Influence of the Application of Different Zinc Oxide Nanoparticles on a Lettuce Crop Grown in an Acidic Mediterranean Soil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis and Characterization of ZnO Nanoparticles (NPs)
2.2. Pot Experiment
2.3. Plant Analysis
2.4. Soil Pore Water Analysis
2.5. Soil Analysis
2.6. Statistical Analysis
3. Results
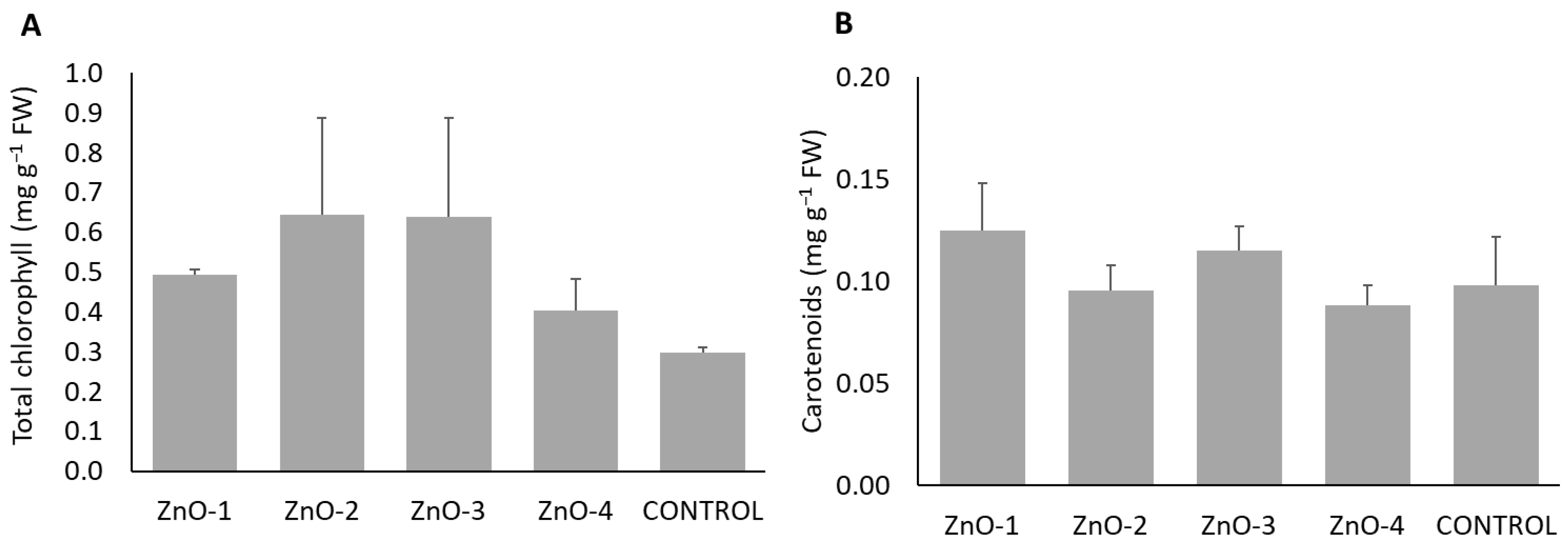
3.1. Plant Analysis
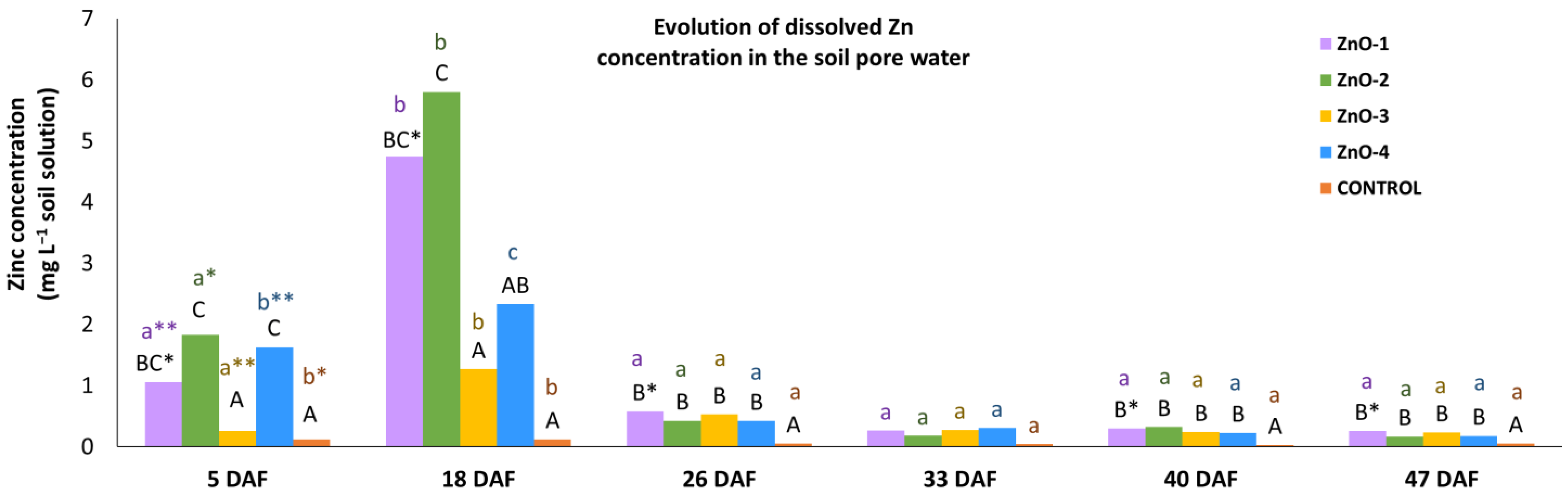
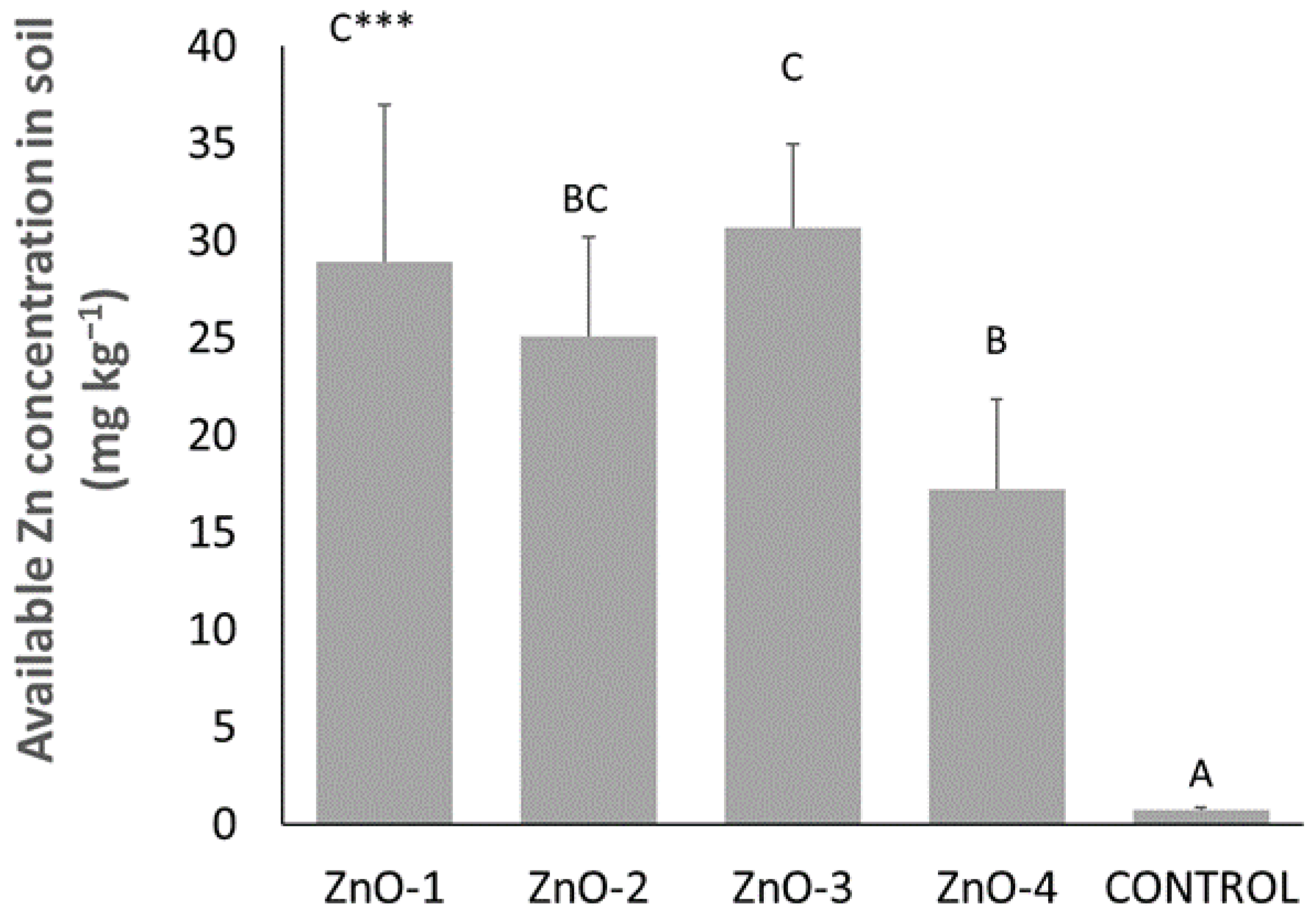
3.2. Soil Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Trujillo-Reyes, J.; Majumdar, S.; Botez, C.E.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Exposure Studies of Core-Shell Fe/Fe3O4 and Cu/CuO NPs to Lettuce (Lactuca sativa) Plants: Are They a Potential Physiological and Nutritional Hazard? J. Hazard. Mater. 2014, 267, 255–263. [Google Scholar] [CrossRef]
- Baslam, M.; Morales, F.; Garmendia, I.; Goicoechea, N. Nutritional Quality of Outer and Inner Leaves of Green and Red Pigmented Lettuces (Lactuca sativa L.) Consumed as Salads. Sci. Hortic. 2013, 151, 103–111. [Google Scholar] [CrossRef]
- Thamburaj, S.; Singh, N. Textbook of Vegetables, Tubercrops and Spices; Thamburaj, S., Singh, N., Eds.; Indian Council of Agricultural Research: New Delhi, India, 2001. [Google Scholar]
- Kathi, S.; Laza, H.; Singh, S.; Thompson, L.; Li, W.; Simpson, C. A Decade of Improving Nutritional Quality of Horticultural Crops Agronomically (2012–2022): A Systematic Literature Review. Sci. Total Environ. 2024, 911, 168665. [Google Scholar] [CrossRef] [PubMed]
- Graham, R.D. Micronutrient Deficiencies in Crops and Their Global Significance. In Micronutrient Deficiencies in Global Crop Production; Springer: Dordrecht, The Netherlands, 2008; pp. 41–61. [Google Scholar] [CrossRef]
- Von Grebmer, K.; Bernstein, J.; Wiemers, M.; Reiner, L.; Bachmeier, M.; Hanano, A.; Towey, O.; Cheilleachair, R.N.; Foley, C.; Sheehan, T.; et al. 2023 Global Hunger Index: The Power of Youth in Shaping Food Systems; Global Hunger Index: Berlin, Germany, 2023; pp. 1–60. [Google Scholar]
- Alloway, B.J. Soil Factors Associated with Zinc Deficiency in Crops and Humans. Environ. Geochem. Health 2009, 31, 537–548. [Google Scholar] [CrossRef] [PubMed]
- Lowe, N.M. The Global Challenge of Hidden Hunger: Perspectives from the Field. Proc. Nutr. Soc. 2021, 80, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Muthayya, S.; Rah, J.H.; Sugimoto, J.D.; Roos, F.F.; Kraemer, K.; Black, R.E. The Global Hidden Hunger Indices and Maps: An Advocacy Tool for Action. PLoS ONE 2013, 8, e67860. [Google Scholar] [CrossRef]
- Caro, C.R.; Del Coronell, M.C.; Arrollo, J.; Martínez, G.; Majana, L.S.; Sarmiento-Rubiano, L.A. La Deficiencia de Zinc: Un Problema Global Que Afecta La Salud y El Desarrollo Cognitivo. Arch. Latinoam. Nutr. 2016, 656, 165–175. [Google Scholar]
- Grantham-McGregor, S.; Cheung, Y.B.; Cueto, S.; Glewwe, P.; Richter, L.; Strupp, B. Developmental Potential in the First 5 Years for Children in Developing Countries. Lancet 2007, 369, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Panel, E.; Nda, A. Scientific Opinion on Dietary Reference Values for Zinc. EFSA J. 2014, 12, 3844. [Google Scholar] [CrossRef]
- Cakmak, I.; Kutman, U.B. Agronomic Biofortification of Cereals with Zinc: A Review. Eur. J. Soil Sci. 2018, 69, 172–180. [Google Scholar] [CrossRef]
- Bhardwaj, A.K.; Chejara, S.; Malik, K.; Kumar, R.; Kumar, A.; Yadav, R.K. Agronomic Biofortification of Food Crops: An Emerging Opportunity for Global Food and Nutritional Security. Front. Plant Sci. 2022, 13, 1055278. [Google Scholar] [CrossRef] [PubMed]
- de Almeida, H.; Carmona, V.M.V.; Inocêncio, M.F.; Furtini Neto, A.E.; Cecílio Filho, A.B.; Mauad, M. Soil Type and Zinc Doses in Agronomic Biofortification of Lettuce Genotypes. Agronomy 2020, 10, 124. [Google Scholar] [CrossRef]
- Fortis-Hernández, M.; García-Delgado, J.D.; Preciado-Rangel, P.; Trejo-Valencia, R.; Sánchez-Estrada, A.; Fortiz-Hernández, J. Commercial and Phytochemical Quality in Biofortified ‘Orejona’ Lettuce with Zinc Oxide Nanoparticles. Not. Bot. Horti Agrobot. 2022, 50, 12969. [Google Scholar] [CrossRef]
- Meneghelli, C.M.; Fontes, P.C.R.; Milagres, C.D.C.; da Silva, J.M.; Junior, E.G. Zinc-Biofortified Lettuce in Aeroponic System. J. Plant Nutr. 2021, 44, 2146–2156. [Google Scholar] [CrossRef]
- Ortiz, R.; Gascó, G.; Méndez, A.; Sanchez-Martín, L.; Obrador, A.; Almendros, P. Comparative Study of Traditional and Environmentally Friendly Zinc Sources Applied in Alkaline Fluvisol Soil: Lettuce Biofortification and Soil Zinc Status. Agronomy 2023, 13, 3014. [Google Scholar] [CrossRef]
- Almendros, P.; Obrador, A.; Gonzalez, D.; Alvarez, J.M. Biofortification of Zinc in Onions (Allium cepa L.) and Soil Zn Status by the Application of Different Organic Zn Complexes. Sci. Hortic. 2015, 186, 254–265. [Google Scholar] [CrossRef]
- Xu, M.; Du, L.; Liu, M.; Zhou, J.; Pan, W.; Fu, H.; Zhang, X.; Ma, Q.; Wu, L. Glycine-Chelated Zinc Rather than Glycine-Mixed Zinc Has Lower Foliar Phytotoxicity than Zinc Sulfate and Enhances Zinc Biofortification in Waxy Corn. Food Chem. 2022, 370, 131031. [Google Scholar] [CrossRef]
- Fontes, R.L.F.; Pereira, J.M.N.; Neves, J.C.L. Uptake and Translocation of Cd and Zn in Two Lettuce Cultivars. Ann. Acad. Bras. Cienc. 2014, 86, 907–922. [Google Scholar] [CrossRef]
- Alloway, B.J. Zinc in Soils and Crop Nutrition, 2nd ed.; International Zinc Association: Brussels, Belgium; Paris, France, 2008. [Google Scholar]
- Navarro-León, E.; Albacete, A.; de la Torre-González, A.; Ruiz, J.M.; Blasco, B. Phytohormone Profile in Lactuca Sativa and Brassica oleracea Plants Grown under Zn Deficiency. Phytochemistry 2016, 130, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Hamzah Saleem, M.; Usman, K.; Rizwan, M.; Al Jabri, H.; Alsafran, M. Functions and Strategies for Enhancing Zinc Availability in Plants for Sustainable Agriculture. Front. Plant Sci. 2022, 13, 1033092. [Google Scholar] [CrossRef]
- Noulas, C.; Tziouvalekas, M.; Karyotis, T. Zinc in Soils, Water and Food Crops. J. Trace Elem. Med. Biol. 2018, 49, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.T.; Malik, A.; Alwarthan, A.; Shaik, M.R. The Enormity of the Zinc Deficiency Problem and Available Solutions; an Overview. Arab. J. Chem. 2022, 15, 103668. [Google Scholar] [CrossRef]
- Al Jabri, H.; Saleem, M.H.; Rizwan, M.; Hussain, I.; Usman, K.; Alsafran, M. Zinc Oxide Nanoparticles and Their Biosynthesis: Overview. Life 2022, 12, 594. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.; Garg, N. Zinc Toxicity in Plants: A Review. Planta 2021, 253, 129. [Google Scholar] [CrossRef] [PubMed]
- Berry, W.L. The Nutrient Status of Zinc in Lettuce Evaluated by Plant Analysis. J. Am. Soc. Hortic. Sci. 1971, 96, 412–414. [Google Scholar] [CrossRef]
- Rengel, Z. Availability of Mn, Zn and Fe in the Rhizosphere. J. Soil Sci. Plant Nutr. 2015, 15, 397–409. [Google Scholar] [CrossRef]
- Moreno-Lora, A.; Delgado, A. Factors Determining Zn Availability and Uptake by Plants in Soils Developed under Mediterranean Climate. Geoderma 2020, 376, 114509. [Google Scholar] [CrossRef]
- FAO; IFAD; UNICEF; WFP; WHO. The State of Food Security and Nutrition in the World 2022; FAO: Rome, Italy, 2022; ISBN 9789251364994. [Google Scholar]
- Tsuzuki, T. Commercial Scale Production of Inorganic Nanoparticles. Int. J. Nanotechnol. 2009, 6, 567–578. [Google Scholar] [CrossRef]
- Yadav, A.; Yadav, K.; Abd-Elsalam, K.A. Exploring the Potential of Nanofertilizers for a Sustainable Agriculture. Plant Nano Biol. 2023, 5, 100044. [Google Scholar] [CrossRef]
- Ahmed, B.; Khan, M.S.; Musarrat, J. Toxicity Assessment of Metal Oxide Nano-Pollutants on Tomato (Solanum Lycopersicon): A Study on Growth Dynamics and Plant Cell. Environ. Pollut. 2020, 240, 802–816. [Google Scholar] [CrossRef]
- Almendros, P.; González, D.; Ibañez, M.A.; Smolders, E.; Fernández, M.D.; García-Gomez, C.; Obrador, A. Influence of ZnO Particle Size and Soil Characteristics on the Estimation of Long-Term Zn Bioavailability by Chemical Extraction Methods and Diffusive Gradients in Thin-Films (DGT). J. Soil Sci. Plant Nutr. 2022, 22, 3901–3913. [Google Scholar] [CrossRef]
- De Francisco, M.; Mira, S.; Duraes, L.; Romeiro, A.; Alvarez-Torrellas, S.; Almendros, P. Zinc Oxide Nanoparticles and Fine Particles: Synthesis, Characterization and Evaluation of the Toxic Effect on Germination and Vigour of Solanum licopersicum L. Agronomy 2024, 14, 980. [Google Scholar] [CrossRef]
- Liu, X.; Wang, F.; Shi, Z. Bioavailability of Zn in ZnO Nanoparticle-Spiked Soil and the Implications to Maize Plants. J. Nanoparticle Res. 2015, 17, 175. [Google Scholar] [CrossRef]
- Milani, N.; Hettiarachchi, G.M.; Kirby, J.K.; Beak, D.G. Fate of Zinc Oxide Nanoparticles Coated onto Macronutrient Fertilizers in an Alkaline Calcareous Soil. PLoS ONE 2015, 10, e0126275. [Google Scholar] [CrossRef] [PubMed]
- Akhil, K.; Jayakumar, J.; Gayathri, G.; Khan, S.S. Effect of Various Capping Agents on Photocatalytic, Antibacterial and Antibiofilm Activities of ZnO Nanoparticles. J. Photochem. Photobiol. B Biol. 2016, 160, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Huy, N.N.; Thanh Thuy, V.T.; Thang, N.H.; Thuy, N.T.; Quynh, L.T.; Khoi, T.T.; Van Thanh, D. Facile One-Step Synthesis of Zinc Oxide Nanoparticles by Ultrasonic-Assisted Precipitation Method and Its Application for H2S Adsorption in Air. J. Phys. Chem. Solids 2019, 132, 99–103. [Google Scholar] [CrossRef]
- Lee, J.; Easteal, A.J.; Pal, U.; Bhattacharyya, D. Evolution of ZnO Nanostructures in Sol–Gel Synthesis. Curr. Appl. Phys. 2009, 9, 792–796. [Google Scholar] [CrossRef]
- Chandrasekaran, P.; Viruthagiri, G.; Srinivasan, N. The Effect of Various Capping Agents on the Surface Modifications of Sol–Gel Synthesised ZnO Nanoparticles. J. Alloys Compd. 2012, 540, 89–93. [Google Scholar] [CrossRef]
- MAPA. Métodos Oficiales de Análisis. Tomo III; Ministerio de Agricultura, Pesca y Alimentación: Madrid, Spain, 1994. [Google Scholar]
- FAO. Base Referencial Mundial Del Recurso Suelo. Sistema Internacional de Clasificación de Suelos; Organización de las Naciones Unidas para la Alimentación y la Agricultura, Ed.; Organización de las Naciones Unidas para la Alimentación y la Agricultura: Roma, Italy, 2015; ISBN 9789253083695. [Google Scholar]
- Ramos, C.; Pomares, F. Abonado de Los Cultivos Hortícolas. In Guía Práctica de la Fertilización Racional de los Cultivos en España Parte II; Ministerio de Medio Ambiente y Medio Rural y Marino: Madrid, Spain, 2010; pp. 181–192. [Google Scholar]
- European Commission. 543/2011/UE Commission Implementing Regulation (EU) No 543/2011 of 7 June 2011. Laying down Detailed Rules for the Application of Council Regulation (EC) No 1234/2007 in Respect of the Fruit and Vegetables and Processed Fruit and Vegetables Sectors. Off. J. Eur. Union 2011, 157, 1–63. [Google Scholar]
- AOAC. Official Methods of Analysis; Association of Official Analytical Chemists: Washington, DC, USA, 1990. [Google Scholar]
- Lichtenthaler, H. Chlorophylls and Carotenoids:Pigments of Photosynthetic Biomembranes. Methods Enzym. 1987, 148, 350–382. [Google Scholar]
- Almendros, P.; Gonzalez, D.; Alvarez, J.M. Long-Term Bioavailability Effects of Synthesized Zinc Chelates Fertilizers on the Yield and Quality of a Flax (Linum usitatissimum L.) Crop. Plant Soil 2013, 368, 251–265. [Google Scholar] [CrossRef]
- Feng, M.H.; Shan, X.Q.; Zhang, S.Z.; Wen, B. A Comparison of the Rhizosphere-Based Method with DTPA, EDTA, CaCl2, and NaNO3 Extraction Methods for Prediction of Bioavailability of Metals in Soil to Barley. Environ. Pollut. 2005, 137, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Luo, X.; Wang, Y.; Feng, Y. Evaluation of Zinc Oxide Nanoparticles on Lettuce (Lactuca sativa L.) Growth and Soil Bacterial Community. Environ. Sci. Pollut. Res. 2017, 25, 6026–6035. [Google Scholar] [CrossRef] [PubMed]
- García-Gómez, C.; Obrador, A.; González, D.; Babín, M.; Fernández, M.D. Comparative Study of the Phytotoxicity of ZnO Nanoparticles and Zn Accumulation in Nine Crops Grown in a Calcareous Soil and an Acidic Soil. Sci. Total Environ. 2018, 644, 770–780. [Google Scholar] [CrossRef] [PubMed]
- Song, U.; Kim, J. Zinc Oxide Nanoparticles: A Potential Micronutrient Fertilizer for Horticultural Crops with Little Toxicity. Hortic. Environ. Biotechnol. 2020, 61, 625–631. [Google Scholar] [CrossRef]
- Hong, J.; Xu, F.; Chen, G.; Huang, X.; Wang, S.; Du, L.; Ding, G. Evaluation of the Effects of Nitrogen, Phosphorus, and Potassium Applications on the Growth, Yield, and Quality of Lettuce (Lactuca sativa L.). Agronomy 2022, 12, 2477. [Google Scholar] [CrossRef]
- de Lima, B.M.; Noboa, C.S.; de Lima, F.M.; da Costa Mello, S.; Purquerio, L.F.V.; Sala, F.C. Agronomic Biofortification with Zinc in Hydroponically Cultivated Lettuce. Aust. J. Crop Sci. 2023, 17, 198–205. [Google Scholar] [CrossRef]
- Graciano, P.D.; Jacinto, A.C.P.; da Silveira, A.J.; Castoldi, R.; de Lima, T.M.; de Oliveira Charlo, H.C.; da Silva, I.G.; Marin, M.V. Agronomic Biofortification with Zinc in Curly Lettuce Cultivars. Rev. Bras. Ciencias Agrar. 2020, 15, 1–9. [Google Scholar] [CrossRef]
- Marschner, H. Mineral Nutrition of Higher Plants, 3rd ed.; Academic Press: Cambridge, MA, USA, 2012. [Google Scholar]
- Ganoe, K.; Ketterings, Q.; Herendeen, N. Zinc. In Agronomy Fact Sheet Series; Cornell University: Ithaca, NY, USA, 2007; Available online: http://nmsp.cals.cornell.edu/publications/factsheets/factsheet32.pdf (accessed on 20 June 2024).
- de Sales Pereira, E.J.; Diniz, M.J.; Pereira, J.O.; da Costa Bandeira, P.P.; Costa, A.C.C.; de Lima Silva, S.; Batista, R.O.; Linhares, P.C.F.; de Sousa, R.P.; de Assis, J.P.; et al. Effect of the Organic Matter from Crop Residues on the Structuration of an Oxisol, at Semi-Arid Region. Aust. J. Crop Sci. 2023, 17, 107–117. [Google Scholar] [CrossRef]
- Longnecker, N.E.; Robson, A.D. Distribution and Transport of Zinc in Plants. In Zinc Soils Plants; Springer: Dordrecht, The Netherlands, 1993; pp. 79–91. [Google Scholar] [CrossRef]
- Cakmak, I.; Marschner, H. Increase in Membrane Permeability and Exudation in Roots of Zinc Deficient Plants. J. Plant Physiol. 1988, 132, 356–361. [Google Scholar] [CrossRef]
- Fairweather-Tait, S.J. Zinc in Human Nutrition. Nutr. Res. Rev. 1988, 1, 23–37. [Google Scholar] [CrossRef] [PubMed]
- Song, U.; Jun, H.; Waldman, B.; Roh, J.; Kim, Y.; Yi, J.; Lee, E.J. Functional Analyses of Nanoparticle Toxicity: A Comparative Study of the Effects of TiO2 and Ag on Tomatoes (Lycopersicon esculentum). Ecotoxicol. Environ. Saf. 2013, 93, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Roosta, H.R.; Estaji, A.; Niknam, F. Effect of Iron, Zinc and Manganese Shortage-Induced Change on Photosynthetic Pigments, Some Osmoregulators and Chlorophyll Fluorescence Parameters in Lettuce. Photosynthetica 2018, 56, 606–615. [Google Scholar] [CrossRef]
- Balashouri, P.; Prameeladevi, Y. Effect of Zinc on Germination, Growth and Pigment Content and Phytomass of Vigna radiata and Sorghum bicolor. J. Ecobiol. 1995, 7, 109–114. [Google Scholar]
- Landa, P. Positive Effects of Metallic Nanoparticles on Plants: Overview of Involved Mechanisms. Plant Physiol. Biochem. 2021, 161, 12–24. [Google Scholar] [CrossRef]
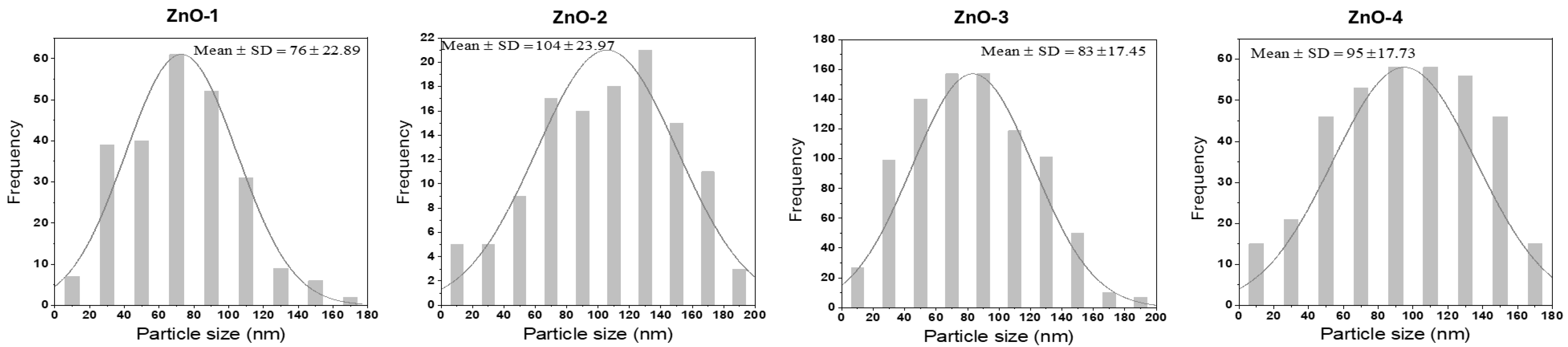


| ZnO-1 | ZnO-2 | ZnO-3 | ZnO-4 | |
|---|---|---|---|---|
| Synthesis method | co-precipitation [40] | co-precipitation [41] | sol–gel [42] | sol–gel [43] |
| Metal precursor | Zn(NO3)2 | ZnSO4 | ZnC4H6O4 | ZnSO4 |
| Hydroxyl group source | NaOH | NH4OH | NaOH | NaOH |
| Hydrodynamic diameter by DLS (nm) | 312.4 ± 7.4 | - | 183.3 ± 1.1 | 573.2 ± 10.0 |
| TEM particle size (nm) | 76 ± 23 | 104 ± 24 | 83 ± 17 | 95 ± 18 |
| Zeta potential (mV) | 6.3 ± 0.5 | −7.4 ± 5.7 | 11.4 ± 0.9 | 16.5 ± 3.5 |
| Bioavailable Zn Concentration (LMWOAs) | Soluble Zn Concentration in Mature Leaves | |
|---|---|---|
| Zn concentrations in the soil pore water (18 days AP) | 0.533 * | 0.598 * |
| Zn concentrations in the soil pore water (47 days AP) | 0.722 * | NS |
| Bioavailable Zn concentration (LMWOAs) | - | 0.808 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Francisco, M.d.; Fernandes-Silva, P.; Durães, L.; Romeiro, A.; Álvarez-Torrellas, S.; Almendros, P. Influence of the Application of Different Zinc Oxide Nanoparticles on a Lettuce Crop Grown in an Acidic Mediterranean Soil. Horticulturae 2024, 10, 681. https://doi.org/10.3390/horticulturae10070681
Francisco Md, Fernandes-Silva P, Durães L, Romeiro A, Álvarez-Torrellas S, Almendros P. Influence of the Application of Different Zinc Oxide Nanoparticles on a Lettuce Crop Grown in an Acidic Mediterranean Soil. Horticulturae. 2024; 10(7):681. https://doi.org/10.3390/horticulturae10070681
Chicago/Turabian StyleFrancisco, Marina de, Pedro Fernandes-Silva, Luisa Durães, Andreia Romeiro, Silvia Álvarez-Torrellas, and Patricia Almendros. 2024. "Influence of the Application of Different Zinc Oxide Nanoparticles on a Lettuce Crop Grown in an Acidic Mediterranean Soil" Horticulturae 10, no. 7: 681. https://doi.org/10.3390/horticulturae10070681





