Metabolomic and Transcriptomic Analysis of Unique Floral Coloration in Osmanthus fragrans Cultivars
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Extraction of Carotenoids
2.3. Detection Conditions of Carotenoids
2.4. Qualitative and Quantitative Analysis of Carotenoids
2.5. Transcriptome Sequencing and Differential Expression Gene Screening
2.6. RT-qPCR Analysis of Genes Related to Carotenoid Synthesis
2.7. Data Analysis
3. Results
3.1. Difference in Carotenoids in ‘Yanzhi Hong’ Petals at the Initial and Peak Flowering Stages
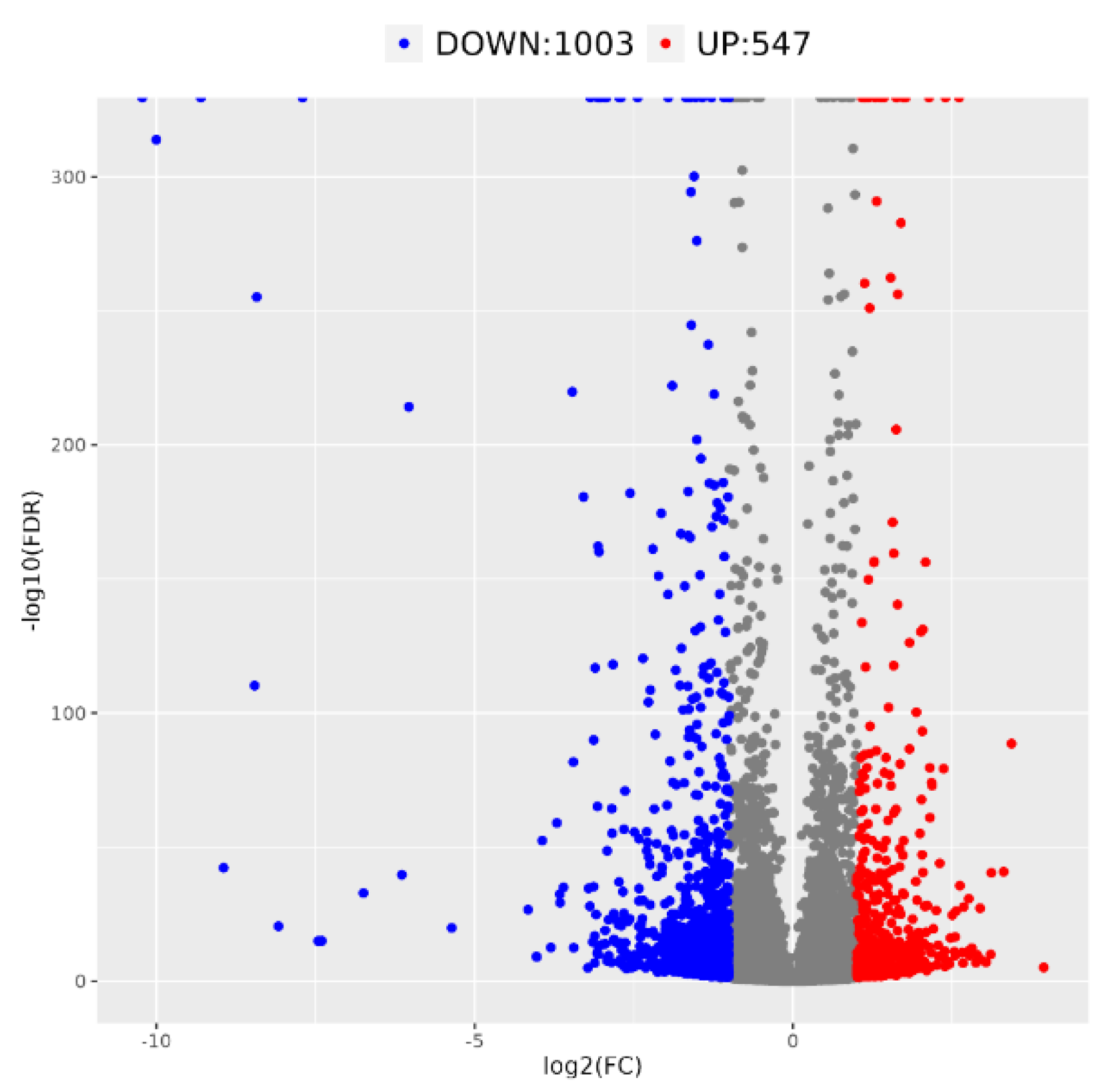
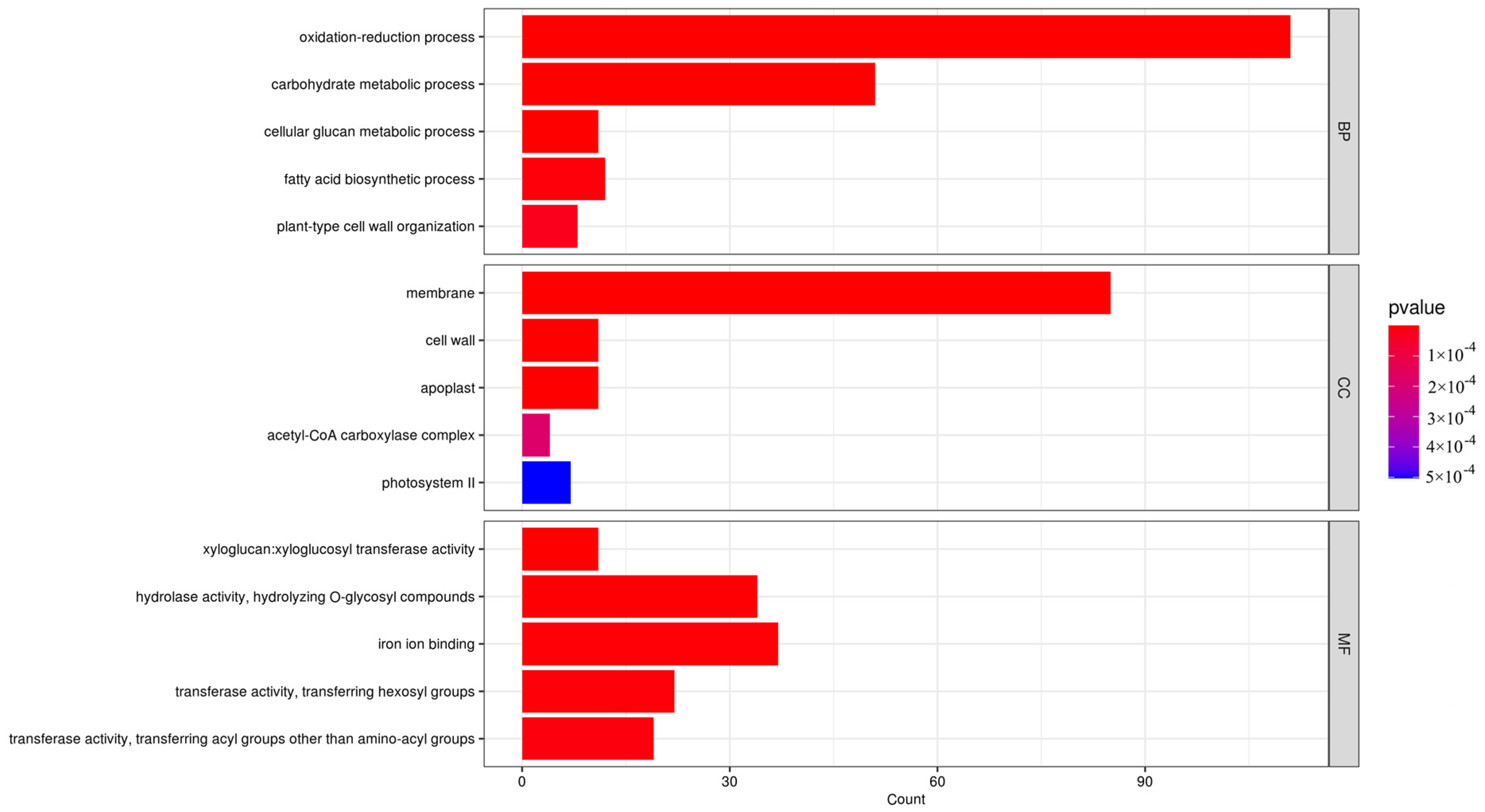
3.2. Transcriptome Sequencing and DEGs Analysis of ‘Yanzhi Hong’ Petals at the Initial and Peak Flowering Stages
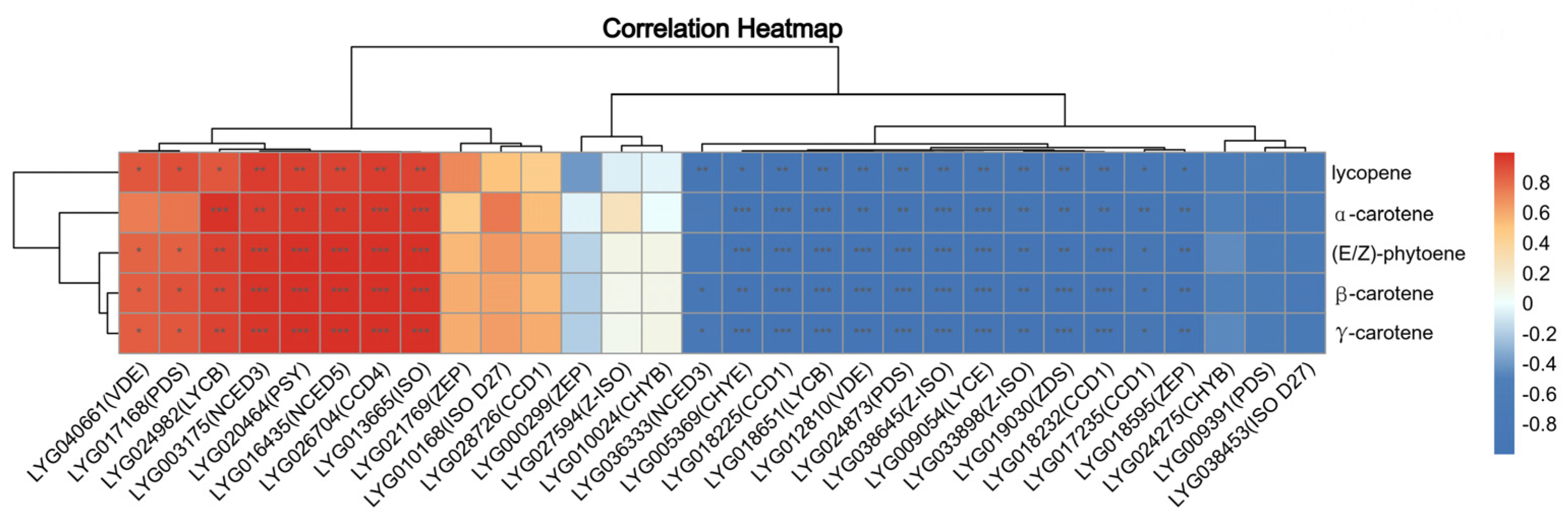
3.3. Association Analysis of Metabolic and Transcriptomic Date of Carotenoid Metabolism Pathway Genes
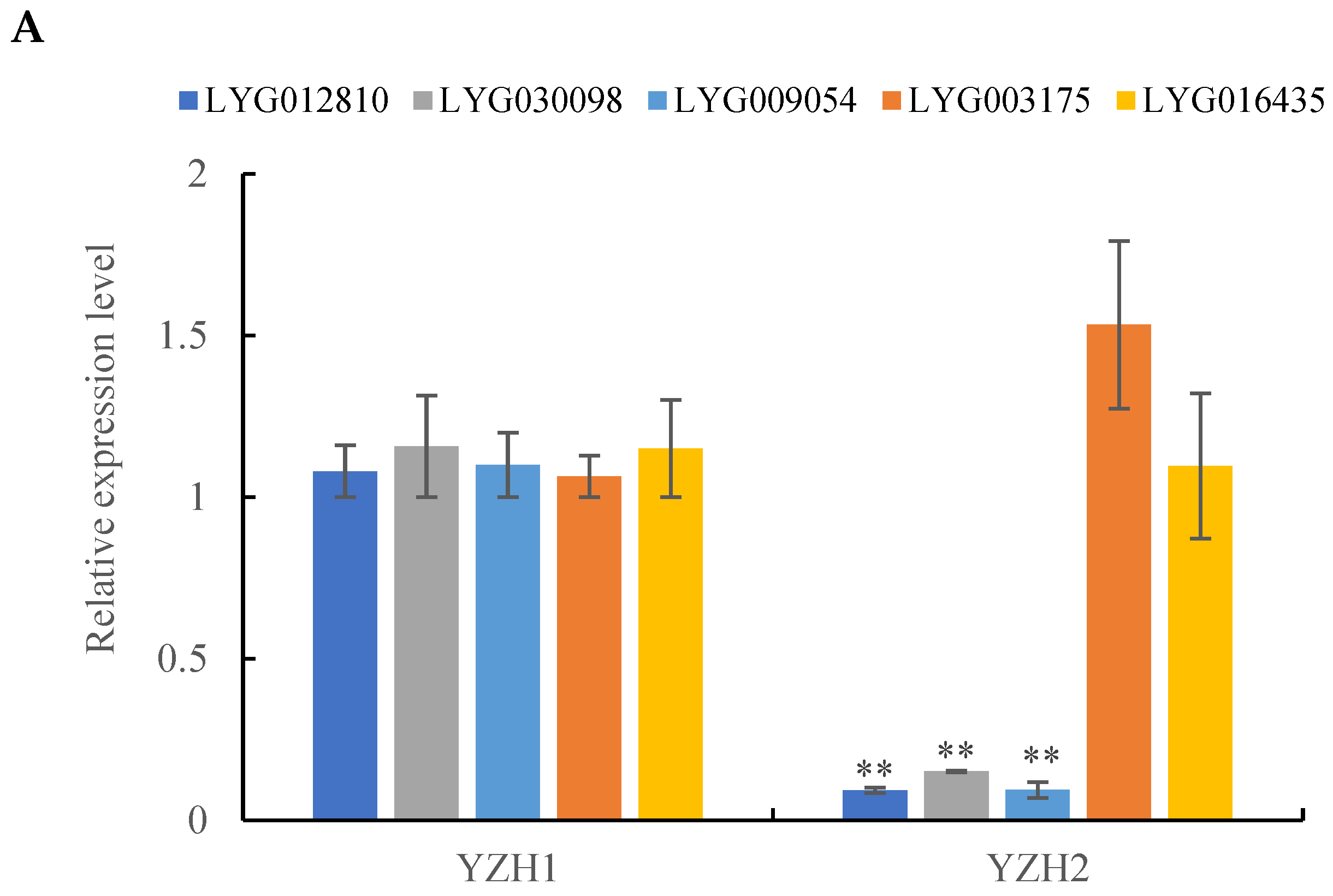
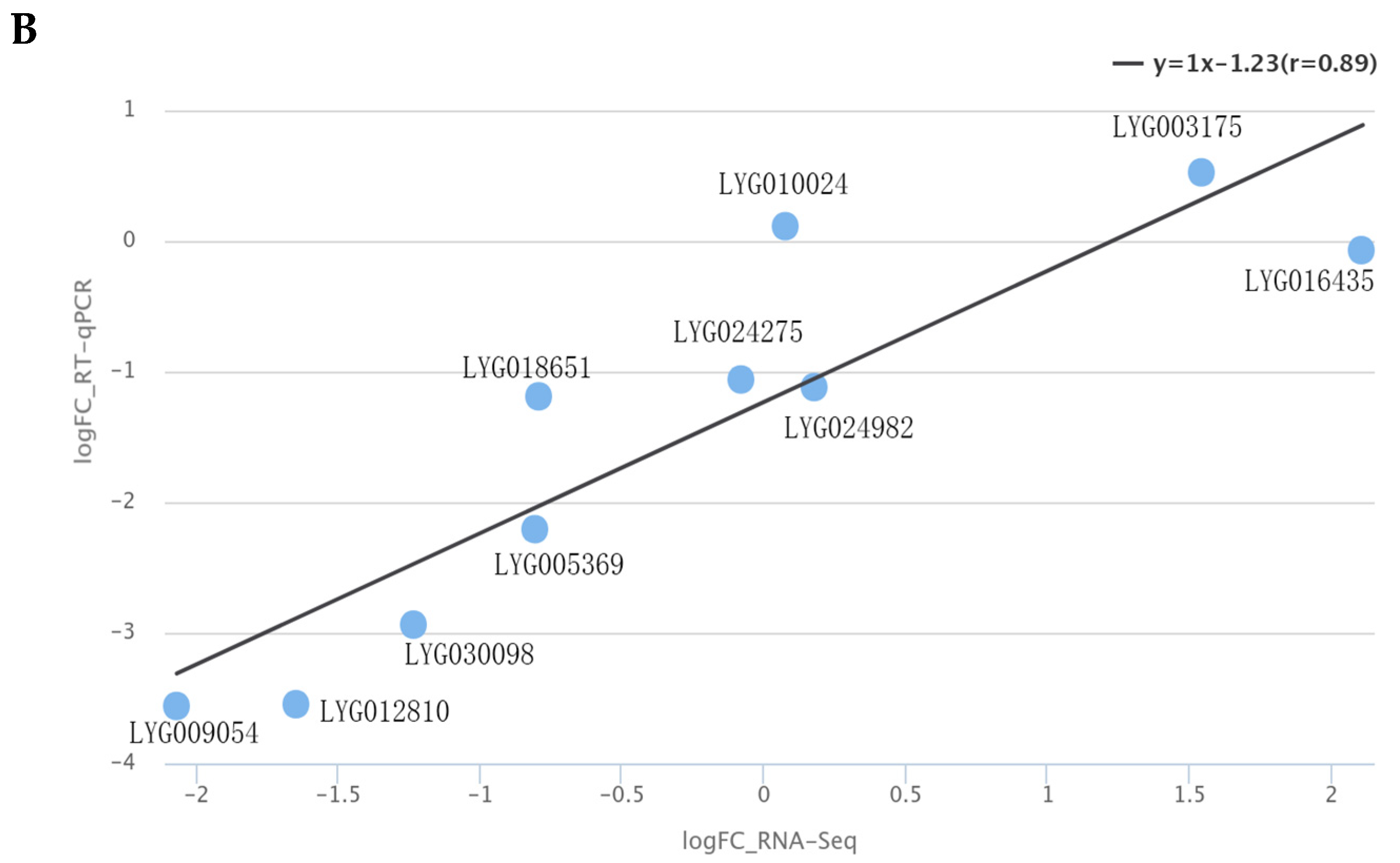
3.4. RT-qPCR Validation of Carotenoid Metabolism Pathway Genes
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, Y. Cultivar classification of Osmanthus fragrans Lour. and the development of germplasm resources of Osmanthus Lour. J. Plant Resour. Environ. 1993, 2, 44–48. [Google Scholar]
- Xiang, Q.; Liu, Y. An Illustrated Monograph of the Sweet Osmanthus Cultivars in China. Chin. Landsc. Archit. 2008, 9, 96. [Google Scholar]
- Yang, K. Chinese Osmanthus; China Forestry Publishing House: Beijing, China, 2020. [Google Scholar]
- Tanaka, Y.; Sasaki, N.; Ohmiya, A. Biosynthesis of plant pigments: Anthocyanins, betalains and carotenoids. Plant J. 2008, 54, 733–749. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Yuan, X.; Gao, J.; Wang, X.; Wang, H.; Li, Y.; Wang, L.; Fu, Z.; Li, B. Mechanism of Flower Petal Coloration and Molecular Breeding. Biotechnol. Bull. 2023, 39, 23–31. [Google Scholar]
- Song, X.; Tian, Y.; Gao, K.; Li, J.; Li, Y.; Wang, J.; Deng, C.; Zhang, F.; Kong, D.; Fan, G.; et al. Genetic and QTL analysis of flower color and pigments in small-flowered chrysanthemum based on high-density genetic map. Ornam. Plant Res. 2023, 3, 17. [Google Scholar] [CrossRef]
- Wen, J.; Wang, C.; Feng, H.; Li, S.; Wang, L.; Wu, R.; Zhao, S. Research Progress on Flower Color of Rose. Acta Hortic. Sinica 2021, 48, 2044–2056. [Google Scholar]
- Cai, X.; Su, F.; Jin, H.; Yao, C.; Wang, C. Components and extraction methods for petal pigments of Osmanthus fragrans ‘Siji Gui’. J. Zhejiang For. Coll. 2010, 27, 559–564. [Google Scholar]
- Zou, J.; Zeng, X.; Chen, H.; Cai, X.; Wang, C. Analysis on characteristic color compounds in different varieties of Osmanthus fragrans Lour. during flowering and senescence. J. South. Agric. 2017, 48, 1683–1690. [Google Scholar]
- Wang, Y.; Luo, Y.; Zhang, C.; Fu, J.; Hu, S.; Zhao, H. Flower Color and Pigment Composition in the Petals of Bud Mutation and its Stock Plant of Osmanthus fragrans‘Jingui’. Acta Hortic. Sinica 2017, 44, 528–536. [Google Scholar]
- Wang, Y.; Zhang, C.; Dong, B.; Fu, J.; Hu, S.; Zhao, H. Carotenoid Accumulation and Its Contribution to Flower Coloration of Osmanthus fragrans. Front. Plant Sci. 2018, 9, 1499. [Google Scholar] [CrossRef]
- Gu, H.; Yang, X.; Wang, L. Progress in Molecular Biological Studies of Osmanthus fragrans. J. Trop. Subtrop. Bot. 2024, in press. [Google Scholar]
- Xiang, Q.; Wang, X.; Liu, Y. Annual Report ICRCO 2014(2) Three new cultivars of Osmanthus fragrans. J. Nanjing For. Univ. 2014, 38, 2+181. [Google Scholar]
- Chai, Z.; Zhang, M.; Li, L.; Zhang, C.; Duan, Y. Non-Targeted Metabolomics Analysis of the Petals of Osmanthus fragrans ‘Yanzhi Hong’ in Different Developmental Phases. J. Northwest For. Univ. 2022, 37, 107–113. [Google Scholar]
- Chen, H.; Zeng, X.; Yang, J.; Cai, X.; Shi, Y.; Zheng, R.; Wang, Z.; Liu, J.; Yi, X.; Xiao, S.; et al. Whole-genome resequencing of Osmanthus fragrans provides insights into flower color evolution. Hortic. Res. 2021, 8, 98. [Google Scholar] [CrossRef]
- Zeng, X. Research of TPS and CCD Function Analysis and Their Influence on Petal Color and Scent in Osmanthus fragrans Lour. Ph.D. Dissertation, Huazhong Agricultural University, Wuhan, China, 2020. [Google Scholar]
- Wang, Y.; Zhang, C.; Xu, B.; Fu, J.; Du, Y.; Fang, Q.; Dong, B.; Zhao, H. Temperature regulation of carotenoid accumulation in the petals of sweet osmanthus via modulating expression of carotenoid biosynthesis and degradation genes. BMC Genom. 2022, 23, 418. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Gao, Y.; Huang, H.; Dai, S. Carotenoid Metabolism and Regulation in Plants. Acta Hortic. Sinica 2022, 49, 2559–2578. [Google Scholar]
- He, J.; Fan, Y. Progress in Composition and Metabolic Regulation of Carotenoids Related to Floral Color. Acta Hortic. Sinica 2022, 49, 1162–1172. [Google Scholar]
- Qing, H.; Chen, J.; Jiang, L.; Qian, J.; Fu, J.; Zhang, C. Functional Characterization of Two Lycopene Cyclases from Sweet Osmanthus (Osmanthus fragrans). Sci. Hortic. 2022, 299, 111062. [Google Scholar] [CrossRef]
- Cai, X.; Mai, R.; Zou, J.; Zhang, H.; Zeng, X.; Zheng, R.; Wang, C. Analysis of aroma-active compounds in three sweet osmanthus (Osmanthus fragrans) cultivars by GC-olfactometry and GC-MS. J. Zhejiang Univ.-Sci. B 2014, 15, 638–648. [Google Scholar] [CrossRef]
- Huang, F.C.; Molnár, P.; Schwab, W. Cloning and functional characterization of carotenoid cleavage dioxygenase 4 genes. J. Exp. Bot. 2009, 60, 3011–3022. [Google Scholar] [CrossRef]
- Baldermann, S.; Kato, M.; Kurosawa, M.; Kurobayashi, Y.; Fujita, A.; Fleischmann, P.; Watanabe, N. Functional characterization of a carotenoid cleavage dioxygenase 1 and its relation to the carotenoid accumulation and volatile emission during the floral development of Osmanthus fragrans Lour. J. Exp. Bot. 2010, 61, 2967–2977. [Google Scholar] [CrossRef] [PubMed]
- Xin, H.; Wu, B.; Zhang, H.; Wang, C.; Li, J.; Yang, B.; Li, S. Characterization of volatile compounds in flowers from four groups of sweet osmanthus (Osmanthus fragrans) cultivars. Can. J. Plant Sci. 2013, 93, 923–931. [Google Scholar] [CrossRef]
- Zou, J.; Cai, X.; Zeng, X.; Zheng, R.; Wang, C. Changes of Aroma-active Compounds in Different Cultivars of Osmanthus fragrans during Flowering. Acta Hortic. Sin. 2017, 44, 1517–1534. [Google Scholar]
- Xia, K.; Jiang, B.; Zhao, Z.; Fan, J.; Wen, G.; Li, F.; Gao, L.; Jiang, Q.; Qiu, S. Comparative analysis of aromatic components from different cultivars of Osmanthus fragrans in Guilin. Guihaia 2018, 38, 1493–1504. [Google Scholar]

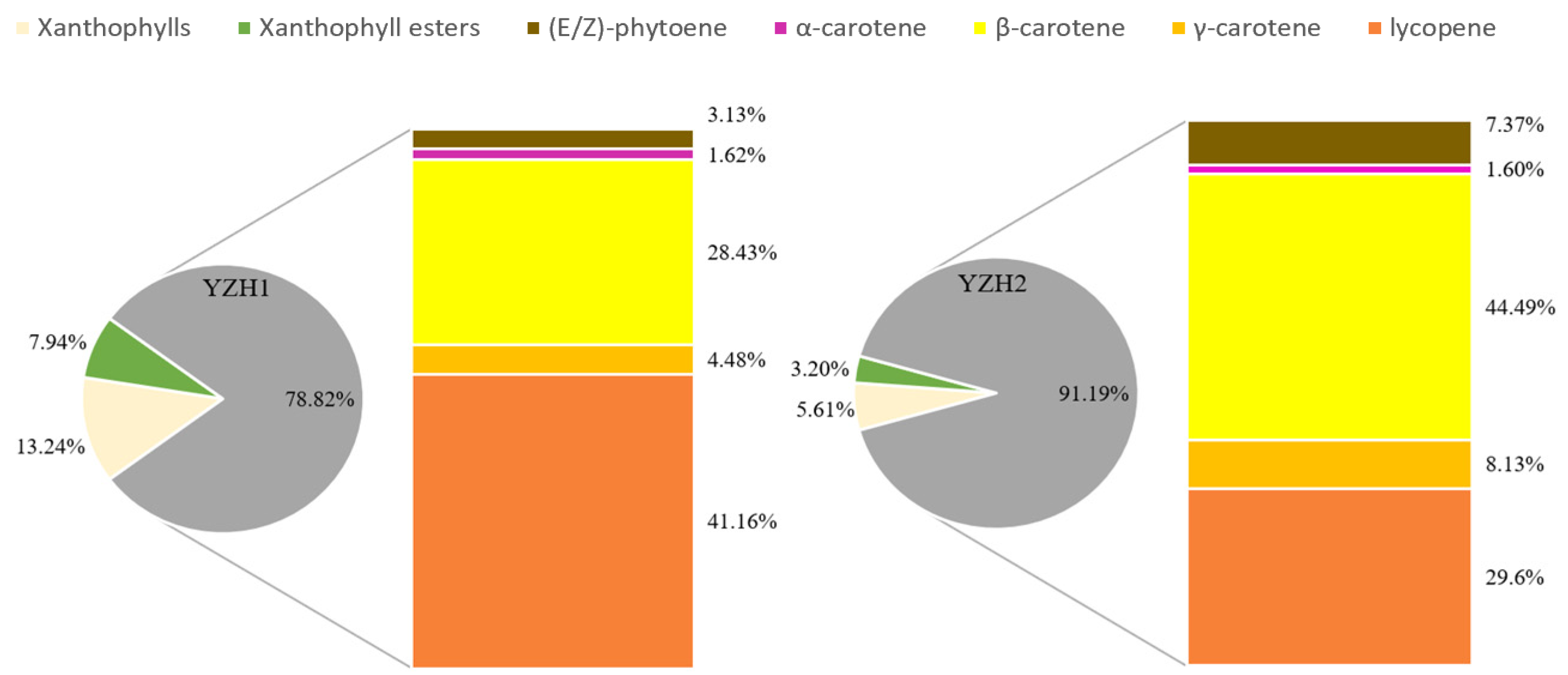



| No | RT | Compound Name | YZH1 | YZH2 |
|---|---|---|---|---|
| Mean µg/g DW | Mean µg/g DW | |||
| Carotenes | ||||
| 1 | 5.00 | (E/Z)-phytoene | 3.72 ± 0.07 b | 15.72 ± 0.42 a |
| 2 | 5.92 | α-carotene | 1.93 ± 0.15 b | 3.40 ± 0.21 a |
| 3 | 6.28 | β-carotene | 33.73 ± 1.33 b | 95.02 ± 4.37 a |
| 4 | 7.38 | γ-carotene | 5.31 ± 0.11 b | 17.35 ± 0.27 a |
| 5 | 8.33 | lycopene | 48.84 ± 2.59 b | 63.23 ± 3.31 a |
| 93.53 ± 4.25 b | 194.72 ± 8.58 a | |||
| Xanthophylls | ||||
| 6 | 1.58 | violaxanthin | 0.12 ± 0.00 a | 0.09 ± 0.01 b |
| 7 | 1.93 | neoxanthin | 0.21 ± 0.01 a | 0.10 ± 0.01 b |
| 8 | 4.04 | lutein | 3.48 ± 0.20 a | 2.51 ± 0.05 b |
| 9 | 4.51 | 8′-apo-β-carotenal | 0.01 ± 0.00 b | 0.08 ± 0.01 a |
| 10 | 4.63 | zeaxanthin | 10.66 ± 0.40 a | 5.77 ± 0.14 b |
| 11 | 5.08 | α-cryptoxanthin | 0.41 ± 0.01 | 0.42 ± 0.02 |
| 12 | 5.52 | β-cryptoxanthin | 0.80 ± 0.06 b | 2.96 ± 0.13 a |
| 13 | 5.54 | echinenone | 0.01 ± 0.00 b | 0.04 ± 0.00 a |
| 15.71 ± 0.69 a | 11.97 ± 0.36 b | |||
| Xanthophyll esters | ||||
| 14 | 6.46 | lutein oleate | 0.05 ± 0.00 | 0.05 ± 0.00 |
| 15 | 6.87 | lutein palmitate | 0.36 ± 0.03 | 0.36 ± 0.03 |
| 16 | 7.18 | zeaxanthin palmitate | 1.09 ± 0.07 a | 0.73 ± 0.07 b |
| 17 | 7.38 | zeaxanthin dilaurate | 0.04 ± 0.01 | 0.03 ± 0.01 |
| 18 | 7.48 | zeaxanthin-laurate-myristate | 0.03 ± 0.00 | 0.02 ± 0.00 |
| 19 | 7.63 | zeaxanthin dimyristate | 0.31 ± 0.01 a | 0.24 ± 0.00 b |
| 20 | 7.96 | zeaxanthin dipalmitate | 0.43 ± 0.02 a | 0.26 ± 0.03 b |
| 21 | 7.77 | zeaxanthin-oleate-palmitate | 0.20 ± 0.01 a | 0.14 ± 0.01 b |
| 22 | 6.95 | β-cryptoxanthin laurate | 0.09 ± 0.02 | 0.07 ± 0.01 |
| 23 | 7.18 | β-cryptoxanthin myristate | 0.16 ± 0.00 a | 0.10 ± 0.01 b |
| 24 | 7.32 | β-cryptoxanthin palmitate | 3.06 ± 0.09 a | 2.25 ± 0.08 b |
| 25 | 6.54 | rubixanthin caprate | 0.05 ± 0.00 | 0.04 ± 0.01 |
| 26 | 6.79 | rubixanthin laurate | 0.64 ± 0.03 a | 0.50 ± 0.01 b |
| 27 | 7.33 | rubixanthin palmitate | 2.92 ± 0.13 a | 2.05 ± 0.10 b |
| 9.42 ± 0.43 a | 6.82 ± 0.35 b | |||
| Sample | RawReads | RawBases | CleanReads | CleanBases | CleanRatio | Q20 | Q30 | GC |
|---|---|---|---|---|---|---|---|---|
| YZH1_1 | 36,021,782 | 5.40 | 35,777,926 | 5.30 | 99.32% | 96.86% | 91.54% | 42.72% |
| YZH1_2 | 50,681,302 | 7.60 | 50,373,046 | 7.48 | 99.39% | 97.19% | 92.17% | 42.80% |
| YZH1_3 | 42,561,224 | 6.38 | 42,258,728 | 6.28 | 99.29% | 96.95% | 91.75% | 42.77% |
| YZH2_1 | 43,146,876 | 6.47 | 42,876,012 | 6.36 | 99.37% | 97.24% | 92.34% | 42.53% |
| YZH2_2 | 46,010,634 | 6.90 | 45,692,536 | 6.77 | 99.31% | 96.99% | 91.81% | 42.53% |
| YZH2_3 | 52,623,910 | 7.89 | 52,210,754 | 7.75 | 99.21% | 96.84% | 91.53% | 42.48% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, S.; Wu, J.; Yu, P.; Tan, Y.; He, Q.; Yang, J.; Cai, X.; Zou, J.; Chen, H.; Zeng, X. Metabolomic and Transcriptomic Analysis of Unique Floral Coloration in Osmanthus fragrans Cultivars. Horticulturae 2024, 10, 801. https://doi.org/10.3390/horticulturae10080801
Wei S, Wu J, Yu P, Tan Y, He Q, Yang J, Cai X, Zou J, Chen H, Zeng X. Metabolomic and Transcriptomic Analysis of Unique Floral Coloration in Osmanthus fragrans Cultivars. Horticulturae. 2024; 10(8):801. https://doi.org/10.3390/horticulturae10080801
Chicago/Turabian StyleWei, Shenni, Jiang Wu, Ping Yu, Yunfei Tan, Qiang He, Jie Yang, Xuan Cai, Jingjing Zou, Hongguo Chen, and Xiangling Zeng. 2024. "Metabolomic and Transcriptomic Analysis of Unique Floral Coloration in Osmanthus fragrans Cultivars" Horticulturae 10, no. 8: 801. https://doi.org/10.3390/horticulturae10080801





