Development and Evaluation of a Trichoderma-Based Bioformulation for Enhancing Sustainable Potato Cultivation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Trichoderma spp. Evaluation and Bioformulation
2.1.1. Morphological Characterisation of Trichoderma Isolates
2.1.2. Molecular Analysis of Trichoderma Isolates
2.1.3. Dual-Plate Bioassay
2.1.4. Trichoderma–Fungicide Interaction
2.1.5. Bioformulation of Trichoderma Isolates
2.2. Experimental Field
2.3. Plant Response Evaluation
2.4. Statistical Analysis
3. Results
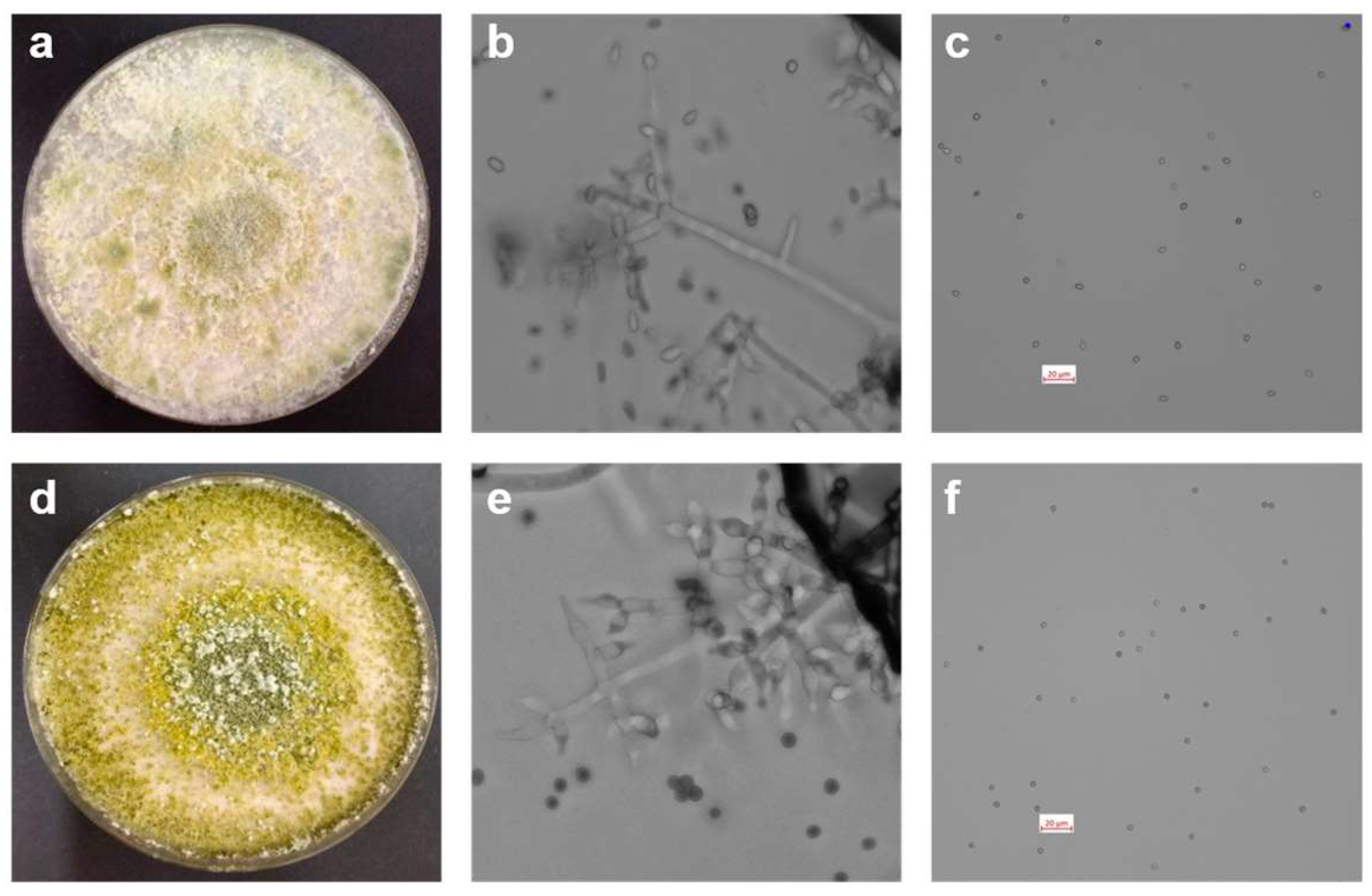
3.1. Morphological and Molecular Characterisation of Trichoderma Isolates
3.2. Dual-Plate Bioassay
3.3. Trichoderma–Fungicide Interaction
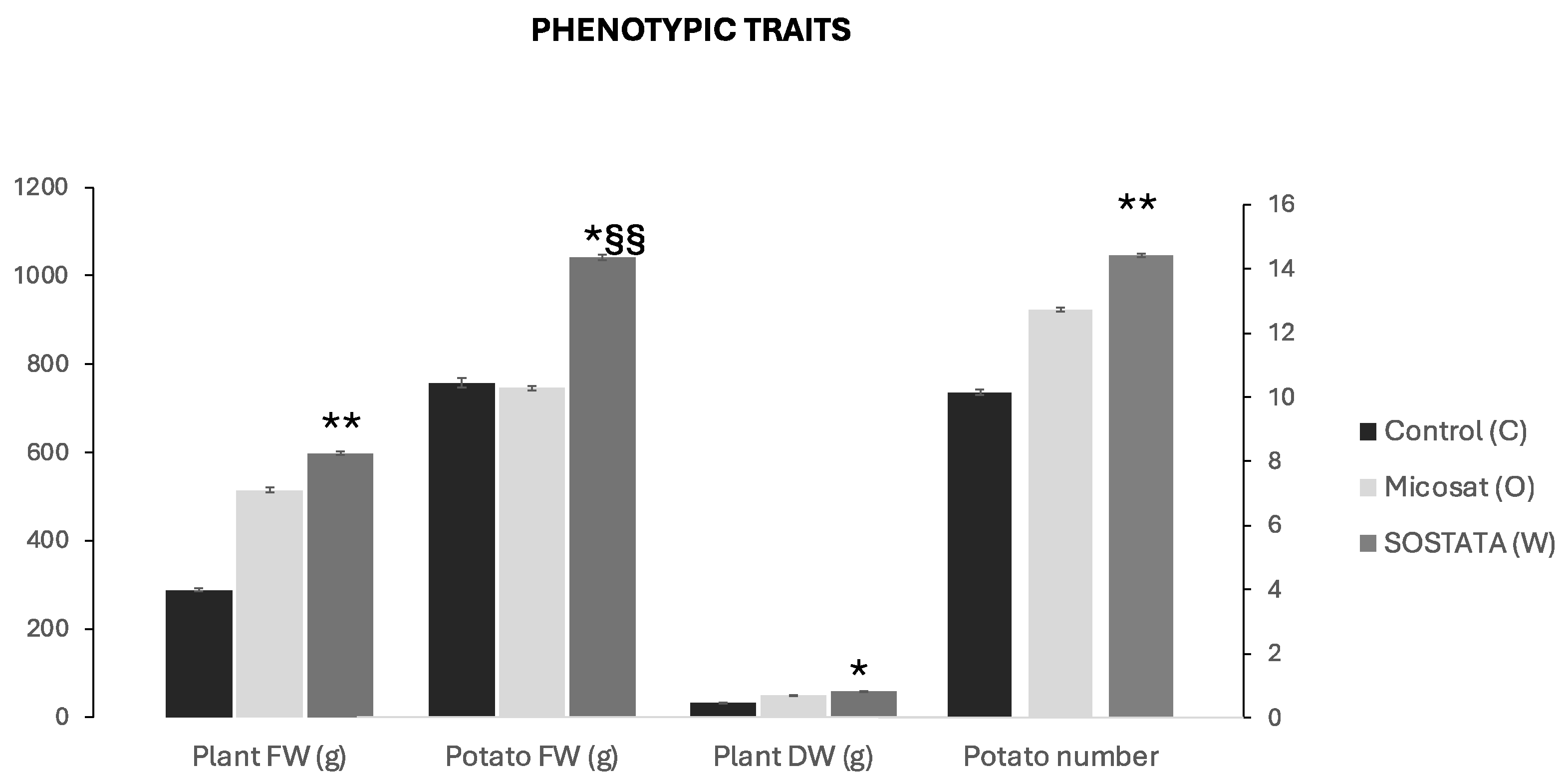
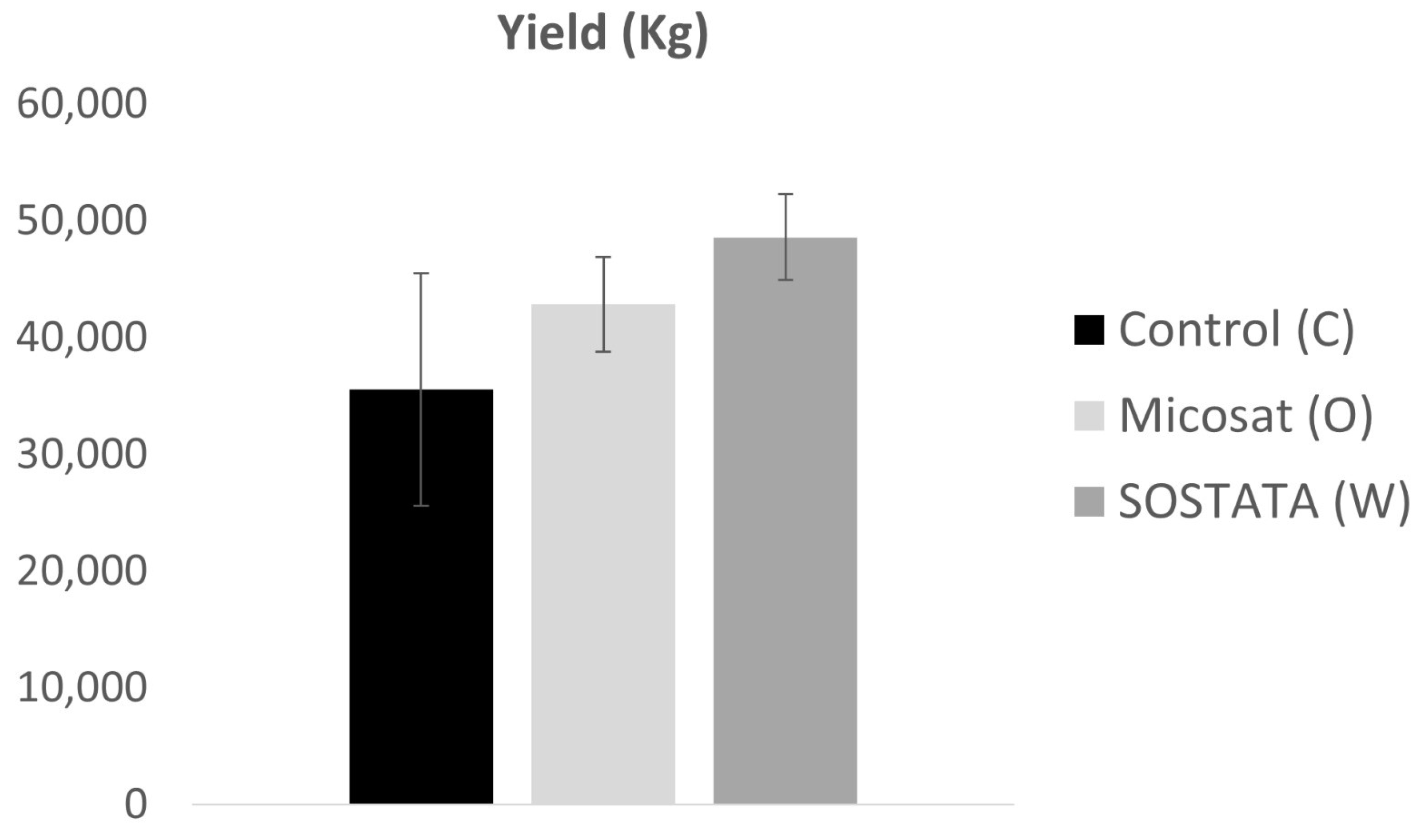
3.4. Evaluation of Morphological Plant Parameters and Yield
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Devaux, A.; Goffart, J.P.; Petsakos, A.; Kromann, P.; Gatto, M.; Okello, J.; Suarez, V.; Hareau, G. Global Food Security, Contributions from Sustainable Potato Agri-Food Systems. In The Potato Crop, 1st ed.; Campos, H., Ortiz, O., Eds.; Springer Nature: Cham, Switzerland, 2020; Volume 1, pp. 3–35. [Google Scholar] [CrossRef]
- Soare, E.; Chiuciu, I.A. Study on the Dynamics of potato Production and worldwide trading during the period 2012–2019. Sci. Pap. Ser. Manag. Econ. Eng. Agric. Rural Dev. 2021, 21, 1–5. [Google Scholar]
- Ramankutty, N.; Mehrabi, Z.; Waha, K.; Jarvis, L.; Kremen, C.; Herrero, M.; Rieseberg, L.H. Trends in Global Agricultural Land Use: Implications for Environmental Health and Food Security. Annu. Rev. Plant Biol. 2019, 69, 789–815. [Google Scholar] [CrossRef] [PubMed]
- Kopittke, P.M.; Menzies, N.W.; Wang, P.; McKenna, B.A.; Lombi, E. Soil and the intensification of agriculture for global food security. Environ. Int. 2019, 132, 105078. [Google Scholar] [CrossRef] [PubMed]
- Szpilko, D.; Ejdys, J. European green deal—Research directions. a systematic literature review. Econ. Environ. 2021, 81, 8–38. [Google Scholar]
- Gustavsen, G.W. Sustainability and Potato Consumption. Potato Res. 2021, 64, 571–586. [Google Scholar] [CrossRef]
- Jennings, S.A.; Koehler, A.K.; Nicklin, K.J.; Deva, C.; Sait, S.M.; Challinor, A.J. Global Potato Yields Increase Under Climate Change with Adaptation and CO2 Fertilisation. Front. Sustain. Food Syst. 2020, 4, 519324. [Google Scholar] [CrossRef]
- Quiroz, R.; Ramírez, D.A.; Kroschel, J.; Andrade-Piedra, J.; Barreda, C.; Condori, B.; Mares, V.; Monneveux, P.; Perez, W. Impact of Climate Change on the Potato Crop and Biodiversity in Its Center of Origin. Open Agric. 2018, 3, 273–283. [Google Scholar] [CrossRef]
- Maqsood, J.; Farooque, A.A.; Wang, X.; Abbas, F.; Acharya, B.; Afzaal, H. Contribution of Climate Extremes to Variation in Potato Tuber Yield in Prince Edward Island. Sustainability 2020, 12, 4937. [Google Scholar] [CrossRef]
- Raymundo, R.; Asseng, S.; Cammarano, D.; Quiroz, R. Potato, Sweet Potato, and Yam Models for Climate Change: A Review. Field Crops Res. 2014, 166, 173–185. [Google Scholar] [CrossRef]
- Haverkort, A.J.; Verhagen, A. Climate Change and Its Repercussions for the Potato Supply Chain. Potato Res. 2008, 51, 223–237. [Google Scholar] [CrossRef]
- Jhariya, M.K.; Banerjee, A.; Meena, R.S.; Yadav, D.K. Agriculture, Forestry and Environmental Sustainability: A Way Forward, 1st ed.; Springer Nature: Singapore, 2019; pp. 1–29. [Google Scholar]
- Woo, S.; Ruocco, M.; Vinale, F.; Nigro, M.; Marra, R.; Lombardi, N.; Pascale, A.; Lanzuise, S.; Manganiello, G.; Lorito, M. Trichoderma-based products and their widespread use in agriculture. Open Mycol. J. 2014, 8, 71–126. [Google Scholar] [CrossRef]
- Harman, G.E. Overview of mechanisms and uses of Trichoderma spp. Phytopathology 2006, 96, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, S.; Pandey, K.; Roshi, Y.R.; Lamichhane, S.K. An overview of multifaceted role of Trichoderma spp. for sustainable agriculture. Arch. Agric. Environ. Sci. 2021, 6, 72–76. [Google Scholar] [CrossRef]
- Waghunde, R.R.; Shelakea, R.M.; Sabalpara, A.N. Trichoderma: A significant fungus for agriculture and environment. Afr. J. Agric. Res. 2016, 11, 1952–1965. [Google Scholar]
- Benítez, T.; Rincón, A.M.; Limón, M.C.; Codón, A.C. Biocontrol mechanisms of Trichoderma strains. Int. Microbiol. 2004, 7, 249–260. [Google Scholar]
- Harman, G.E.; Howell, C.R.; Viterbo, A.; Chet, I.; Lorito, M. Trichoderma species—Opportunistic, avirulent plant symbionts. Nat. Rev. Microbiol. 2004, 2, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Rouphael, Y.; Carillo, P.; Colla, G.; Fiorentino, N.; Sabatino, L.; El-Nakhel, C.; Giordano, M.; Pannico, A.; Cirillo, V.; Shabani, E.; et al. Appraisal of Combined Applications of Trichoderma Virens and a Biopolymer-Based Biostimulant on Lettuce Agronomical, Physiological, and Qualitative Properties under Variable N Regimes. Agronomy 2020, 10, 196. [Google Scholar] [CrossRef]
- Martínez-Medina, A.; del Mar Alguacil, M.; Pascual, J.A.; van Wees, S.C.M. Phytohormone Profiles Induced by Trichoderma Isolates Correspond with Their Biocontrol and Plant Growth-Promoting Activity on Melon Plants. J. Chem. Ecol. 2014, 40, 804–815. [Google Scholar] [CrossRef]
- Gravel, V.; Antoun, H.; Tweddell, R.J. Growth Stimulation and Fruit Yield Improvement of Greenhouse Tomato Plants by Inoculation with Pseudomonas Putida or Trichoderma Atroviride: Possible Role of Indole Acetic Acid (IAA). Soil. Biol. Biochem. 2007, 39, 1968–1977. [Google Scholar] [CrossRef]
- MacKenzie, A.J.; Starman, T.W.; Windham, M.T. Enhanced Root and Shoot Growth of Chrysanthemum Cuttings Propagated with the Fungus Trichoderma harzianum. HortScience 1995, 30, 496–498. [Google Scholar] [CrossRef]
- Ousley, M.A.; Lynch, J.M.; Whipps, J.M. Potential of Trichoderma spp. as Consistent Plant Growth Stimulators. Biol. Fertil. Soils 1994, 17, 85–90. [Google Scholar] [CrossRef]
- Yedidia, I.; Benhamou, N.; Chet, I. Induction of Defense Responses in Cucumber Plants (Cucumis sativus L.) by the Biocontrol Agent Trichoderma Harzianum. Appl. Environ. Microbiol. 1999, 65, 1061–1070. [Google Scholar] [CrossRef] [PubMed]
- Caruso, G.; El-Nakhel, C.; Rouphael, Y.; Comite, E.; Lombardi, N.; Cuciniello, A.; Woo, S.L. Diplotaxis tenuifolia (L.) DC. Yield and Quality as Influenced by Cropping Season, Protein Hydrolysates, and Trichoderma Applications. Plants 2020, 9, 697. [Google Scholar] [CrossRef]
- Visconti, D.; Fiorentino, N.; Cozzolino, E.; Woo, S.L.; Fagnano, M.; Rouphael, Y. Can Trichoderma-Based Biostimulants Optimize N Use Efficiency and Stimulate Growth of Leafy Vegetables in Greenhouse Intensive Cropping Systems. Agronomy 2020, 10, 121. [Google Scholar] [CrossRef]
- Abdullah, N.S.; Doni, F.; Mispan, M.S.; Saiman, M.Z.; Yusuf, Y.M.; Oke, M.A.; Suhaimi, N.S.M. Harnessing Trichoderma in Agriculture for Productivity and Sustainability. Agronomy 2021, 11, 2559. [Google Scholar] [CrossRef]
- Amuza, A.C.; Zaharia, R. Trichoderma spp. Mechanisms of action in the control of storage pathogens review. Horticulture 2022, 66, 781–790. [Google Scholar]
- Risoli, R.; Cotrozzi, L.; Sarrocco, S.; Nuzzaci, M.; Pellegrini, E.; Vitti, E. Trichoderma-Induced Resistance to Botrytis cinerea in Solanum Species: A Meta-Analysis. Plants 2022, 11, 180. [Google Scholar] [CrossRef]
- Ons, L.; Bylemans, D.; Thevissen, K.; Cammue, B.P.A. Combining Biocontrol Agents with Chemical Fungicides for Integrated Plant Fungal Disease Control. Microorganisms 2020, 8, 1930. [Google Scholar] [CrossRef]
- Gams, W.; Bissett, J. Morphology and Identification of Trichoderma. Trichoderma and Gliocladium. In Basic Biology, Taxonomy and Genetics, 1st ed.; Kubicek, C.P., Harman, G.E., Taylor & Francis Ltd., Eds.; Gunpowder Square: London, UK, 2002; pp. 3–31. [Google Scholar]
- Siddiquee, S. Morphology-Based Characterization of Trichoderma Species. In Practical Handbook of the Biology and Molecular Diversity of Trichoderma Species from Tropical Regions, 1st ed.; Gupta, V.K., Tuohy, M.G., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 41–69. [Google Scholar] [CrossRef]
- Błaszczyk, L.; Basińska-Barczak, A.; Ćwiek-Kupczyńska, H.; Gromadzka, K.; Popiel, D.; Stępień, Ł. Suppressive effect of Trichoderma spp. on toxigenic Fusarium Species. Pol. J. Microbiol. 2017, 66, 85–100. [Google Scholar] [CrossRef]
- García Núñez, H.G.; Martínez Campos, Á.R.; Hermosa Prieto, M.R.; Vázquez, E.M.; Aguilar Ortigoza, C.J.; González Esquivel, C.E. Morphological and molecular characterization of native isolates of Trichoderma and its potential biocontrol against Phytophthora infestans. Rev. Mex. Fitopatol. 2017, 35, 58–79. [Google Scholar]
- Chaverri, P.; Branco Rocha, F.; Jaklitsch, W.; Gazis, R.; Degenkolb, T.; Samuels, G.J. Systematics of the Trichoderma harzianum species complex and the re-identification of commercial biocontrol strains. Eur. PMC Funders Group Mycol. 2015, 107, 558–590. [Google Scholar]
- Metz, N.; Hausladen, H. Trichoderma spp. As potential biological control agent against Alternaria solani in potato. Biol. Control 2022, 166, 104820. [Google Scholar] [CrossRef]
- Consolo, V.F.; Monaco, C.I.; Cordo, C.A.; Salerno, G.L. Characterization of novel Trichoderma spp. isolates as a search for effective biocontrollers of fungal diseases of economically important crops in Argentina. World J. Microbiol. Biotechnol. 2012, 28, 1389–1398. [Google Scholar] [CrossRef] [PubMed]
- El-gamal, N.; Atalla, S.M.M.; El-Mohamedy, R.R.S. Improvement in potential of enzymes from Chaetomium globosum and Trichoderma harzianum using different agricultural wastes and its applications. Biosci. Res. 2018, 15, 3977–3987. [Google Scholar]
- Bhale, U.N.; Rajkonda, J.N. Compatibility of Fungicides and Antagonistic Activity of Trichoderma spp. against Plant Pathogens. Biosci. Methods 2015, 6, 1–9. [Google Scholar]
- Zhang, S.; Xu, B.; Zhang, J.; Gan, Y. Identification of the antifungal activity of Trichoderma longibrachiatum T6 and assessment of bioactive substances in controlling phytopathgens. Pestic. Biochem. Physiol. 2018, 147, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Qualhato, T.F.; Lopes, F.A.C.; Steindorff, A.S.; Brandão, R.S.; Jesuino, R.S.A.; Ulhoa, C. Mycoparasitism studies of Trichoderma species against three phytopathogenic fungi. Evaluation of antagonism and hydrolytic enzyme production. Biotechnol. Lett. 2013, 35, 1573–6776. [Google Scholar] [CrossRef] [PubMed]
- Amin, F.; Razdan, V.K. Potential of Trichoderma species as biocontrol agents of soil borne fungal propagules. J. Phytol. 2010, 2, 38–41. [Google Scholar]
- Russell, P.E. Sensitivity baselines in fungicide resistance research and management. In Frac Monograph, 1st ed.; Crop Life International: Brussels, Belgium, 2002; pp. 3–54. [Google Scholar]
- Yang, C.; Hamel, C.; Vujanovic, V.; Gan, Y. Fungicide: Modes of Action and Possible Impact on Non target microorganisms. Int. Sch. Res. Netw. 2011, 2011, 130289. [Google Scholar] [CrossRef]
- Meyer, S.L.F.; Roberts, D.P. Combinations of Biocontrol Agents for Management of Plant-Parasitic Nematodes and Soilborne Plant-Pathogenic Fungi. J. Nematol. 2002, 34, 1–8. [Google Scholar]
- Myresiotis, C.K.; Karaoglanidis, G.S.; Vryzas, Z.; Papadopoulou-Mourkidou, E. Evaluation of plant-growth-promoting rhizobacteria, acibenzolar-S-methyl and hymexazol for integrated control of Fusarium crown and root rot on tomato. Pest. Manag. Sci. 2012, 68, 404–411. [Google Scholar] [CrossRef] [PubMed]
- Selvakumar, G.; Saha, S.; Kundu, S. Inhibitory activity of pine needle tannin extracts on some agriculturally resourceful microbes. Indian J. Microbiol. 2007, 47, 70–267. [Google Scholar] [CrossRef] [PubMed]
- Rakibuzzaman, M.; Akand, M.; Siddika, M.; Uddin, A. Impact of Trichoderma application as bio-stimulator on disease suppression, growth and yield of potato. J. Biosci. Agric. Res. 2021, 27, 2252–2257. [Google Scholar] [CrossRef]
- Şesan, T.E.; Oancea, A.O.; Ştefan, L.M.; Mănoiu, V.S.; Ghiurea, M.; Răut, I.; Constantinescu-Aruxandei, D.; Toma, A.; Savin, S.; Bira, A.F.; et al. Effects of Foliar Treatment with a Trichoderma Plant Biostimulant Consortium on Passiflora caerulea L. Yield and Quality. Microorganisms 2020, 8, 123. [Google Scholar] [CrossRef] [PubMed]
- Aljboori, Q.H.; Babenko, A.; Gulik, E.; Kurovsky, A.V.; Mikhailova, S. Effect Trichoderma spp on Wheat (Triticum aestivum). In Proceedings of the IOP Conference Series: Earth and Environmental Science, Bandung, Indonesia, 7–8 March 2023; Volume 1158, p. 072020. [Google Scholar] [CrossRef]
- Contreras Liza, S.E.; Ramírez, R.J.M.; Olivas, D.B.L. Production of potato seed tubers under the effect of Trichoderma sp. and rhizobacteria in greenhouse conditions. Rev. Ciências Agroveterinárias 2022, 21, 419–427. [Google Scholar] [CrossRef]
- Galindo, M.; Rueda, D.; Romero, P.; Medina, M.; Bangeppagari, M.; Gangireddygari, V.S.R.; Mulla, S.I. Evaluation of the interaction of arbuscular mycorrhizal fungi and Trichoderma harzianum in the development and nutrition of potato plants. Asian J. Agric. Biol. 2018, 6, 403–416. [Google Scholar]
- Wang, Z.; Li, Y.; Zhuang, L.; Yu, Y.; Liu, J.; Zhang, L.; Gao, Z.; Wu, Y.; Gao, W.; Ding, G.C.; et al. A Rhizosphere-Derived Consortium of Bacillus subtilis and Trichoderma harzianum Suppresses Common Scab of Potato and Increases Yield. Comput. Struct. Biotechnol. J. 2019, 17, 645–653. [Google Scholar] [CrossRef]
- Constantia, J.; Jannah, S.N.; Wijanarka, W.; Purwantisari, S. The Potential Of Potato Cultivation (Solanum tuberosum L.) With the application of plant growth promoting rhizobacteria (PGPR) And Tricho Powder commercial on medium land. Agric 2023, 35, 133–148. [Google Scholar] [CrossRef]
- Ommati, F.; Zaker, M.; Mohammadi, A. Biological control of Fusarium wilt of potato (Fusarium oxysporum f. sp. tuberosi) by Trichoderma isolates under field condition and their effect on yield. J. Crop Prot. 2013, 2, 435–442. [Google Scholar]
- Manzar, N.; Kashyap, A.S.; Goutam, R.S.; Rajawat, M.V.S.; Sharma, P.K.; Sharma, S.K.; Singh, H.V. Trichoderma: Advent of Versatile Biocontrol Agent, Its Secrets and Insights into Mechanism of Biocontrol Potential. Sustainability 2022, 14, 12786. [Google Scholar] [CrossRef]
- Tyśkiewicz, R.; Nowak, A.; Ozimek, E.; Jaroszuk-Ściseł, J. Trichoderma: The Current Status of Its Application in Agriculture for the Biocontrol of Fungal Phytopathogens and Stimulation of Plant Growth. Int. J. Mol. Sci. 2022, 23, 2329. [Google Scholar] [CrossRef] [PubMed]
- Lahlali, R.; Ezrari, S.; Radouane, N.; Kenfaoui, J.; Esmaeel, Q.; El Hamss, H.; Belabess, Z.; Barka, E.A. Biological Control of Plant Pathogens: A Global Perspective. Microorganisms 2022, 10, 596. [Google Scholar] [CrossRef] [PubMed]
- Pertot, I.; Puopolo, G.; Giovannini, O.; Angeli, D.; Sicher, C.; Perazzolli, M. Advantages and limitations involved in the use of microbial biofungicides for the control of root and foliar phytopathogens of fruit crops. Italus Hortus 2016, 23, 3–12. [Google Scholar]
- Kubiak, A.; Wolna-Maruwka, A.; Niewiadomska, A.; Pilarska, A.A. The Problem of Weed Infestation of Agricultural Plantations vs. the Assumptions of the European Biodiversity Strategy. Agronomy 2022, 12, 1808. [Google Scholar] [CrossRef]



| Primer Name | Gene | Primer Direction | Sequence (5′-3′) |
|---|---|---|---|
| Internal Transcribed Spacer | ITS | ITS1 | TCCGTAGGTGAACCTGCGG |
| ITS4 | TCCTCCGCTTATTGATATGC | ||
| Translation elongation factor 1 alpha | Tef-1 | EF1-728 | CATCGAGAAGTTCGAGAAGG |
| TEF1R | GCCATCCTTGGGAGATACCAGC | ||
| Beta-actin | β-actin | TRI-ACT1 | TGGCACCACACCTTCTACAATGA |
| TRI-ACT2 | TCTCCTTCTGCATACGGTCGGA |
| Experimental Fields | |||||
|---|---|---|---|---|---|
| Site | Airola | Cervinara | Maddaloni | Acerra | Avezzano |
| (41°03′10.2″ N 14°35′05.8″ E) | (41°01′49.1″ N 14°37′05.0″ E) | (41°01′03.6″ N 14°21′42.4″ E) | (40°56′20.2″ N 14°24′25.8″ E) | (42°03′53.5″ N 13°31′54.2″ E) | |
| Cultivated area | 4 ha | 4 ha | 2 ha | 2 ha | 2 ha |
| Cultivar | Agata | Agata | Inova | Colomba | Colomba & Cicero |
| % of Growth Inhibition | ||||||
|---|---|---|---|---|---|---|
| Lidal (Tetraconazole) | Talendo (Proquinazid) | Biomic (Tannins) | ||||
| % inhib. 7 dpi | SD | % inhib. 7 dpi | SD | % inhib. 7 dpi | SD | |
| Trichoderma asperelloides (1A) | 100 | 0 | 0 | 0 | 0 | 0 |
| Trichoderma harzianum (1B) | 54.4 | 2.8 | 0 | 0 | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Napolitano, A.; Senatore, M.; Coluccia, S.; Palomba, F.; Castaldo, M.; Spasiano, T.; Avino, A.G.; Vitale, A.; Bonfante, A.; Sacco, A.; et al. Development and Evaluation of a Trichoderma-Based Bioformulation for Enhancing Sustainable Potato Cultivation. Horticulturae 2024, 10, 664. https://doi.org/10.3390/horticulturae10070664
Napolitano A, Senatore M, Coluccia S, Palomba F, Castaldo M, Spasiano T, Avino AG, Vitale A, Bonfante A, Sacco A, et al. Development and Evaluation of a Trichoderma-Based Bioformulation for Enhancing Sustainable Potato Cultivation. Horticulturae. 2024; 10(7):664. https://doi.org/10.3390/horticulturae10070664
Chicago/Turabian StyleNapolitano, Angelo, Mauro Senatore, Simone Coluccia, Francesca Palomba, Margherita Castaldo, Teresa Spasiano, Alessio Giovanni Avino, Andrea Vitale, Antonello Bonfante, Adriana Sacco, and et al. 2024. "Development and Evaluation of a Trichoderma-Based Bioformulation for Enhancing Sustainable Potato Cultivation" Horticulturae 10, no. 7: 664. https://doi.org/10.3390/horticulturae10070664






