Genome-Wide Identification, Characterization, and Expression Analysis of the DMP Gene Family in Pepper (Capsicum annuum L.)
Abstract
:1. Introduction
2. Material and Methods
2.1. Identification and Physicochemical Analysis of DMP Gene Family in Pepper
2.2. Multiple Sequence Alignment and Phylogenetic Analysis of DMP Genes
2.3. Analysis of Conserved Domain, Gene Structure, and Conserved Protein Motif of DMP Family Genes
2.4. Chromosomal Locations, Cis-Acting Elements Analysis, and Collinearity Analysis of DMP Genes in Pepper
2.5. Gene Expression Analysis and Sample Stress Treatment
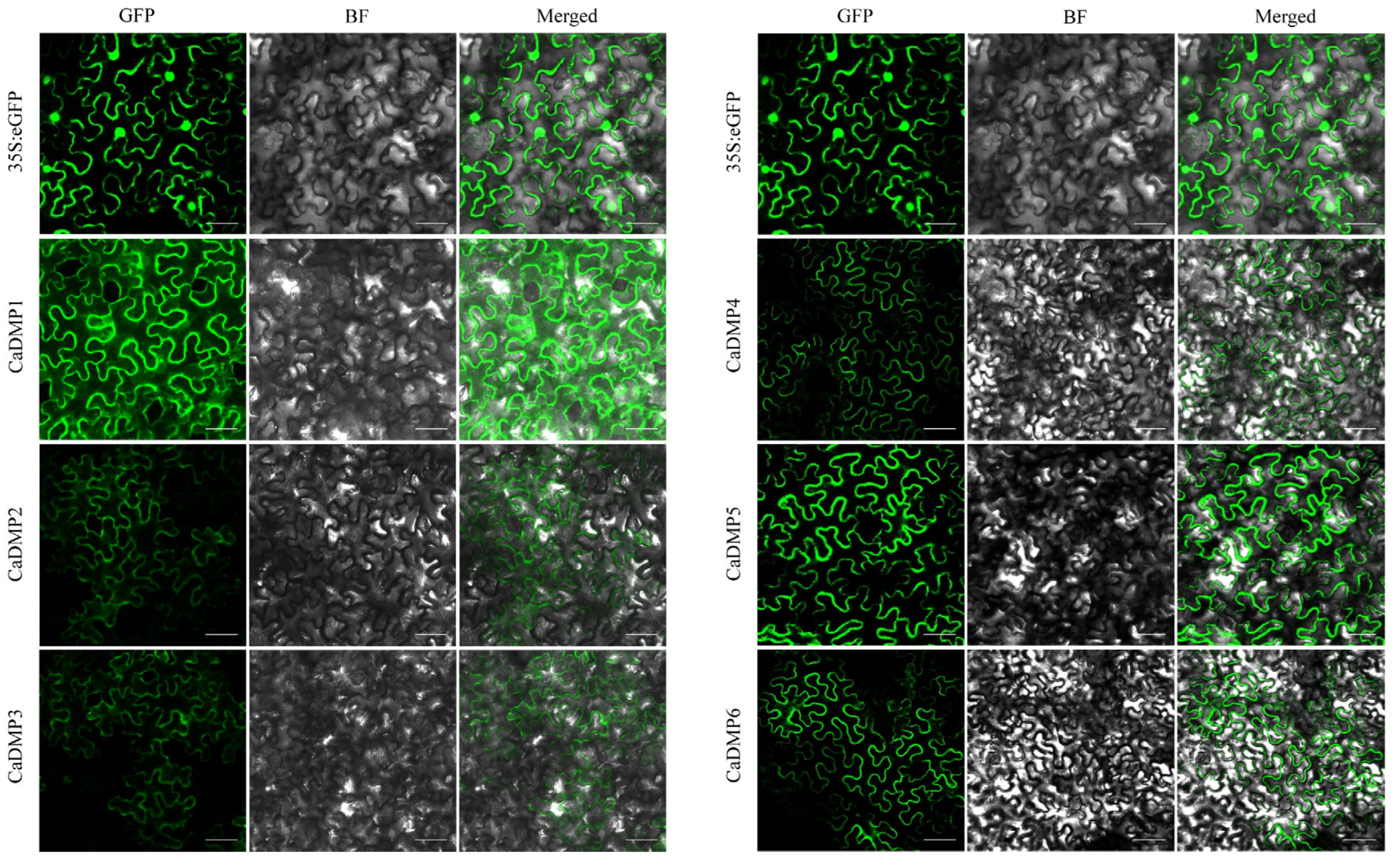
2.6. Transient Expression of the CaDMP Genes in Nicotiana benthamiana
3. Results
3.1. Identification of DMP Genes and Analysis of Physicochemical Properties in Pepper
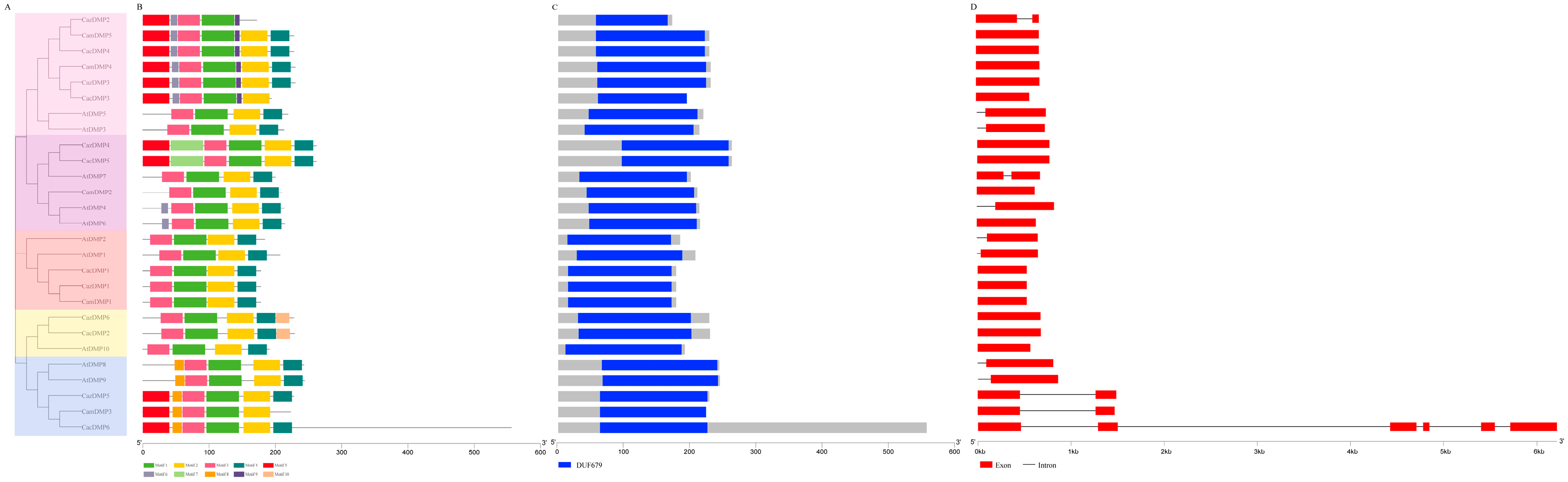
3.2. Phylogenetic Analysis of CaDMP Genes
3.3. Analysis of Conserved Domain, Gene Structure, and Conserved Protein Motif of DMP Family Genes
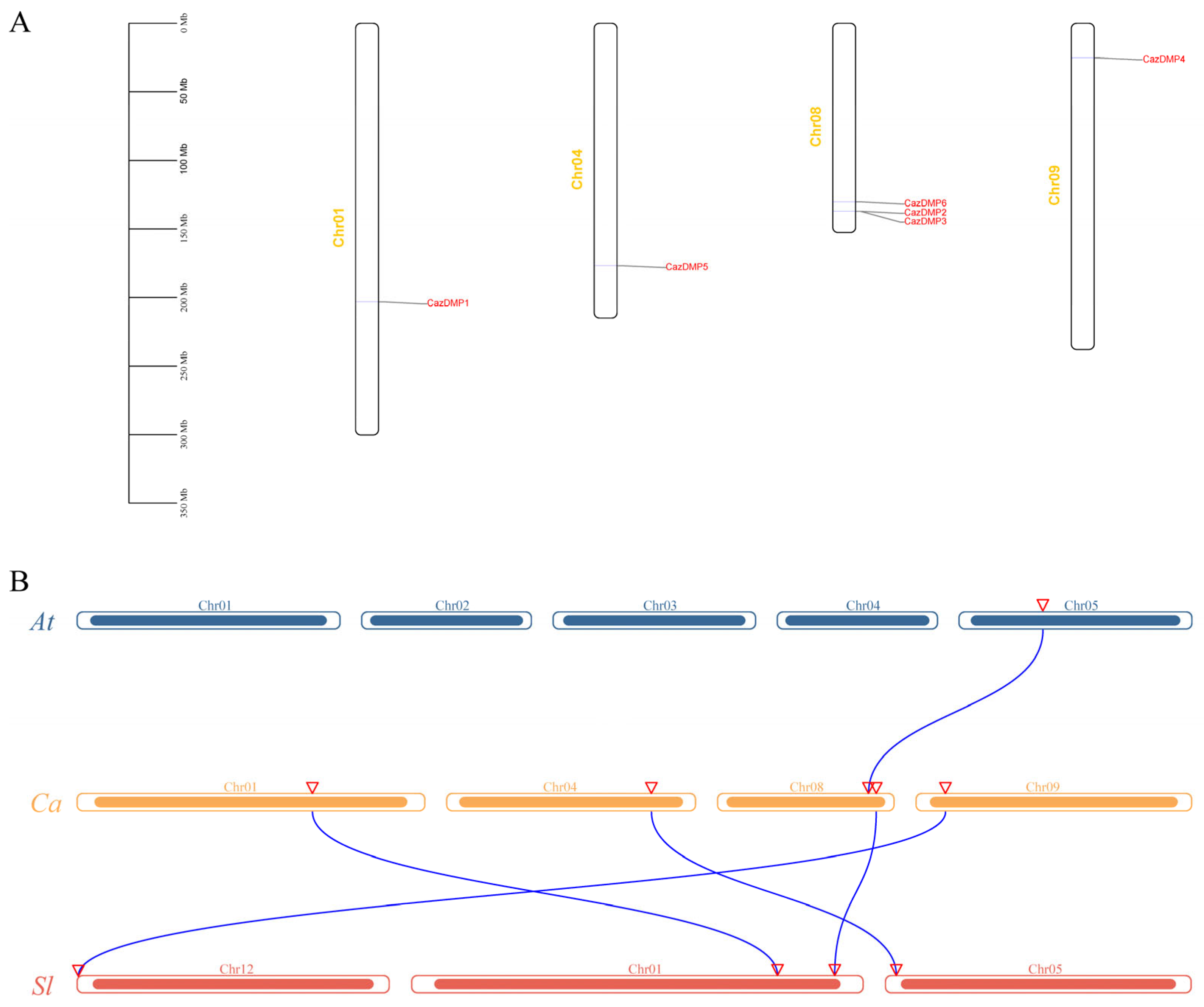
3.4. Chromosomal Locations and Collinearity of CaDMP
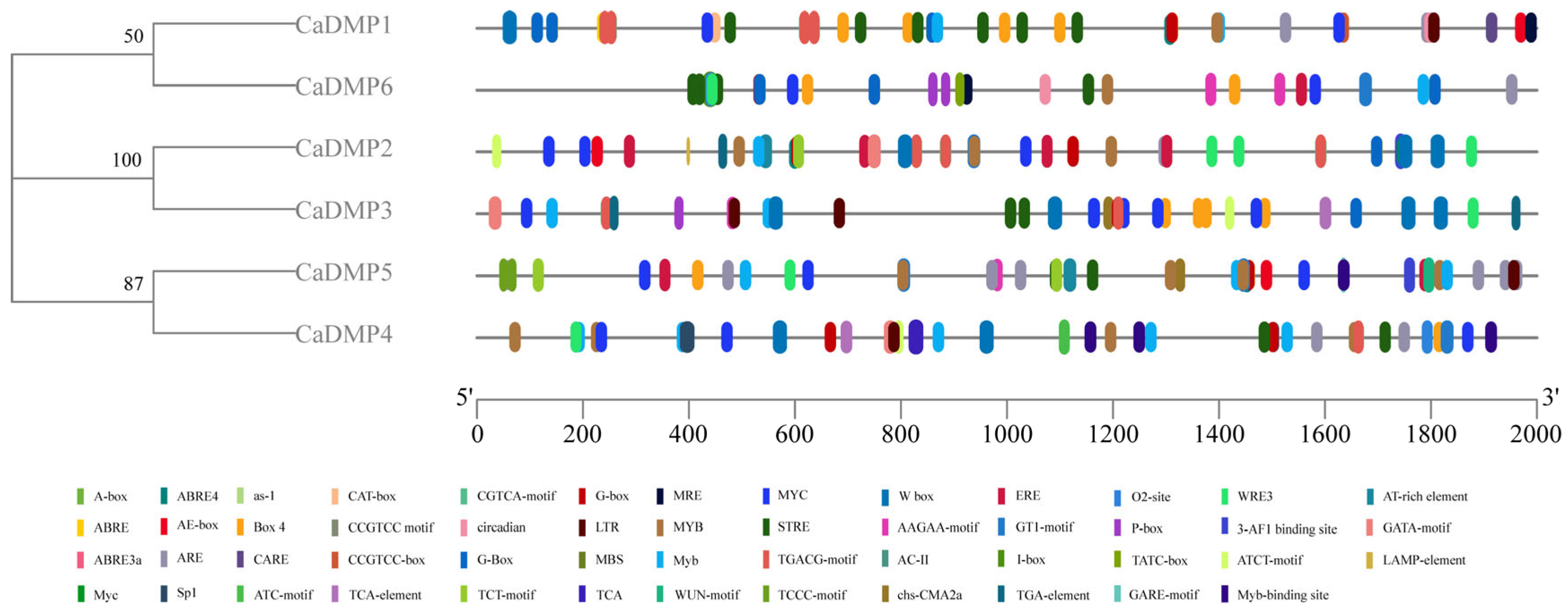
3.5. Analysis of Cis-Acting Elements of DMP Gene Promoter in Pepper
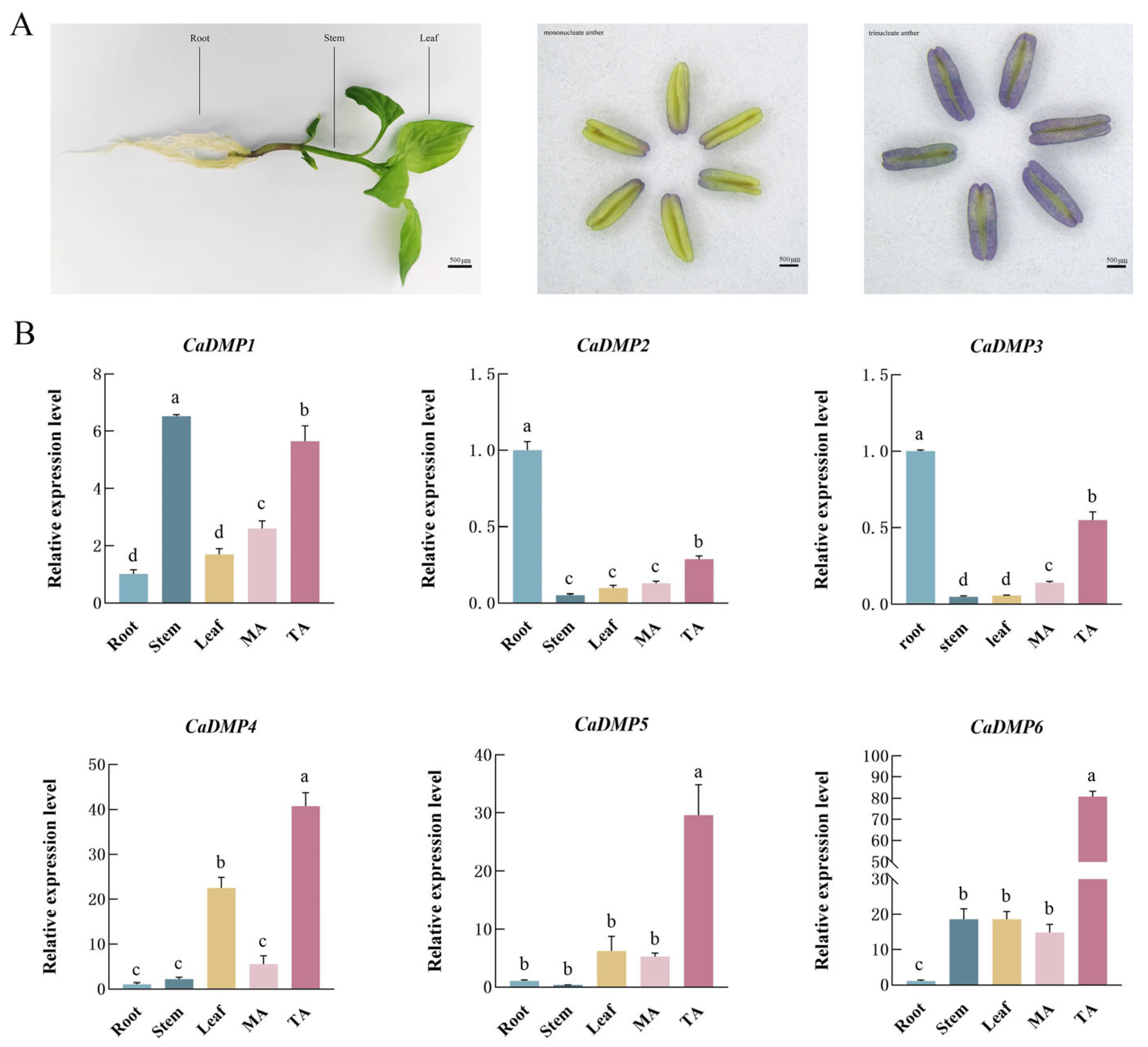
3.6. Expression of DMP Gene in Different Tissues of Pepper
3.7. Expression Analysis of CaDMP Genes under Different Abiotic Stresses
3.8. Transient Expression of the CaDMP Genes in Nicotiana benthamiana
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mori, T.; Kawai-Toyooka, H.; Igawa, T.; Nozaki, H. Gamete Dialogs in Green Lineages. Mol. Plant 2015, 8, 1442–1454. [Google Scholar] [CrossRef] [PubMed]
- Dresselhaus, T.; Sprunck, S.; Wessel, G.M. Fertilization Mechanisms in Flowering Plants. Curr. Biol. 2016, 26, R125–R139. [Google Scholar] [CrossRef] [PubMed]
- Mori, T.; Kuroiwa, H.; Higashiyama, T.; Kuroiwa, T. GENERATIVE CELL SPECIFIC 1 is essential for angiosperm fertilization. Nat. Cell Biol. 2005, 8, 64–71. [Google Scholar] [CrossRef] [PubMed]
- von Besser, K.; Frank, A.C.; Johnson, M.A.; Preuss, D. Arabidopsis HAP2(GCS1) is a sperm-specific gene required for pollen tube guidance and fertilization. Development 2006, 133, 4761–4769. [Google Scholar] [CrossRef]
- Sprunck, S.; Hackenberg, T.; Englhart, M.; Vogler, F. Same same but different: Sperm-activating EC1 and ECA1 gametogenesis-related family proteins. Biochem. Soc. Trans. 2014, 42, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Liu, C.; Qi, X.; Jiao, Y.; Wang, D.; Wang, Y.; Liu, Z.; Chen, C.; Chen, B.; Tian, X.; et al. Mutation of ZmDMP enhances haploid induction in maize. Nat. Plants 2019, 5, 575–580. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Chen, B.; Li, M.; Wang, D.; Jiao, Y.; Qi, X.; Wang, M.; Liu, Z.; Chen, C.; Wang, Y.; et al. A DMP-triggered in vivo maternal haploid induction system in the dicotyledonous Arabidopsis. Nat. Plants 2020, 6, 466–472. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Wang, X.; Chen, W.; Yao, J.; Li, Y.; Fang, S.; Lv, Y.; Li, X.; Pan, J.; Liu, C.; et al. Cotton DMP gene family: Characterization, evolution, and expression profiles during development and stress. Int. J. Biol. Macromol. 2021, 183, 1257–1269. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Mori, T.; Ueda, K.; Yamada, L.; Nagahara, S.; Higashiyama, T.; Sawada, H.; Igawa, T. The male gamete membrane protein DMP9/DAU2 is required for double fertilization in flowering plants. Development 2018, 145, dev170076. [Google Scholar] [CrossRef]
- Dwivedi, S.L.; Britt, A.B.; Tripathi, L.; Sharma, S.; Upadhyaya, H.D.; Ortiz, R. Haploids: Constraints and opportunities in plant breeding. Biotechnol. Adv. 2015, 33, 812–829. [Google Scholar] [CrossRef]
- Supena, E.D.J.; Suharsono, S.; Jacobsen, E.; Custers, J.B.M. Successful development of a shed-microspore culture protocol for doubled haploid production in Indonesian hot pepper (Capsicum annuum L.). Plant Cell Rep. 2005, 25, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ochoa-Alejo, N. Anther Culture of Chili Pepper (Capsicum spp.). In Plant Cell Culture Protocols; Humana Press: Totowa, NJ, USA, 2012; pp. 227–231. [Google Scholar]
- Kim, M.; Jang, I.-C.; Kim, J.-A.; Park, E.-J.; Yoon, M.; Lee, Y. Embryogenesis and plant regeneration of hot pepper (Capsicum annuum L.) through isolated microspore culture. Plant Cell Rep. 2007, 27, 425–434. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Wu, P.; Trampe, B.; Tian, X.; Lübberstedt, T.; Chen, S. Novel technologies in doubled haploid line development. Plant Biotechnol. J. 2017, 15, 1361–1370. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Pérez, T.; Gómez-García, M.d.R.; Valverde, M.E.; Paredes-López, O. Capsicum annuum(hot pepper): An ancient Latin-American crop with outstanding bioactive compounds and nutraceutical potential. A review. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2972–2993. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Gou, B.; Wang, Y.; Yang, N.; Duan, P.; Wei, M.; Zhang, G.; Wei, B. Identification and relative expression analysis of CaFRK gene family in pepper. 3 Biotech 2022, 12, 137. [Google Scholar] [CrossRef]
- Reiser, L.; Bakker, E.; Subramaniam, S.; Chen, X.; Sawant, S.; Khosa, K.; Prithvi, T.; Berardini, T.Z.; Harris, T. The Arabidopsis Information Resource in 2024. Genetics 2024, 227, iyae027. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, D.; Xiao, Q.; Wang, H.; Wen, J.; Tu, J.; Shen, J.; Fu, T.; Yi, B. An in planta haploid induction system in Brassica napus. J. Integr. Plant Biol. 2022, 64, 1140–1144. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.D.; Clements, J.; Eddy, S.R. HMMER web server: Interactive sequence similarity searching. Nucleic Acids Res. 2011, 39 (Suppl. 2), W29–W37. [Google Scholar] [CrossRef] [PubMed]
- Letunic, I.; Khedkar, S.; Bork, P. SMART: Recent updates, new developments and status in 2020. Nucleic Acids Res. 2021, 49, D458–D460. [Google Scholar] [CrossRef]
- Wang, J.; Chitsaz, F.; Derbyshire, M.K.; Gonzales, N.R.; Gwadz, M.; Lu, S.; Marchler, G.H.; Song, J.S.; Thanki, N.; Yamashita, R.A.; et al. The conserved domain database in 2023. Nucleic Acids Res. 2023, 51, D384–D388. [Google Scholar] [CrossRef]
- Artimo, P.; Jonnalagedda, M.; Arnold, K.; Baratin, D.; Csardi, G.; de Castro, E.; Duvaud, S.; Flegel, V.; Fortier, A.; Gasteiger, E.; et al. ExPASy: SIB bioinformatics resource portal. Nucleic Acids Res. 2012, 40, W597–W603. [Google Scholar] [CrossRef]
- Martin, D.P.; Chou, K.-C.; Shen, H.-B. A New Method for Predicting the Subcellular Localization of Eukaryotic Proteins with Both Single and Multiple Sites: Euk-mPLoc 2.0. PLoS ONE 2010, 5, e9931. [Google Scholar]
- Krogh, A.; Larsson, B.; von Heijne, G.; Sonnhammer, E.L.L. Predicting transmembrane protein topology with a hidden markov model: Application to complete genomes. J. Mol. Biol. 2001, 305, 567–580. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Wang, J.; Wang, W.; Hu, T.; Hu, H.; Bao, C. A high-quality chromosome-level genome assembly reveals genetics for important traits in eggplant. Hortic. Res. 2020, 7, 153. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Chen, B.; Wang, D.; Zhu, X.; Li, M.; Zhang, J.; Chen, M.; Wang, M.; Riksen, T.; Liu, J.; et al. In vivo maternal haploid induction in tomato. Plant Biotechnol. J. 2021, 20, 250–252. [Google Scholar] [CrossRef] [PubMed]
- Hall, B.G. Building Phylogenetic Trees from Molecular Data with MEGA. Mol. Biol. Evol. 2013, 30, 1229–1235. [Google Scholar] [CrossRef]
- Bailey, T.L.; Boden, M.; Buske, F.A.; Frith, M.; Grant, C.E.; Clementi, L.; Ren, J.; Li, W.W.; Noble, W.S. MEME SUITE: Tools for motif discovery and searching. Nucleic Acids Res. 2009, 37, W202–W208. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Jin, J.; Guo, A.-Y.; Zhang, H.; Luo, J.; Gao, G. GSDS 2.0: An upgraded gene feature visualization server. Bioinformatics 2015, 31, 1296–1297. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Lescot, M.; Déhais, P.; Thijs, G.; Marchal, K.; Moreau, Y.; Van de Peer, Y.; Rouzé, P.; Rombauts, S. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002, 30, 325–327. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Cyprys, P.; Lindemeier, M.; Sprunck, S. Gamete fusion is facilitated by two sperm cell-expressed DUF679 membrane proteins. Nat. Plants 2019, 5, 253–257. [Google Scholar] [CrossRef]
- Kelliher, T.; Starr, D.; Su, X.; Tang, G.; Chen, Z.; Carter, J.; Wittich, P.E.; Dong, S.; Green, J.; Burch, E.; et al. One-step genome editing of elite crop germplasm during haploid induction. Nat. Biotechnol. 2019, 37, 287–292. [Google Scholar] [CrossRef]
- Kim, H.J.; Ok, S.H.; Bahn, S.C.; Jang, J.; Oh, S.A.; Park, S.K.; Twell, D.; Ryu, S.B.; Shin, J.S. Endoplasmic Reticulum– and Golgi-Localized Phospholipase A2 Plays Critical Roles in Arabidopsis Pollen Development and Germination. Plant Cell 2011, 23, 94–110. [Google Scholar] [CrossRef]
- Kelliher, T.; Starr, D.; Richbourg, L.; Chintamanani, S.; Delzer, B.; Nuccio, M.L.; Green, J.; Chen, Z.; McCuiston, J.; Wang, W.; et al. MATRILINEAL, a sperm-specific phospholipase, triggers maize haploid induction. Nature 2017, 542, 105–109. [Google Scholar] [CrossRef]
- Yao, L.; Zhang, Y.; Liu, C.X.; Liu, Y.; Wang, Y.L.; Liang, D.W.; Liu, J.T.; Sahoo, G.; Kelliher, T. OsMATL mutation induces haploid seed formation in indica rice. Nat. Plants 2018, 4, 530–533. [Google Scholar] [CrossRef]
- Liu, C.X.; Zhong, Y.; Oi, X.L.; Chen, M.; Li, Z.K.; Chen, C.; Tian, X.L.; Li, I.L.; Jiao, Y.Y.; Wang, D.; et al. Extension of the in vivo haploid induction system from diploid maize to hexaploid wheat. Plant Biotechnol. J. 2020, 18, 316–318. [Google Scholar] [CrossRef]
- Cheng, Z.X.; Sun, Y.; Yang, S.H.; Zhi, H.; Yin, T.; Ma, X.J.; Zhang, H.S.; Diao, X.M.; Guo, Y.; Li, X.H.; et al. Establishing in planta haploid inducer line by edited SiMTL in foxtail millet (Setaria italica). Plant Biotechnol. J. 2021, 19, 1089–1091. [Google Scholar] [CrossRef]
- Kelliher, T.; Starr, D.; Wang, W.; McCuiston, J.; Zhong, H.; Nuccio, M.L.; Martin, B. Maternal Haploids Are Preferentially Induced by CENH3-tailswap Transgenic Complementation in Maize. Front. Plant Sci. 2016, 7, 414. [Google Scholar] [CrossRef]
- Long, L.; Feng, Y.M.; Shang, S.Z.; Zhao, J.R.; Hu, G.Y.; Xu, F.C.; Song, C.P.; Jin, S.X.; Gao, W. In vivo maternal haploid induction system in cotton. Plant Physiol. 2024, 194, 1286–1289. [Google Scholar] [CrossRef]
- Yin, S.; Li, S.; Sun, L.; Shi, K.X.; Fan, S.S.; Liu, X.W.; Ren, H.Z. Mutating the maternal haploid inducer gene CsDMP in cucumber produces haploids in planta. Plant Physiol. 2024, 194, 1282–1285. [Google Scholar] [CrossRef]


| Gene Rename | Sequence ID | ORF | Chromosome | Number of aa | Molecular Weight (kDa) | pI | Subcellular Prediction | TM Domain |
|---|---|---|---|---|---|---|---|---|
| CazDMP1 | Capana01g003066 | 537 | Chr01 | 178 | 19,505.76 | 8.2 | Cell membrane | 4 |
| CazDMP2 | Capana08g001833 | 519 | Chr08 | 172 | 18,884.65 | 7.76 | Cell membrane | 3 |
| CazDMP3 | Capana08g001834 | 693 | Chr08 | 230 | 25,311.34 | 8.3 | Cell membrane | 5 |
| CazDMP4 | Capana09g000598 | 789 | Chr09 | 262 | 28,696.4 | 7.48 | Cell membrane | 4 |
| CazDMP5 | Capana04g002148 | 687 | Chr04 | 228 | 24,854.16 | 8.19 | Cell membrane Extracellular | 4 |
| CazDMP6 | Capana08g001404 | 687 | Chr08 | 228 | 25,069.12 | 9.17 | Cell membrane | 4 |
| CamDMP1 | CA.PGAv.1.6.scaffold889.3 | 537 | Chr01 | 178 | 19,505.76 | 8.2 | Cell membrane | 2 |
| CamDMP2 | CA.PGAv.1.6.scaffold529.59 | 633 | Chr02 | 210 | 22,959.81 | 5.6 | Extracellular | 3 |
| CamDMP3 | CA.PGAv.1.6.scaffold1604.5 | 669 | Chr11 | 223 | 24,404.62 | 8.25 | Cell membrane Extracellular | 4 |
| CamDMP4 | CA.PGAv.1.6.scaffold855.1 | 693 | PGAv.1.6.scaffold855 | 230 | 25,283.28 | 8.3 | Cell membrane | 3 |
| CamDMP5 | CA.PGAv.1.6.scaffold4196.1 | 687 | PGAv.1.6.scaffold4196 | 228 | 25,082.04 | 8.3 | Cell membrane Extracellular Nucleus | 4 |
| CacDMP1 | CC.CCv1.2.scaffold838.12 | 537 | Chr01 | 178 | 19,538.77 | 6.79 | Cell membrane | 4 |
| CacDMP2 | CC.CCv1.2.scaffold1256.1 | 690 | Chr08 | 229 | 25,188.22 | 9.03 | Cell membrane Endoplasmic reticulum | 4 |
| CacDMP3 | CC.CCv1.2.scaffold605.8 | 582 | Chr08 | 194 | 21,384.51 | 6.81 | Cell membrane | 3 |
| CacDMP4 | CC.CCv1.2.scaffold605.10 | 687 | Chr08 | 228 | 25,009.98 | 8.64 | Cell membrane Extracellular Nucleus | 4 |
| CacDMP5 | CC.CCv1.2.scaffold233.37 | 789 | Chr09 | 262 | 28,683.4 | 7.5 | Extracellular | 4 |
| CacDMP6 | CC.CCv1.2.scaffold439.5 | 1671 | Chr11 | 556 | 61,532.35 | 8.94 | Chloroplast | 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Zhang, D.; Li, X.; He, J.; Chen, Z.; Xu, N.; Zhong, Y.; Yao, S.; Qu, L.; Li, B.; et al. Genome-Wide Identification, Characterization, and Expression Analysis of the DMP Gene Family in Pepper (Capsicum annuum L.). Horticulturae 2024, 10, 679. https://doi.org/10.3390/horticulturae10070679
Zhang Y, Zhang D, Li X, He J, Chen Z, Xu N, Zhong Y, Yao S, Qu L, Li B, et al. Genome-Wide Identification, Characterization, and Expression Analysis of the DMP Gene Family in Pepper (Capsicum annuum L.). Horticulturae. 2024; 10(7):679. https://doi.org/10.3390/horticulturae10070679
Chicago/Turabian StyleZhang, Yamin, Doudou Zhang, Xinru Li, Jie He, Zhuona Chen, Nan Xu, Yike Zhong, Shuqian Yao, Lingbo Qu, Bo Li, and et al. 2024. "Genome-Wide Identification, Characterization, and Expression Analysis of the DMP Gene Family in Pepper (Capsicum annuum L.)" Horticulturae 10, no. 7: 679. https://doi.org/10.3390/horticulturae10070679





