Foliar Spraying of Brassinolide Affects Leaf Quality and Secondary Metabolite Profiles of Cold-Stressed Tea Plants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Culture and Exogenous BR Treatments
2.2. Experimental Design
2.3. Analysis of Variables
2.4. Non-Targeted Metabolome Assay
2.5. Data Analysis
3. Results
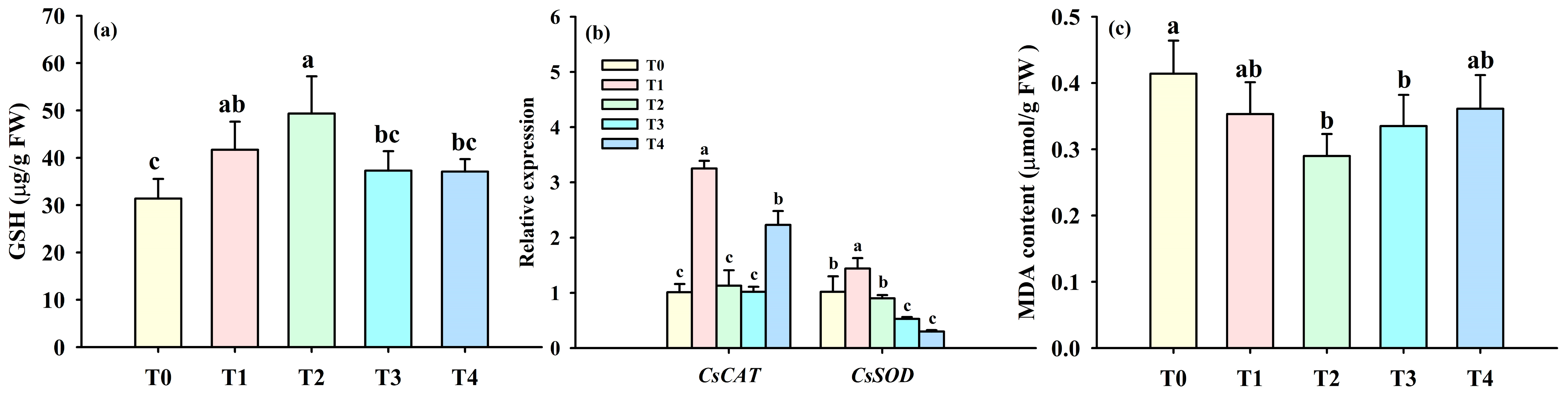
3.1. Changes in Leaf Physiological Indexes
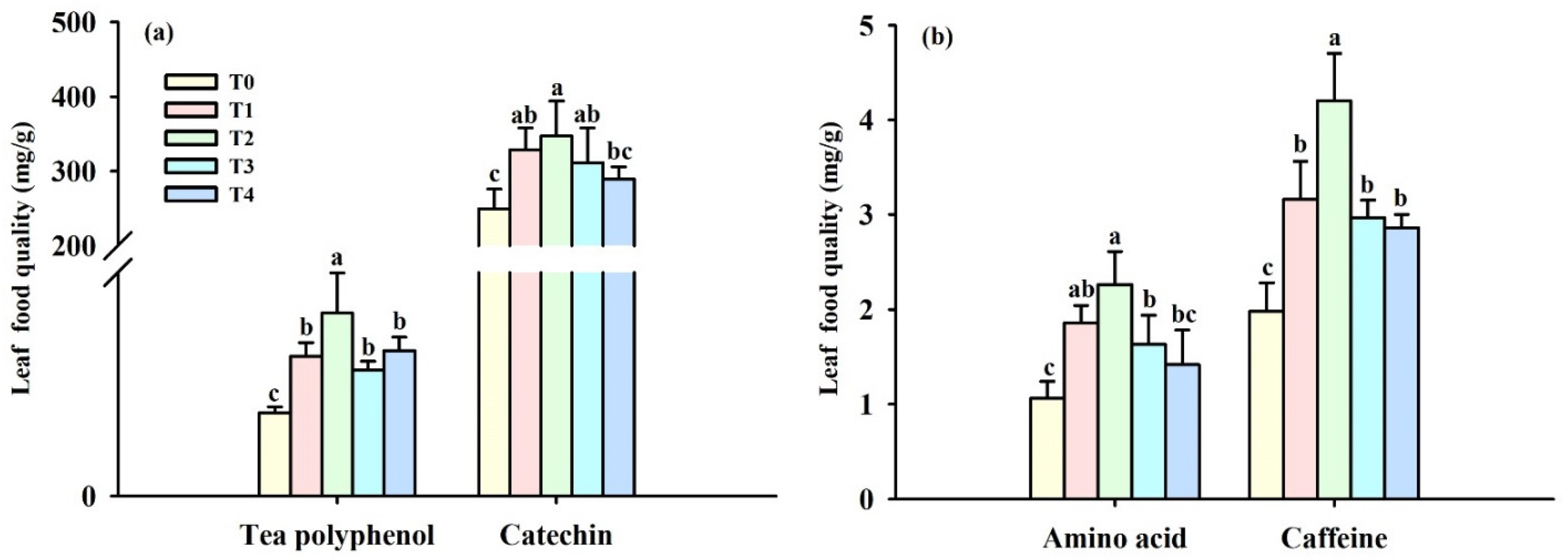
3.2. Changes in Leaf Quality
3.3. Changes in Leaf Quality-Associated Gene Expression
3.4. Changes in Leaf Antioxidant Defense Systems
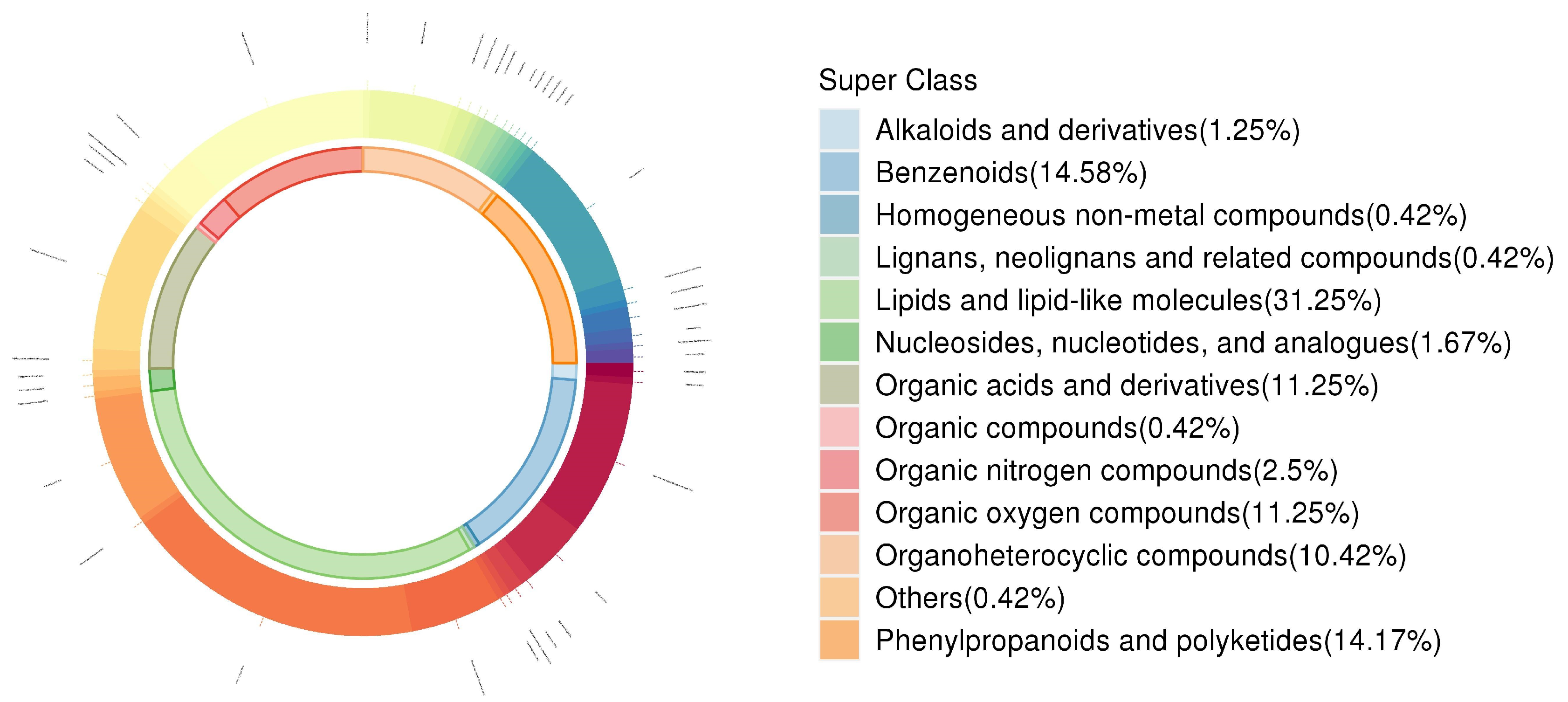
3.5. Changes in Leaf Secondary Metabolite Profiles
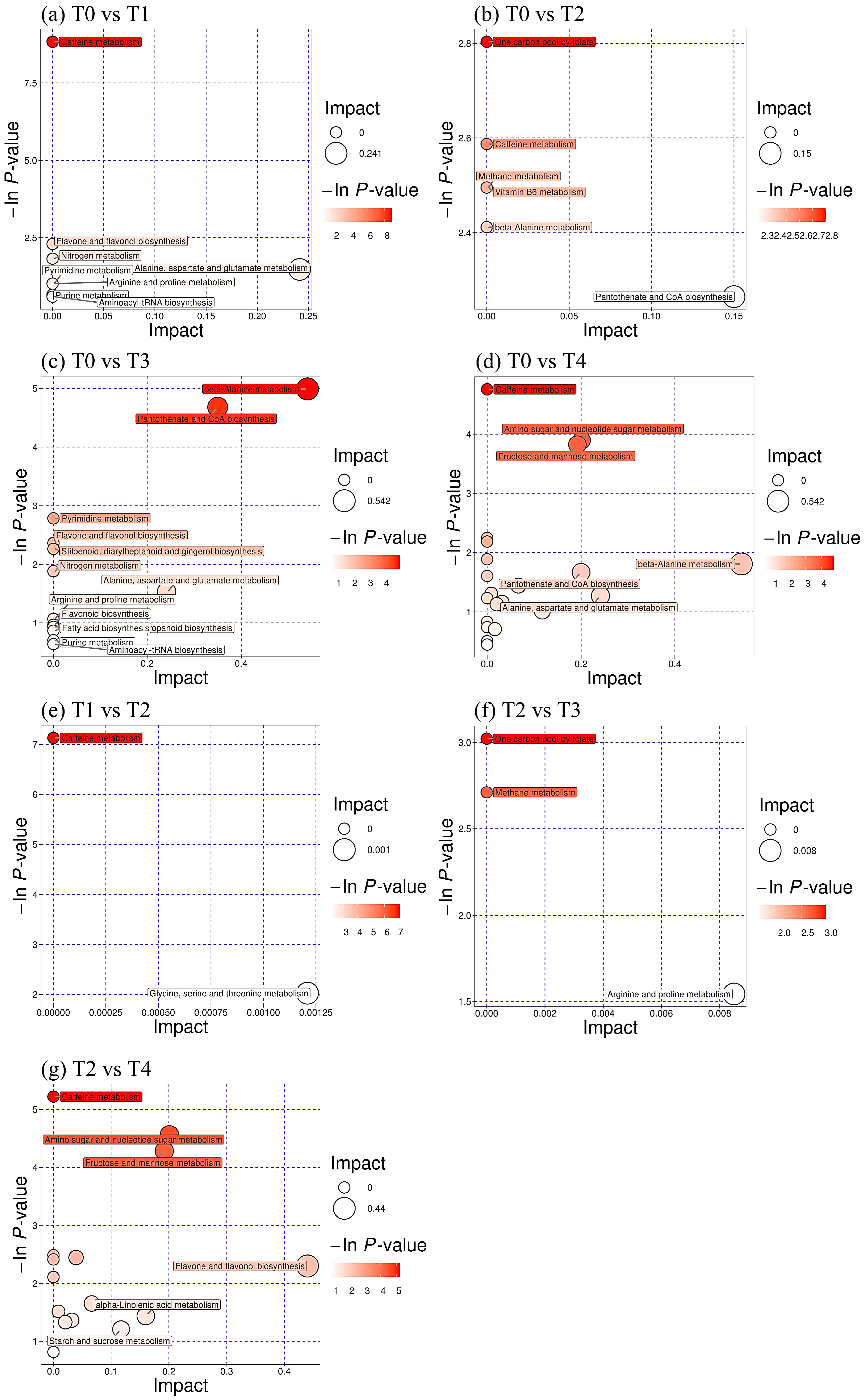
3.6. Changes in Differential Metabolite Pathways
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, Q.; Wang, X.; Wang, Q. Requirements of environmental conditions for tea plant growth and development. Sichuan Agric. Sci. Technol. 2011, 12, 28–29, (In Chinese with English Abstract). [Google Scholar]
- Wang, X.C.; Wang, L.; Hao, X.Y.; Li, N.N.; Ding, C.Q.; Huang, J.Y.; Yang, Y.J. Progress and prospective of cold resistance mechanism in tea plants. J. Tea Commun. 2022, 49, 139–148, (In Chinese with English Abstract). [Google Scholar]
- Han, W.Y.; LI, X.; Yan, P.; Zhang, L.P.; Zhang, L. Prevention and control technology of “cold spell in later spring” in tea garden. China Tea 2018, 40, 9–12, (In Chinese with English Abstract). [Google Scholar]
- Chen, F.; Liu, Y.P.; Gu, X.P.; Hu, J.M.; Yu, F.; Zhang, B. Effects of low temperature on photosynthetic characteristics and yield of tea (Camellia sinensis L.). Crops 2018, 3, 155–161, (In Chinese with English Abstract). [Google Scholar]
- Wu, J.; Nadeem, M.; Galagedara, L.; Thomas, R.; Cheema, M. Recent insights into cell responses to cold stress in plants: Signaling, defence, and potential functions of phosphatidic acid. Environ. Exp. Bot. 2022, 203, 105068. [Google Scholar] [CrossRef]
- Ritonga, F.N.; Chen, S. Physiological and molecular mechanism involved in cold stress tolerance in plants. Plants 2020, 9, 560. [Google Scholar] [CrossRef] [PubMed]
- Akula, R.; Ravishankar, G.A. Influence of abiotic stress signals on secondary metabolites in plants. Plant Signal. Behav. 2011, 6, 1720–1731. [Google Scholar] [CrossRef]
- Zhang, Z.; Song, C.; Zhao, J.; Xia, E.; Wen, W.; Zeng, L.; Benedito, V.A. Editorial: Secondary metabolites and metabolism in tea plants. Front. Plant Sci. 2023, 14, 1143022. [Google Scholar] [CrossRef]
- Clouse, S.D.; Sasse, J.M. Brassinosteroids: Essential regulators of plant growth and development. Annu. Rev. Plant Biol. 1998, 49, 427–451. [Google Scholar] [CrossRef]
- Sadura, I.; Janeczko, A. Physiological and molecular mechanisms of brassinosteroid-induced tolerance to high and low temperature in plants. Biol. Plant. 2018, 62, 601–616. [Google Scholar] [CrossRef]
- Zhou, W.J.; Wu, W.P.; Tang, C.B.; Xiao, Z.F.; Chen, G.H.; Wang, Y. Effect of exogenous 2,4-epibrassinolide on germination and physiological characteristics of rice seedlings under chilling stress. Acta Agric. Boreali-Occid. Sin. 2020, 29, 1410–1416, (In Chinese with English Abstract). [Google Scholar]
- Singh, I.; Kumar, U.; Singh, S.K.; Gupta, C.; Singh, M.; Kushwaha, S.R. Physiological and biochemical effect of 24-epibrassinoslide on cold tolerance in maize seedlings. Physiol. Mol. Boil Plants 2012, 18, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yang, P.; Xie, J.M.; Yu, J.H. Effects of 2,4-epibrassinolide on growth and antioxidant enzymes system in pepper roots under chilling stress. J. Nuclear Agric. Sci. 2015, 29, 1001–1008, (In Chinese with English Abstract). [Google Scholar]
- Zhang, F.; Lu, K.; Gu, Y.; Zhang, L.; Li, W.; Li, Z. Effects of low-temperature stress and brassinolide application on the photosynthesis and leaf structure of tung tree seedlings. Front. Plant Sci. 2020, 10, 497266. [Google Scholar] [CrossRef] [PubMed]
- Ye, K.; Shen, W.; Zhao, Y. External application of brassinolide enhances cold resistance of tea plants (Camellia sinensis L.) by integrating calcium signals. Planta 2023, 258, 114. [Google Scholar] [CrossRef] [PubMed]
- Jin, Q.; Wang, Z.; Chen, Y.; Luo, Y.; Tian, N.; Liu, Z.; Huang, J.; Liu, S. Transcriptomics analysis reveals the signal transduction mechanism of brassinolides in tea leaves and its regulation on the growth and development of Camellia sinensis. BMC Genom. 2022, 23, 29. [Google Scholar] [CrossRef] [PubMed]
- Han, W.Y.; Huang, J.G.; Li, X.; Li, Z.X.; Ahammed, G.J.; Yan, P.; Stepp, J.R. Altitudinal effects on the quality of green tea in east China: A climate change perspective. Eur. Food Res. Technol. 2017, 243, 323–330. [Google Scholar] [CrossRef]
- Ahmed, S.; Griffin, T.S.; Kraner, D.; Schaffner, M.K.; Sharma, D.; Hazel, M.; Leitch, A.R.; Orians, C.M.; Han, W.Y.; Stepp, J.R.; et al. Environmental factors variably impact tea secondary metabolites in the context of climate change. Front. Plant Sci. 2019, 10, 939. [Google Scholar] [CrossRef] [PubMed]
- Qin, Q.; Wang, B.H.; Wang, J.Y.; Chang, M.M.; Xia, T.; Shi, X.Z.; Xu, G.W. A comprehensive strategy for studying protein-metabolite interactions by metabolomics and native mass spectrometry. Talanta 2019, 194, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Chaleckis, R.; Meister, I.; Zhang, P.; Wheelock, C.E. Challenges, progress and promises of metabolite annotation for LC-MS-based me tabolomics. Curr. Opin. Biotechnol. 2019, 55, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.N.; Liang, Q.L. Advance in metabolomics based on Mass Spectrometry. J. Instrum. Anal. 2017, 36, 161–169. [Google Scholar]
- Wang, S.T.; Li, X.N.; Wang, J.; Ling, X.M. Metabonomics and its analytical technique. Chin. J. Pharm. Anal. 2010, 30, 1792–1799, (In Chinese with English Abstract). [Google Scholar]
- Wang, M.M.; Cheng, H.T.; Xue, M. Recent development of LC-MS-based analytical procedures and techniques in metabonomics. J. Int. Pharm. Res. 2011, 38, 130–136. [Google Scholar]
- Zhou, B.; Ma, C.; Ren, X.; Xia, T.; Li, X. LC–MS/MS-based metabolomic analysis of caffeine-degrading fungus Aspergillus sydowii during tea fermentation. J. Food Sci. 2020, 85, 477–485. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; He, Y.; Irfan, A.R.; Liu, X.; Yu, Q.; Zhang, Q.; Yang, D. Exogenous brassinolide enhances the growth and cold resistance of maize (Zea mays L.) seedlings under chilling stress. Agronomy 2020, 10, 488. [Google Scholar] [CrossRef]
- Wu, Q.S. Experimental Guideline in Plant Physiology; China Agricultural Press: Beijing, China, 2019. (In Chinese) [Google Scholar]
- Li, Q.S.; Xie, Y.C.; Rahman, M.M.; Hashem, A.; Abd_Allah, E.F.; Wu, Q.S. Arbuscular mycorrhizal fungi and endophytic fungi activate leaf antioxidant defense system of lane late navel orange. J. Fungi 2022, 8, 282. [Google Scholar] [CrossRef] [PubMed]
- Sudhakar, C.; Lakshmi, A.; Giridarakumar, S. Changes in the antioxidant enzymes efficacy in two high yielding genotypes of mulberry (Morus alba L.) under NaCl salinity. Plant Sci. 2001, 161, 613–619. [Google Scholar] [CrossRef]
- Cao, J.L.; Shao, Y.D.; Zou, Y.N.; Wu, Q.S.; Yang, T.Y.; Kuča, K. Inoculation with Clariodeoglomus etunicatum improves leaf food quality of tea exposed to P stress. Not. Bot. Horti Agrobot. 2021, 49, 12166. [Google Scholar] [CrossRef]
- Liu, C.Y.; Wang, Y.J.; WU, Q.S.; Yang, Y.T.; Kuča, K. Arbuscular mycorrhizal fungi improve the antioxidant capacity of tea (Camellia sinensis) seedlings under drought stress. Not. Bot. Horti Agrobot. 2020, 48, 1993–2005. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and 2−ΔΔCt method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Doppler, M.; Kluger, B.; Bueschl, C.; Schneider, C.; Krska, R.; Delcambre, S.; Karsten, H.; Lemmens, M.; Schuhmacher, R. Stable isotope-assisted evaluation of different extraction solvents for untargeted metabolomics of plants. Int. J. Mol. Sci. 2016, 17, 1017. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.Z.; Liu, Y.F.; Xiao, Y.; Zhou, L.Y.; Zeng, Y.X.; Song, S.X.; Zhao, E.; Qing, Z. Effect of different light ratio on physiology and main amino acid accumulation of Fuding-dabaicha leaf. Sci. Technol. Food Ind. 2021, 42, 29–35, (In Chinese with English Abstract). [Google Scholar]
- Zhou, W.L.; Lu, Y.L.; AO, J.H.; Chen, D.W.; Huang, Y.; Shen, D.C.; Huang, H.J.; Jiang, Y. Effect of compound bacterial manure on the growth of sugarcane. Sugarcane Canesugar 2016, 6, 14–17, (In Chinese with English Abstract). [Google Scholar]
- Li, M.; Shu, S.; Guo, S.R.; Du, J.; Wang, J.W. Effect of 24-brassinolides on photosynthetic characteristics and fruit quality of cherry tomato. Acta Bot. Boreali-Occident. Sin. 2015, 35, 138–145, (In Chinese with English Abstract). [Google Scholar]
- Quan, M.P.; Xu, J.H.; Yin, J.M.; Yang, Z.K.; Tan, W.M. The physiological mechanisms of exogenous brassinolide regulating abiotic stresses in plants: A review. J. Plant Prot. 2023, 50, 22–31, (In Chinese with English Abstract). [Google Scholar]
- Wang, L.C. Study on Cold Resistance of Different Tea Cultivars Introduced in Linyi Area. Master’s Thesis, Shandong Normal University, Jinan, China, 2007. (In Chinese). [Google Scholar]
- Ma, R.; Li, Y.; Ma, T.T. Influences of arbuscular mycorrhizal fungi on quality and related gene expression of Liubao tea. Jiangsu Agric. Sci. 2022, 50, 157–163, (In Chinese with English Abstract). [Google Scholar]
- Chu, W.; Liu, Y.Y.; LI, Y.B.; Chu, X.S. Advances on plant 3-hydroxy-3-methylglutaryl coenzyme a reductase (HMGR) genes. Curr. Biotechnol. 2018, 8, 93–102, (In Chinese with English Abstract). [Google Scholar]
- Chen, L.; Zhang, Z.Z.; Chen, J.; Zhang, Y.G.; Wan, X.C. Review on enzymatic biosynthesis of theanine. J. Tea Sci. 2011, 31, 1–10, (In Chinese with English Abstract). [Google Scholar]
- Liu, Z.; Cao, M.A.; Kuča, K.; Alqahtani, M.D.; Muthuramalingam, P.; Wu, Q.S. Cloning of CAT genes in Satsuma mandarin and their expression characteristics in response to environmental stress and arbuscular mycorrhizal fungi. Plant Cell Rep. 2024, 43, 123. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.S.; Manish, K. Effect of 24-Epibrassinolide on lipid peroxidation and proline in three Brassica species under temperature stress. J. Stress Physiol. Biochem. 2013, 9, 376–384. [Google Scholar]
- Kaur, H.; Sirhindi, G.; Bhardwaj, R.; Alyemeni, M.N.; Siddique, K.H.M.; Ahmad, P. 28-homobrassinolide regulates antioxidant enzyme activities and gene expression in response to salt- and temperature-induced oxidative stress in Brassica juncea. Sci. Rep. 2018, 8, 8735. [Google Scholar] [CrossRef] [PubMed]
- Lei, A.Q.; Zhou, J.H.; Rong, Z.Y.; Alqahtani, M.D.; Gao, X.B.; Wu, Q.S. Mycorrhiza-triggered changes in leaf food quality and secondary metabolite profile in tea at low temperatures. Rhizosphere 2024, 29, 100840. [Google Scholar] [CrossRef]
- Li, S.F.; Liu, J.H.; Yin, H.Y.; Liu, D.M.; Guo, X.M.; Yang, Y.G.; Wang, H.F.; Yu, Y.J. Study on difference of tea cultivars planted in Xinyang by non-targeted metabolomics with chemometrics based on Ultrahigh Performance Liquid Chromatography-High Resolution Mass Spectrometry. J. Instrum. Anal. 2022, 41, 149–155, (In Chinese with English Abstract). [Google Scholar]
- Paiva, L.; Lima, E.; Motta, M.; Marcone, M.; Baptista, J. Variability of antioxidant properties, catechins, caffeine, L-theanine and other amino acids in different plant parts of Azorean Camellia sinensis. Curr. Res. Food Sci. 2020, 3, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Qiao, J.; Hu, J.; Li, Y.P.; REN, G.X.; Xiang, Y.; Zang, Y.M.; Liu, Y.; Liu, C.S. Effect of exogenous brassinolide on morphological characters and contents of seven chemical constituents of Glycyrrhiza uralensis. China J. Chin. Mat. Med. 2016, 41, 197–204, (In Chinese with English Abstract). [Google Scholar]
- Ashihara, H.; Crozier, A. Biosynthesis and metabolism of caffeine and related purine alkaloids in plants. Adv. Bot. Res. 1999, 30, 118–205. [Google Scholar]
- Mohanpuria, P.; Kumar, V.; Yadav, S.K. Tea caffeine: Metabolism, functions, and reduction strategies. Food Sci. Biotechnol. 2010, 19, 275–287. [Google Scholar] [CrossRef]


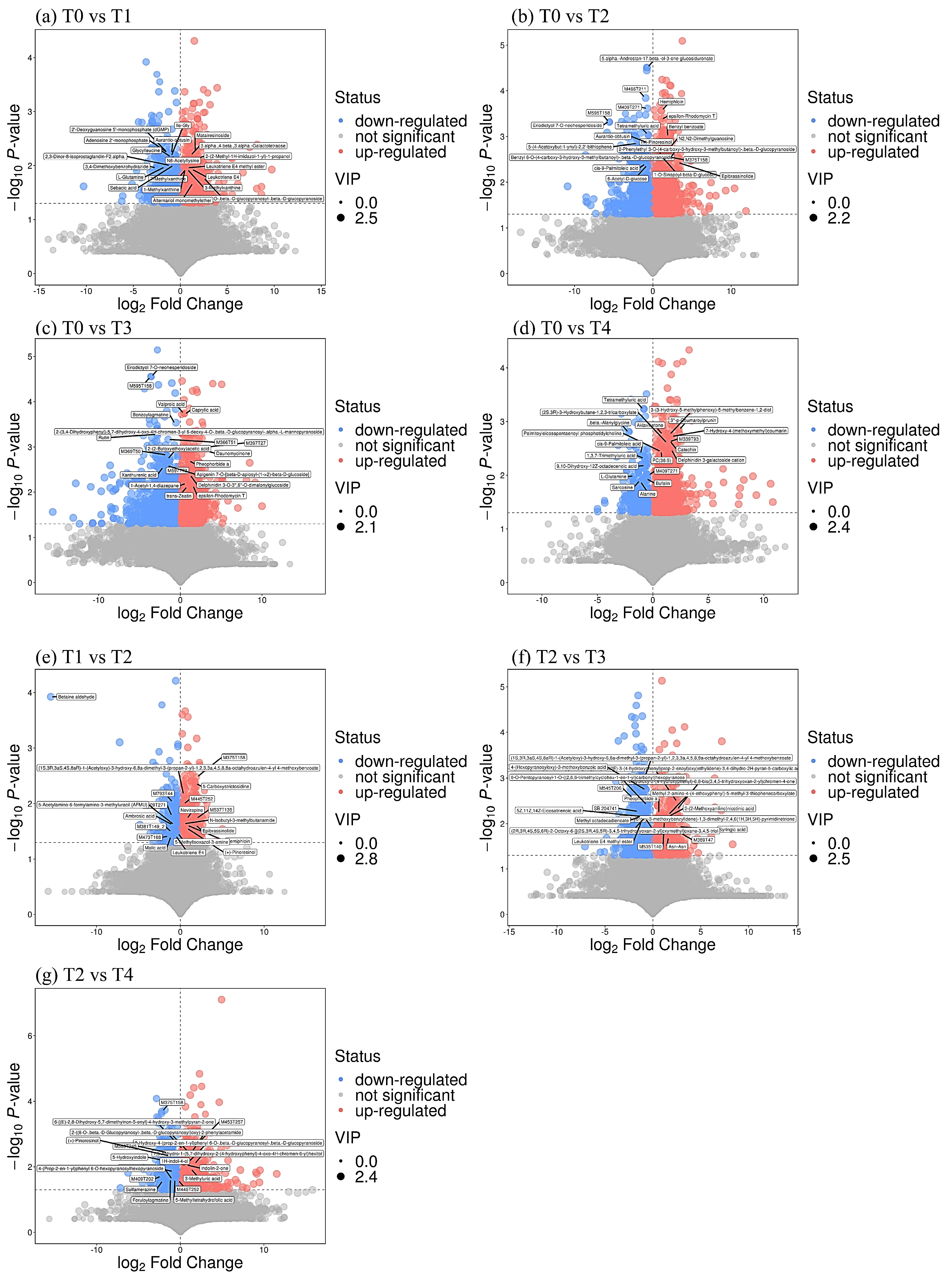
| Comparison of Treatments | Metabolite Name | Category | KEGG ID | VIP | p-Value | Fold Change |
|---|---|---|---|---|---|---|
| T0 vs. T1 | L-Glutamine | Amino acid | C00064 | 2.089 | 0.0107 | 0.32 ↓ |
| Rutin | Flavonoid | C05625 | 1.982 | 0.0110 | 0.26 ↓ | |
| T0 vs. T2 | 7-Methylxanthine | Caffeine | C16353 | 1.760 | 0.0123 | 1.57 ↑ |
| 3-Methylxanthine | C16357 | 1.760 | 0.0123 | 1.57 ↑ | ||
| T0 vs. T3 | L-Glutamine | Amino acid | C00064 | 1.194 | 0.0164 | 0.30 ↓ |
| Chlorogenic acid | Flavonoid | C00852 | 1.997 | 0.0233 | 2.93 ↑ | |
| Rutin | Flavone | C05625 | 2.000 | 0.0005 | 0.13 ↓ | |
| T0 vs. T4 | Catechin | Flavonoid | C06562 | 1.951 | 0.0023 | 2.75 ↑ |
| Epicatechin | C09727 | 1.779 | 0.0050 | 2.44 ↑ | ||
| L-Glutamine | Amino acid | C00064 | 2.057 | 0.0104 | 0.30 ↓ | |
| T1 vs. T2 | 7-Methyluric acid | Caffeine | C16355 | 2.034 | 0.0483 | 0.38 ↓ |
| 1-Methyluric acid | C16359 | 2.034 | 0.0483 | 0.38 ↓ | ||
| Baclofen | Amino acid | 1.990 | 0.0436 | 0.58 ↓ | ||
| T2 vs. T3 | N-Acetyl-L-glutamate 5-semialdehyde | Amino acid | C01250 | 1.805 | 0.0334 | 1.77 ↑ |
| T2 vs. T4 | Quercetin | Flavone | C00389 | 1.919 | 0.0264 | 1.43 ↑ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wen, Y.; Lei, A.-Q.; Hashem, A.; Abd_Allah, E.F.; Wu, Q.-S.; Gao, X.-B. Foliar Spraying of Brassinolide Affects Leaf Quality and Secondary Metabolite Profiles of Cold-Stressed Tea Plants. Horticulturae 2024, 10, 639. https://doi.org/10.3390/horticulturae10060639
Wen Y, Lei A-Q, Hashem A, Abd_Allah EF, Wu Q-S, Gao X-B. Foliar Spraying of Brassinolide Affects Leaf Quality and Secondary Metabolite Profiles of Cold-Stressed Tea Plants. Horticulturae. 2024; 10(6):639. https://doi.org/10.3390/horticulturae10060639
Chicago/Turabian StyleWen, Yue, An-Qi Lei, Abeer Hashem, Elsayed Fathi Abd_Allah, Qiang-Sheng Wu, and Xiu-Bing Gao. 2024. "Foliar Spraying of Brassinolide Affects Leaf Quality and Secondary Metabolite Profiles of Cold-Stressed Tea Plants" Horticulturae 10, no. 6: 639. https://doi.org/10.3390/horticulturae10060639







