Salicylic Acid Improves Yield, Fruit Quality, and Post-Harvest Storage in Sweet Cherry (Prunus avium L.) cv. Lapins Subjected to Late-Deficit Irrigation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Description of the Study Site and Plant Material
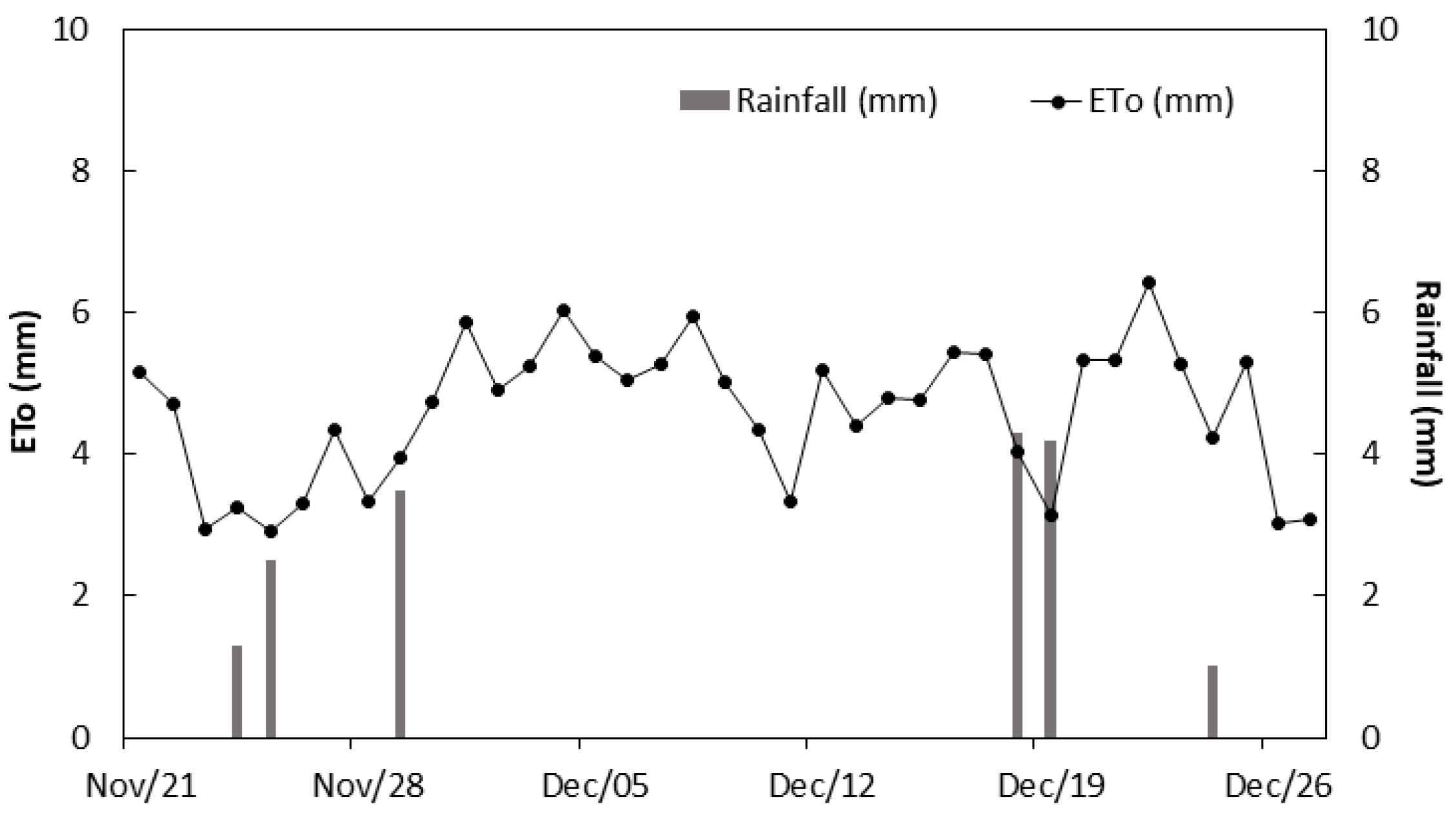
2.2. Treatments and Weather Conditions
2.3. Leaf Water Potential Measurement
2.4. Yield and Fruit Quality Analysis
2.5. Experimental Design and Statistical Analyses
3. Results
3.1. Environmental Conditions
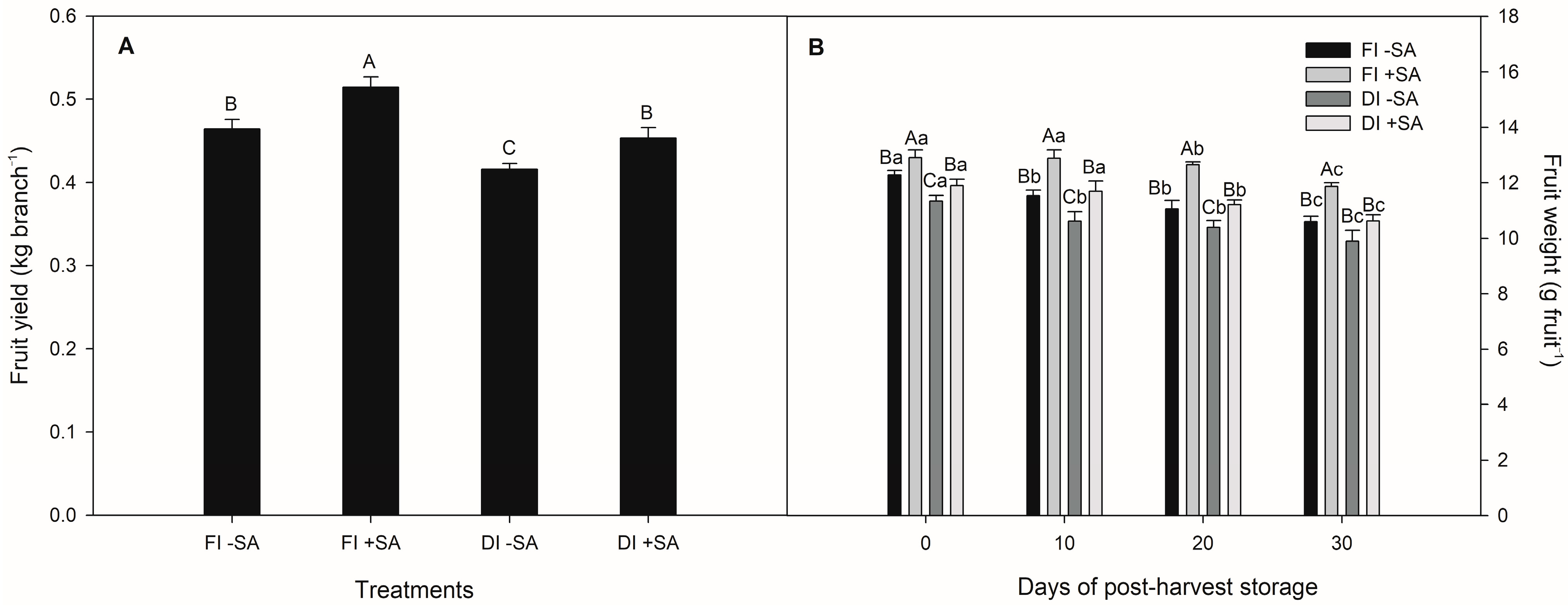
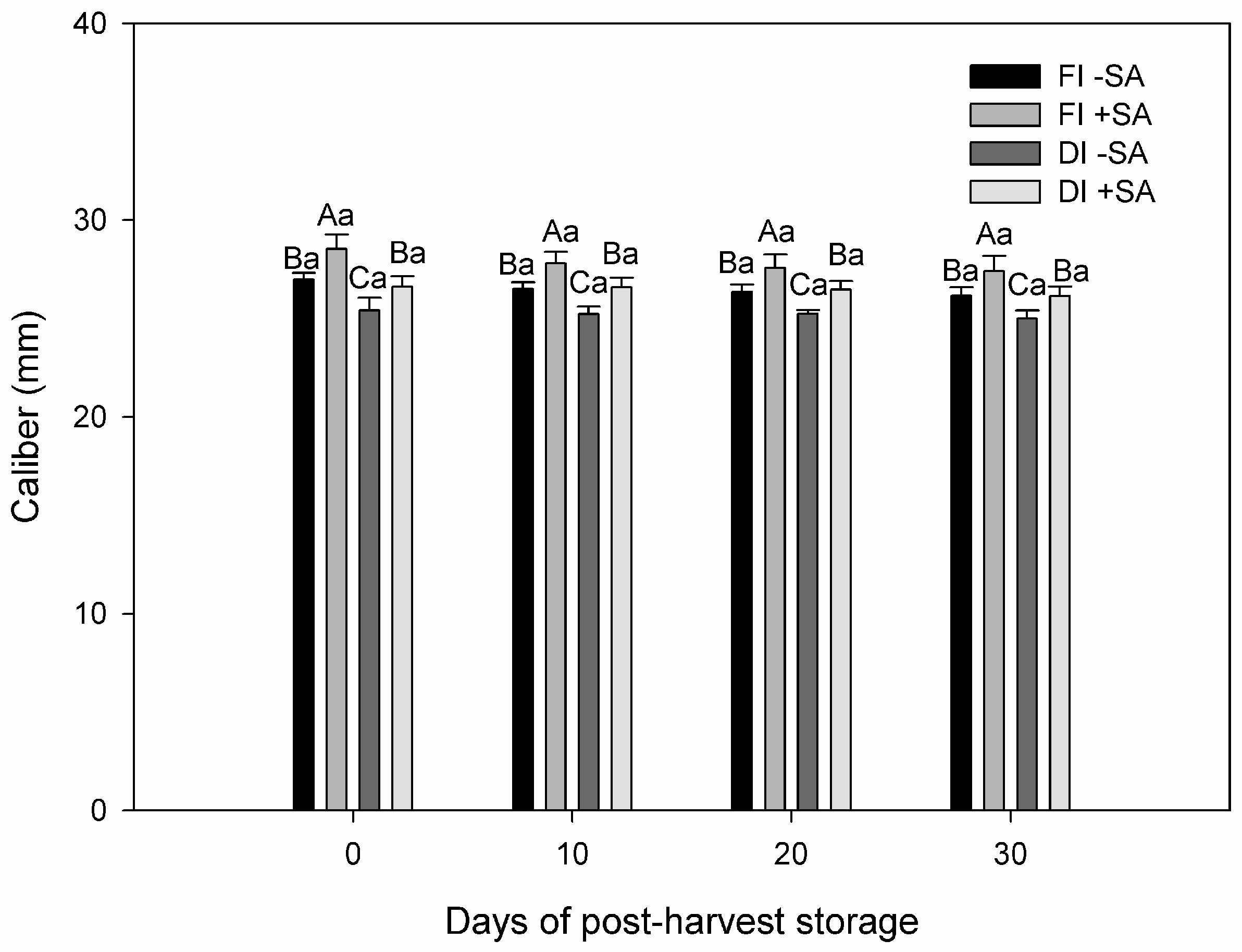
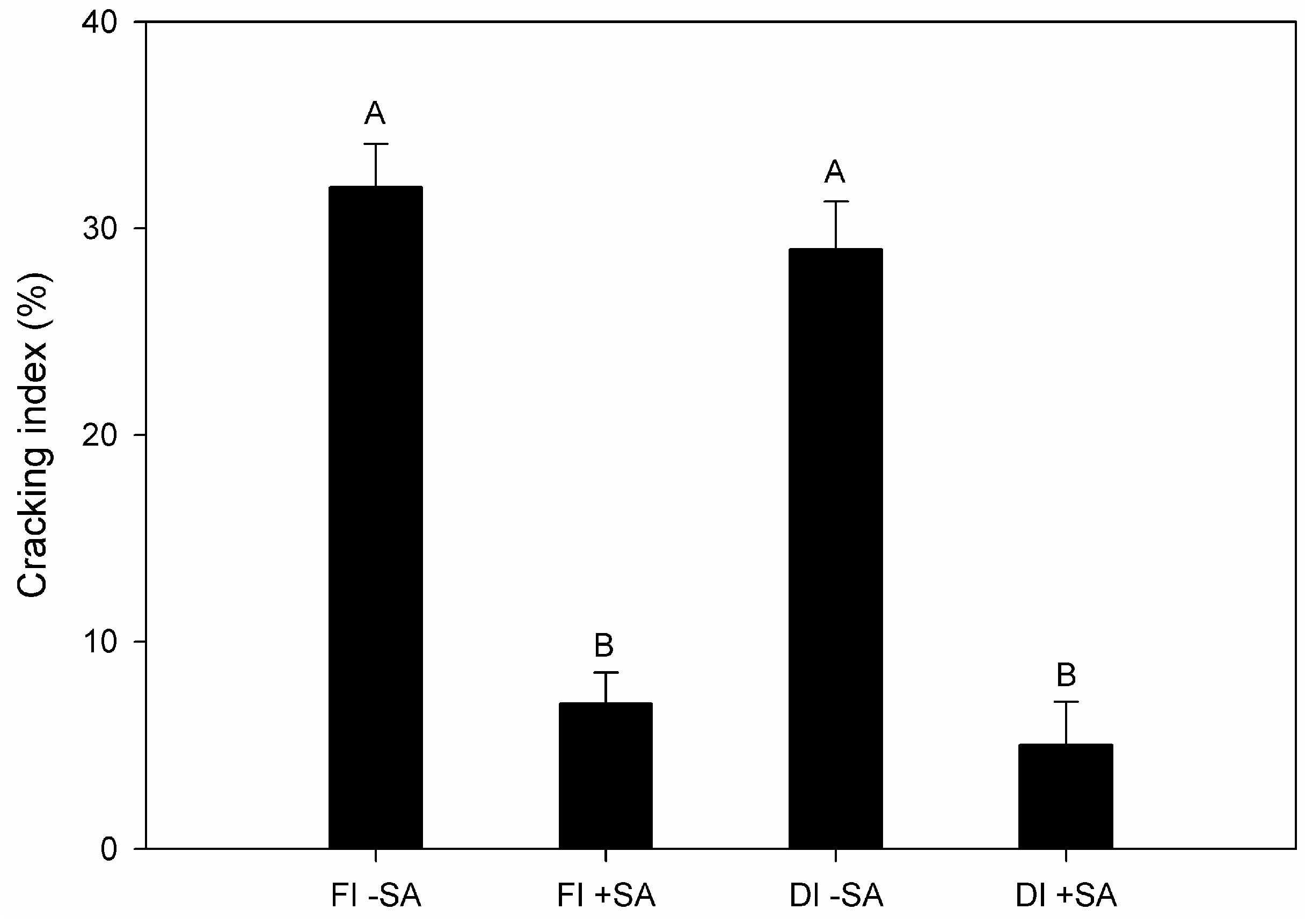
3.2. Fruit Yield and Quality of P. avium
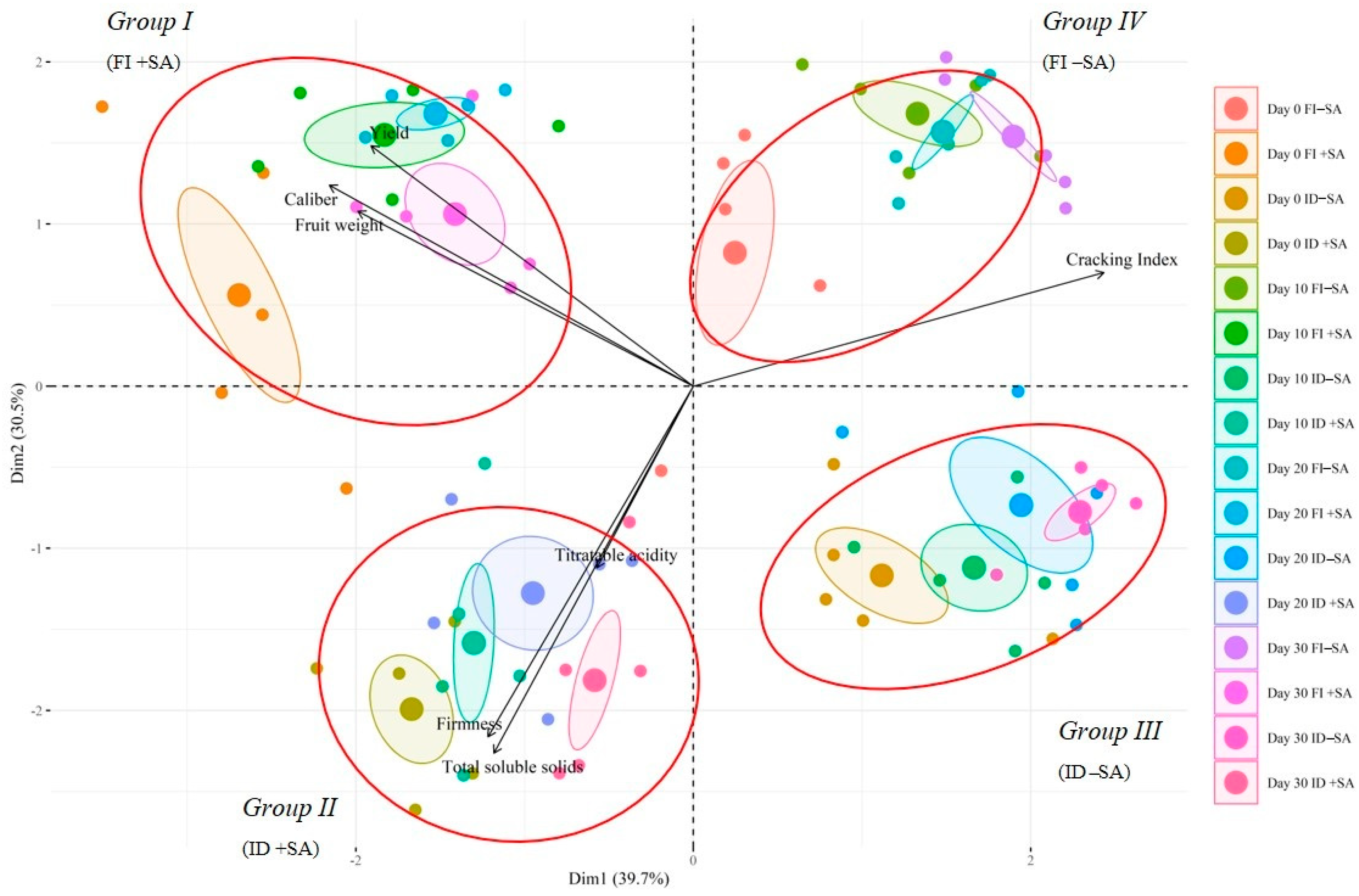
3.3. Multivariate Analysis Based on Quality Parameters Measured in P. avium cv. Lapins Fruit during Post-Harvest Storage under Different Treatments
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sheffield, J.; Wood, E. Drought: Past Problems and Future Scenarios; Routledge: London, UK, 2012. [Google Scholar]
- IPCC. Climate Change and Land: An IPCC Special Report on Climate Change, Desertification, Land Degradation, Sustainable Land Management, Food Security, and Greenhouse Gas Fluxes in Terrestrial Ecosystems; Shukla, P.R., Skea, J., Buendia, E.C., Masson-Delmotte, V., Pörtner, H.-O., Roberts, D.C., Zhai, P., Slade, R., Connors, S., van Diemen, R., et al., Eds.; IPCC: Geneva, Switzerland, 2019. [Google Scholar]
- Khan, Z.; Jan, R.; Asif, S.; Farooq, M.; Jang, Y.; Kim, E.; Kim, N.; Kim, K. Exogenous melatonin induces salt and drought stress tolerance in rice by promoting plant growth and defense system. Sci. Rep. 2024, 14, 1214. [Google Scholar] [CrossRef] [PubMed]
- Bertamini, M.; Zulini, L.; Muthuchelian, K.; Nedunchezhian, N. Effect of water deficit on photosynthetic and other physiological responses in grapevine (Vitis vinifera L. cv. Riesling) plants. Photosynthetica 2006, 44, 151–154. [Google Scholar] [CrossRef]
- Ribera-Fonseca, A.; Jorquera-Fontena, E.; Castro, M.; Acevedo, P.; Parra, J.C.; Reyes-Díaz, M. Exploring VIS/NIR reflectance indices for the estimation of water status in highbush blueberry plants grown under full and deficit irrigation. Sci. Hortic. 2019, 256, 108557. [Google Scholar] [CrossRef]
- González-Villagra, J.; Bravo, L.A.; Reyes-Díaz, M.; Cohen, J.D.; Ribera-Fonseca, A.; López-Olivari, R.; Jorquera-Fontena, E.; Tighe-Neira, R. Pre-harvest salicylic acid application affects fruit quality and yield under deficit irrigation in Aristotelia chilensis (Mol.) Plants. Plants 2023, 12, 3279. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Gao, H.; Jia, X.; Wei, J.; Mao, K.; Ma, F. MdHB-7 regulates water use efficiency in transgenic apple (Malus domestica) Under Long-Term Moderate Water Deficit. Front. Plant Sci. 2021, 12, 740492. [Google Scholar] [CrossRef] [PubMed]
- Marsal, J.; Lopez Velasco, G.; del Campo, J.; Mata, M.; Arbones, A.; Girona, J. Postharvest regulated deficit irrigation in ‘Summit’ sweet cherry: Fruit yield and quality in the following season. Irrig. Sci. 2010, 28, 181–189. [Google Scholar] [CrossRef]
- Blanco, V.; Martínez-Hernández, G.B.; Artés-Hernández, F.; Blaya-Ros, P.J.; Torres-Sánchez, R.; Domingo, R. Water relations and quality changes throughout fruit development and shelf life of sweet cherry grown under regulated deficit irrigation. Agric. Water Manag. 2019, 217, 243–254. [Google Scholar] [CrossRef]
- Jorquera-Fontena, E.; Tighe-Neira, R.; Bota, J.; Inostroza-Blancheteau, C.; Pastenes, C. Response of sink manipulation in Lapins sweet cherry (Prunus avium L.) branches to late-deficit irrigation. Sci. Hortic. 2022, 304, 111323. [Google Scholar] [CrossRef]
- Faienza, M.F.; Corbo, F.; Carocci, A.; Catalano, A.; Clodoveo, M.L.; Grano, M.; Wang, D.Q.; D’Amato, G.; Muragli, M.; Franchini, C.; et al. Novel insights in health-promoting properties of sweet cherries. J. Funct. Foods 2020, 69, 103945. [Google Scholar] [CrossRef]
- Salvadores, Y.; Bastías, R.M. Environmental factors and physiological responses of sweet cherry production under protective cover systems: A review. Chil. J. Agric. Res. 2023, 83, 484–498. [Google Scholar] [CrossRef]
- ODEPA. Boletín de Fruta. Oficina de Estudios y Políticas Agrarias: Santiago de Chile, Chile. 2024. Available online: https://www.odepa.gob.cl/ (accessed on 5 March 2024).
- FAOSTAT. Statistical Databases on Global Food Production and Trade. Food and Agriculture Organization. 2024. Available online: https://www.fao.org/faostat/en/#home (accessed on 5 March 2024).
- Santibáñez, F.; Santibáñez, P.; González, P. Elaboración de una Base Digital del Clima Comunal de Chile: Línea Base (1980–2010) y Proyección Al Año 2050; Ministerio del Medio Ambiente de Chile: Santiago, Chile, 2016. [Google Scholar]
- Garreaud, R.D.; Boisier, J.P.; Rondanelli, R.; Montecinos, A.; Sepúlveda, H.H.; Veloso-Aguila, D. The Central Chile Mega Drought (2010–2018): A climate dynamics perspective. Int. J. Clim. 2020, 40, 421–439. [Google Scholar] [CrossRef]
- Alvarez-Garreton, C.; Boisier, J.P.; Garreaud, R.; Seibert, J.; Vis, M. Progressive water deficitis during multiyear droughts in basins with long hydrological memory in Chile. Hydrol. Earth Syst. Sci. 2021, 25, 429–446. [Google Scholar] [CrossRef]
- Correia, S.; Schouten, R.; Silva, A.; Gonçalves, B. Factors affecting quality and health promoting compounds during growth and postharvest life of sweet cherry (Prunus avium L.). Front. Plant Sci. 2017, 8, 2166. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Jiang, Y.; Zhang, Z. The role of different natural organic acids in postharvest fruit quality management and its mechanism. Food Front. 2023, 4, 1127–1143. [Google Scholar] [CrossRef]
- Correia, S.; Schouten, R.; Silva, A.; Gonçalves, B. Sweet cherry fruit cracking mechanisms and prevention strategies: A review. Sci. Hortic. 2018, 240, 369–377. [Google Scholar] [CrossRef]
- Winkler, A.; Bunger, P.; Lang, P.; Schumann, C.; Brüggenwith, M.; Knoche, M. Mode of action of calcium in reducing macrocraking of sweet cherry fruit. J. Am. Soc. Hort. 2024, 149, 61–74. [Google Scholar] [CrossRef]
- Pereira, S.; Silva, V.; Bacelar, E.; Guedes, F.; Silva, A.P.; Ribeiro, C.; Gonçalves, B. Cracking in sweet cherry cultivars early Bigi and Lapins: Correlation with quality attributes. Plants 2020, 9, 1557. [Google Scholar] [CrossRef] [PubMed]
- Blanco, V.; Blaya-Ros, P.J.; Torres-Sánchez, R.; Domingo, R. Irrigation and crop load management lessen rain-induced cherry cracking. Plants 2022, 11, 3249. [Google Scholar] [CrossRef] [PubMed]
- La Spada, P.; Dominguez, E.; Continella, A.; Heredia, A.; Gentile, A. Factors influencing fruit cracking: An environmental and agronomic perspective. Front. Plant Sci. 2024, 15, 1343452. [Google Scholar] [CrossRef]
- Giménez, M.J.; Valverde, J.M.; Valero, D.; Guillén, F.; Martínez-Romero, D.; Serrano, M.; Castillo, S. Quality and antioxidant properties on sweet cherries as affected by pre-harvest salicylic and acetylsalicylic acids treatments. Food Chem. 2014, 160, 226–232. [Google Scholar] [CrossRef]
- Balbontín, C.; Gutiérrez, C.; Wolff, M.; Figueroa, C.R. Effect of abscisic acid and methyl jasmonate preharvest applications on fruit quality and cracking tolerance of sweet cherry. Chil. J. Agric. Res. 2018, 78, 438–446. [Google Scholar] [CrossRef]
- Santos, M.; Maia, C.; Meireles, I.; Pereira, S.; Egea-Cortines, M.; Sousa, J.R.; Raimundo, F.; Matos, M.; Gonçalves, B. Effects of calcium- and seaweed-based biostimulants on sweet cherry profitability and quality. Biol. Life Sci. Forum 2023, 27, 45. [Google Scholar]
- Hayat, Q.; Hayat, S.; Irfan, M.; Ahmad, A. Effect of exogenous salicylic acid under changing environment: A review. Environ. Exp. Bot. 2010, 68, 14–25. [Google Scholar] [CrossRef]
- Ahmed, S.; Faruk-Ahmed, S.; Biswas, A.; Sultana, A.; Issak, M. Salicylic acid and chitosan mitigate high temperature stress of rice via growth improvement, physio-biochemical adjustments and enhanced antioxidant activity. Plant Stress 2024, 11, 100343. [Google Scholar] [CrossRef]
- Karantzi, A.D.; Kafkaletou, M.; Tsaniklidis, G.; Bai, J.; Christopoulos, M.V.; Fanourakis, D.; Tsantili, E. Preharvest foliar salicylic acid sprays reduce cracking of Fig fruit at harvest. Appl. Sci. 2021, 11, 11374. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, F.; Wang, J.; Yang, Q.; Wang, P.; Zhao, H.; Wang, J.; Wang, C.; Xu, X.H. Salicylic acid inhibits the postharvest decay of goji berry (Lycium barbarum L.) by modulating the antioxidant system and phenylpropanoid metabolites. Postharvest Biol. Technol. 2021, 178, 111558. [Google Scholar] [CrossRef]
- Jiang, B.; Liu, R.; Fang, X.; Tong, C.; Chen, H.; Gao, H. Effects of salicylic acid treatment on fruit quality and wax composition of blueberry (Vaccinium virgatum Ait). Food Chem. 2022, 30, 130757. [Google Scholar] [CrossRef] [PubMed]
- Retamal-Salgado, J.; Adaos, G.; Cedeño-García, G.; Ospino-Olivella, S.C.; Vergara-Retamales, R.; Lopéz, M.D.; Olivares, R.; Hirzel, J.; Olivares-Soto, H.; Betancur, M. Preharvest applications of oxalic acid and salicylic acid increase fruit firmness and polyphenolic content in blueberry (Vaccinium corymbosum L.). Horticulturae 2023, 9, 639. [Google Scholar] [CrossRef]
- Giménez, M.; Serrano, M.; Valverde, J.; Martínez-Romero, D.; Castillo, S.; Valero, D.; Guillén, F. Preharvest salicylic acid and acetylsalicylic acid treatments preserve quality and enhance antioxidant systems during postharvest storage of sweet cherry cultivars. J. Sci. Food Agric. 2017, 97, 1220–1228. [Google Scholar] [CrossRef]
- CIREN. Estudio Agológico. In Descripciones de Suelos, Materiales y Símbolos. IX Región; Centro de Información de Recursos Naturales: Santiago, Chile, 2022; Volume 122, p. 343. [Google Scholar]
- Balbontín, C.; Ayala, H.; Rubilar, J.; Cote, J.; Figueroa, C.R. Transcriptional analysis of cell wall and cuticle related genes during fruit development of two sweet cherry cultivars with contrasting levels of cracking tolerance. Chil. J. Agric. Res. 2014, 74, 162–169. [Google Scholar] [CrossRef]
- Bustamante, M.; Muñoz, A.; Romero, I.; Osorio, P.; Mánquez, S.; Arriola, R.; Reyes-Díaz, M.; Ribera-Fonseca, A. Impact of potassium pre-harvest applications on fruit quality and condition of sweet cherry (Prunus avium L.) cultivated under plastic covers in Southern Chile orchards. Plants 2023, 10, 2778. [Google Scholar] [CrossRef] [PubMed]
- Christensen, J.V. Cracking in cherries. III. Determination of cracking susceptibility. Acta Agric. Scand. 1972, 22, 128–136. [Google Scholar] [CrossRef]
- Ulloa-Inostroza, E.; Córdova, C.; Campos, M.; Reyes-Díaz, M. Methyl jasmonate improves antioxidants, protecting photosynthetic apparatus in blueberry plants under water deficit. Horticulturae 2024, 10, 259. [Google Scholar] [CrossRef]
- González-Villagra, J.; Reyes-Díaz, M.M.; Tighe-Neira, R.; Inostroza-Blancheteau, C.; Luengo-Escobar, A.; Bravo, L.A. Salicylic acid improves antioxidant defense system and photosynthetic performance in Aristotelia chilensis plants subjected to moderate drought stress. Plants 2022, 11, 639. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Sun, X.; Liu, L. Action of salicylic acid on plant growth. Front. Plant Sci. 2022, 13, 878076. [Google Scholar] [CrossRef]
- Iqbal, N.; Fatma, M.; Gautam, H.; Sehar, Z.; Rasheed, F.; Khan, M.; Sofo, A.; Khan, N.A. Salicylic acid increases photosynthesis of drought grown mustard plants effectively with sufficient-N via regulation of ethylene, abscisic acid, and nitrogen-use efficiency. J. Plant Growth Regul. 2022, 41, 1966–1977. [Google Scholar] [CrossRef]
- Xin, L.; Wang, J.; Yang, Q. Exogenous salicylic acid alleviates water deficit stress by protecting photosynthetic system in Maize seedlings. Agronomy 2023, 13, 2443. [Google Scholar] [CrossRef]
- Mimouni, H.; Wasti, S.; Manaa, A.; Gharbi, E.; Chalh, A.; Vandoorne, B.; Lutts, S.; Ben Ahmed, H. Does salicylic acid (SA) improve tolerance to salt stress in plants? A study of SA effects on tomato plant growth, water dynamics, photosynthesis, and biochemical parameters. OMICS 2016, 20, 180–910. [Google Scholar] [CrossRef]
- Gao, Q.; Liu, Y.; Liu, Y.; Dai, C.; Zhang, Y.; Zhou, F.; Zhu, Y. Salicylic acid modulates the osmotic system and photosynthesis rate to enhance the drought tolerance of Toona ciliate. Plants 2023, 12, 4187. [Google Scholar] [CrossRef]
- Díaz-Mula, H.M.; Serrano, M.; Valero, D. Alginate coatings preserve fruit quality and bioactive compounds during storage of sweet cherry fruit. Food Bioprocess Technol. 2012, 5, 2990–2997. [Google Scholar] [CrossRef]
- Mandal, D.; Laldingliana, W.; Hazarika, T.; Nautiyal, B.P. Salicylic acid delayed postharvest ripening and enhanced shelf life of tomato fruits at ambient storage. Acta Hortic. 2018, 1213, 115–121. [Google Scholar] [CrossRef]
- Haider, S.; Ahmad, S.; Sattar, A.; Akbar, M.; Nasir, M.; Naz, S. Effects of salicylic acid on postharvest fruit quality of “Kinnow” mandarin under cold storage. Sci. Hortic. 2020, 259, 108843. [Google Scholar] [CrossRef]
- Sharma, S.; Sharma, R.R. Impact of staggered treatments of novel molecules and ethylene absorbents on postharvest fruit physiology and enzyme activity of ‘Santa Rosa’ plums. Sci. Hortic. 2016, 198, 242–248. [Google Scholar] [CrossRef]
- Ruiz-Aracil, M.C.; Valverde, J.M.; Lorente-Mento, J.M.; Carrión-Antolí, A.; Castillo, S.; Martínez-Romero, D.; Guillén, F. Sweet cherry (Prunus avium L.) Cracking during development on the tree and at harvest: The impact of methyl jasmonate on four different growing seasons. Agriculture 2023, 13, 1244. [Google Scholar] [CrossRef]
- Correia, S.; Santos, M.; Glinska, S.; Gapinska, M.; Matos, M.; Carnide, V.; Schouten, R.; Silva, A.; Gonçalves, B. Effect of exogenous compound spray son cherry cracking: Skin properties and gene expression. J. Sci. Food Agric. 2020, 100, 2911–2921. [Google Scholar] [CrossRef]
- Wang, L.; Chen, Y.; Wu, M.; Dai, F.; Ye, M.; Chen, F.; Qi, Y.; Luo, Z.; Huang, H. Involvement of lignin deposition and cell wall degradation in stem senescence of Chinese flowering cabbage during storage. Postharvest Biol. Technol. 2023, 198, 112256. [Google Scholar] [CrossRef]


| Irrigation Treatment | Applied Water | Mean Midday Leaf Water Potential (MPa) | |||
|---|---|---|---|---|---|
| (m3 ha−1) | 11 November | 5 December | 20 December | 3 January † | |
| Full irrigation (FI) | 1085 | −0.61 | −0.86 | −1.01 | −0.94 |
| Deficit irrigation (DI) | 778 | -- | −1.12 ** | −1.35 ** | −1.09 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Villagra, J.; Chicahual, C.; Jorquera-Fontena, E.; Falquetto-Gomes, P.; Nunes-Nesi, A.; Reyes-Díaz, M. Salicylic Acid Improves Yield, Fruit Quality, and Post-Harvest Storage in Sweet Cherry (Prunus avium L.) cv. Lapins Subjected to Late-Deficit Irrigation. Horticulturae 2024, 10, 707. https://doi.org/10.3390/horticulturae10070707
González-Villagra J, Chicahual C, Jorquera-Fontena E, Falquetto-Gomes P, Nunes-Nesi A, Reyes-Díaz M. Salicylic Acid Improves Yield, Fruit Quality, and Post-Harvest Storage in Sweet Cherry (Prunus avium L.) cv. Lapins Subjected to Late-Deficit Irrigation. Horticulturae. 2024; 10(7):707. https://doi.org/10.3390/horticulturae10070707
Chicago/Turabian StyleGonzález-Villagra, Jorge, Camila Chicahual, Emilio Jorquera-Fontena, Priscilla Falquetto-Gomes, Adriano Nunes-Nesi, and Marjorie Reyes-Díaz. 2024. "Salicylic Acid Improves Yield, Fruit Quality, and Post-Harvest Storage in Sweet Cherry (Prunus avium L.) cv. Lapins Subjected to Late-Deficit Irrigation" Horticulturae 10, no. 7: 707. https://doi.org/10.3390/horticulturae10070707
APA StyleGonzález-Villagra, J., Chicahual, C., Jorquera-Fontena, E., Falquetto-Gomes, P., Nunes-Nesi, A., & Reyes-Díaz, M. (2024). Salicylic Acid Improves Yield, Fruit Quality, and Post-Harvest Storage in Sweet Cherry (Prunus avium L.) cv. Lapins Subjected to Late-Deficit Irrigation. Horticulturae, 10(7), 707. https://doi.org/10.3390/horticulturae10070707








