Abstract
The combination of high or low temperatures and high salt may cause significant harm to the yield, quality, and overall productivity of forage pea crops. The germination process, a crucial phase in the life cycle of forage peas, may be greatly influenced by varying temperature and salinity conditions. To comprehend the influence of these elements on the germination of forage peas, one must use many tactics, including the choice of resilient forage pea cultivars. The experiment aimed to evaluate the response of four forage pea cultivars (Arda, Ozkaynak, Taskent, and Tore) caused by various temperature (10 °C, 15 °C, and 20 °C) and salt (0, 5, 10, 15, and 20 dS m−1) conditions at the germination stage using multivariate analysis and machine learning methods. An observation of statistical significance (p < 0.01) was made regarding the variations between genotypes, temperature–salt levels, and the interaction of the observed factors: germination percentage (GP), shoot length (SL), root length (RL), fresh weight (FW), and dry weight (DW). The cultivar Tore had the best values for SL (1.63 cm), RL (5.38 cm), FW (1.10 g), and DW (0.13 g) among all the cultivars. On the other hand, the Ozkaynak cultivar had the highest value for GP (89.13%). The values of all of the parameters that were investigated decreased as the salt level rose, whereas the values increased when the temperature level increased. As a result, the Tore cultivar exhibited the highest values for shoot length, root length, fresh weight, and dry weight variables when exposed to a maximum temperature of 20 °C and a saline level of 0 dS m−1. It was determined that temperature treatment of fodder peas can reduce salt stress if kept at optimum levels. The effects of temperature and salt treatments on the germination data of several fodder pea cultivars were analyzed and predicted. Three distinct machine learning algorithms were used to create predictions. Based on R2 (0.899), MSE (5.344), MAPE (6.953), and MAD (4.125) measures, the MARS model predicted germination power (GP) better. The GPC model performed better in predicting shoot length (R2 = 0.922, MSE = 0.602, MAPE = 11.850, and MAD = 0.326) and root length (R2 = 0.900, MSE = 0.719, MAPE = 12.673, and MAD = 0.554), whereas the Xgboost model performed better in estimating fresh weight (R2 = 0.966, MSE = 0.130, MAPE = 11.635, and MAD = 0.090) and dry weight (R2 = 0.895, MSE = 0.021, MAPE = 12.395, and MAD = 0.013). The results of the research show that the techniques and analyses used can estimate stress tolerance, susceptibility levels, and other plant parameters, making it a cost-effective and reliable way to quickly and accurately study forage peas and related species.
1. Introduction
Abiotic stress is a pervasive issue that significantly constrains agricultural output on a global scale [1]. Simultaneously, it has a detrimental impact on the growth and development of plants, thereby impairing their output [2]. Plants are susceptible to salinity at any stage of their growth cycle, beginning from the point when the seeds are deposited into the soil [3,4]. Nevertheless, plants exhibit heightened sensitivity, particularly throughout the stages of germination and seedling growth [5,6].
In the process of seed imbibition, salinity reduces the osmotic potential of the environment in comparison to the osmotic potential of the seed itself, which stops the seed from absorbing water [7]. Consequently, the rate of seed germination is decreased, and the time of seed germination is postponed. Post-germination, salinity may harm the viability of the embryo by causing an excessive buildup of Na+ and Cl− ions [8,9]. In addition, salt stress induces the production of reactive oxygen species (ROS) and oxidative damage, leading to the disruption of many macromolecules. Hence, a decline in α-amylase activity leads to a notable drop in the transportation of sugars, thereby limiting the growth and development of the embryo [10].
The treatment of seeds with temperature may have a considerable impact on the germination and early development phases of seeds that have been subjected to salt stress [11,12]. Elevated temperatures have the potential to compound the negative consequences of salinity by further reducing the osmotic potential and increasing the evaporation rate, which ultimately results in more severe dehydration stress [13]. On the other hand, it has been discovered that certain temperature regimes may mitigate the negative consequences of salt stress [9]. An example of this would be that appropriate heat conditions have the potential to boost the enzyme activity associated with germination, such as α-amylase, which in turn promotes greater mobilization of stored reserves. Furthermore, temperature treatments have the potential to affect the production and activity of heat shock proteins (HSPs) and antioxidant enzymes. These proteins and enzymes play essential roles in reducing the oxidative damage caused by reactive oxygen species (ROS) when they are subjected to salt stress [14,15]. In order to boost seedling vigor and overall plant resistance to salt, appropriate temperature treatments may be used. These treatments work by stabilizing cellular structures and increasing processes that allow plants to tolerate stress [16].
There is a need for changes to be made to the existing systems in agricultural production because of factors such as population expansion, rising temperatures, unpredictable weather patterns, and erratic rainfall [17]. Traditional feed crops such as alfalfa and common vetch from legumes, as well as traditional forage crops like sainfoin, are farmed in Türkiye. The use of natural rangelands, stubble, and cereal straw are the primary components of sustainable livestock production [18]. As a result of the diverse climatic and soil features that exist throughout the nation, forage crops have the potential to be grown as primary and secondary crops in coastal areas as well as in central transitional regions [19]. Not only are legume forage crops notable for their many benefits, which include the fixation of nitrogen, the enhancement of soil quality, and the favorable contribution they provide to the primary crop, but they are also a source of high-quality roughage [20]. One of the most important cool-season leguminous forage species is the forage pea, also known as Pisum sativum ssp. arvense (L.) Moench. It is grown to produce grain or hay in climate zones that are classified as temperate. Furthermore, the plant has the potential to be grown as an intermediate crop in addition to the primary crop in regions with cold weather [21]. However, its variety-level reaction to temperature and salinity fluctuations, notably during germination, is unclear. Since there is little research on this topic, forage pea varieties’ germination responses to abiotic stressors must be examined. Furthermore, optimization and forecasting are particularly desired in agricultural research, and solid findings may be produced by using modern computer techniques such as machine learning algorithms that employ fewer inputs [22,23]. These approaches are advantageous because they are easily accessible and can be implemented without requiring extensive resources or specialized equipment. However, the usefulness of each model may change depending on the data used and the way the experiment is set up [24,25]. As a result, for plants grown on a large scale, like forage peas, it is crucial to anticipate how the plant will react to its surroundings during the germination period [26].
In light of this knowledge, the primary objective of this study was to analyze the growth of various forage pea cultivars under salt stress during the germination stage, with temperature treatment applied. Germination is a complex yet crucial process for plant growth and development. The secondary objective was to utilize machine learning algorithms to predict and optimize the germination parameters of fodder peas exposed to salt stress and temperature treatment.
2. Materials and Methods
2.1. Material
Arda, Ozkaynak, Taskent, and Tore forage pea cultivars were used as materials in the experiments. The seeds were obtained from the Erciyes University Faculty of Agriculture, and the experiment was set up in its laboratory. The seeds were stored dry in cloth bags at room temperature until use. The seeds were first sterilized with 5% commercial bleach (ACE) for five minutes, then rinsed with distilled water three times. NaCl (Merck) concentrations at electrical conductivities (EC) of 5, 10, 15, and 20 dS m−1 were adjusted before the start of the experiment. Distilled water served as a control (0 dS m−1). A total of 25 seeds for each cultivar were placed into three-layer filter papers (20 × 20 cm) irrigated with 7 mL of the respective solutions for each paper. After filter papers with seeds were rolled, they were placed into a sealed plastic bag to prevent moisture loss with 4 replications. The packages were incubated at 10 °C, 15 °C, and 20 °C in the dark. The seeds were considered germinated with the emergence of the radicle ≥ 2 mm. Root length (RL) (cm), shoot length (SL) (cm), fresh weight (FW) (g), and dry weight (DW) (g) were measured by randomly selecting 10 seedlings per replication. Germination percentage (GP), RL, SL, and FW values were recorded on the 14th day. To calculate the dry weight, the fresh shoots were dried for 72 h at 60 °C.
2.2. Analysis of Variance
Germination experiments were evaluated using analysis of variance (ANOVA) in accordance with a completely randomized trial design comprising a 4 (cultivar) × 3 (temperature) × 5 (EC) factorial arrangement with four replicates. Disparities among applications were detected utilizing the Duncan multiple comparison test, with a significance level set at 5% (Supplementary Tables S1–S4). XLSTAT (version 2023.1.3, Addinsoft, Paris, France) was utilized to assess the data.
2.3. Machine Learning (ML) Analysis
The objective was to estimate the output variables (germination percentage, shoot length, root length, fresh weight, and dry weight) by modeling the input variables (cultivars, temperature, and EC). The study included three machine learning (ML) algorithms: the multivariate adaptive regression spline (MARS) model [27], the extreme gradient boosting (XGBoost) model [28], and the Gaussian processes classifier (GPC) model [29]. These ML algorithms were chosen for their ability to handle complex and nonlinear relationships between variables, as well as their robustness in making accurate predictions based on multiple input factors, which is essential for optimizing the germination parameters under varying conditions of salt stress and temperature treatment.
The statistical notation for the MARS method, which was utilized in this study to predict output variables based on independent factors, is as follows:
The mathematical expression hkm(Xv(k,m)) denotes the basis function, where represents a constant (intercept) and denotes the coefficient of basis functions. The v(k,m) is an index of the independent variable in the mth component of the kth product. Km is the parameter that restricts the order of interaction.
Utilizing a gradient boosting framework, the XGBoost model is an algorithm of the gradient boosting decision tree variety that has been optimized for speed and performance. One notable benefit of XGBoost is its ability to learn from mistakes and reduce error rates through iterative processes. The mathematical formula of the XGBoost model is as follows:
where represents the value that the model predicts for the ith observation. The K denotes the number of decision trees in total. The is the kth decision tree. The feature vector corresponds to the ith observation.
The random variable distribution is described by the Gaussian probability density function. The statistical representation of the Gaussian process method used in the study is as follows:
The symbol m(x) represents the mean function of the Gaussian process, while k(x,x′) signifies the kernel function. Similarities between data points are identified by the covariance function (kernel). A constant value, such as zero or the mean of the training dataset, is generally assigned to the mean function. The mean of the training dataset was utilized in this investigation.
The evaluation of the effectiveness of the algorithm incorporated the use of four essential metrics: mean absolute deviation (MAD), mean absolute percentage error (MAPE), root mean square error (RMSE), and R-squared (R2). Denoted as R2, the coefficient of determination measures the extent to which the model accurately reproduces the empirical data (Equation (4)). RMSE, represented by Equation (5), measures the degree of proximity that exists between the predicted and actual values. One of the beneficial characteristics of MAPE is its independence from scale, which enables the representation of changes in percentage form (Equation (6)). In addition, the root mean absolute deviation (MAD), which is denoted by Equation (7), establishes the comprehensive pattern of errors in predictions [27,30]. The R package caret was utilized to randomly partition the dataset into two sets: 30% for the testing set and 70% for the training set. As shown in Equation (8), the optimal hyperparameters for each machine learning model were determined utilizing the Grid Search Cross-Validation (GCV) technique [31]. Additionally, standard deviations were calculated (Equation (9)). The training/testing sample size in the dataset is denoted by n; the measured real value is ; the predicted value is ; and the mean of the measured values is . The penalty function for the complexity of the model encompassing λ terms is denoted as M(λ). The sm is the standard deviation of the model errors, and the sd is the standard deviation of outputs. The computation of performance metrics and machine learning algorithms was conducted utilizing the R (version 4.3.2) programming language [27,32].
3. Results
The variance analysis findings for the main factors of the research are shown in Table 1. The analysis findings indicated statistically significant (p < 0.001) effects of cultivar, temperature, and EC on the germination percentage (GP), shoot length (SL), root length (RL), fresh weight (FW), and dry weight (DW). Among the observed variables, cultivar Tore had higher values for SL, RL, FW, and DW, whereas cultivar Ozkaynak had the highest value for the GP variable. There was a positive correlation between the temperature and the values of the observed variables. As the temperature increased, the values of the variables also increased. The highest values for all variables were seen at the maximum temperature treatment (20 °C). There was a negative correlation between the EC and the values of the observed variables. With the rise in EC, there was a corresponding decrease in the values of the variables. The highest values were determined for all parameters observed in conditions without salt stress.

Table 1.
Results of variance analysis for the effects of the main factors.
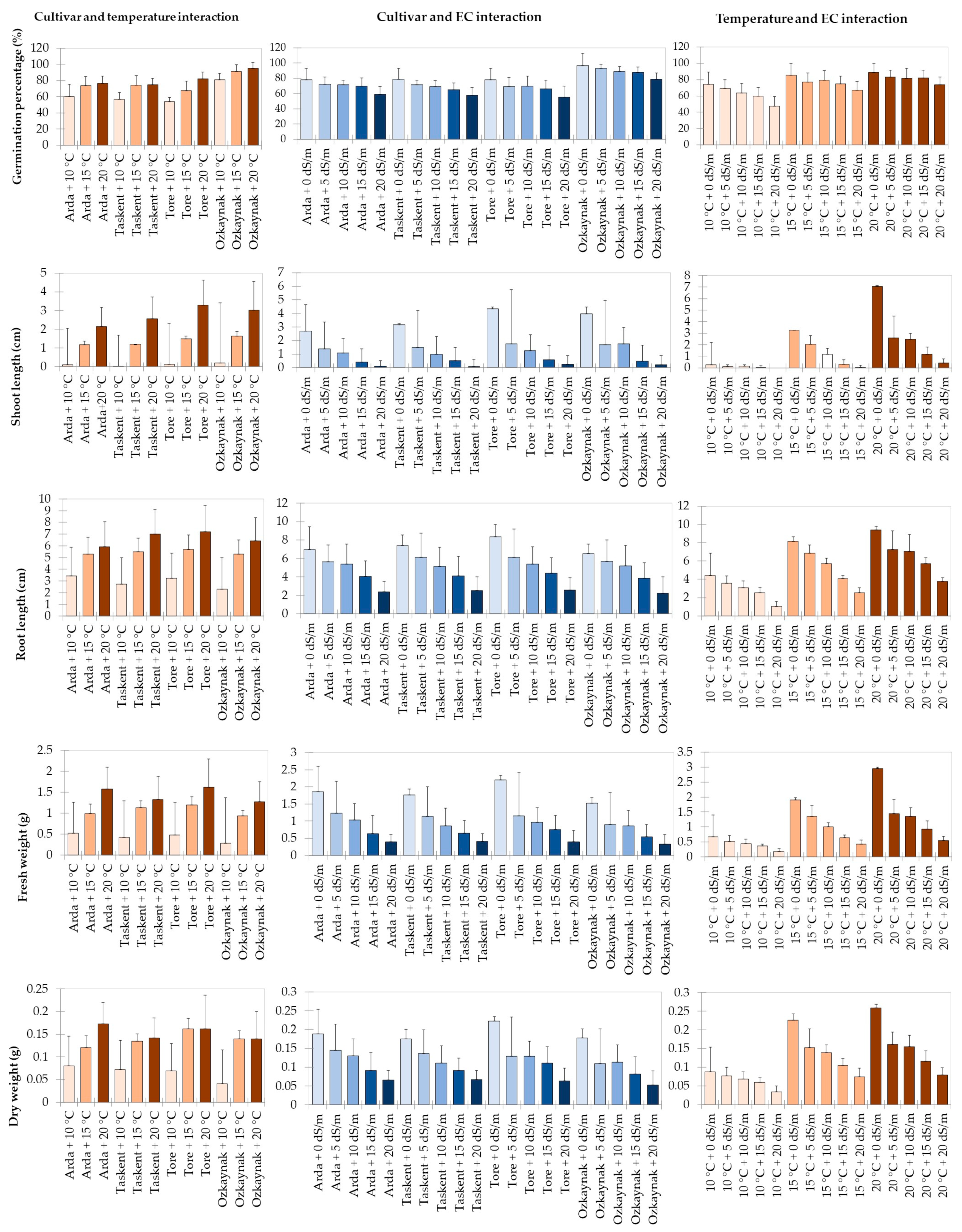
Figure 1 displays the results of the analysis for the interaction between cultivar and temperature, cultivar and EC, and temperature and EC in the investigation. The research revealed a statistically significant (p < 0.001) influence of the interaction between cultivar and temperature on the GP, SL, RL, FW, and DW variables. Among the cultivars that sprouted under the highest temperature (20 °C) conditions, it was found that the Ozkaynak cultivar had the highest percentage of germination. The Tore cultivar exhibited the greatest root length as well as the highest values for both root length and fresh weight. On the other hand, the Arda cultivar had the highest value in terms of dry weight.
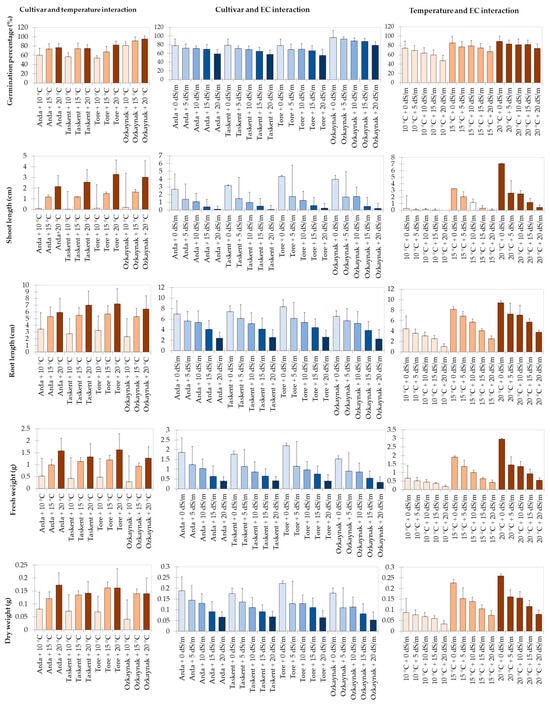
Figure 1.
Results of the analysis for effects of interaction.
The investigation revealed statistically significant effects of cultivar and EC interaction on shoot length (p < 0.001), root length (p < 0.05), fresh weight (p < 0.001), and dry weight (p < 0.001). The Tore cultivar had the greatest values for SL, RL, FW, and DW observed variables. These values were seen in the absence of salt stress.
The findings of the study indicated that the interaction between temperature and EC had a statistically significant impact (p < 0.001) on the GP, SL, RL, FW, and DW variables. The maximum values for all observed variables were obtained when temperature (20 °C) circumstances reached their peak and salinity conditions were at 0 EC.
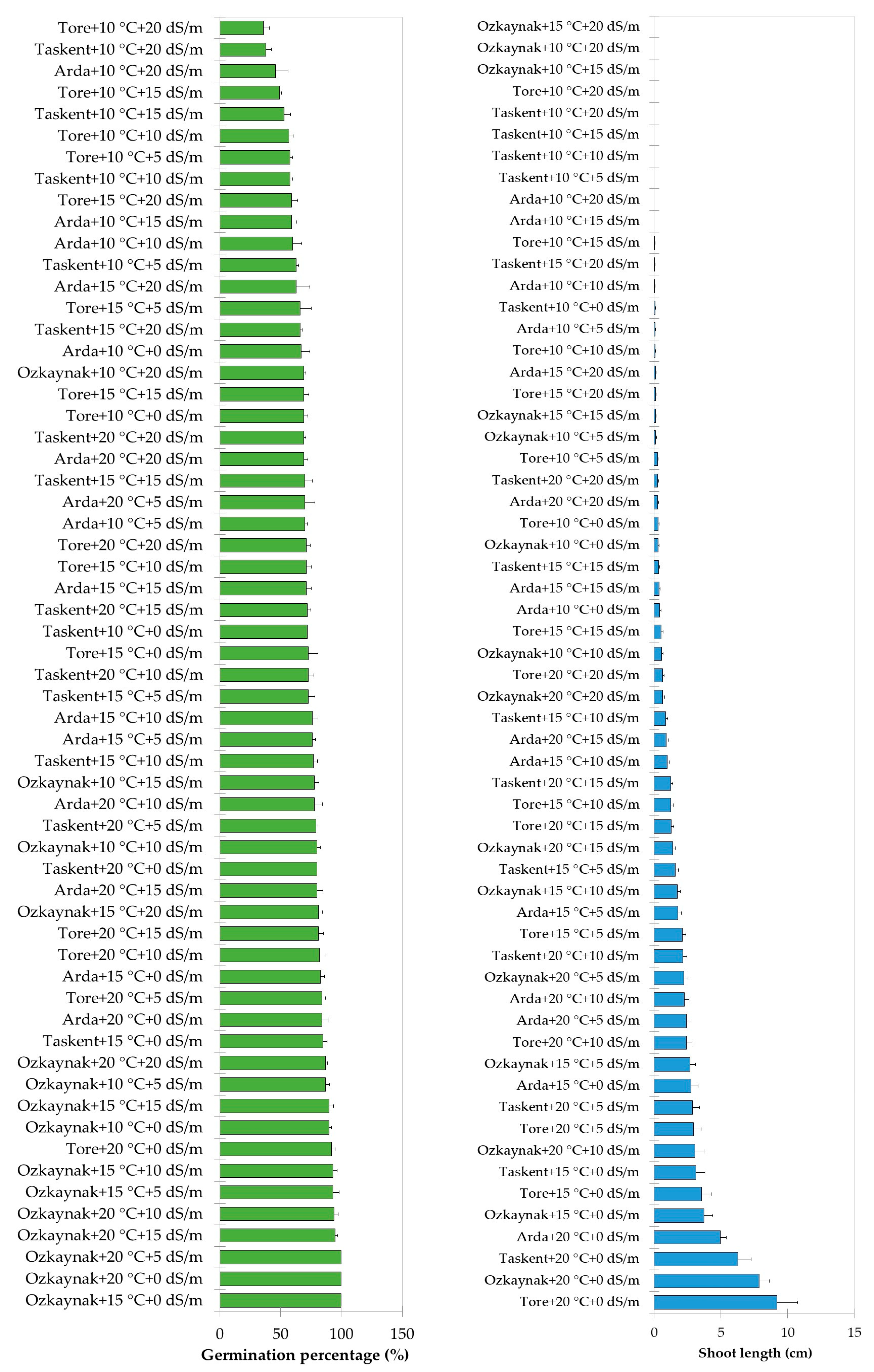
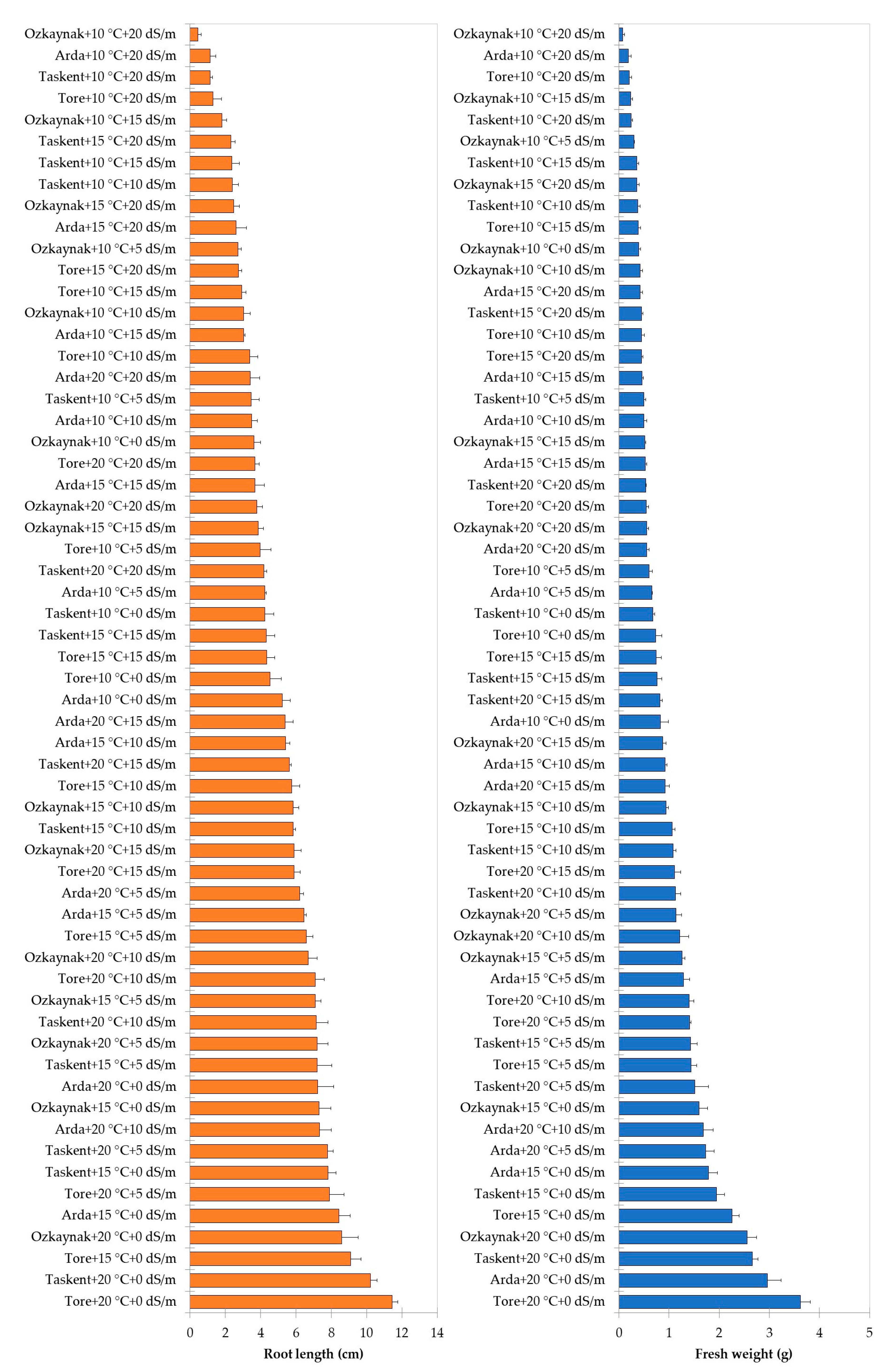
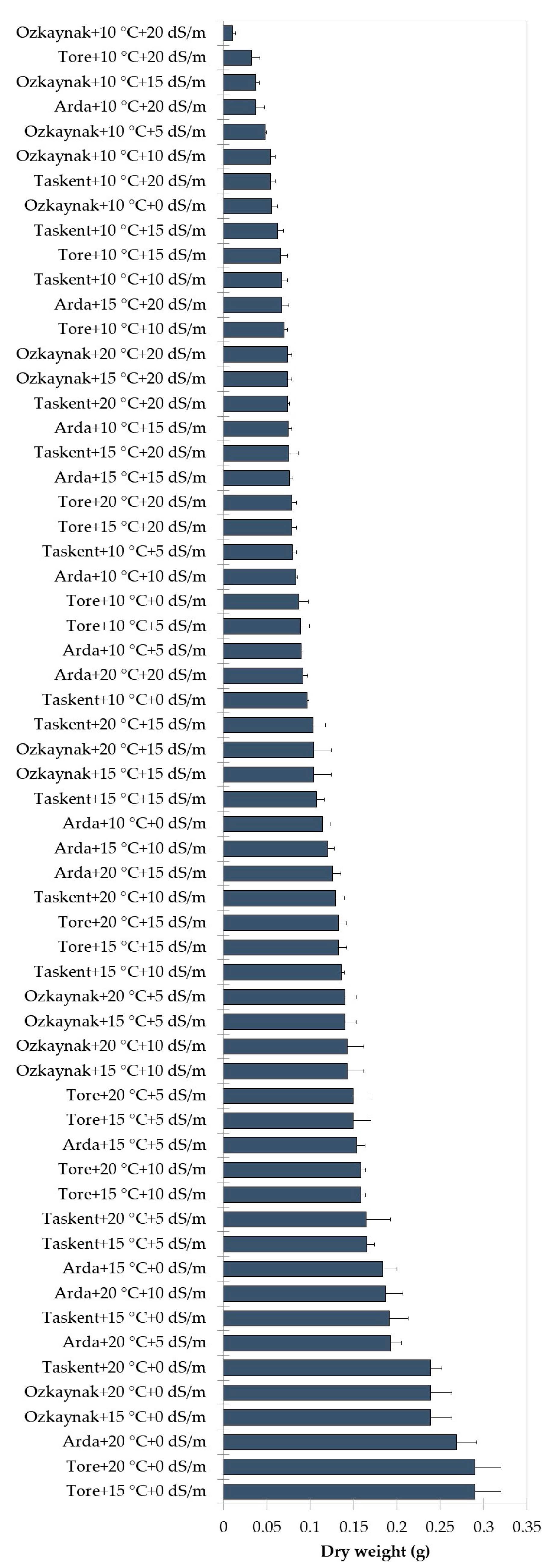
The findings of the analysis, examining the interaction among cultivar, temperature, and EC, are shown in Figure 2, Figure 3 and Figure 4. The investigation revealed statistically significant effects of cultivar, temperature, and EC interaction on shoot length (p < 0.001), root length (p < 0.01), fresh weight (p < 0.001), and dry weight (p < 0.001). The Tore cultivar had the greatest values for the observed shoot length, root length, fresh weight, and dry weight variables under conditions of maximum temperature (20 °C) and a saline level of 0 EC.
Figure 2.
Effects of interaction among the cultivar, temperature, and EC for germination percentage and shoot length.
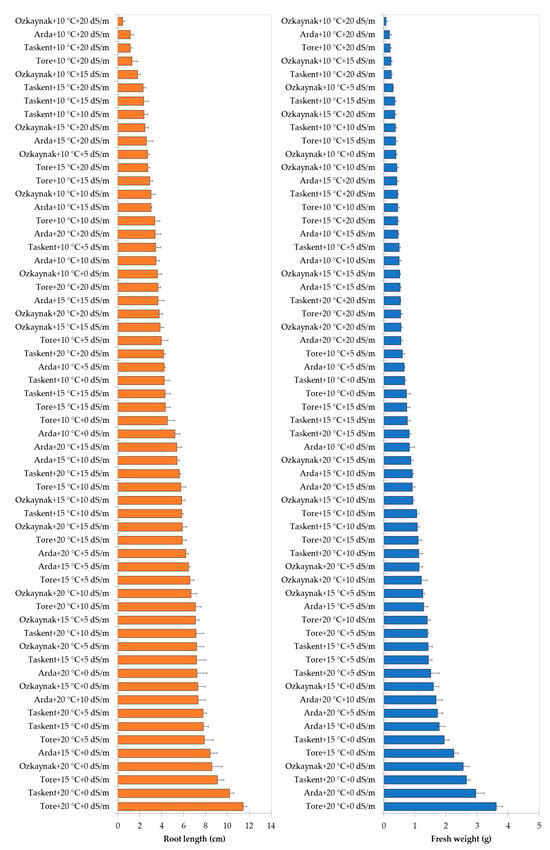
Figure 3.
Effects of interaction among the cultivar, temperature, and EC for root length and fresh weight.
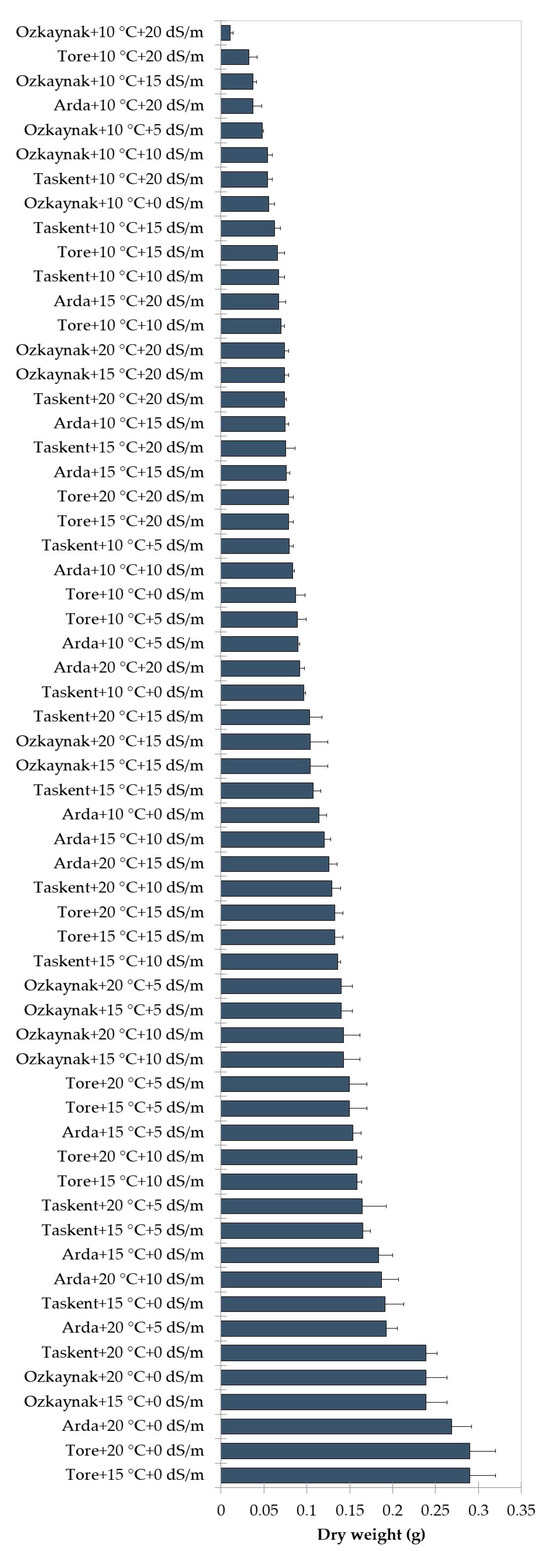
Figure 4.
Effects of interaction among the cultivar, temperature, and EC for dry weight.
ML Analysis
The effects of varying salinity and temperature conditions on forage pea were predicted by modeling the variables GP, SL, RL, FW, and DW using three distinct machine learning algorithms (MARS, Xgboost, and GPC). The modeling technique included four distinct fodder pea cultivars (Arda, Ozkaynak, Taskent, and Tore), three varying temperature (10 °C, 15 °C, and 20 °C) settings, and five varied salinity (0 EC, 5 EC, 10 EC, 15 EC, and 20 EC) levels as input factors. The observed variables (GP, SL, RL, FW, and DW) were used as output parameters. Table 2 provides a thorough summary of the study’s results and displays the results produced by the machine learning models used in the research. Metrics such as the root mean squared error (MSE), mean absolute percentage error (MAPE), and mean absolute deviation (MAD) are often used to assess the overall performance of an algorithm. A decrease in the values of these measures indicates that the model’s predictions are becoming more accurate and aligning with the observed values. Moreover, it evaluates the degree to which the R-squared (R2) model can explain the variation seen between the independent components and the dependent variable being studied.

Table 2.
Algorithm goodness-of-fit criterions for prediction of variables.
A grid search cross-validation (GCV) technique was used to assess the performance of the MARS, XGBoost, and GPC models throughout the evaluation process. The XGBoost model had the lowest MSE, MAPE, and MAD values, which indicates that it has greater prediction accuracy for training data of all observable parameters. For the variables GP, SL, RL, FW, and DW, the root mean square error (MSE) values were determined to be 3.990, 0.264, 0.657, 0.123, and 0.013, respectively. According to the findings, the mean absolute percentage error (MAPE) values for the same variables were found to be 4.360, 13.319, 12.643, 9.694, and 8.294, respectively. In addition, the values of the root mean absolute deviation (MAD) for the same variables were examined and found to be 2.948, 0.155, 0.401, 0.078, and 0.009, respectively. Furthermore, the model displayed the greatest R2 coefficient when generating predictions for the variables (GP, SL, RL, FW, and DW) using the training dataset (0.926, 0.980, 0.933, 0.973, and 0.960, respectively). When compared to other models, the XGBoost model was shown to have the highest level of performance for the training dataset (Table 2).
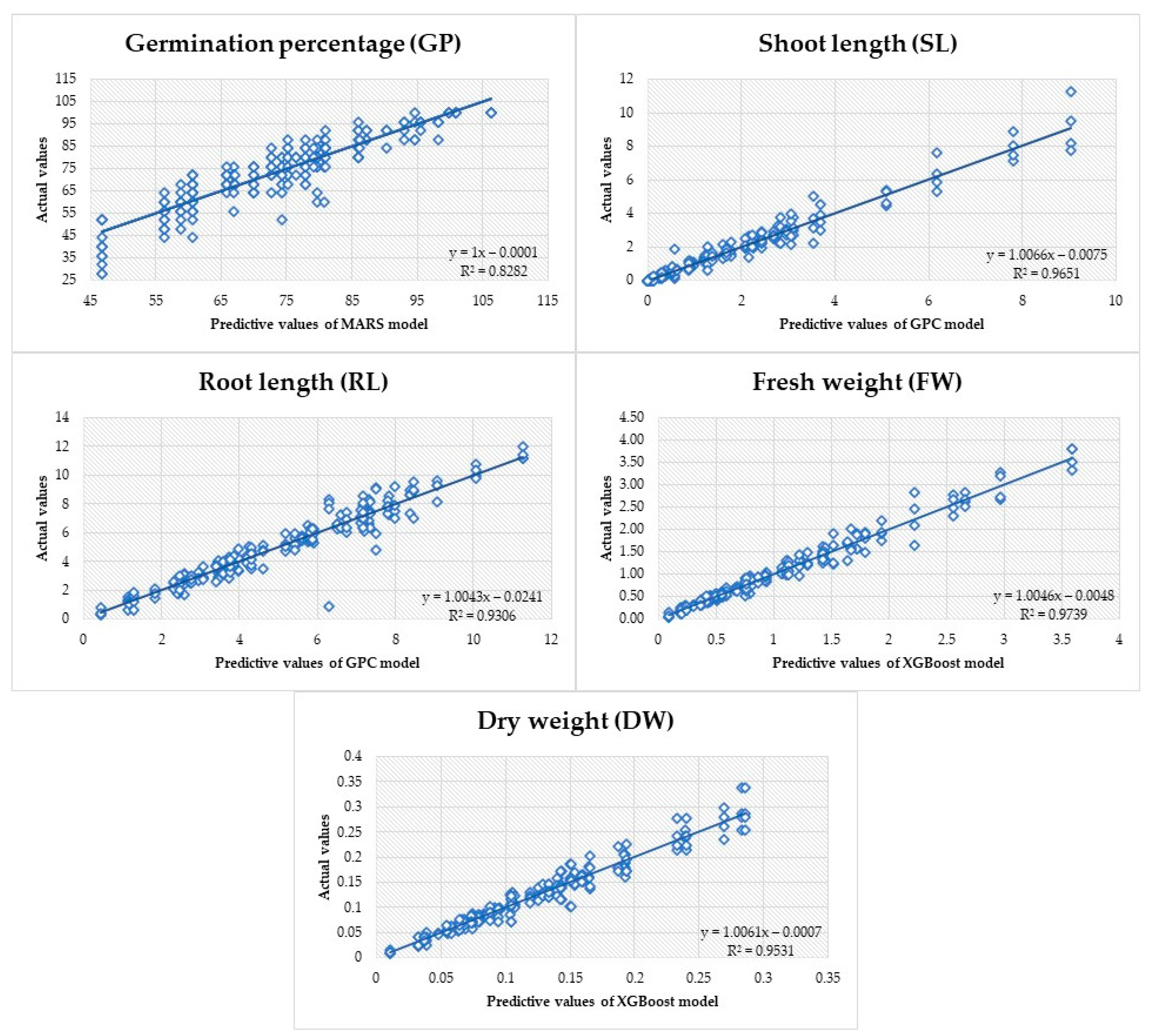
Each ML model underwent assessment by generating predictions using the test dataset (Table 2). This aided in predicting the potential performance of the model on unfamiliar data. The MARS model exhibited higher predictive ability for germination power (GP) based on the examination of the R2, MSE, MAPE, and MAD metrics. Moreover, when assessing the criterion metrics, it was determined that the GPC model had superior performance in predicting shoot length (SL) and root length (RL), while the Xgboost model showed stronger performance in estimating fresh weight (FW) and dry weight (DW). Figure 5 displays a linear regression graph illustrating the models’ predicted values, which offer the most precise prediction for the variables being studied, along with the observed actual values.
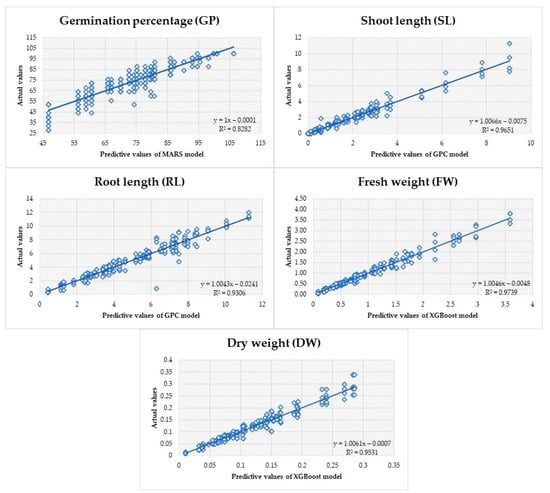
Figure 5.
Observed values for variables and anticipated values using machine learning models.
The MARS analysis identified the most effective models, which consisted of 11 terms for GP, 10 terms for SL, 8 terms for RL, 11 terms for FW, and 11 terms for DW (Table 3). These models included intercept terms without any interaction effects. Upon analyzing the ninth term with the lowest coefficient and the tenth term with the highest coefficient for GP, it was anticipated that the 20 EC treatment would result in the greatest loss, while the Ozkan cultivar would result in the greatest rise. When examining the tenth term with the lowest coefficient and the third term with the highest coefficient for shoot length, it was predicted that the 20 °C × 20 EC interaction would lead to the most significant decrease, while the 20 °C treatment alone would result in the most significant increase. When examining the eighth term with the smallest coefficient and the fourth term with the largest coefficient for root length, it was predicted that the 20 EC treatment would lead to the most significant decrease, while the 20 °C treatment would result in the most significant increase. By examining the eleventh term with the lowest coefficient and the third term with the highest coefficient for fresh weight, it was predicted that the 20 °C × 20 EC interaction would lead to the most significant decrease, while the 20 °C treatment alone would result in the most significant increase. When examining the eighth term with the smallest coefficient and the fourth term with the largest coefficient for fresh weight, it was predicted that the 20 °C × 5 EC interaction would lead to the most significant decrease, while the 20 °C treatment alone would lead to the most significant increase.

Table 3.
Outcome of the MARS algorithm for the observed variables.
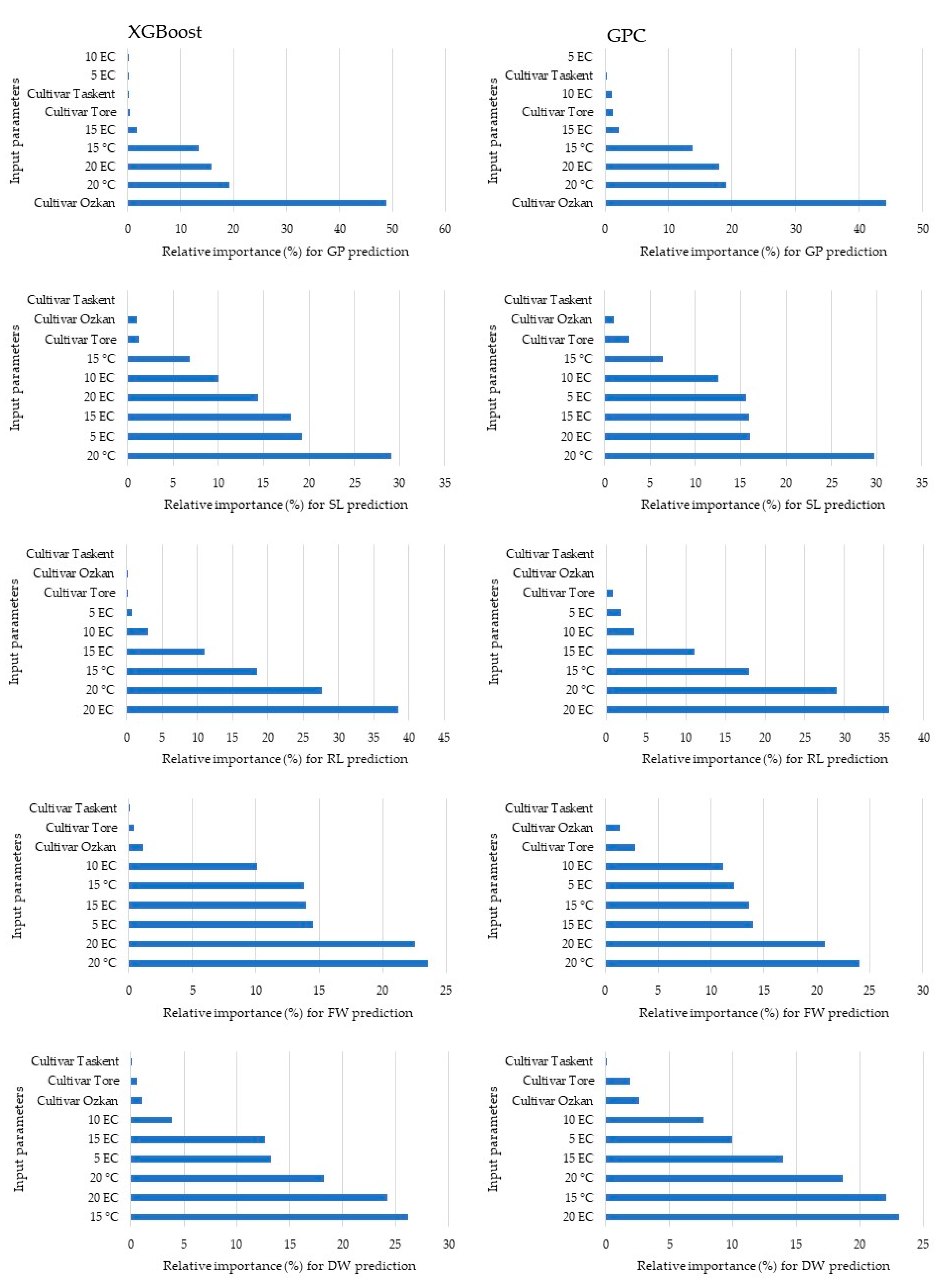
Figure 6 presents the variable importance rankings for predicting the output variable using input variables in the XGBoost and GPC models. The analysis revealed that the most effective factors determining the germination percentage, shoot length, root length, fresh weight, and dry weight in the XGBoost model were cultivar Ozkan at 20 °C, 20 EC, 20 °C, and 15 °C, respectively. Within the GPC model, the variable that had the greatest impact on dry weight was 20 °C, although the outcomes for other factors had similarities with those of the XGBoost model.
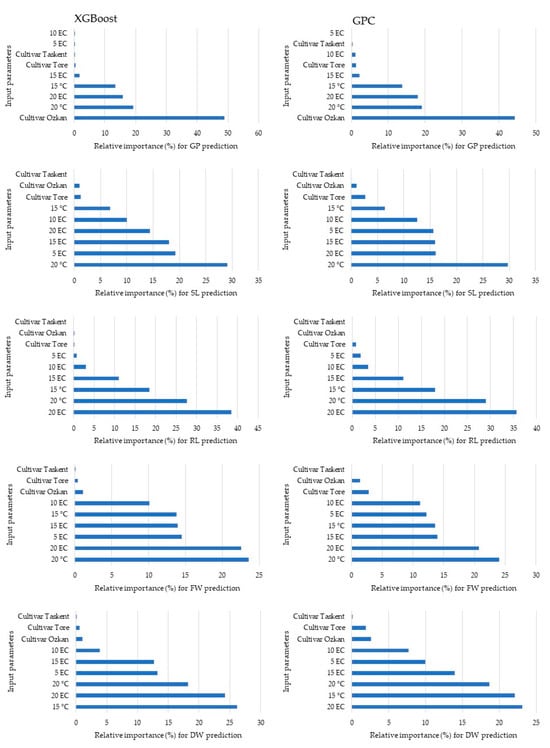
Figure 6.
The significance of the variables in making predictions.
Using the predictive capabilities of machine learning algorithms, expected germination parameters between 0 and 20 °C were predicted to optimize germination parameters under changing salt stress and temperature treatment conditions and to enable more effective agricultural practices (Supplementary Table S5). The aim was to predict the expected values of the temperature process on unobserved data based on the findings. According to the results, it was predicted that the application of machine learning algorithms might result in the failure of fodder pea plants to germinate at temperatures of 9 °C or lower for the SL parameter, 1 °C or lower for the RL parameter, 5 °C or lower for the FW parameter, and 1 °C or lower for the DW parameter.
4. Discussion
The current research demonstrates that salt has a negative impact on the germination and development of fodder pea cultivar seeds, as shown in Table 1. The germination and seedling phases are crucial for the survival and successful development of plants, especially under challenging conditions. The results of this investigation revealed that the germination and establishment of fodder pea seeds were progressively hindered as the salt stress increased. Several studies have found that higher salinity levels have a negative impact on germination percentage and speed in field pea [33], chickpea [34], wheat [35], and other legumes [36,37,38]. There are a few reasons that contribute to salinity’s ability to delay and impede seed germination. These variables include a reduction in water intake, alterations in the mobilization of stored food, and disruption of the structural organization of proteins [39]. The findings of this study were in line with studies that had been discussed and published in the past. It is possible that the osmotic impact of salt stress, which results in a reduction in growth promoters, is responsible for the shortening of growth characteristics [8]. There is also the possibility that the growth suppression is caused by a lack of water, ion toxicity, and nutritional imbalance because of the obstruction of other nutrients, including nitrogen, phosphorus, potassium, calcium, and nitrogen oxides [40].
As demonstrated in Table 1, the fodder pea cultivars that were treated to germination at temperatures of 10, 15, and 20 °C had the greatest germination values at the 20 °C temperature. Moreover, it was discovered that the Ozkaynak cultivar had the greatest percentage of germination among the cultivars that sprouted under the circumstances of the maximum temperature (20 °C). High ambient temperatures pose a substantial threat to agricultural productivity worldwide throughout plant growth and germination phases [38]. Additionally, plants exposed to low temperatures within the temperature range of 0 to 15 °C face challenges in productivity due to osmotic and oxidative stress, which necessitates the activation of various stress-related signaling pathways to restore cellular balance [41,42]. For plants that are susceptible to heat limitations, germination may provide early research for determining the threshold tolerance of the plant. Çaçan et al. [43] investigated the germination abilities of several fodder pea lines and kinds under varied temperature conditions (10, 23, 27, and 30 °C). According to their study, the rate of germination typically fell at a temperature of 30 °C, but the time it took for germination to occur was reduced at a temperature of 10 °C. Sivritepe [44] declared that the optimal temperature for conducting germination experiments on Pisum sativum seedlings is 20 °C. According to Brar et al. [45], the maximum germination rate of Pisum arvense seeds was seen at a temperature of 20 °C. The results of our investigation were consistent with those of earlier research.
The results of our research shed light on the significance of using machine learning (ML) strategies, in particular ML algorithms like XGBoost, MARS, and GPC, to enhance the comprehension and optimization of germination in forage pea when it is subjected to salt stress and temperature treatment. The outcomes of our study indicated that these computational methods are useful in predicting and improving important factors associated with germination. In the process of analyzing the prediction performances and R2 of the models, it was found that the model performances were comparable to those of earlier research that has been published in the literature. Benlioğlu et al. [23] effectively used machine learning methods to clarify the germination traits of tetraploid wheat in the presence of drought-induced stress. In their investigation, they reported that the elastic net (ELNET) model showed more efficiency in predicting germination speed (R2 = 0.762, RMSE = 4.873, MAD = 3.755), germination power (R2 = 0.514, RMSE = 4.946, MAD = 3.581), and water absorption capacity (R2 = 0.902, RMSE = 5.140, MAD = 2.492), the Gaussian processes classifier (GPC) model showed superior performance in predicting root length (R2 = 0.981, RMSE = 0.552, MAD = 0.407), and the XGBoost model showed better prediction performance for shoot length (R2 = 0.962, RMSE = 0.761, MAD = 0.457), fresh weight (R2 = 0.962, RMSE = 0.077, MAD = 0.056), and dry weight (R2 = 0.944, RMSE = 0.018, MAD = 0.014). In their investigation, Aasim et al. [46] examined the potential impact of different concentrations of hydrogen peroxide (H2O2) on the germination and morphological attributes of in vitro-grown cannabis seedlings. The researchers employed four distinct machine learning algorithms to analyze the data: random forest, extreme gradient boosting, support vector classifier, and Gaussian process. The results of the research demonstrated that the RF model demonstrated a higher level of accuracy in forecasting the output variable. Machine learning models, in contrast to more conventional approaches, provide a strategy that is both more effective and more precise for predicting the outcomes of complex and nonlinear biological processes in the fields of agriculture and applied sciences [25,47,48]. All the germination parameters that were observed in our research showed that the XGboost model had the greatest performance in terms of prediction. Previous research has also indicated that the XGBoost model, which is one of the machine learning algorithms, has higher prediction performance when it comes to optimizing tissue culture parameters and forecasting the outcomes of regeneration under tissue culture circumstances [25,30,49]. Chen and Guestrin [28] developed the XGBoost algorithm, a powerful tool for addressing regression and classification problems. It is a member of the gradient boosting decision tree category and is well-known for its exceptional performance and speed [28]. Within a gradient boosting architecture, XGBoost excels at leveraging errors to iteratively reduce the error rate over several iterations.
The efficiency of an algorithm might fluctuate based on the attributes of the dataset and the complexity of the issue being addressed [25]. Within this particular context, some algorithms may be better suited for certain sorts of data and issues, while others may be more efficient for distinct data formats and difficulties. For instance, while certain methods may perform well in datasets with many dimensions, algorithms that have a tendency to overfit may not be the best choice for tiny datasets. In order to obtain the best possible performance, it is often essential to experiment with various methods and carefully scrutinize model assessment criteria [27,30,48,49].
Based on the findings and unobserved data, the expected estimated values of the temperature process were determined. According to the results, it was predicted that ML algorithms at 9 degrees or less according to the SL parameter, 1 degree or less according to the RL parameter, 5 degrees or less according to the FW parameter, and 1 degree or less according to the DW parameter may result in the failure of fodder pea plants to germinate. When the temperature drops below the ideal range for germination, fodder pea plants either do not germinate at all or have a very poor germination rate [3,45].
5. Conclusions
Overall, the study’s results indicate that when temperature (from 10 °C to 20 °C) conditions rise, the cultivars become less sensitive to salt stress in terms of germination characteristics such as shoot length, root length, fresh weight, and dry weight. When compared to the cultivars Arda, Ozkaynak, and Taskent, the Tore demonstrated a higher tolerance to salt stress. In addition, the results of this research demonstrate that making it possible for forage pea cultivars to overcome the constraints that are imposed by salt stress and temperature treatment is essential, as is the ability to identify the abiotic stress conditions that are associated with the plant species and variety. In addition to these achievements, the incorporation of machine learning (ML) methods into the process of optimization and prediction, as well as the prediction of germination for cultivars such as fodder peas based on environmental circumstances, may positively boost efficiency in terms of both time allocation and workload. From this point, XGBoost, MARS, and GPC are examples of machine learning models that have successfully predicted crucial parameters in procedures that include complicated biological processes. As more research is conducted to investigate the interaction between machine learning and seed germination, there is a possibility that unique insights and strategies could be discovered. These discoveries might be used to enhance the sustainability and efficiency of plant propagation methods.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/horticulturae10060656/s1, Table S1: The mean values for effects of interaction between cultivar and temperature; Table S2: The mean values for effects of interaction between cultivar and EC; Table S3: The mean values for effects of interaction between temperature and EC; Table S4: The mean values for effects of interaction among cultivar, temperature, and EC; Table S5: The temperature values that the model is intended to predict and the expected prediction values; Table S6: Abbreviations and explanations.
Author Contributions
Conceptualization, S.U. and O.O.; methodology, F.D. and B.E.; software, F.D., B.E. and M.Y.; validation, A.S. and S.U.; formal analysis, M.Y.; investigation, F.D.; resources, S.U.; data curation, F.D., B.E. and M.Y.; writing—original draft preparation, F.D., B.E. and M.Y.; writing—review and editing, O.O., F.D., B.E., M.Y. and A.A.; visualization, M.Y.; supervision, A.S.; project administration, S.U.; funding acquisition, M.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
All data supporting the conclusions of this article are included in this article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Kopecká, R.; Kameniarová, M.; Černý, M.; Brzobohatý, B.; Novák, J. Abiotic stress in crop production. Int. J. Mol. Sci. 2023, 24, 6603. [Google Scholar] [CrossRef]
- Prasad, P.V.V.; Staggenborg, S.A.; Ristic, Z. Impacts of drought and/or heat stress on physiological, developmental, growth, and yield processes of crop plants. In Response of Crops to Limited Water; Ahuja, L.R., Reddy, V.R., Saseendran, S.A., Yu, Q., Eds.; ASA-CSSA-SSSA: Madison, WI, USA, 2008; Volume 1, pp. 301–355. [Google Scholar]
- Küçüközcü, G.; Avcı, S. Tolerance of forage pea cultivars to salinity and drought stress during germination and seedling growth. Int. J. Agric. Environ. Food Sci. 2020, 4, 368–375. [Google Scholar] [CrossRef]
- Lamichaney, A.; Parihar, A.K.; Hazra, K.K.; Dixit, G.P.; Katiyar, P.K.; Singh, D.; Singh, N.P. Untangling the influence of heat stress on crop phenology, seed set, seed weight, and germination in field pea (Pisum sativum L.). Front. Plant Sci. 2021, 12, 635868. [Google Scholar] [CrossRef]
- Bayuelo-Jiménez, J.S.; Craig, R.; Lynch, J.P. Salinity tolerance of Phaseolus species during germination and early seedling growth. Crop Sci. 2002, 42, 1584–1594. [Google Scholar] [CrossRef]
- Wahid, A.; Gelani, S.; Ashraf, M.; Foolad, M.R. Heat tolerance in plants: An overview. Environ. Exp. Bot. 2007, 61, 199–223. [Google Scholar] [CrossRef]
- Munns, R.; Passioura, J.B.; Colmer, T.D.; Byrt, C.S. Osmotic adjustment and energy limitations to plant growth in saline soil. New Phytol. 2020, 225, 1091–1096. [Google Scholar] [CrossRef]
- Desoky, E.M.; Merwad, A.M.; Elrys, A.S. Response of pea plants to natural bio-stimulants under soil salinity stress. Am. J. Plant Physiol. 2017, 12, 28–37. [Google Scholar]
- El Sabagh, A.; Islam, M.S.; Skalicky, M.; Ali, R.M.; Singh, K.; Anwar, H.M.; Hossain, A.; Mahboob, W.; Aamir, I.M.; Ratnasekera, D.; et al. Salinity stress in wheat (Triticum aestivum L.) in the changing climate: Adaptation and management strategies. Front. Agron. 2021, 3, 661932. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Raihan, M.R.H.; Masud, A.A.C.; Rahman, K.; Nowroz, F.; Rahman, M.; Nahar, K.; Fujita, M. Regulation of reactive oxygen species and antioxidant defense in plants under salinity. Int. J. Mol. Sci. 2021, 22, 9326. [Google Scholar] [CrossRef]
- Kataria, S.; Baghel, L.; Guruprasad, K.N. Pre-treatment of Seeds with Static Magnetic Field Improves Germination and Early Growth Characteristics under Salt Stress in Maize and Soybean. Biocatal. Agric. Biotechnol. 2017, 10, 83–90. [Google Scholar] [CrossRef]
- Cirka, M.; Kaya, A.R.; Eryigit, T. Influence of Temperature and Salinity Stress on Seed Germination and Seedling Growth of Soybean (Glycine max L.). Legume Res. Int. J. 2021, 44, 1053–1059. [Google Scholar]
- George, T.S.; Taylor, M.A.; Dodd, I.C.; White, P.J. Climate Change and Consequences for Potato Production: A Review of Tolerance to Emerging Abiotic Stress. Potato Res. 2017, 60, 239–268. [Google Scholar] [CrossRef]
- Khan, Z.; Shahwar, D. Role of Heat Shock Proteins (HSPs) and Heat Stress Tolerance in Crop Plants. In Sustainable Agriculture in the Era of Climate Change; Springer: Cham, Switzerland, 2020; pp. 211–234. [Google Scholar]
- Jahan, M.S.; Shu, S.; Wang, Y.; Chen, Z.; He, M.; Tao, M.; Guo, S. Melatonin Alleviates Heat-Induced Damage of Tomato Seedlings by Balancing Redox Homeostasis and Modulating Polyamine and Nitric Oxide Biosynthesis. BMC Plant Biol. 2019, 19, 414. [Google Scholar] [CrossRef]
- Maryum, Z.; Luqman, T.; Nadeem, S.; Khan, S.M.U.D.; Wang, B.; Ditta, A.; Khan, M.K.R. An Overview of Salinity Stress, Mechanism of Salinity Tolerance and Strategies for Its Management in Cotton. Front. Plant Sci. 2022, 13, 907937. [Google Scholar] [CrossRef]
- Altieri, M.A.; Nicholls, C.I.; Henao, A.; Lana, M.A. Agroecology and the design of climate change-resilient farming systems. Agron. Sustain. Dev. 2015, 35, 869–890. [Google Scholar] [CrossRef]
- Tan, M.; Yolcu, H. Current status of forage crops cultivation and strategies for the future in Turkey: A review. J. Agric. Sci. 2021, 27, 114–121. [Google Scholar] [CrossRef]
- Kara, E.; Sürmen, M. Yield and quality characteristics of forage pea varieties at different phenological stages. Adnan Menderes Univ. J. Fac. Agric. 2023, 20, 295–301. [Google Scholar] [CrossRef]
- Avci, S. Potential impact of annual forage legumes on sustainable cropping systems in Turkey. In Sustainable Agriculture Reviews 51. Legume Agriculture and Biotechnology; Lichtfouse, E., Ed.; Springer: Cham, Switzerland, 2021; Volume 2, pp. 97–118. [Google Scholar]
- Sarıkaya, M.F.; İleri, O.; Erkovan, Ş.; Erkovan, H.; Koç, A. Growing forage pea (Pisum arvense L.) for hay: Different sowing dates and plant densities in Central Anatolia. J. Atatürk Univ. Fac. Agric. 2023, 54, 75–80. [Google Scholar] [CrossRef]
- Yasam, S.; Nair, S.A.H.; Kumar, K.S. Machine learning based robust model for seed germination detection and classification. Int. J. Intell. Syst. Appl. Eng. 2023, 11, 116–124. [Google Scholar]
- Benlioğlu, B.; Demirel, F.; Türkoğlu, A.; Haliloğlu, K.; Özaktan, H.; Kujawa, S.; Niedbała, G. Insights into drought tolerance of tetraploid wheat genotypes in the germination stage using machine learning algorithms. Agriculture 2024, 14, 206. [Google Scholar] [CrossRef]
- Cetin, N.; Ozaktan, H.; Uzun, S.; Uzun, O.; Ciftci, C.Y. Machine learning based mass prediction and discrimination of chickpea (Cicer arietinum L.) cultivars. Euphytica 2023, 219, 20. [Google Scholar] [CrossRef]
- Türkoğlu, A.; Bolouri, P.; Haliloğlu, K.; Eren, B.; Demirel, F.; Işık, M.I.; Niedbała, G. Modeling callus induction and regeneration in hypocotyl explant of fodder pea (Pisum sativum var. arvense L.) using machine learning algorithm method. Agronomy 2023, 13, 2835. [Google Scholar] [CrossRef]
- Türkoğlu, A.; Haliloğlu, K.; Demirel, F.; Aydin, M.; Çiçek, S.; Yiğider, E.; Niedbała, G. Machine Learning Analysis of the Impact of Silver Nitrate and Silver Nanoparticles on Wheat (Triticum aestivum L.): Callus Induction, Plant Regeneration, and DNA Methylation. Plants 2023, 12, 4151. [Google Scholar] [CrossRef]
- Demirel, F.; Eren, B.; Yilmaz, A.; Türkoğlu, A.; Haliloğlu, K.; Niedbała, G.; Nowosad, K. Prediction of grain yield in wheat by CHAID and MARS algorithms analyses. Agronomy 2023, 13, 1438. [Google Scholar] [CrossRef]
- Chen, T.; Guestrin, C. Xgboost: A scalable tree boosting system. In Proceedings of the 22nd ACM SIGKDD International Conference on Knowledge Discovery and Data Mining, San Francisco, CA, USA, 13–17 August 2016; pp. 785–794. [Google Scholar]
- Rasmussen, C.E. Gaussian processes in machine learning. In Summer School on Machine Learning; MIT Press: Cambridge, MA, USA, 2003; pp. 63–71. [Google Scholar]
- Demirel, F.; Uğur, R.; Popescu, G.C.; Demirel, S.; Popescu, M. Usage of Machine learning algorithms for establishing an effective protocol for the in vitro micropropagation ability of black chokeberry (Aronia melanocarpa (Michx.) Elliott). Horticulturae 2023, 9, 1112. [Google Scholar] [CrossRef]
- Camacho-Pérez, E.; Lugo-Quintal, J.M.; Tirink, C.; Aguilar-Quiñonez, J.A.; Gastelum-Delgado, M.A.; Lee-Rangel, H.A.; Chay-Canul, A.J. Predicting carcass tissue composition in Blackbelly sheep using ultrasound measurements and machine learning methods. Trop. Anim. Health Prod. 2023, 55, 300. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria. Available online: https://www.R-project.org/ (accessed on 1 December 2023).
- Wolde, G.; Adamu, C. Impact of salinity on seed germination and biomass yields of field pea (Pisum sativum L.). Asian J. Sci. Technol. 2018, 9, 7565–7569. [Google Scholar]
- Özaktan, H.; Çiftçi, C.Y.; Kaya, M.D.; Uzun, S.; Akdogan, G. Chloride salts inhibit emergence and seedling growth of chickpea rather than germination. Legum. Res. Int. J. 2017, 40, 60–66. [Google Scholar] [CrossRef]
- Majid, A.; Mohsen, S.; Mandana, A.; Saeid, J.H.; Ezatollah, E.; Fariborz, S. The effects of different levels of salinity and indole-3-acetic acid (IAA) on early growth and germination of wheat seedling. J. Stress Physiol. Biochem. 2013, 9, 206–212. [Google Scholar]
- Esechie, H.A. Partitioning of chloride ion in the germinating seed of two forage legumes under varied salinity and temperature regimes. Commun. Soil Sci. Plant Anal. 1995, 26, 3357–3370. [Google Scholar] [CrossRef]
- Morais, M.C.; Panuccio, M.R.; Muscolo, A.; Freitas, H. Does salt stress increase the ability of the exotic legume Acacia longifolia to compete with native legumes in sand dune ecosystems? Environ. Exp. Bot. 2012, 82, 74–79. [Google Scholar] [CrossRef]
- Piwowarczyk, B.; Tokarz, K.; Kamińska, I. Responses of grass pea seedlings to salinity stress in in vitro culture conditions. Plant Cell Tissue Organ Cult. 2016, 124, 227–240. [Google Scholar] [CrossRef]
- Ibrahim, E.A. Seed priming to alleviate salinity stress in germinating seeds. J. Plant Physiol. 2016, 192, 38–46. [Google Scholar] [CrossRef]
- Hasegawa, P.M.; Bressan, R.A.; Zhu, J.K.; Bohnert, H.J. Plant cellular and molecular responses to high salinity. Annu. Rev. Plant Biol. 2020, 51, 463–499. [Google Scholar] [CrossRef]
- Aslam, M.; Fakher, B.; Ashraf, M.A.; Cheng, Y.; Wang, B.; Qin, Y. Plant low-temperature stress: Signaling and response. Agronomy 2022, 12, 702. [Google Scholar] [CrossRef]
- Jankovska-Bortkevič, E.; Katerova, Z.; Todorova, D.; Jankauskienė, J.; Mockevičiūtė, R.; Sergiev, I.; Jurkonienė, S. Effects of auxin-type plant growth regulators and cold stress on the endogenous polyamines in pea plants. Horticulturae 2023, 9, 244. [Google Scholar] [CrossRef]
- Çaçan, E.; Özbay, N.; Kökten, K. Determination of germination and emergence performances of some forage pea lines and varieties at different temperatures. Nevsehir J. Sci. Technol. 2016, 5, 62–68. [Google Scholar]
- Sivritepe, H.Ö. Assessment of Seed Viability. Alatarım J. 2011, 10, 94–105. [Google Scholar]
- Brar, G.S.; Gomez, J.F.; McMichael, B.L.; Matches, A.G.; Taylor, H.M. Germination of twenty forage legumes as influenced by temperature. Agron. J. 1991, 83, 173–175. [Google Scholar] [CrossRef]
- Aasim, M.; Katırcı, R.; Akgur, O.; Yildirim, B.; Mustafa, Z.; Nadeem, M.A.; Baloch, F.S.; Karakoy, T.; Yılmaz, G. Machine learning (ML) algorithms and artificial neural network for optimizing in vitro germination and growth indices of industrial hemp (Cannabis sativa L.). Ind. Crops Prod. 2022, 181, 114801. [Google Scholar] [CrossRef]
- Gökmen, F.; Uygur, V.; Sukuşu, E. Extreme gradient boosting regression model for soil available boron. Eurasian Soil Sci. 2023, 56, 738–746. [Google Scholar] [CrossRef]
- Tırınk, C.; Piwczyński, D.; Kolenda, M.; Önder, H. Estimation of body weight based on biometric measurements by using random forest regression, support vector regression and cart algorithms. Animals 2023, 13, 798. [Google Scholar] [CrossRef] [PubMed]
- Şimşek, Ö.; Dalda Şekerci, A.; Isak, M.A.; Bulut, F.; İzgü, T.; Tütüncü, M.; Dönmez, D. Optimizing micropropagation and rooting protocols for diverse lavender genotypes: A Synergistic Approach Integrating Machine Learning Techniques. Horticulturae 2024, 10, 52. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).