google Scholar] [CrossRef]
- Flore, J.A.; Hancock, J.F. Blueberry fruit photosynthesis. In Proceedings of the ‘Carbon Economy of Fruit’ Congress, 1990: Bonn-Röttgen, Germany, 2–4 May 1995; Blanke, M.M., Ed.; Bonn University Press: Bonn, Germany, 1995; p. 7. [Google Scholar]
- Birkhold, K.; Damell, R.; Koch, K. Carbon exchange and carbon cost of developing rabbiteye blueberry fruit. In Proceedings of the ‘Carbon Economy of Fruit’ Congress, August 1990: Bonn-Röttgen, Germany, 9–13 July 1990; Blanke, M.M., Ed.; Bonn University Press: Bonn, Germany, 1990; p. 8. [Google Scholar]
- Loypimai, P.; Paewboonsom, S.; Damerow, L.; Blanke, M. The wax bloom on blueberry: Application of luster sensor technology to assess glossiness and the effect of polishing as a fruit quality parameter. J. Appl. Bot. 2017, 90, 154–158. [Google Scholar] [CrossRef]
- Yan, Y.; Dossett, M.; Castellarin, S.D. Cuticular waxes affect fruit surface color in blueberries. Plant People Planet 2023, 5, 736–751. [Google Scholar] [CrossRef]
- Blanke, M.; Höfer, M.; Pring, R.J. Stomata of tetraploid apples leaves cultured in vitro. Ann. Bot. 1994, 73, 651–655. [Google Scholar] [CrossRef]
- Baker, E.A. Chemistry and morphology of plant epicuticular waxes. In Plant Cuticle; Cutler, D.F., Alvin, K.L., Price, C.E., Eds.; Academic Press: London, UK, 1982; pp. 139–166. [Google Scholar]
- Blanke, M.; Cooke, D. Respiration and plasma membrane ATPase in strawberry stolons. Plant Growth Regul. 2000, 30, 163–170. [Google Scholar] [CrossRef]
- Blanke, M.M. Determination of chlorophyll using DMSO. Wein-Wiss. 1992, 47, 32–35. [Google Scholar]
- Blanke, M.M.; Leyhe, A. Stomatal activity of the grape berry cv. Riesling, Müller-Thurgau and Ehrenfelser. J. Plant Physiol. 1986, 127, 451–460. [Google Scholar] [CrossRef]
- Gough, R.E.; Shutak, V.G. Effect of SADH on leaves of cultivated highbush blueberry. HortScience 1976, 11, 514–515. [Google Scholar] [CrossRef]
- Blanke, M.M.; Lovatt, C.J. Anatomy and transpiration of the avocado inflorescence. Ann. Bot. 1993, 71, 543–547. [Google Scholar] [CrossRef]
- Milivojević, J.; Radivojević, D.; Ruml, M.; Dimitrijević, M.; Maksimović, J.D. Does microclimate under grey colored hail protection net affect biological and nutritional properties of ‘Duke’ highbush blueberry (V. corymbosum L.)? Fruits 2016, 71, 161–170. [Google Scholar] [CrossRef]

| Flower Part | Number of Stomata [mm−2] | Number of Stomata [berry−1] | Percentage of Fruit Stomata |
|---|---|---|---|
| Petals | 0 | 0 | 0 |
| Ring enclosed by calyx | 42–45 | 375–400 | 5–7 |
| Sepals | |||
| Adaxial sepals | 0 | 0 | 0 |
| Abaxial sepals | 170–200 | 5200–7650 | 93–95 |
| Content of Chlorophyll | [µg/g] | [µg/cm2] | [µg/Berry] | Chl a:b |
|---|---|---|---|---|
| Ovary in May | 100–120 | 52–72 | 24–29 | 3.9–4.2 |
| Ovary in June | 190–210 | 43–48 | 67–72 | 4.0–4.3 |
| Ovary in July | 370–390 | 27–29 | 38–42 | 4.7–5.9 |
| Sepals in July | 590–610 | 17–18 | 93–108 | 5.0–5.2 |
| Blueberry leaf | 1800–2100 | 41–43 | 26–40 | 5.9–6.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blanke, M. Structure and Function of Blueberry Fruit and Flowers: Stomata, Transpiration and Photoassimilation. Horticulturae 2024, 10, 606. https://doi.org/10.3390/horticulturae10060606
Blanke M. Structure and Function of Blueberry Fruit and Flowers: Stomata, Transpiration and Photoassimilation. Horticulturae. 2024; 10(6):606. https://doi.org/10.3390/horticulturae10060606
Chicago/Turabian StyleBlanke, Michael. 2024. "Structure and Function of Blueberry Fruit and Flowers: Stomata, Transpiration and Photoassimilation" Horticulturae 10, no. 6: 606. https://doi.org/10.3390/horticulturae10060606
APA StyleBlanke, M. (2024). Structure and Function of Blueberry Fruit and Flowers: Stomata, Transpiration and Photoassimilation. Horticulturae, 10(6), 606. https://doi.org/10.3390/horticulturae10060606





