Gene-Based Developments in Improving Quality of Tomato: Focus on Firmness, Shelf Life, and Pre- and Post-Harvest Stress Adaptations
Abstract
:1. Introduction
2. Enhancement of Firmness
3. Improving Tomato Quality
4. Upgrading of Nutritional and Flavor Values
5. Developments in Fruit Ripening
6. Progress in Improving the Bioactive Components
7. Improving the Quality and Quantity
8. Updates on Genetic Engineering Technology Used in the Development of Tomato
8.1. Prime Editing Technology
8.2. Mitochondria-Based Editing Technology (mitoTALENs)
8.3. Zinc Finger Nucleases (ZFN)
8.4. APOBEC-Cas9 Fusion-Induced Deletion Systems (AFIDs)
8.5. Possible Risks of the Genetic Technologies
8.6. Ethical Considerations in Genetic Technology
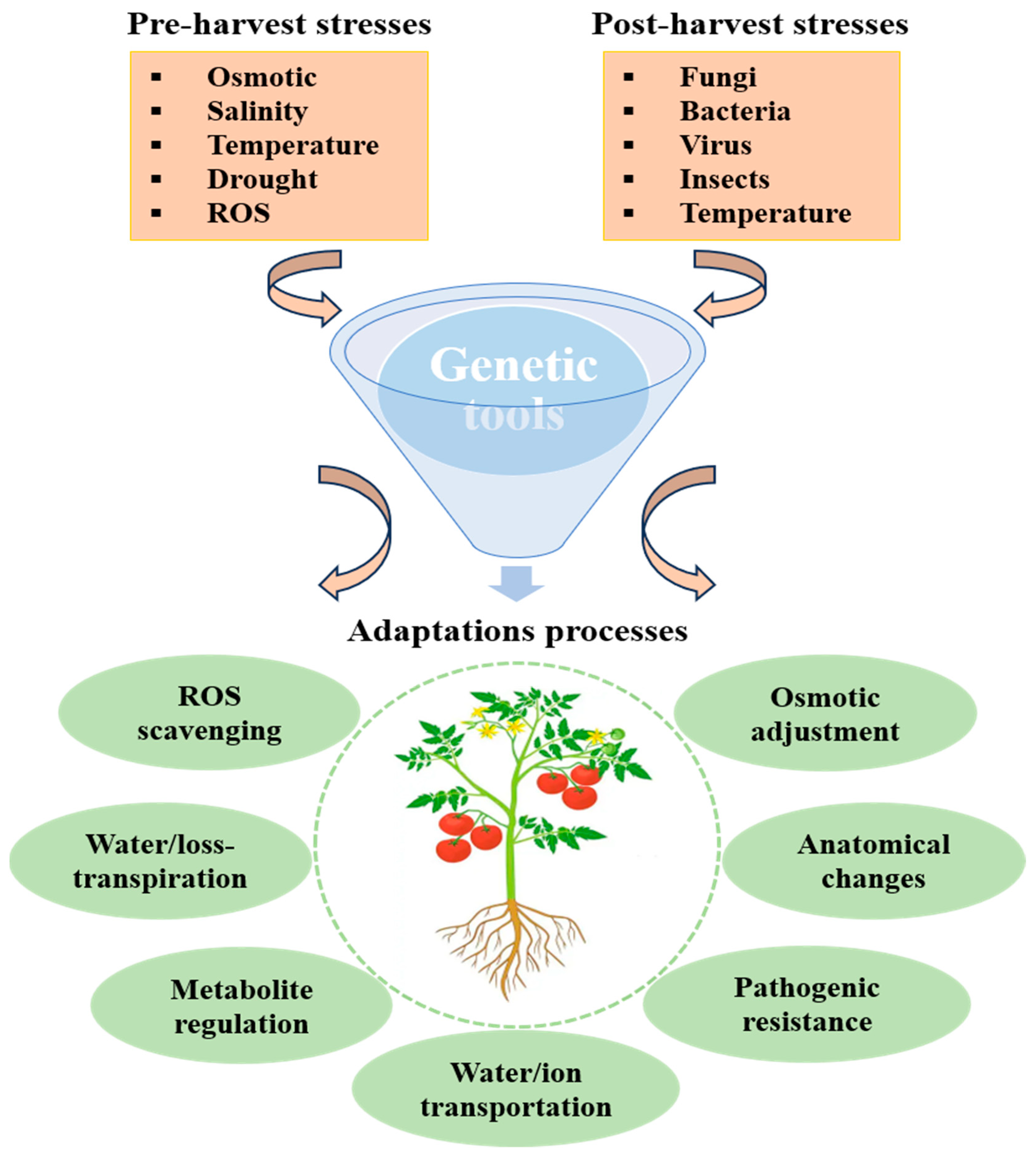
9. Genetic Tool Applied to Alleviate Pre- and Post-Harvest Challenges
9.1. Alleviating Biotic Stress
9.2. Controlling Abiotic Stresses
9.3. Improving Post-Harvest Shelf Life
9.4. Post-Harvest Pathogen Resistance
10. Conclusions
Funding
Conflicts of Interest
References
- Kimura, S.; Sinha, N. Tomato (Solanum lycopersicum): A Model Fruit-Bearing Crop. CSH Protoc. 2008, 2008, pdb-emo105. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Sun, S.; Wang, H.; Wang, K.; Yu, H.; Zhou, Z.; Xin, P.; Chu, J.; Zhao, T.; Wang, H.; et al. FIS1 Encodes a GA2-Oxidase That Regulates Fruit Firmness in Tomato. Nat. Commun. 2020, 11, 5844. [Google Scholar] [CrossRef] [PubMed]
- Chapman, N.H.; Bonnet, J.; Grivet, L.; Lynn, J.; Graham, N.; Smith, R.; Sun, G.; Walley, P.G.; Poole, M.; Causse, M.; et al. High-Resolution Mapping of a Fruit Firmness-Related Quantitative Trait Locus in Tomato Reveals Epistatic Interactions Associated with a Complex Combinatorial Locus. Plant Physiol. 2012, 159, 1644–1657. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Wang, K.; Shu, X.; Liang, J.; Fan, X.; Sun, L. Changes in Fruit Firmness, Quality Traits and Cell Wall Constituents of Two Highbush Blueberries (Vaccinium corymbosum L.) during Postharvest Cold Storage. Sci. Hortic. 2019, 246, 557–562. [Google Scholar] [CrossRef]
- Lara, I. The Fruit Cuticle: Actively Tuning Postharvest Quality. In Preharvest Modulation of Postharvest Fruit and Vegetable Quality; Academic Press: Cambridge, MA, USA, 2018; pp. 93–120. [Google Scholar] [CrossRef]
- Lara, I.; Heredia, A.; Domínguez, E. Shelf Life Potential and the Fruit Cuticle: The Unexpected Player. Front. Plant Sci. 2019, 10, 460894. [Google Scholar] [CrossRef]
- Quinet, M.; Angosto, T.; Yuste-Lisbona, F.J.; Blanchard-Gros, R.; Bigot, S.; Martinez, J.P.; Lutts, S. Tomato Fruit Development and Metabolism. Front. Plant Sci. 2019, 10, 1554. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Yeats, T.H.; Uluisik, S.; Rose, J.K.C.; Seymour, G.B. Fruit Softening: Revisiting the Role of Pectin. Trends Plant Sci. 2018, 23, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Paniagua, C.; Posé, S.; Morris, V.J.; Kirby, A.R.; Quesada, M.A.; Mercado, J.A. Fruit Softening and Pectin Disassembly: An Overview of Nanostructural Pectin Modifications Assessed by Atomic Force Microscopy. Ann. Bot. 2014, 114, 1375–1383. [Google Scholar] [CrossRef] [PubMed]
- Thole, V.; Vain, P.; Yang, R.Y.; Almeida Barros da Silva, J.; Enfissi, E.M.A.; Nogueira, M.; Price, E.J.; Alseekh, S.; Fernie, A.R.; Fraser, P.D.; et al. Analysis of Tomato Post-Harvest Properties: Fruit Color, Shelf Life, and Fungal Susceptibility. Curr. Protoc. Plant Biol. 2020, 5, e20108. [Google Scholar] [CrossRef]
- Adaskaveg, J.A.; Blanco-Ulate, B. Targeting Ripening Regulators to Develop Fruit with High Quality and Extended Shelf Life. Curr. Opin. Biotechnol. 2023, 79, 102872. [Google Scholar] [CrossRef]
- Aljabali, A.A.A.; El-Tanani, M.; Tambuwala, M.M. Principles of CRISPR-Cas9 Technology: Advancements in Genome Editing and Emerging Trends in Drug Delivery. J. Drug Deliv. Sci. Technol. 2024, 92, 105338. [Google Scholar] [CrossRef]
- Li, H.; Yang, Y.; Hong, W.; Huang, M.; Wu, M.; Zhao, X. Applications of Genome Editing Technology in the Targeted Therapy of Human Diseases: Mechanisms, Advances and Prospects. Signal Transduct. Target. Ther. 2020, 5, 1. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Peng, C.; Mao, A.; Zhong, M.; Hu, Z. An Overview of Microbial Enzymatic Approaches for Pectin Degradation. Int. J. Biol. Macromol. 2024, 254, 127804. [Google Scholar] [CrossRef] [PubMed]
- Shiferaw Terefe, N.; Buckow, R.; Versteeg, C. Quality-Related Enzymes in Plant-Based Products: Effects of Novel Food-Processing Technologies Part 3: Ultrasonic Processing. Crit. Rev. Food Sci. Nutr. 2015, 55, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Bird, C.R.; Smith, C.J.S.; Ray, J.A.; Moureau, P.; Bevan, M.W.; Bird, A.S.; Hughes, S.; Morris, P.C.; Grierson, D.; Schuch, W. The Tomato Polygalacturonase Gene and Ripening-Specific Expression in Transgenic Plants. Plant Mol. Biol. 1988, 11, 651–662. [Google Scholar] [CrossRef]
- Ren, H.; Zhao, Q.; Feng, Y.; Tang, P.; Wang, Y.; Jiang, J.; Hu, C.; Wang, Y.; Cui, B.; Xie, X.; et al. Gene-Specific Silencing of SlPL16, a Pectate Lyase Coding Gene, Extends the Shelf Life of Tomato Fruit. Postharvest Biol. Technol. 2023, 201, 112368. [Google Scholar] [CrossRef]
- Mengstie, M.A.; Wondimu, B.Z. Mechanism and Applications of CRISPR/Cas-9-Mediated Genome Editing. Biologics 2021, 15, 353. [Google Scholar] [CrossRef]
- Egea, I.; Estrada, Y.; Flores, F.B.; Bolarín, M.C. Improving Production and Fruit Quality of Tomato under Abiotic Stress: Genes for the Future of Tomato Breeding for a Sustainable Agriculture. Environ. Exp. Bot. 2022, 204, 105086. [Google Scholar] [CrossRef]
- Meza, S.L.R.; Egea, I.; Massaretto, I.L.; Morales, B.; Purgatto, E.; Egea-Fernández, J.M.; Bolarin, M.C.; Flores, F.B. Traditional Tomato Varieties Improve Fruit Quality without Affecting Fruit Yield under Moderate Salt Stress. Front. Plant Sci. 2020, 11, 587754. [Google Scholar] [CrossRef]
- Wai, A.H.; Naing, A.H.; Lee, D.J.; Kim, C.K.; Chung, M.Y. Molecular Genetic Approaches for Enhancing Stress Tolerance and Fruit Quality of Tomato. Plant Biotechnol. Rep. 2020, 14, 515–537. [Google Scholar] [CrossRef]
- Kapoor, D.; Singh, S.; Kumar, V.; Romero, R.; Prasad, R.; Singh, J. Antioxidant Enzymes Regulation in Plants in Reference to Reactive Oxygen Species (ROS) and Reactive Nitrogen Species (RNS). Plant Gene 2019, 19, 100182. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, Y.; Zhu, J.K. Thriving under Stress: How Plants Balance Growth and the Stress Response. Dev. Cell 2020, 55, 529–543. [Google Scholar] [CrossRef] [PubMed]
- Botella, M.Á.; Hernández, V.; Mestre, T.; Hellín, P.; García-Legaz, M.F.; Rivero, R.M.; Martínez, V.; Fenoll, J.; Flores, P. Bioactive Compounds of Tomato Fruit in Response to Salinity, Heat and Their Combination. Agric. 2021, 11, 534. [Google Scholar] [CrossRef]
- Massaretto, I.L.; Albaladejo, I.; Purgatto, E.; Flores, F.B.; Plasencia, F.; Egea-Fernández, J.M.; Bolarin, M.C.; Egea, I. Recovering Tomato Landraces to Simultaneously Improve Fruit Yield and Nutritional Quality against Salt Stress. Front. Plant Sci. 2018, 9, 1778. [Google Scholar] [CrossRef] [PubMed]
- Tungmunnithum, D.; Thongboonyou, A.; Pholboon, A.; Yangsabai, A. Flavonoids and Other Phenolic Compounds from Medicinal Plants for Pharmaceutical and Medical Aspects: An Overview. Medicines 2018, 5, 93. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, N.; Zhang, B.; Chachar, Z.; Li, J.; Xiao, G.; Wang, Q.; Hayat, F.; Deng, L.; Narejo, M.-U.; Bozdar, B.; et al. Micronutrients and Their Effects on Horticultural Crop Quality, Productivity and Sustainability. Sci. Hortic. 2024, 323, 112512. [Google Scholar] [CrossRef]
- Salgotra, R.K.; Chauhan, B.S. Genetic Diversity, Conservation, and Utilization of Plant Genetic Resources. Genes 2023, 14, 174. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Irfan, M.; Siddiqui, M.W.; Thankappan, R.; Liao, W. From Classical Breeding to Modern Biotechnological Advancement in Horticultural Crops—Trait Improvement and Stress Resilience. Front. Plant Sci. 2023, 14, 1293682. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, Z.H.; Ahmad, F.; Ibrahim, S.A. Biotechnology Approaches to Food Security: Risks and Solutions. In Microbial Biotechnology in the Food Industry: Advances, Challenges, and Potential Solutions; Ahmad, F., Mohammad, Z.H., Ibrahim, S.A., Zaidi, S., Eds.; Springer International Publishing: Cham, Switzerland, 2024; pp. 1–13. ISBN 978-3-031-51417-3. [Google Scholar]
- Singh, A.; Behera, C. Strategies, Opportunities, and Challenges in Crop Genetic Diversity Conservation: A Plant Breeder’s Perspective. In Molecular Genetics and Genomics Tools in Biodiversity Conservation; Kumar, A., Choudhury, B., Dayanandan, S., Khan, M.L., Eds.; Springer Nature: Singapore, 2022; pp. 151–169. ISBN 978-981-16-6005-4. [Google Scholar]
- Wang, T.; Zhang, H.; Zhu, H. CRISPR Technology Is Revolutionizing the Improvement of Tomato and Other Fruit Crops. Hortic. Res. 2019, 6, 77. [Google Scholar] [CrossRef]
- Gaj, T.; Gersbach, C.A.; Barbas, C.F. ZFN, TALEN and CRISPR/Cas-Based Methods for Genome Engineering. Trends Biotechnol. 2013, 31, 397. [Google Scholar] [CrossRef]
- Li, S.; Li, K.; Ju, Z.; Cao, D.; Fu, D.; Zhu, H.; Zhu, B.; Luo, Y. Genome-Wide Analysis of Tomato NF-Y Factors and Their Role in Fruit Ripening. BMC Genom. 2016, 17, 36. [Google Scholar] [CrossRef]
- Li, H.; Brouwer, M.; Del Pup, E.; van Lieshout, N.; Finkers, R.; Bachem, C.W.B.; Visser, R.G.F. Allelic Variation in the Autotetraploid Potato: Genes Involved in Starch and Steroidal Glycoalkaloid Metabolism as a Case Study. BMC Genom. 2024, 25, 274. [Google Scholar] [CrossRef]
- Jaganathan, D.; Ramasamy, K.; Sellamuthu, G.; Jayabalan, S.; Venkataraman, G. CRISPR for Crop Improvement: An Update Review. Front. Plant Sci. 2018, 9, 985. [Google Scholar] [CrossRef]
- Liu, Q.; Yang, F.; Zhang, J.; Liu, H.; Rahman, S.; Islam, S.; Ma, W.; She, M. Application of CRISPR/Cas9 in Crop Quality Improvement. Int. J. Mol. Sci. 2021, 22, 4206. [Google Scholar] [CrossRef] [PubMed]
- Şimşek, Ö.; Isak, M.A.; Dönmez, D.; Dalda Şekerci, A.; İzgü, T.; Kaçar, Y.A. Advanced Biotechnological Interventions in Mitigating Drought Stress in Plants. Plants 2024, 13, 717. [Google Scholar] [CrossRef] [PubMed]
- Tsatsakis, A.M.; Nawaz, M.A.; Kouretas, D.; Balias, G.; Savolainen, K.; Tutelyan, V.A.; Golokhvast, K.S.; Lee, J.D.; Yang, S.H.; Chung, G. Environmental Impacts of Genetically Modified Plants: A Review. Environ. Res. 2017, 156, 818–833. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, X.; Zhang, Y.; Zhang, A.; You, C.X. Regulation of Fleshy Fruit Ripening: From Transcription Factors to Epigenetic Modifications. Hortic. Res. 2022, 9, uhac013. [Google Scholar] [CrossRef]
- Li, S.; Chen, K.; Grierson, D. Molecular and Hormonal Mechanisms Regulating Fleshy Fruit Ripening. Cells 2021, 10, 1136. [Google Scholar] [CrossRef] [PubMed]
- Klee, H.J.; Giovannoni, J.J. Genetics and Control of Tomato Fruit Ripening and Quality Attributes. Annu. Rev. Genet. 2011, 45, 41–59. [Google Scholar] [CrossRef]
- Wang, R.; Lammers, M.; Tikunov, Y.; Bovy, A.G.; Angenent, G.C.; de Maagd, R.A. The Rin, nor and Cnr Spontaneous Mutations Inhibit Tomato Fruit Ripening in Additive and Epistatic Manners. Plant Sci. 2020, 294, 110436. [Google Scholar] [CrossRef]
- Ito, Y.; Nishizawa-Yokoi, A.; Endo, M.; Mikami, M.; Shima, Y.; Nakamura, N.; Kotake-Nara, E.; Kawasaki, S.; Toki, S. Re-Evaluation of the Rin Mutation and the Role of RIN in the Induction of Tomato Ripening. Nat. Plants 2017, 3, 866–874. [Google Scholar] [CrossRef] [PubMed]
- Giovannoni, J. Ripening Activator Turned Repressor. Nat. Plants 2017, 3, 920–921. [Google Scholar] [CrossRef]
- Li, S.; Xu, H.; Ju, Z.; Cao, D.; Zhu, H.; Fu, D.; Grierson, D.; Qin, G.; Luo, Y.; Zhu, B. The RIN-MC Fusion of MADS-Box Transcription Factors Has Transcriptional Activity and Modulates Expression of Many Ripening Genes. Plant Physiol. 2018, 176, 891–909. [Google Scholar] [CrossRef] [PubMed]
- Brumos, J. Gene Regulation in Climacteric Fruit Ripening. Curr. Opin. Plant Biol. 2021, 63, 102042. [Google Scholar] [CrossRef]
- Chaudhary, J.; Alisha, A.; Bhatt, V.; Chandanshive, S.; Kumar, N.; Sonah, H.; Deshmukh, R.; Mir, Z.; Kumar, A.; Yadav, S.; et al. Mutation Breeding in Tomato: Advances, Applicability and Challenges. Plants 2019, 8, 128. [Google Scholar] [CrossRef]
- Li, S.; Zhu, B.; Pirrello, J.; Xu, C.; Zhang, B.; Bouzayen, M.; Chen, K.; Grierson, D. Roles of RIN and Ethylene in Tomato Fruit Ripening and Ripening-Associated Traits. New Phytol. 2020, 226, 460–475. [Google Scholar] [CrossRef] [PubMed]
- Dubois, M.; Van den Broeck, L.; Inzé, D. The Pivotal Role of Ethylene in Plant Growth. Trends Plant Sci. 2018, 23, 311–323. [Google Scholar] [CrossRef]
- Kapoor, L.; Simkin, A.J.; George Priya Doss, C.; Siva, R. Fruit Ripening: Dynamics and Integrated Analysis of Carotenoids and Anthocyanins. BMC Plant Biol. 2022, 22, 27. [Google Scholar] [CrossRef]
- Saini, R.K.; Prasad, P.; Lokesh, V.; Shang, X.; Shin, J.; Keum, Y.-S.; Lee, J.-H. Carotenoids: Dietary Sources, Extraction, Encapsulation, Bioavailability, and Health Benefits—A Review of Recent Advancements. Antioxidants 2022, 11, 795. [Google Scholar] [CrossRef]
- Bin-Jumah, M.N.; Nadeem, M.S.; Gilani, S.J.; Mubeen, B.; Ullah, I.; Alzarea, S.I.; Ghoneim, M.M.; Alshehri, S.; Al-Abbasi, F.A.; Kazmi, I. Lycopene: A Natural Arsenal in the War against Oxidative Stress and Cardiovascular Diseases. Antioxidants 2022, 11, 232. [Google Scholar] [CrossRef]
- Bhagwat, A.C.; Patil, A.M.; Saroj, S.D. CRISPR/Cas 9—Based Editing in the Production of Bioactive Molecules. Mol. Biotechnol. 2022, 64, 245–251. [Google Scholar] [CrossRef]
- Koh, W.; Kwak, H.; Cheong, E.; Lee, C.J. GABA Tone Regulation and Its Cognitive Functions in the Brain. Nat. Rev. Neurosci. 2023, 24, 523–539. [Google Scholar] [CrossRef]
- Nagamine, A.; Ezura, H. Genome Editing for Improving Crop Nutrition. Front. Genome Ed. 2022, 4, 850104. [Google Scholar] [CrossRef]
- Kumar, D.; Yadav, A.; Ahmad, R.; Dwivedi, U.N.; Yadav, K. CRISPR-Based Genome Editing for Nutrient Enrichment in Crops: A Promising Approach toward Global Food Security. Front. Genet. 2022, 13, 932859. [Google Scholar] [CrossRef]
- Ariizumi, T.; Shinozaki, Y.; Ezura, H. Genes That Influence Yield in Tomato. Breed. Sci. 2013, 63, 3–13. [Google Scholar] [CrossRef]
- Sethi, S. CRISPR/Cas-Mediated Multiplex Gene Editing in Tomato (Solanum lycopersicum L.). Gene Ed. Plants 2024, 795–815. [Google Scholar] [CrossRef]
- Yu, Q.H.; Wang, B.; Li, N.; Tang, Y.; Yang, S.; Yang, T.; Xu, J.; Guo, C.; Yan, P.; Wang, Q.; et al. CRISPR/Cas9-Induced Targeted Mutagenesis and Gene Replacement to Generate Long-Shelf Life Tomato Lines. Sci. Rep. 2017, 7, 11874. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, S.; Zhu, X.; Chang, Y.; Wang, C.; Ma, N.; Wang, J.; Zhang, X.; Lyu, J.; Xie, J. A Comprehensive Evaluation of Tomato Fruit Quality and Identification of Volatile Compounds. Plants 2023, 12, 2947. [Google Scholar] [CrossRef]
- Yang, T.; Deng, L.; Zhao, W.; Zhang, R.; Jiang, H.; Ye, Z.; Li, C.B.; Li, C. Rapid Breeding of Pink-Fruited Tomato Hybrids Using the CRISPR/Cas9 System. J. Genet. Genom. 2019, 46, 505–508. [Google Scholar] [CrossRef]
- Uluisik, S.; Chapman, N.H.; Smith, R.; Poole, M.; Adams, G.; Gillis, R.B.; Besong, T.M.D.; Sheldon, J.; Stiegelmeyer, S.; Perez, L.; et al. Genetic Improvement of Tomato by Targeted Control of Fruit Softening. Nat. Biotechnol. 2016, 34, 950–952. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, L.; Zhao, R.; Yu, W.; Li, R.; Li, Y.; Sheng, J.; Shen, L. Knockout of SlMAPK3 Reduced Disease Resistance to Botrytis Cinerea in Tomato Plants. J. Agric. Food Chem. 2018, 66, 8949–8956. [Google Scholar] [CrossRef]
- Hilioti, Z.; Ganopoulos, I.; Ajith, S.; Bossis, I.; Tsaftaris, A. A Novel Arrangement of Zinc Finger Nuclease System for in Vivo Targeted Genome Engineering: The Tomato LEC1-LIKE4 Gene Case. Plant Cell Rep. 2016, 35, 2241–2255. [Google Scholar] [CrossRef]
- Lü, P.; Yu, S.; Zhu, N.; Chen, Y.R.; Zhou, B.; Pan, Y.; Tzeng, D.; Fabi, J.P.; Argyris, J.; Garcia-Mas, J.; et al. Genome Encode Analyses Reveal the Basis of Convergent Evolution of Fleshy Fruit Ripening. Nat. Plants 2018, 4, 784–791. [Google Scholar] [CrossRef]
- Jung, Y.J.; Lee, G.J.; Bae, S.; Kang, K.K. Reduced Ethylene Production in Tomato Fruits upon CRSPR/Cas9-Mediated Lemads-Rin Mutagenesis. Hortic. Sci. Technol. 2018, 36, 396–405. [Google Scholar] [CrossRef]
- Gao, Y.; Zhu, N.; Zhu, X.; Wu, M.; Cai-Zhong, J.; Grierson, D.; Luo, Y.; Shen, W.; Zhong, S.; Fu, D.Q.; et al. Diversity and Redundancy of the Ripening Regulatory Networks Revealed by the FruitENCODE and the New CRISPR/Cas9 CNR and NOR Mutants. Hortic. Res. 2019, 6, 39. [Google Scholar] [CrossRef]
- Gago, C.; Drosou, V.; Paschalidis, K.; Guerreiro, A.; Miguel, G.; Antunes, D.; Hilioti, Z. Targeted Gene Disruption Coupled with Metabolic Screen Approach to Uncover the LEAFY COTYLEDON1-LIKE4 (L1L4) Function in Tomato Fruit Metabolism. Plant Cell Rep. 2017, 36, 1065–1082. [Google Scholar] [CrossRef]
- Andersson, M.; Turesson, H.; Nicolia, A.; Fält, A.S.; Samuelsson, M.; Hofvander, P. Efficient Targeted Multiallelic Mutagenesis in Tetraploid Potato (Solanum tuberosum) by Transient CRISPR-Cas9 Expression in Protoplasts. Plant Cell Rep. 2017, 36, 117–128. [Google Scholar] [CrossRef]
- Tuncel, A.; Corbin, K.R.; Ahn-Jarvis, J.; Harris, S.; Hawkins, E.; Smedley, M.A.; Harwood, W.; Warren, F.J.; Patron, N.J.; Smith, A.M. Cas9-Mediated Mutagenesis of Potato Starch-Branching Enzymes Generates a Range of Tuber Starch Phenotypes. Plant Biotechnol. J. 2019, 17, 2259–2271. [Google Scholar] [CrossRef]
- Waltz, E. GABA-Enriched Tomato Is First CRISPR-Edited Food to Enter Market. Nat. Biotechnol. 2022, 40, 9–11. [Google Scholar] [CrossRef]
- Deng, L.; Wang, H.; Sun, C.; Li, Q.; Jiang, H.; Du, M.; Li, C.B.; Li, C. Efficient Generation of Pink-Fruited Tomatoes Using CRISPR/Cas9 System. J. Genet. Genom. 2018, 45, 51–54. [Google Scholar] [CrossRef]
- Rodríguez-Leal, D.; Lemmon, Z.H.; Man, J.; Bartlett, M.E.; Lippman, Z.B. Engineering Quantitative Trait Variation for Crop Improvement by Genome Editing. Cell 2017, 171, 470–480.e8. [Google Scholar] [CrossRef]
- Dahan-Meir, T.; Filler-Hayut, S.; Melamed-Bessudo, C.; Bocobza, S.; Czosnek, H.; Aharoni, A.; Levy, A.A. Efficient in Planta Gene Targeting in Tomato Using Geminiviral Replicons and the CRISPR/Cas9 System. Plant J. 2018, 95, 5–16. [Google Scholar] [CrossRef]
- Čermák, T.; Baltes, N.J.; Čegan, R.; Zhang, Y.; Voytas, D.F. High-Frequency, Precise Modification of the Tomato Genome. Genome Biol. 2015, 16, 232. [Google Scholar] [CrossRef]
- Zsögön, A.; Čermák, T.; Naves, E.R.; Notini, M.M.; Edel, K.H.; Weinl, S.; Freschi, L.; Voytas, D.F.; Kudla, J.; Peres, L.E.P. De Novo Domestication of Wild Tomato Using Genome Editing. Nat. Biotechnol. 2018, 36, 1211–1216. [Google Scholar] [CrossRef]
- Kwon, C.T.; Heo, J.; Lemmon, Z.H.; Capua, Y.; Hutton, S.F.; Van Eck, J.; Park, S.J.; Lippman, Z.B. Rapid Customization of Solanaceae Fruit Crops for Urban Agriculture. Nat. Biotechnol. 2020, 38, 182–188. [Google Scholar] [CrossRef]
- Yuste-Lisbona, F.J.; Fernández-Lozano, A.; Pineda, B.; Bretones, S.; Ortíz-Atienza, A.; García-Sogo, B.; Müller, N.A.; Angosto, T.; Capel, J.; Moreno, V.; et al. ENO Regulates Tomato Fruit Size through the Floral Meristem Development Network. Proc. Natl. Acad. Sci. USA 2020, 117, 8187–8195. [Google Scholar] [CrossRef]
- Nonaka, S.; Arai, C.; Takayama, M.; Matsukura, C.; Ezura, H. Efficient Increase of Γ-Aminobutyric Acid (GABA) Content in Tomato Fruits by Targeted Mutagenesis. Sci. Rep. 2017, 7, 7057. [Google Scholar] [CrossRef]
- Galeano, M.; Polito, F.; Bitto, A.; Irrera, N.; Campo, G.M.; Avenoso, A.; Calò, M.; Lo Cascio, P.; Minutoli, L.; Barone, M.; et al. Systemic Administration of High-Molecular Weight Hyaluronan Stimulates Wound Healing in Genetically Diabetic Mice. Biochim. Biophys. Acta 2011, 1812, 752–759. [Google Scholar] [CrossRef]
- Li, X.; Wang, Y.; Chen, S.; Tian, H.; Fu, D.; Zhu, B.; Luo, Y.; Zhu, H. Lycopene Is Enriched in Tomato Fruit by CRISPR/Cas9-Mediated Multiplex Genome Editing. Front. Plant Sci. 2018, 9, 559. [Google Scholar] [CrossRef]
- Wang, R.; Tavano, E.C.d.R.; Lammers, M.; Martinelli, A.P.; Angenent, G.C.; de Maagd, R.A. Re-Evaluation of Transcription Factor Function in Tomato Fruit Development and Ripening with CRISPR/Cas9-Mutagenesis. Sci. Rep. 2019, 9, 1696. [Google Scholar] [CrossRef]
- Yang, Y.; Zhu, G.; Li, R.; Yan, S.; Fu, D.; Zhu, B.; Tian, H.; Luo, Y.; Zhu, H. The RNA Editing Factor SLORRM4 Is Required for Normal Fruit Ripening in Tomato1. Plant Physiol. 2017, 175, 1690–1702. [Google Scholar] [CrossRef]
- Zhou, L.; Tian, S.; Qin, G. RNA Methylomes Reveal the M6A-Mediated Regulation of DNA Demethylase Gene SlDML2 in Tomato Fruit Ripening. Genome Biol. 2019, 20, 156. [Google Scholar] [CrossRef]
- Klap, C.; Yeshayahou, E.; Bolger, A.M.; Arazi, T.; Gupta, S.K.; Shabtai, S.; Usadel, B.; Salts, Y.; Barg, R. Tomato Facultative Parthenocarpy Results from SlAGAMOUS-LIKE 6 Loss of Function. Plant Biotechnol. J. 2017, 15, 634–647. [Google Scholar] [CrossRef]
- Li, R.; Li, R.; Li, X.; Fu, D.; Zhu, B.; Tian, H.; Luo, Y.; Zhu, H. Multiplexed CRISPR/Cas9-Mediated Metabolic Engineering of γ-Aminobutyric Acid Levels in Solanum lycopersicum. Plant Biotechnol. J. 2018, 16, 415–427. [Google Scholar] [CrossRef]
- Hu, J.; Israeli, A.; Ori, N.; Sun, T.P. The Interaction between DELLA and ARF/IAA Mediates Crosstalk between Gibberellin and Auxin Signaling to Control Fruit Initiation in Tomato. Plant Cell 2018, 30, 1710–1728. [Google Scholar] [CrossRef]
- Ye, J.; Wang, X.; Hu, T.; Zhang, F.; Wang, B.; Li, C.; Yang, T.; Li, H.; Lu, Y.; Giovannoni, J.J.; et al. An InDel in the Promoter of AI-ACTIVATED MALATE TRANSPORTER9 Selected during Tomato Domestication Determines Fruit Malate Contents and Aluminum Tolerance. Plant Cell 2017, 29, 2249–2268. [Google Scholar] [CrossRef]
- Chen, L.; Yang, D.; Zhang, Y.; Wu, L.; Zhang, Y.; Ye, L.; Pan, C.; He, Y.; Huang, L.; Ruan, Y.L.; et al. Evidence for a Specific and Critical Role of Mitogen-Activated Protein Kinase 20 in Uni-to-Binucleate Transition of Microgametogenesis in Tomato. New Phytol. 2018, 219, 176–194. [Google Scholar] [CrossRef]
- Collins, E.J.; Bowyer, C.; Tsouza, A.; Chopra, M. Tomatoes: An Extensive Review of the Associated Health Impacts of Tomatoes and Factors That Can Affect Their Cultivation. Biology 2022, 11, 239. [Google Scholar] [CrossRef]
- Xu, X.; Yuan, Y.; Feng, B.; Deng, W. CRISPR/Cas9-Mediated Gene-Editing Technology in Fruit Quality Improvement. Food Qual. Saf. 2020, 4, 159–166. [Google Scholar] [CrossRef]
- Chen, P.J.; Liu, D.R. Prime Editing for Precise and Highly Versatile Genome Manipulation. Nat. Rev. Genet. 2023, 24, 161–177. [Google Scholar] [CrossRef]
- Lu, C.; Kuang, J.; Shao, T.; Xie, S.; Li, M.; Zhu, L.L.; Zhu, L.L. Prime Editing: An All-Rounder for Genome Editing. Int. J. Mol. Sci. 2022, 23, 9862. [Google Scholar] [CrossRef]
- Zong, Y.; Liu, Y.; Xue, C.; Li, B.; Li, X.; Wang, Y.; Li, J.; Liu, G.; Huang, X.; Cao, X.; et al. An Engineered Prime Editor with Enhanced Editing Efficiency in Plants. Nat. Biotechnol. 2022, 40, 1394–1402. [Google Scholar] [CrossRef]
- Tingting, L.; Jinpeng, Z.; Xi, Y.; Kejian, W.; Yuchun, R.; Chun, W. Development and Application of Prime Editing in Plants. Rice Sci. 2023, 30, 509–522. [Google Scholar] [CrossRef]
- Alberts, B.; Johnson, A.; Lewis, J.; Raff, M.; Roberts, K.; Walter, P. The Genetic Systems of Mitochondria and Plastids. In Molecular Biology of the Cell, 4th ed.; Garland Science: New York, NY, USA, 2002. [Google Scholar]
- Xia, X.; Cheng, X.; Li, R.; Yao, J.; Li, Z.; Cheng, Y. Advances in Application of Genome Editing in Tomato and Recent Development of Genome Editing Technology. Theor. Appl. Genet. 2021, 134, 2727–2747. [Google Scholar] [CrossRef]
- Arimura, S. ichi MitoTALENs: A Method for Targeted Gene Disruption in Plant Mitochondrial Genomes. Methods Mol. Biol. 2022, 2363, 335–340. [Google Scholar] [CrossRef]
- Bi, R.; Li, Y.; Xu, M.; Zheng, Q.; Zhang, D.F.; Li, X.; Ma, G.; Xiang, B.; Zhu, X.; Zhao, H.; et al. Direct Evidence of CRISPR-Cas9-Mediated Mitochondrial Genome Editing. Innovation 2022, 3, 100329. [Google Scholar] [CrossRef]
- Urnov, F.D.; Rebar, E.J.; Holmes, M.C.; Zhang, H.S.; Gregory, P.D. Genome Editing with Engineered Zinc Finger Nucleases. Nat. Rev. Genet. 2010, 11, 636–646. [Google Scholar] [CrossRef]
- Kim, S.; Kim, J.S. Targeted Genome Engineering via Zinc Finger Nucleases. Plant Biotechnol. Rep. 2011, 5, 9–17. [Google Scholar] [CrossRef]
- Shipman, E.N.; Yu, J.; Zhou, J.; Albornoz, K.; Beckles, D.M. Can Gene Editing Reduce Postharvest Waste and Loss of Fruit, Vegetables, and Ornamentals? Hortic. Res. 2021, 8, 1. [Google Scholar] [CrossRef]
- Pacesa, M.; Pelea, O.; Jinek, M. Past, Present, and Future of CRISPR Genome Editing Technologies. Cell 2024, 187, 1076–1100. [Google Scholar] [CrossRef]
- Rodríguez-Rodríguez, D.R.; Ramírez-Solís, R.; Garza-Elizondo, M.A.; Garza-Rodríguez, M.D.L.; Barrera-Saldaña, H.A. Genome Editing: A Perspective on the Application of CRISPR/Cas9 to Study Human Diseases (Review). Int. J. Mol. Med. 2019, 43, 1559–1574. [Google Scholar] [CrossRef]
- Jin, S.; Fei, H.; Zhu, Z.; Luo, Y.; Liu, J.; Gao, S.; Zhang, F.; Chen, Y.H.; Wang, Y.; Gao, C. Rationally Designed APOBEC3B Cytosine Base Editors with Improved Specificity. Mol. Cell 2020, 79, 728–740.e6. [Google Scholar] [CrossRef]
- Liu, G.; Lin, Q.; Jin, S.; Gao, C. The CRISPR-Cas Toolbox and Gene Editing Technologies. Mol. Cell 2022, 82, 333–347. [Google Scholar] [CrossRef] [PubMed]
- Tian, T.; Zhou, X. CRISPR-Based Biosensing Strategies: Technical Development and Application Prospects. Annu. Rev. Anal. Chem. (Palo Alto. Calif). 2023, 16, 311–332. [Google Scholar] [CrossRef] [PubMed]
- Ghimire, B.K.; Yu, C.Y.; Kim, W.-R.; Moon, H.-S.; Lee, J.; Kim, S.H.; Chung, I.M. Assessment of Benefits and Risk of Genetically Modified Plants and Products: Current Controversies and Perspective. Sustainability 2023, 15, 1722. [Google Scholar] [CrossRef]
- Cappetta, E.; Andolfo, G.; Guadagno, A.; Di Matteo, A.; Barone, A.; Frusciante, L.; Ercolano, M.R. Tomato Genomic Prediction for Good Performance under High-Temperature and Identification of Loci Involved in Thermotolerance Response. Hortic. Res. 2021, 8, 212. [Google Scholar] [CrossRef]
- Access, O.; Achparaki, M.; Thessalonikeos, E.; Tsoukali, H.; Mastrogianni, O.; Zaggelidou, E.; Chatzinikolaou, F.; Vasilliades, N.; Raikos, N.; Isabirye, M.; et al. Stresses in Plants: Biotic and Abiotic; Intech: London, UK, 2012; p. 13. [Google Scholar]
- Ahmad, S.; Wei, X.; Sheng, Z.; Hu, P.; Tang, S. CRISPR/Cas9 for Development of Disease Resistance in Plants: Recent Progress, Limitations and Future Prospects. Brief. Funct. Genom. 2018, 19, 26–39. [Google Scholar] [CrossRef] [PubMed]
- Tashkandi, M.; Ali, Z.; Aljedaani, F.; Shami, A.; Mahfouz, M.M. Engineering Resistance against Tomato Yellow Leaf Curl Virus via the CRISPR/Cas9 System in Tomato. Plant Signal. Behav. 2018, 13, e1525996. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Tian, X.; Xu, H.; Zhan, B.; Zhou, C.; Li, S.; Zhang, Z. Knockout of SlDCL2b Attenuates the Resistance of Tomato to Potato Spindle Tuber Viroid Infection. Mol. Plant Pathol. 2024, 25, e13441. [Google Scholar] [CrossRef] [PubMed]
- Djennane, S.; Gersch, S.; Le-Bohec, F.; Piron, M.-C.; Baltenweck, R.; Lemaire, O.; Merdinoglu, D.; Hugueney, P.; Nogué, F.; Mestre, P. CRISPR/Cas9 Editing of Downy Mildew Resistant 6 (DMR6-1) in Grapevine Leads to Reduced Susceptibility to Plasmopara viticola. J. Exp. Bot. 2023, 75, 2100–2112. [Google Scholar] [CrossRef]
- Li, H.; Men, W.; Ma, C.; Liu, Q.; Dong, Z.; Tian, X.; Wang, C.; Liu, C.; Gill, H.; Ma, P.; et al. Wheat Powdery Mildew Resistance Gene Pm13 from Aegilops Longissima Encodes a Unique Mixed Lineage Kinase Domain-like Protein. Nat. Commun. 2024, 15, 2449. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Chen, G.; Tang, B.; Zhang, J.; Zhou, S.; Hu, Z. The Jasmonate ZIM-Domain Protein Gene SlJAZ2 Regulates Plant Morphology and Accelerates Flower Initiation in Solanum lycopersicum Plants. Plant Sci. 2018, 267, 65–73. [Google Scholar] [CrossRef]
- Liu, H.; Able, A.J.; Able, J.A. Priming Crops for the Future: Rewiring Stress Memory. Trends Plant Sci. 2022, 27, 699–716. [Google Scholar] [CrossRef] [PubMed]
- Benitez-Alfonso, Y.; Soanes, B.K.; Zimba, S.; Sinanaj, B.; German, L.; Sharma, V.; Bohra, A.; Kolesnikova, A.; Dunn, J.A.; Martin, A.C.; et al. Enhancing Climate Change Resilience in Agricultural Crops. Curr. Biol. 2023, 33, R1246–R1261. [Google Scholar] [CrossRef]
- Lee, H.S.; Kim, Y.; Pham, G.; Kim, J.W.; Song, J.H.; Lee, Y.; Hwang, Y.S.; Roux, S.J.; Kim, S.H. Brassinazole Resistant 1 (BZR1)-Dependent Brassinosteroid Signalling Pathway Leads to Ectopic Activation of Quiescent Cell Division and Suppresses Columella Stem Cell Differentiation. J. Exp. Bot. 2015, 66, 4835–4849. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Qin, K.; Song, X.; Zhang, Q.; Zhou, Y.; Xia, X.; Yu, J. BZR1 Transcription Factor Regulates Heat Stress Tolerance through FERONIA Receptor-like Kinase-Mediated Reactive Oxygen Species Signaling in Tomato. Plant Cell Physiol. 2018, 59, 2239–2254. [Google Scholar] [CrossRef] [PubMed]
- Yun, S.D.; Kim, M.H.; Oh, S.A.; Soh, M.S.; Park, S.K. Overexpression of C-Repeat Binding Factor1 (CBF1) Gene Enhances Heat Stress Tolerance in Arabidopsis. J. Plant Biol. 2022, 65, 253–260. [Google Scholar] [CrossRef]
- Huang, X.; Wei, J.M.; Feng, W.Z.; Luo, Q.; Tan, G.F.; Li, Y.Z. Interaction between SlMAPK3 and SlASR4 Regulates Drought Resistance in Tomato (Solanum lycopersicum L.). Mol. Breed. 2023, 43, 73. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Hardcastle, T.J.; Pastor, A.C.; Yip, W.H.; Tang, S.; Baulcombe, D.C. A Novel DCL2-Dependent MiRNA Pathway in Tomato Affects Susceptibility to RNA Viruses. Genes Dev. 2018, 32, 1155–1160. [Google Scholar] [CrossRef] [PubMed]
- Nekrasov, V.; Wang, C.; Win, J.; Lanz, C.; Weigel, D.; Kamoun, S. Rapid Generation of a Transgene-Free Powdery Mildew Resistant Tomato by Genome Deletion. Sci. Rep. 2017, 7, 482. [Google Scholar] [CrossRef]
- Koseoglou, E. The Study of SlPMR4 CRISPR/Cas9 Mediated Tomato Allelic Series for Resistance against Powdery Mildew Eleni Koseoglou; Wageningen University: Wageningen, The Netherlands, 2017; pp. 1–29. [Google Scholar]
- de Toledo Thomazella, D.P.; Brail, Q.; Dahlbeck, D.; Staskawicz, B.J. CRISPR-Cas9 Mediated Mutagenesis of a DMR6 Ortholog in Tomato Confers Broad-Spectrum Disease Resistance. bioRxiv 2016, 064824. [Google Scholar] [CrossRef]
- Prihatna, C.; Barbetti, M.J.; Barker, S.J. A Novel Tomato Fusarium Wilt Tolerance Gene. Front. Microbiol. 2018, 9, 1226. [Google Scholar] [CrossRef]
- Ortigosa, A.; Gimenez-Ibanez, S.; Leonhardt, N.; Solano, R. Design of a Bacterial Speck Resistant Tomato by CRISPR/Cas9-Mediated Editing of SlJAZ2. Plant Biotechnol. J. 2019, 17, 665–673. [Google Scholar] [CrossRef] [PubMed]
- Catanzariti, A.M.; Lim, G.T.T.; Jones, D.A. The Tomato I-3 Gene: A Novel Gene for Resistance to Fusarium Wilt Disease. New Phytol. 2015, 207, 106–118. [Google Scholar] [CrossRef] [PubMed]
- Fradin, E.F.; Zhang, Z.; Juarez Ayala, J.C.; Castroverde, C.D.M.; Nazar, R.N.; Robb, J.; Liu, C.-M.; Thomma, B.P.H.J. Genetic Dissection of Verticillium Wilt Resistance Mediated by Tomato Ve1. Plant Physiol. 2009, 150, 320–332. [Google Scholar] [CrossRef]
- Cui, J.; Jiang, N.; Zhou, X.; Hou, X.; Yang, G.; Meng, J.; Luan, Y. Tomato MYB49 Enhances Resistance to Phytophthora Infestans and Tolerance to Water Deficit and Salt Stress. Planta 2018, 248, 1487–1503. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.R.; Mukherjee, P.K.; Eapen, S. Expression of a Fungal Endochitinase Gene in Transgenic Tomato and Tobacco Results in Enhanced Tolerance to Fungal Pathogens. Physiol. Mol. Biol. Plants Int. J. Funct. Plant Biol. 2010, 16, 39–51. [Google Scholar] [CrossRef]
- Horvath, D.M.; Stall, R.E.; Jones, J.B.; Pauly, M.H.; Vallad, G.E.; Dahlbeck, D.; Staskawicz, B.J.; Scott, J.W. Transgenic Resistance Confers Effective Field Level Control of Bacterial Spot Disease in Tomato. PLoS ONE 2012, 7, e42036. [Google Scholar] [CrossRef]
- Martin, G.B.; Brommonschenkel, S.H.; Chunwongse, J.; Frary, A.; Ganal, M.W.; Spivey, R.; Wu, T.; Earle, E.D.; Tanksley, S.D. Map-Based Cloning of a Protein Kinase Gene Conferring Disease Resistance in Tomato. Science 1993, 262, 1432–1436. [Google Scholar] [CrossRef]
- Li, R.; Zhang, L.; Wang, L.; Chen, L.; Zhao, R.; Sheng, J.; Shen, L. Reduction of Tomato-Plant Chilling Tolerance by CRISPR-Cas9-Mediated SlCBF1 Mutagenesis. J. Agric. Food Chem. 2018, 66, 9042–9051. [Google Scholar] [CrossRef]
- Wang, L.; Chen, L.; Li, R.; Zhao, R.; Yang, M.; Sheng, J.; Shen, L. Reduced Drought Tolerance by CRISPR/Cas9-Mediated SlMAPK3 Mutagenesis in Tomato Plants. J. Agric. Food Chem. 2017, 65, 8674–8682. [Google Scholar] [CrossRef] [PubMed]
- Moghaieb, R.E.A.; Tanaka, N.; Saneoka, H.; Hussein, H.A.; Yousef, S.S.; Ewada, M.A.F.; Aly, M.A.M.; Fujita, K. Expression of Betaine Aldehyde Dehydrogenase Gene in Transgenic Tomato Hairy Roots Leads to the Accumulation of Glycine Betaine and Contributes to the Maintenance of the Osmotic Potential under Salt Stress. Soil Sci. Plant Nutr. 2000, 46, 873–883. [Google Scholar] [CrossRef]
- Wang, G.; Kong, F.; Zhang, S.; Meng, X.; Wang, Y.; Meng, Q. A Tomato Chloroplast-Targeted DnaJ Protein Protects Rubisco Activity under Heat Stress. J. Exp. Bot. 2015, 66, 3027–3040. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.X.; Blumwald, E. Transgenic Salt-Tolerant Tomato Plants Accumulate Salt in Foliage but Not in Fruit. Nat. Biotechnol. 2001, 19, 765–768. [Google Scholar] [CrossRef] [PubMed]
- Seong, E.S.; Cho, H.S.; Choi, D.; Joung, Y.H.; Lim, C.K.; Hur, J.H.; Wang, M.H. Tomato Plants Overexpressing CaKR1 Enhanced Tolerance to Salt and Oxidative Stress. Biochem. Biophys. Res. Commun. 2007, 363, 983–988. [Google Scholar] [CrossRef] [PubMed]
- Moghaieb, R.E.A.; Nakamura, A.; Saneoka, H.; Fujita, K. Evaluation of Salt Tolerance in Ectoine-Transgenic Tomato Plants (Lycopersicon esculentum) in Terms of Photosynthesis, Osmotic Adjustment, and Carbon Partitioning. GM Crops 2011, 2, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Huertas, R.; Olías, R.; Eljakaoui, Z.; Gálvez, F.J.; Li, J.; De Morales, P.A.; Belver, A.; Rodríguez-Rosales, M.P. Overexpression of SlSOS2 (SlCIPK24) Confers Salt Tolerance to Transgenic Tomato. Plant. Cell Environ. 2012, 35, 1467–1482. [Google Scholar] [CrossRef]
- Goel, D.; Singh, A.K.; Yadav, V.; Babbar, S.B.; Bansal, K.C. Overexpression of Osmotin Gene Confers Tolerance to Salt and Drought Stresses in Transgenic Tomato (Solanum lycopersicum L.). Protoplasma 2010, 245, 133–141. [Google Scholar] [CrossRef]
- Lu, W.; Guo, C.; Li, X.; Duan, W.; Ma, C.; Zhao, M.; Gu, J.; Du, X.; Liu, Z.; Xiao, K. Overexpression of TaNHX3, a Vacuolar Na+/H+ Antiporter Gene in Wheat, Enhances Salt Stress Tolerance in Tobacco by Improving Related Physiological Processes. Plant Physiol. Biochem. 2014, 76, 17–28. [Google Scholar] [CrossRef]
- Wei, D.; Zhang, W.; Wang, C.; Meng, Q.; Li, G.; Chen, T.H.H.; Yang, X. Genetic Engineering of the Biosynthesis of Glycinebetaine Leads to Alleviate Salt-Induced Potassium Efflux and Enhances Salt Tolerance in Tomato Plants. Plant Sci. 2017, 257, 74–83. [Google Scholar] [CrossRef]
- García-Abellan, J.O.; Egea, I.; Pineda, B.; Sanchez-Bel, P.; Belver, A.; Garcia-Sogo, B.; Flores, F.B.; Atares, A.; Moreno, V.; Bolarin, M.C. Heterologous Expression of the Yeast HAL5 Gene in Tomato Enhances Salt Tolerance by Reducing Shoot Na+ Accumulation in the Long Term. Physiol. Plant. 2014, 152, 700–713. [Google Scholar] [CrossRef]
- Hu, D.-G.; Ma, Q.-J.; Sun, C.-H.; Sun, M.-H.; You, C.-X.; Hao, Y.-J. Overexpression of MdSOS2L1, a CIPK Protein Kinase, Increases the Antioxidant Metabolites to Enhance Salt Tolerance in Apple and Tomato. Physiol. Plant. 2016, 156, 201–214. [Google Scholar] [CrossRef]
- Iqbal, N.; Khan, N.A.; Ferrante, A.; Trivellini, A.; Francini, A.; Khan, M.I.R. Ethylene Role in Plant Growth, Development and Senescence: Interaction with Other Phytohormones. Front. Plant Sci. 2017, 8, 475. [Google Scholar] [CrossRef]
- Liu, M.; Pirrello, J.; Chervin, C.; Roustan, J.P.; Bouzayen, M. Ethylene Control of Fruit Ripening: Revisiting the Complex Network of Transcriptional Regulation. Plant Physiol. 2015, 169, 2380–2390. [Google Scholar] [CrossRef]
- Shahwar, D.; Ahn, N.; Kim, D.; Ahn, W.; Park, Y. Mutagenesis-Based Plant Breeding Approaches and Genome Engineering: A Review Focused on Tomato. Mutat. Res. Rev. Mutat. Res. 2023, 792, 108473. [Google Scholar] [CrossRef]
- Ranjan, A.; Ichihashi, Y.; Sinha, N.R. The Tomato Genome: Implications for Plant Breeding, Genomics and Evolution. Genome Biol. 2012, 13, 167. [Google Scholar] [CrossRef]
- Youssef, K.; Ippolito, A.; Roberto, S.R. Editorial: Post-Harvest Diseases of Fruit and Vegetable: Methods and Mechanisms of Action. Front. Microbiol. 2022, 13, 900060. [Google Scholar] [CrossRef]
- Alexandre, Y.; Le Berre, R.; Barbier, G.; Le Blay, G. Screening of Lactobacillus Spp. for the Prevention of Pseudomonas Aeruginosa Pulmonary Infections. BMC Microbiol. 2014, 14, 107. [Google Scholar] [CrossRef]
- Lewicki, P.P.; Lenart, A. Contamination, Detection and Control of Mycotoxins in Fruits and Vegetables. Most 2006, 375–400. [Google Scholar]
- Zhang, Z.Q.; Chen, T.; Li, B.Q.; Qin, G.Z.; Tian, S.P. Molecular Basis of Pathogenesis of Postharvest Pathogenic Fungi and Control Strategy in Fruits: Progress and Prospect. Mol. Hortic. 2021, 1, 2. [Google Scholar] [CrossRef]
- Qadri, R.; Azam, M.; Khan, I.; Yang, Y.; Ejaz, S.; Akram, M.T.; Khan, M.A. Conventional and Modern Technologies for the Management of Post-Harvest Diseases. In Plant Disease Management Strategies for Sustainable Agriculture through Traditional and Modern Approaches; Sustainability in Plant and Crop Protection; Springer: Cham, Switzerland, 2020; Volume 13, pp. 137–172. [Google Scholar] [CrossRef]
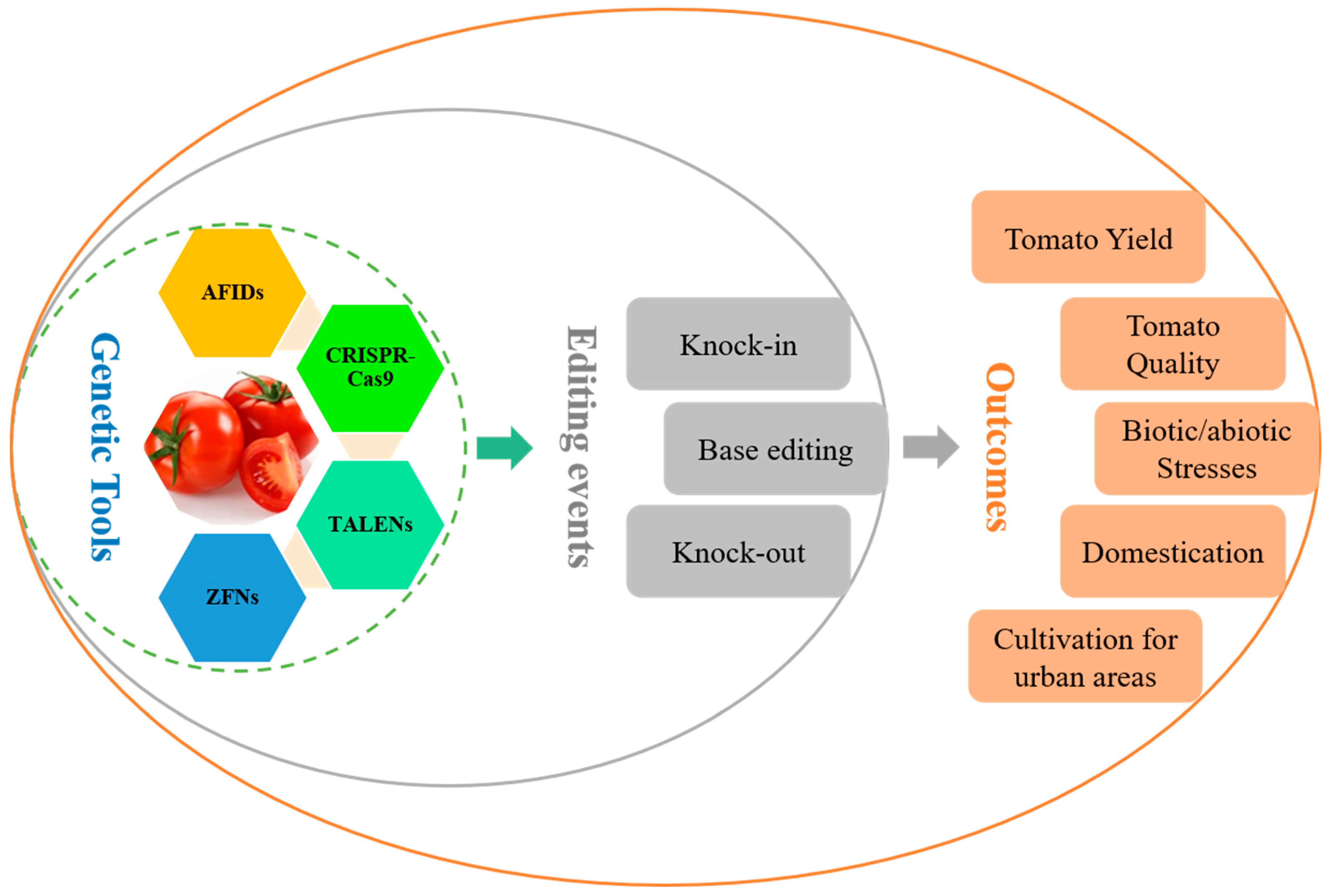
| Gene | Phenotypic Property | Genetics Editing Tool | Reference |
|---|---|---|---|
| PL | Firmness | CRISPR/Cas9 | [63] |
| SIMAPK3 | Alleviate biotic/abiotic stress | CRISPR/Cas9 | [64] |
| L1L4 | Increase size and stress resistance | ZFN | [65] |
| ALC | Shelf life | CRISPR/Cas9 | [66] |
| RIN | Fruit ripening | CRISPR/Cas9 | [67] |
| SBP-CNR and NAC-NOR | Fruit ripening | CRISPR/Cas9 | [68] |
| SBE1 and INV2 | Amylopectin, osmosis | CRISPR/Cas9 | [69] |
| StGBSS | Amylase synthesis | CRISPR/Cas9 | [70] |
| SBE1, SBE2 | Starch regulator | CRISPR/Cas9 | [71] |
| CaMBD | GABA regulator | CRISPR/Cas9 | [72] |
| MYB12 | Colorization | CRISPR/Cas9 | [73] |
| CLV3, S or SP | Tomato yield | CRISPR/Cas9 (Cis-regulatory mutations) | [74] |
| PSY1 | Colorization | [75] | |
| SLANT1 | Colorization | CRISPR/Cas9, (targeted insertion of the strong promoter) | [76] |
| FW2.2, FAS, MULT and CycB | Fruit size and number | CRISPR/Cas9 | [77] |
| SP, SP5G and SIER | Firmness | CRISPR/Cas9 | [78] |
| ENO | Fruit size | CRISPR/Cas9 | [79] |
| GABA-TP1, TP2, TP3, SSADH, CAT9 | GABA content | CRISPR/Cas9 | [80] |
| SLGAD2, 3 | GABA content | CRISPR/Cas9 | [81] |
| SGR1, LCY-E, LCY-B1, B2, Blc | Lycopene synthesis | CRISPR/Cas9 | [82] |
| AP2a, FUL1, FUL2 | Fruit ripening | CRISPR/Cas9 | [83] |
| SLORRM4 | Fruit ripening | CRISPR/Cas9 | [84] |
| SIDML2 | Fruit ripening | CRISPR/Cas9 | [85] |
| SIAGL6 | Parthenocarpic fruit | CRISPR/Cas9 | [86] |
| PG2a, TBG4 | Fruit firmness | CRISPR/Cas9 | [83] |
| GGP1 | Ascorbic acid | CRISPR/Cas9 | [87] |
| ARF7 | Parthenocarpic fruit | CRISPR/Cas9 | [88] |
| ALMT9 | Regulate malate content | CRISPR/Cas9 | [89] |
| MAPK20 | Regulate sugar content | CRISPR/Cas9 | [90] |
| Stress-Related | Genes | Phenotypic Traits | References |
|---|---|---|---|
| Biotic stress | DCL2 | Vulnerability to tobacco mosaic virus, tomato mosaic virus, and potato virus. | [124] |
| MLO1 | Protection from powdery mildews. | [125] | |
| CP and Rep of virus | Protection from tomato yellow leaf curl virus | [113] | |
| PMR4 | Protection from powdery mildews | [126] | |
| DMR6 | Protection from powdery mildews | [127] | |
| Solyc08g075077 | Vulnerability to fusarium wilt disease | [128] | |
| MAPK3 | Related to gray mold disease | [64] | |
| JAZ2 | Protection against bacterial speck disease | [129] | |
| SRLK5 | Resilience against fusarium wilt | [130] | |
| Ve1 | Tolerance to verticillium wilt disease | [131] | |
| SIMYB49 | Ability to withstand the fungal pathogen phytophthora infestans | [132] | |
| ech42 | Resistance to fungal pathogen Alternaria alternata | [133] | |
| Bs2 | Tolerance to bacterial spot disease | [134] | |
| PPo, Pto, Prf | Tolerance to bacterial pathogen, Pseudomonas syringae pv. tomato | [135] | |
| Abiotic stress | BZR1 | Reduced tolerance to heat stress | [121] |
| CBF1 | Reduced tolerance to cold stress | [136] | |
| MAPK3 | Reduced tolerance to drought stress | [137] | |
| BADH1 | Convert betaine aldehyde to glycine betaine | [138] | |
| cAPX | Minimize cellular damage by scavenging super oxides | [139] | |
| NHX1 | Overexpressed NHX1 vacuolar NA+/H+ antiporter | [140] | |
| CaKR1 | Elevated expression of antioxidant enzyme that eliminate antioxidants | [141] | |
| Ectoine (ectA, ectB, ectC) | Enhances peroxidase activity and decreases MDA contents by the accumulation of ectoine | [142] | |
| AtSIS0S2 | Regulates the Na+/H+ and endosomal vacular K+, Na+/H+, mainly responsible for Na+ extrusion out of the roots, loading of Na+ into xylem, and the compartmentalization of Na+, and K+ | [143] | |
| ToOsmotin | When overexpressed, leads to the accumulation of solutes and protects the native protein’s structure | [144] | |
| TaNHX2 | Regulates the Na+, pH, and K+ homeostasis | [145] | |
| coda | Enhances the NaCl-induced expression of genes encoding the K+ transporter, Na+/H+ antiporter, and H+-ATPase | [146] | |
| HAl5 | Regulate and maintain the homeostasis of Na+ and K+, and SIHKT1, SIHKT2, and SIHKT5 | [147] | |
| MdSOS2L1 | Interact with MdCBL1, MdCBL4 and MdCBL10 proteins to increase tolerance | [148] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nie, H.; Yang, X.; Zheng, S.; Hou, L. Gene-Based Developments in Improving Quality of Tomato: Focus on Firmness, Shelf Life, and Pre- and Post-Harvest Stress Adaptations. Horticulturae 2024, 10, 641. https://doi.org/10.3390/horticulturae10060641
Nie H, Yang X, Zheng S, Hou L. Gene-Based Developments in Improving Quality of Tomato: Focus on Firmness, Shelf Life, and Pre- and Post-Harvest Stress Adaptations. Horticulturae. 2024; 10(6):641. https://doi.org/10.3390/horticulturae10060641
Chicago/Turabian StyleNie, Hongmei, Xiu Yang, Shaowen Zheng, and Leiping Hou. 2024. "Gene-Based Developments in Improving Quality of Tomato: Focus on Firmness, Shelf Life, and Pre- and Post-Harvest Stress Adaptations" Horticulturae 10, no. 6: 641. https://doi.org/10.3390/horticulturae10060641
APA StyleNie, H., Yang, X., Zheng, S., & Hou, L. (2024). Gene-Based Developments in Improving Quality of Tomato: Focus on Firmness, Shelf Life, and Pre- and Post-Harvest Stress Adaptations. Horticulturae, 10(6), 641. https://doi.org/10.3390/horticulturae10060641






