Establishment of a Comprehensive Evaluation System for Low Nitrogen and Screening of Nitrogen-Efficient Germplasm in Peanut
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Experimental Design
2.2.1. Seedling Experiment
2.2.2. Field Experiment
2.3. Measurements and Data Analysis
2.3.1. Indicators for Peanut Seedling Evaluation
2.3.2. Indicators of Peanut Field Evaluation
2.4. Data Analysis and D Value Calculation
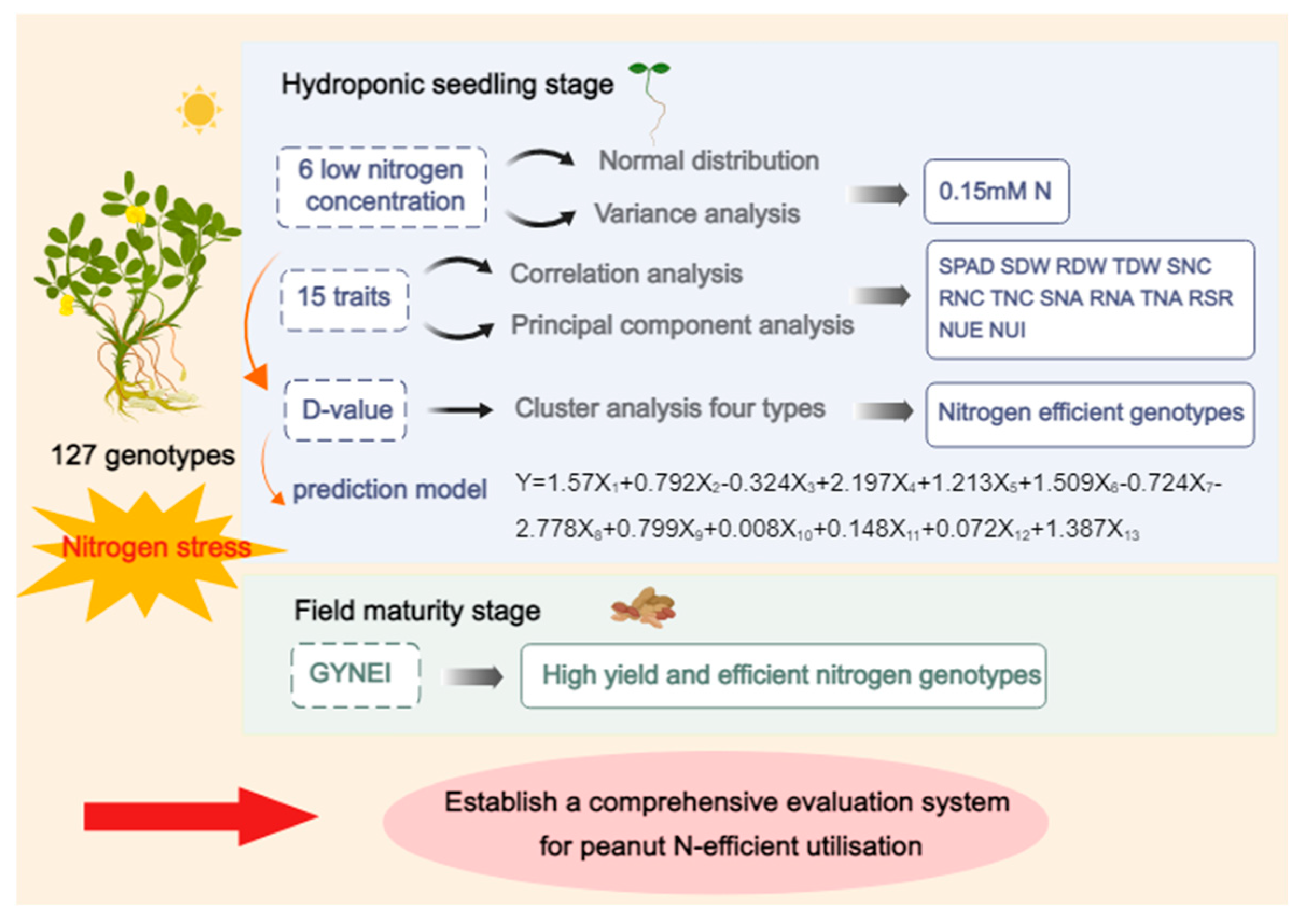
3. Results
3.1. Evaluation of N Efficiency in Peanut Seedlings
3.1.1. Leaf Etiolation Degree and SPAD Changes among Peanut Genotypes
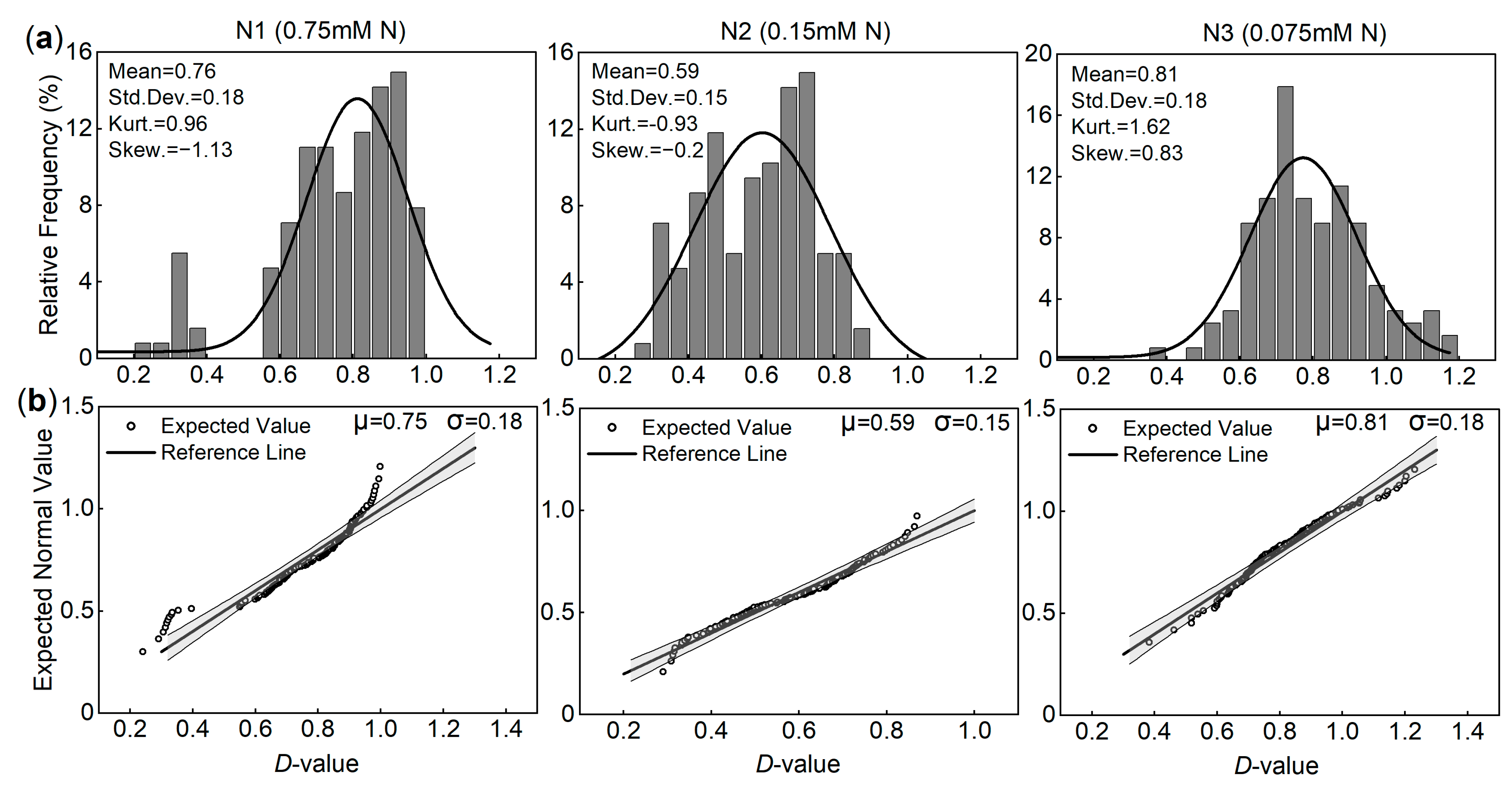
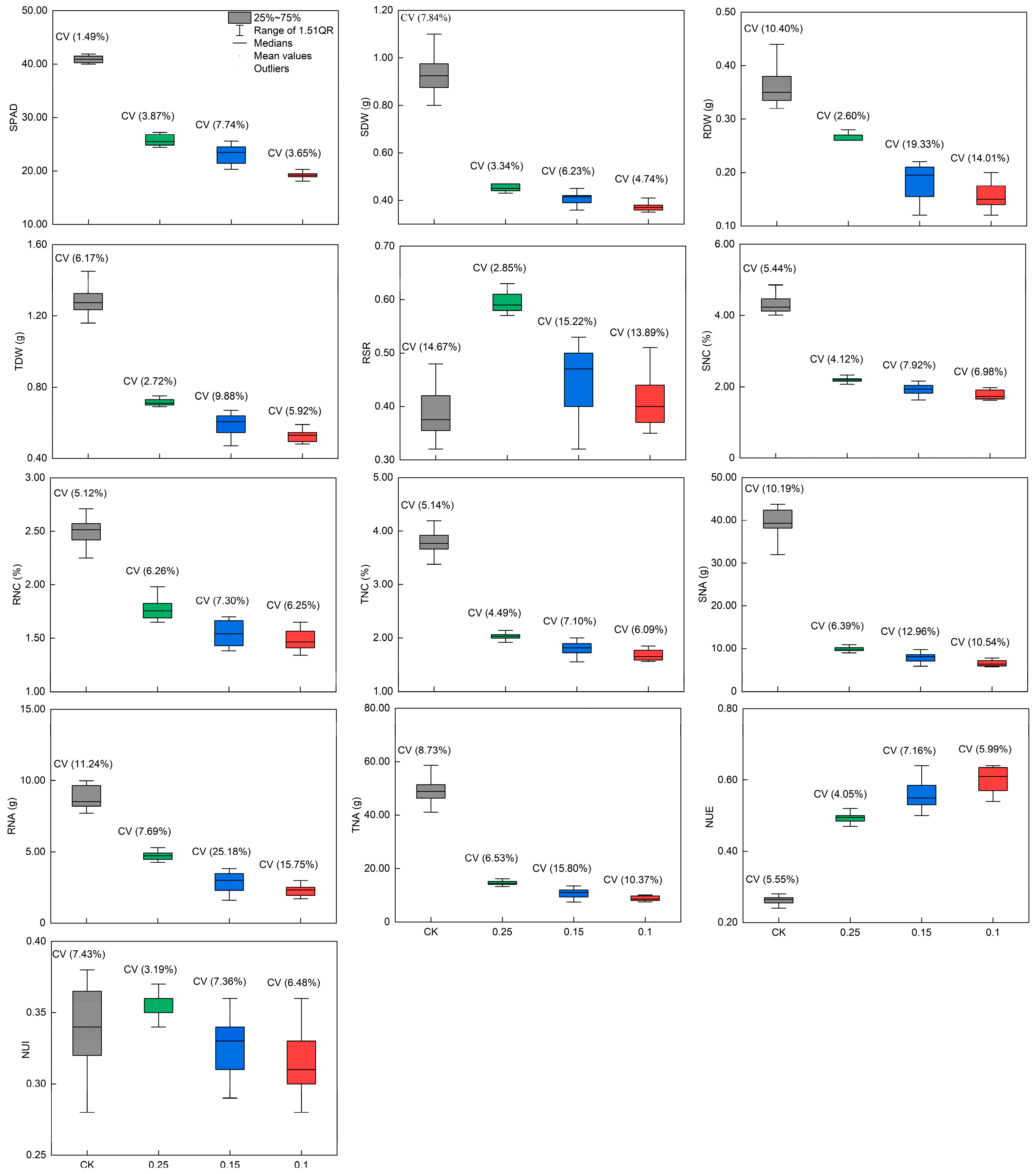
3.1.2. Determination of the N Concentration Indicative of Low-N Tolerance in Peanut Plants
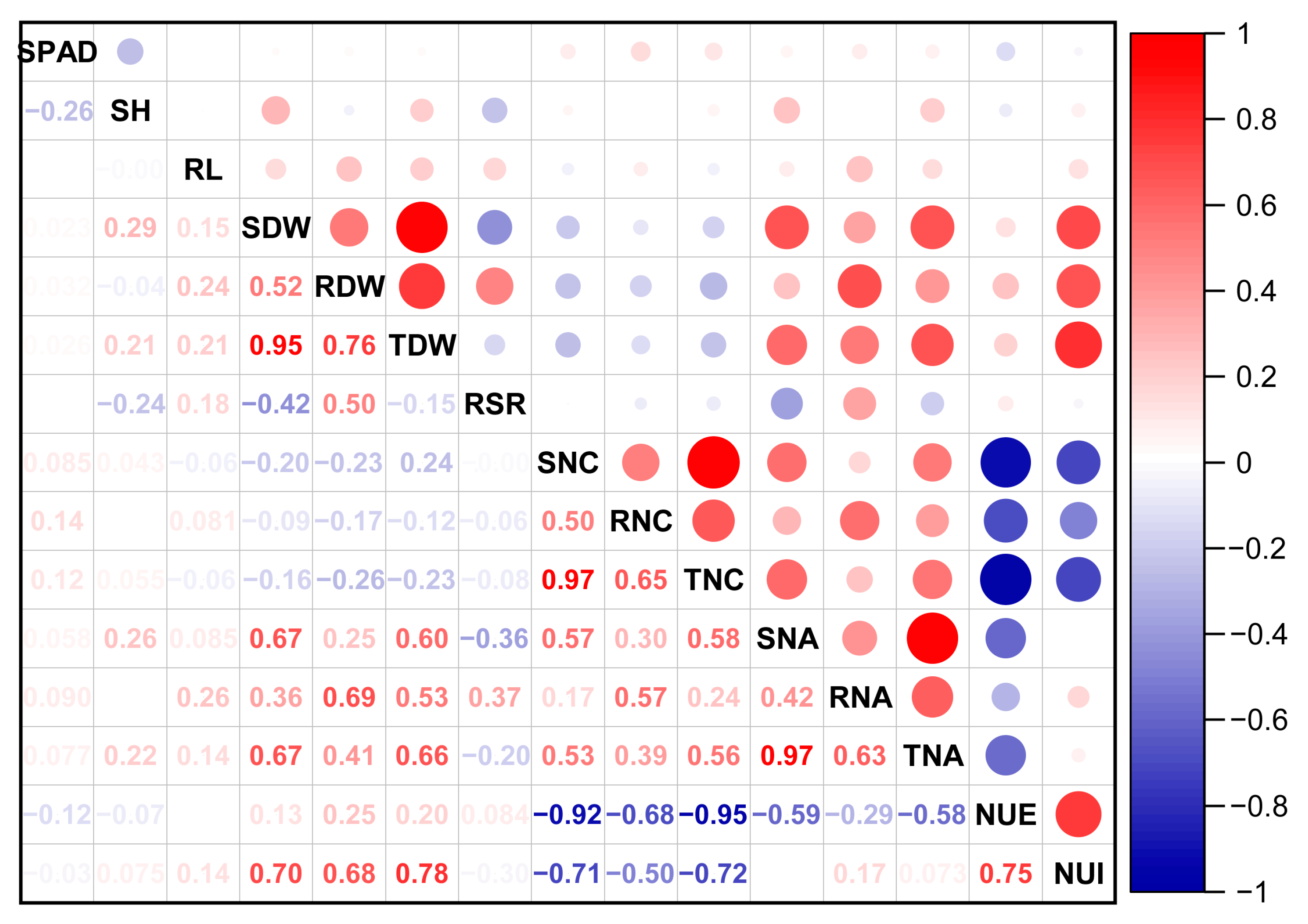
3.1.3. Pearson’s Correlation Analysis and PCA Analysis among Traits of the Peanut Germplasm
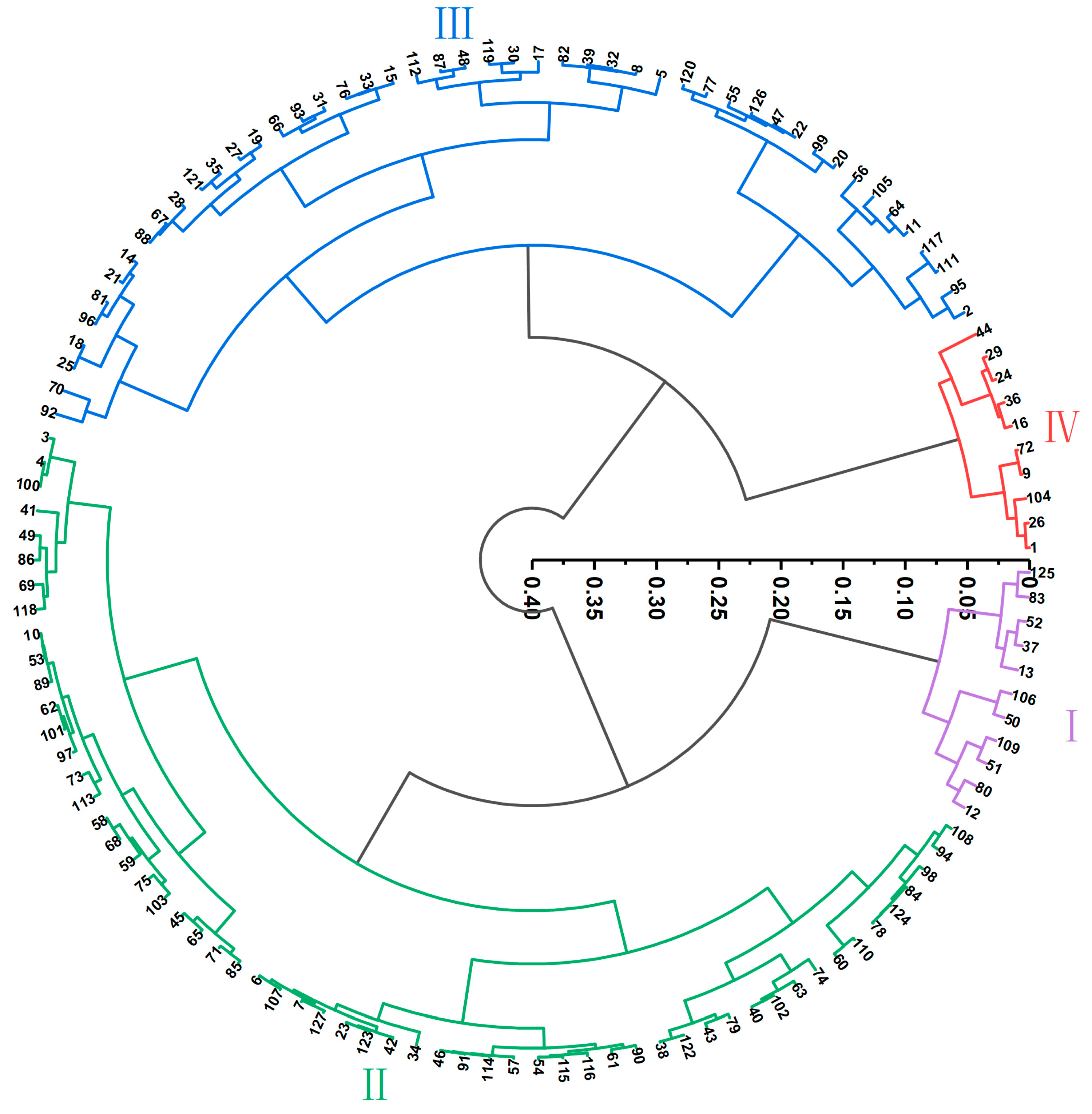
3.1.4. Cluster Analysis of the N Efficiency of the Peanut Materials
3.2. Evaluation of N Efficiency in the Field
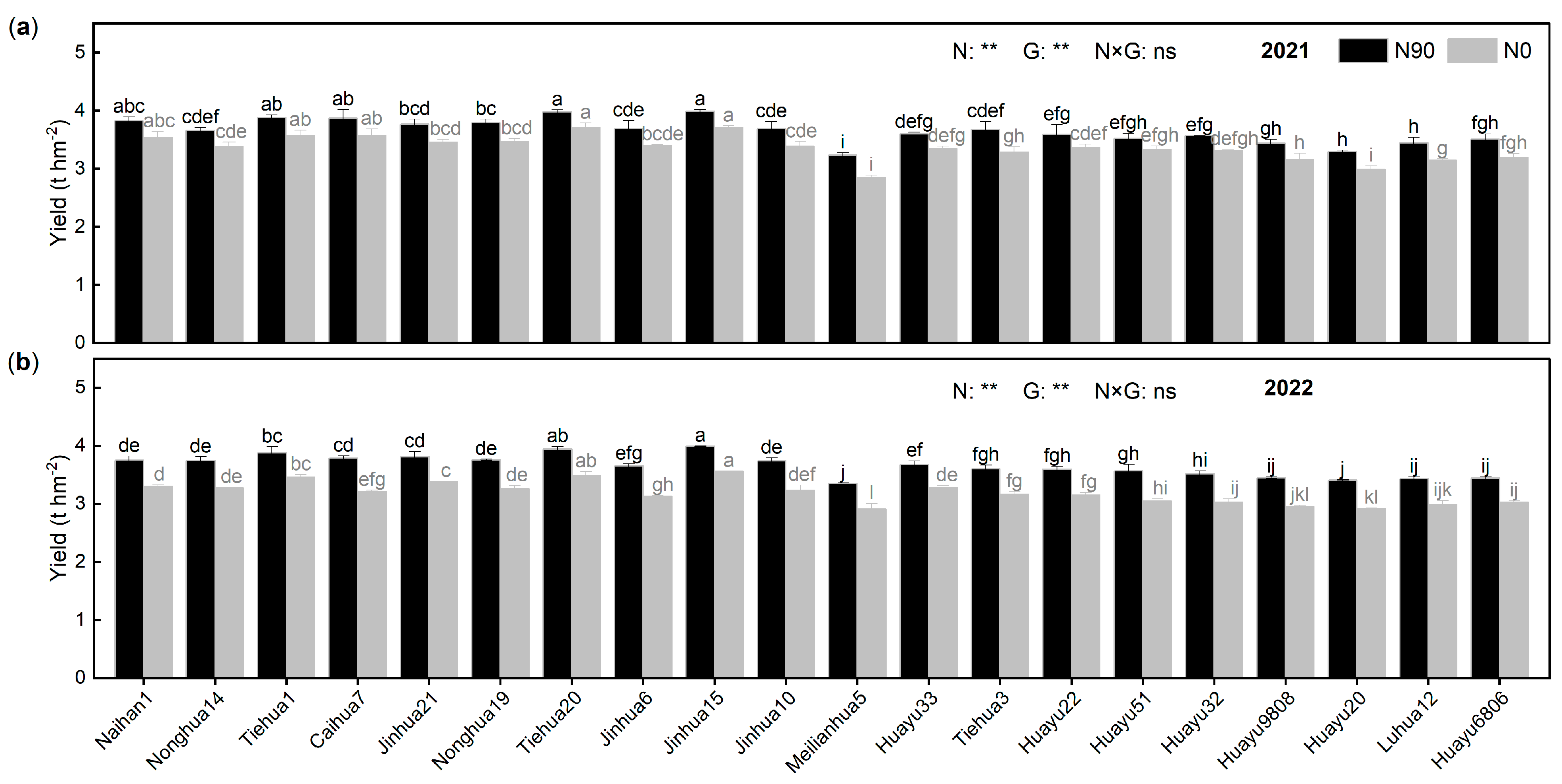
3.2.1. Response of Peanut Yield and Related Factors to N Stress
3.2.2. Response of Peanut N Efficiency to N Stress
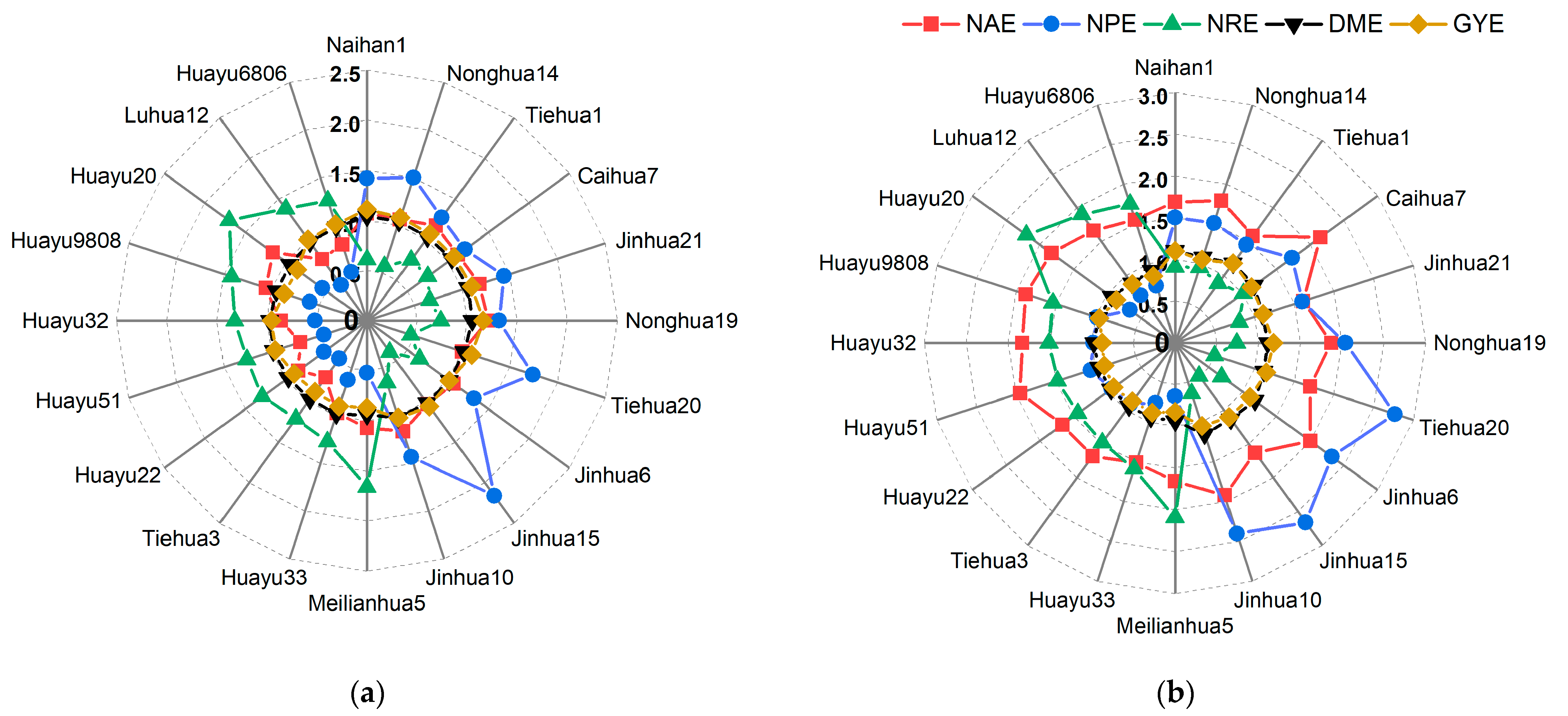
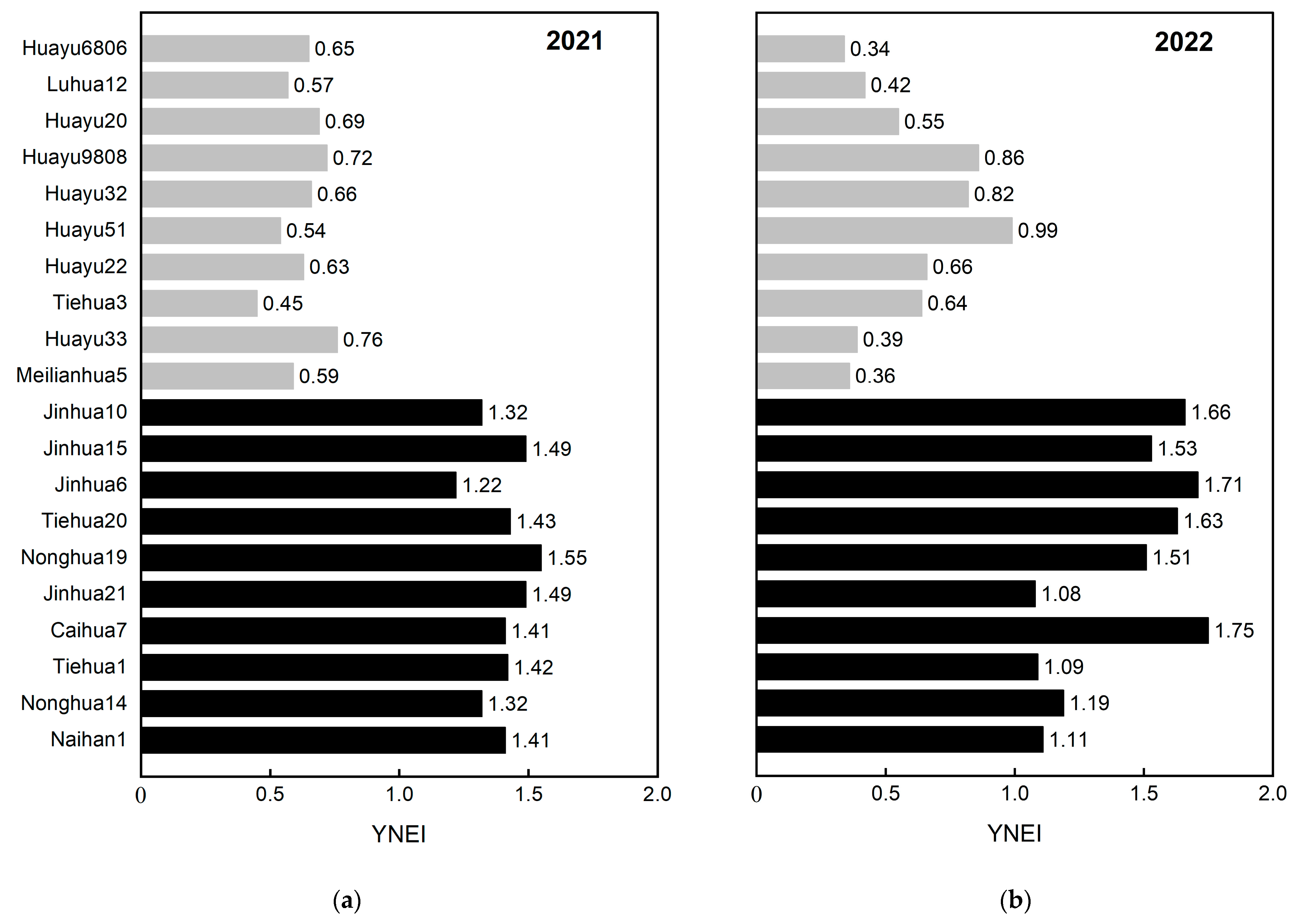
3.2.3. Screening for N-Efficient Genotypes in the Field
3.3. Identification of N Efficiency in Peanuts
3.3.1. Characterization of N-Efficient Peanut Seedlings
3.3.2. Complementary Validation of N Efficiency for Peanut Genotypes in Different Conditions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Arya, S.S.; Salve, A.R.; Chauhan, S. Peanuts as functional food: A review. J. Food Sci. Technol. 2016, 53, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Y.; Cheng, Y.H.; Chen, K.E.; Tsay, Y.F. Nitrate Transport, Signaling, and Use Efficiency. Annu. Rev. Plant Biol. 2018, 69, 85–122. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Wu, K.; Song, W.Z.; Zhong, N.; Wu, Y.Z.; Fu, X.D. Improving Crop Nitrogen Use Efficiency toward Sustainable Green Revolution. Annu. Rev. Plant Biol. 2022, 73, 523–551. [Google Scholar] [CrossRef] [PubMed]
- Anas, M.; Liao, F.; Verma, K.K.; Sarwar, M.A.; Mahmood, A.; Chen, Z.L.; Li, Q.; Zeng, X.P.; Liu, Y.; Li, Y.R. Fate of nitrogen in agriculture and environment: Agronomic, eco-physiological and molecular approaches to improve nitrogen use efficiency. Biol. Res. 2020, 53, 20. [Google Scholar] [CrossRef] [PubMed]
- Zuluaga, D.L.; Sonnante, G. The Use of Nitrogen and Its Regulation in Cereals: Structural Genes, Transcription Factors, and the Role of miRNAs. Plants 2019, 8, 294. [Google Scholar] [CrossRef] [PubMed]
- Moll, R.H.; Kamprath, E.J.; Jackson, W.A. Analysis and Interpretation of Factors Which Contribute to Efficiency of Nitrogen Utilization. Agron. J. 1962, 74, 562–564. [Google Scholar] [CrossRef]
- Kessel, B.; Schierholt, A.; Becker, H.C. Nitrogen Use Efficiency in a Genetically Diverse Set of Winter Oilseed Rape (Brassica napus L.). Crop Sci. 2012, 52, 2546–2554. [Google Scholar] [CrossRef]
- Wu, Z.M.; Luo, J.S.; Han, Y.L.; Hua, Y.P.; Guan, C.Y.; Zhang, Z.H. Low Nitrogen Enhances Nitrogen Use Efficiency by Triggering NO3− Uptake and Its Long-Distance Translocation. J. Agric. Food Chem. 2019, 67, 6736–6747. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.J.; Wang, D.M.; Tao, Z.Q.; Yang, Y.S.; Gao, Z.X.; Zhao, G.C.; Chang, X.H. Impacts of Nitrogen Deficiency on Wheat (Triticum aestivum L.) Grain during the Medium Filling Stage: Transcriptomic and Metabolomic Comparisons. Front. Plant Sci. 2021, 12, 20. [Google Scholar] [CrossRef]
- Sinha, S.K.; Kumar, A.; Tyagi, A.; Venkatesh, K.; Paul, D.; Singh, N.K.; Mandal, P.K. Root architecture traits variation and nitrate-influx responses in diverse wheat genotypes under different external nitrogen concentrations. Plant Physiol. Biochem. 2020, 148, 246–259. [Google Scholar] [CrossRef]
- Chen, J.; Liu, L.T.; Wang, Z.B.; Zhang, Y.J.; Sun, H.C.; Song, S.J.; Bai, Z.Y.; Lu, Z.Y.; Li, C.D. Nitrogen Fertilization Increases Root Growth and Coordinates the Root-Shoot Relationship in Cotton. Front. Plant Sci. 2020, 11, 13. [Google Scholar] [CrossRef] [PubMed]
- The, S.V.; Snyder, R.; Tegeder, M. Targeting Nitrogen Metabolism and Transport Processes to Improve Plant Nitrogen Use Efficiency. Front. Plant Sci. 2021, 11, 22. [Google Scholar] [CrossRef] [PubMed]
- Masclaux-Daubresse, C.; Daniel-Vedele, F.; Dechorgnat, J.; Chardon, F.; Gaufichon, L.; Suzuki, A. Nitrogen uptake, assimilation and remobilization in plants: Challenges for sustainable and productive agriculture. Ann. Bot. 2010, 105, 1141–1157. [Google Scholar] [CrossRef]
- Liu, Y.; Yan, Z.H.; Wang, J.G.; Zhao, J.H.; Liu, Y.Y.; Zou, J.; Li, L.; Zhang, J.L.; Wan, S.B. Optimizing Initial Nitrogen Application Rates to Improve Peanut (Arachis hypogaea L.) Biological Nitrogen Fixation. Agronomy 2023, 13, 3020. [Google Scholar] [CrossRef]
- Rathore, V.S.; Nathawat, N.S.; Bhardwaj, S.; Yadav, B.M.; Kumar, M.; Santra, P.; Kumar, P.; Reager, M.L.; Yadava, N.D.; Yadav, O.P. Optimization of deficit irrigation and nitrogen fertilizer management for peanut production in an arid region. Sci. Rep. 2021, 11, 14. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Song, P.H.; Zhang, Y.C.; Zhou, D.Y.; Dong, Q.Q.; Jia, P.Y.; Liu, Z.H.; Zhao, X.H.; Yu, H.Q. Physiological Mechanism of Photosynthetic, Nutrient, and Yield Responses of Peanut Cultivars with Different Tolerances under Low K Stress. Agronomy 2023, 13, 185. [Google Scholar] [CrossRef]
- Ren, J.Y.; Guo, P.; Zhang, H.; Shi, X.L.; Ai, X.; Wang, J.; Jiang, C.J.; Zhao, X.H.; Liu, X.B.; Yu, H.Q. Comparative physiological and coexpression network analyses reveal the potential drought tolerance mechanism of peanut. BMC Plant Biol. 2022, 22, 16. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, Y.; Han, Z.K.; Chen, Y.N.; Huai, D.X.; Kang, Y.P.; Wang, Z.H.; Yan, L.Y.; Jiang, H.F.; Lei, Y.; et al. Integrated Transcriptomics and Metabolomics Analysis Reveal Key Metabolism Pathways Contributing to Cold Tolerance in Peanut. Front. Plant Sci. 2021, 12, 15. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, Z.M.; Ding, H.; Wen, S.Q.; Zhang, G.C.; Qin, F.F.; Dai, L.X. Comprehensive effects of salt stress and peanut cultivars on the rhizosphere bacterial community diversity of peanut. Arch. Microbiol. 2022, 204, 11. [Google Scholar] [CrossRef]
- Wang, C.B.; Zheng, Y.M.; Shen, P.; Zheng, Y.P.; Wu, Z.F.; Sun, X.W.; Yu, T.Y.; Feng, H. Determining N supplied sources and N use efficiency for peanut under applications of four forms of N fertilizers labeled by isotope 15N. J. Integr. Agric. 2016, 15, 432–439. [Google Scholar] [CrossRef]
- Rakotoson, T.; Dusserre, J.; Letourmy, P.; Ramonta, I.R.; Cao, T.V.; Ramanantsoanirina, A.; Roumet, P.; Ahmadi, N.; Raboin, L.M. Genetic variability of nitrogen use efficiency in rainfed upland rice. Field Crop. Res. 2017, 213, 194–203. [Google Scholar] [CrossRef]
- Miao, J.J.; Shi, F.; Li, W.; Zhong, M.; Li, C.; Chen, S.S. Comprehensive screening of low nitrogen tolerant maize based on multiple traits at the seedling stage. Peerj 2022, 10, 17. [Google Scholar] [CrossRef]
- Liu, C.J.; Gong, X.W.; Wang, H.L.; Dang, K.; Deng, X.P.; Feng, B.L. Low-nitrogen tolerance comprehensive evaluation and physiological response to nitrogen stress in broomcorn millet (Panicum miliaceum L.) seedling. Plant Physiol. Biochem. 2020, 151, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Sinha, V.B.; Gupta, N.; Rajpal, S.; Kuchi, S.; Sitaramam, V.; Parsad, R.; Raghuram, N. Phenotyping for Nitrogen Use Efficiency: Rice Genotypes Differ in N-Responsive Germination, Oxygen Consumption, Seed Urease Activities, Root Growth, Crop Duration, and Yield at Low N. Front. Plant Sci. 2018, 9, 16. [Google Scholar] [CrossRef] [PubMed]
- Schum, A.; Jansen, G. In vitro method for early evaluation of nitrogen use efficiency associated traits in potato. J. Appl. Bot. Food Qual. 2014, 87, 256–264. [Google Scholar]
- Mu, X.H.; Chen, Y.L. The physiological response of photosynthesis to nitrogen deficiency. Plant Physiol. Biochem. 2021, 158, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.J.; Zhao, Y.J.; Hao, F. Development of nitrogen efficiency screening system in alfalfa (Medicago sativa L.) and analysis of alfalfa nitrogen efficiency types. Peerj 2022, 10, 21. [Google Scholar] [CrossRef] [PubMed]
- Tantray, A.Y.; Hazzazi, Y.; Ahmad, A. Physiological, Agronomical, and Proteomic Studies Reveal Crucial Players in Rice Nitrogen Use Efficiency under Low Nitrogen Supply. Int. J. Mol. Sci. 2022, 23, 6410. [Google Scholar] [CrossRef]
- Aninbon, C.; Jogloy, S.; Vorasoot, N.; Nuchadomrong, S.; Holbrook, C.C.; Kvien, C.; Puppala, N.; Patanothai, A. Variability of arginine content and yield components in Valencia peanut germplasm. Breed. Sci. 2017, 67, 207–212. [Google Scholar] [CrossRef]
- Li, G.H.; Guo, X.; Sun, W.; Hou, L.; Wang, G.H.; Tian, R.Z.; Wang, X.J.; Qu, C.J.; Zhao, C.Z. Nitrogen application in pod zone improves yield and quality of two peanut cultivars by modulating nitrogen accumulation and metabolism. BMC Plant Biol. 2024, 24, 12. [Google Scholar] [CrossRef]
- Zhang, G.C.; Liu, Q.B.; Zhang, Z.M.; Ci, D.W.; Zhang, J.L.; Xu, Y.; Guo, Q.; Xu, M.L.; He, K. Effect of Reducing Nitrogen Fertilization and Adding Organic Fertilizer on Net Photosynthetic Rate, Root Nodules and Yield in Peanut. Plants 2023, 12, 2902. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.Y.; Xu, K.; Li, J.; Li, F.; Qiao, J.W.; Li, H.; Gao, G.Z.; Yan, G.X.; Wu, X.M. Evaluation of yield and agronomic traits and their genetic variation in 488 global collections of Brassica napus L. Genet. Resour. Crop Evol. 2014, 61, 979–999. [Google Scholar] [CrossRef]
- He, H.Y.; Yang, R.; Li, Y.J.; Ma, A.S.; Cao, L.Q.; Wu, X.M.; Chen, B.Y.; Tian, H.; Gao, Y.J. Genotypic Variationin Nitrogen Utilization Efficiency of Oilseed Rape (Brassica napus) under Contrasting N Supply in Pot and Field Experiments. Front. Plant Sci. 2017, 8, 15. [Google Scholar] [CrossRef] [PubMed]
- Gaudinier, A.; Rodriguez-Medina, J.; Zhang, L.F.; Olson, A.; Liseron-Monfils, C.; Bagman, A.M.; Foret, J.; Abbitt, S.; Tang, M.; Li, B.H.; et al. Transcriptional regulation of nitrogen-associated metabolism and growth. Nature 2018, 563, 259. [Google Scholar] [CrossRef]
- Limpens, J.; Granath, G.; Aerts, R.; Heijmans, M.M.P.D.; Sheppard, L.J.; Bragazza, L.; Williams, B.L.; Rydin, H.; Bubier, J.; Moore, T. Glasshouse vs field experiments: Do they yield ecologically similar results for assessing N impacts on peat mosses? New Phytol. 2012, 195, 408–418. [Google Scholar] [CrossRef]

| Items | Indices | Principle Component | |||
|---|---|---|---|---|---|
| Factor 1 | Factor 2 | Factor 3 | Factor 4 | ||
| Characteristic vector | SPAD | 0.108 | 0.134 | −0.096 | 0.846 |
| SH | 0.062 | 0.281 | −0.129 | −0.717 | |
| RL | −0.007 | 0.097 | 0.460 | −0.079 | |
| SDW | −0.184 | 0.971 | 0.028 | −0.079 | |
| RDW | −0.263 | 0.500 | 0.774 | 0.092 | |
| TDW | −0.242 | 0.918 | 0.303 | −0.027 | |
| RSR | −0.047 | −0.443 | 0.813 | 0.108 | |
| SNC | 0.938 | −0.010 | −0.045 | −0.016 | |
| RNC | 0.745 | 0.019 | 0.161 | 0.092 | |
| TNC | 0.978 | 0.031 | −0.063 | 0.010 | |
| SNA | 0.555 | 0.794 | −0.017 | −0.088 | |
| RNA | 0.325 | 0.414 | 0.764 | 0.099 | |
| TNA | 0.554 | 0.794 | 0.200 | −0.046 | |
| NUE | −0.980 | −0.046 | 0.036 | −0.004 | |
| NUI | −0.761 | 0.576 | 0.233 | 0.012 | |
| Eigen value | 4.830 | 4.107 | 2.301 | 1.291 | |
| Contribution percentage (%) | 32.202 | 27.379 | 15.342 | 8.606 | |
| Accumulative contribution percentage (%) | 83.529 | ||||
| Year | Traits | N90 | N0 | ||||
|---|---|---|---|---|---|---|---|
| Range | Mean | CV (%) | Range | Mean | CV (%) | ||
| 2021 | Pod number per plant | 17–24 | 21 | 9.59 | 15–23 | 18 | 12.98 |
| Full-pod number per plant | 14–23 | 18 | 12.29 | 12–22 | 16 | 15.98 | |
| 100 pods weight (g) | 152.85–231.62 | 181.86 | 11.43 | 139.15–214.01 | 171.23 | 10.90 | |
| 100 kernels weight (g) | 57.78–94.99 | 74.21 | 15.00 | 57.67–90.05 | 72.06 | 14.45 | |
| Pod yield (t hm−2) | 3.23–3.98 | 3.65 | 5.71 | 2.85–3.71 | 3.36 | 6.53 | |
| 2022 | Pod number per plant | 14–23 | 18 | 13.87 | 12–22 | 16 | 20.02 |
| Full-pod number per plant | 11–23 | 16 | 21.97 | 8–21 | 13 | 28.33 | |
| 100 pods weight (g) | 152.66–238.24 | 182.58 | 11.82 | 138.38–219.77 | 173.00 | 12.05 | |
| 100 kernels weight (g) | 59.26–97.23 | 74.38 | 14.80 | 58.93–93.21 | 72.19 | 14.39 | |
| Pod yield (t hm−2) | 3.35–3.99 | 3.65 | 5.05 | 2.91–3.56 | 3.19 | 6.00 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, P.; Gang, D.; Wang, Y.; Guo, P.; Zhao, X.; Jiang, C.; Yu, H. Establishment of a Comprehensive Evaluation System for Low Nitrogen and Screening of Nitrogen-Efficient Germplasm in Peanut. Horticulturae 2024, 10, 669. https://doi.org/10.3390/horticulturae10070669
Zhang P, Gang D, Wang Y, Guo P, Zhao X, Jiang C, Yu H. Establishment of a Comprehensive Evaluation System for Low Nitrogen and Screening of Nitrogen-Efficient Germplasm in Peanut. Horticulturae. 2024; 10(7):669. https://doi.org/10.3390/horticulturae10070669
Chicago/Turabian StyleZhang, Ping, Dongming Gang, Yanliang Wang, Pei Guo, Xinhua Zhao, Chunji Jiang, and Haiqiu Yu. 2024. "Establishment of a Comprehensive Evaluation System for Low Nitrogen and Screening of Nitrogen-Efficient Germplasm in Peanut" Horticulturae 10, no. 7: 669. https://doi.org/10.3390/horticulturae10070669
APA StyleZhang, P., Gang, D., Wang, Y., Guo, P., Zhao, X., Jiang, C., & Yu, H. (2024). Establishment of a Comprehensive Evaluation System for Low Nitrogen and Screening of Nitrogen-Efficient Germplasm in Peanut. Horticulturae, 10(7), 669. https://doi.org/10.3390/horticulturae10070669





