Abstract
Podophyllum hexandrum Royle, also known as Podophyllum emodi Wall, holds significant ecological, ornamental, and medicinal values. However, it has become endangered due to overexploitation, prolonged seed dormancy, slow natural regeneration, and climate change. This study developed an efficient in vitro protocol for callogenesis and micropropagation of P. hexandrum to conserve germplasm in in vitro conditions. Callus formation from various plant parts, including the leaf, stem, rhizome, radicle, and cotyledon, was induced using Murashige and Skoog (MS) medium supplemented with different plant growth regulators. The combination of benzyladenine at 1 mg L−1 and 4-dichlorophenoxy acetic acid at 3 mg L−1 was optimal for biomass production, yielding 215.88 ± 0.31 mg, with growth per gram at 8.32 ± 0.32 and a growth rate of 13.62 ± 0.25 mg/day on MS medium. For shoot proliferation, benzyladenine (3.5 mg L−1) and naphthalene acetic acid (0.5 mg L−1) combined with activated charcoal showed the highest shoot induction percentage per explant. For shoot regeneration from calluses, 6-benzylaminopurine (0.5 mg L−1) and thidiazuron (2 mg L−1) were most effective, producing superior shoot length, number of regenerations, and regeneration percentage. Root induction was successful with α-naphthalene acetic acid supplementation (0.5 to 1.5 mg L−1) in MS medium, resulting in the highest number per explant (4.08 ± 0.08), length (5.45 ± 0.15 cm), and rooting rate (87.00 ± 1.66%) of roots in plantlets. Subculturing for callus culture was performed every 28 days for up to four subcultures to prevent nutrient depletion and toxic metabolite accumulation, ensuring tissue health and viability. Continuous subculturing of callus on MS medium maintained healthy P. hexandrum germplasm in vitro. Overall, this micropropagation protocol provides a rapid system for conserving P. hexandrum germplasm.
1. Introduction
Podophyllum hexandrum Royle, synonym P. emodi Wall, is an important ornamental and medicinal plant. P. hexandrum is cherished for its large, deeply lobed leaves, that resemble elegant umbrellas. These leaves emerge in spring, adding a lush and exotic appearance to shaded garden areas. These leaves, with their waxy texture and rich green color, span up to 12 inches (30 cm) in diameter, creating a striking focal point. In late spring to early summer, the plant produces charming nodding flowers with six white to pink petals, adding an element of mystery and allure beneath the large foliage [1]. Following the flowers, decorative fruit resembling miniature apples adorn the plant, enhancing its ornamental value, especially in fall when they ripen and add vibrant color to the landscape [2,3]. P. hexandrum’s ability to thrive in shaded environments makes it a valuable addition to shade gardens, where it can fill in under trees or alongside other shade-loving plants. Its low maintenance requirements and historical significance in traditional medicine practices further elevate its appeal among gardeners interested in ethnobotany or cultural gardening. Overall, the combination of its attractive foliage, charming flowers and fruit, shade tolerance, and cultural significance solidifies P. hexandrum’s status as an esteemed ornamental plant in garden landscapes [4].
P. hexandrum belongs to the well-known plant family Berberidaceae, thriving in the northern Himalayan regions, including various parts of Pakistan. Its distribution spans altitudes ranging from 2300 m to 3700 m above mean sea level [5,6]. P. hexandrum has been an immensely significant source of nutrition, medicinal substances, and other healthcare products since ancient times. It has been a revered Aindri (a divine medicine), dating back to prehistoric eras [7]. It contains various bioactive compounds, such as lignans, proteins, glycosides, flavonoids, saponins, and terpenes [8].
One of the most notable natural secondary metabolites found in P. hexandrum is podophyllotoxin (PTOX), or podophyllin. It is used in the treatment of various cancers [9]. Additionally, PTOX and its derivatives exhibit antimicrobial, antiviral, antifungal, antineoplastic, insecticidal, antiparasitic, and radioprotective properties. They are used in the treatment of leukemia and rheumatoid arthritis [10]. Podophyllotoxin, or podophyllin, can also be used as a hepatic stimulant, purgative, emetic, antipyretic, antiseptic for wound healing, and a strong analgesic. Additionally, PTOX exhibits strong antioxidant properties, helping to neutralize free radicals and reduce oxidative stress. It can reduce inflammation, modulate the immune system, improve digestion, and alleviate gastrointestinal issues such as bloating and indigestion [11]. Podophyllotoxin can be incorporated into skincare products for its healing and protective effects. It is useful in treating respiratory conditions by reducing congestion and soothing the respiratory tract and can relieve muscle spasms and cramps [12]. P. hexandrum has several interesting cultivars and variants, including P. hexandrum var. axillare, P. hexandrum var. bhootanense, P. hexandrum var. emodi, and P. hexandrum var. Jaeschkei. Among these, P. hexandrum var. Emodi (the Emodi variety) is known for its relatively higher PTOX content compared to other cultivars [5,6,7].
The wild P. hexandrum population has drastically declined in recent years due to overexploitation, habitat destruction, prolonged winter conditions, slow regeneration, and limited fruit production. Deforestation and land use changes further contribute to habitat loss and fragmentation [13]. Climate change triggers phenological mismatch, altering the plant life-cycle events of P. hexandrum. Factors like temperature, precipitation, and day length impact flowering, fruiting, and leafing [14]. Limited awareness and incentives among local communities and stakeholders lead to unsustainable exploitation and neglect of the plant’s conservation status [15].
Multiple factors collectively drive the P. hexandrum species to endangered status. Studies report a population reduction of 68% to 97% in the Swat region of Pakistan, categorizing it as critically endangered [5,10,16]. Despite being internationally recognized as a rare plant on the IUCN Red List, excessive harvesting persists, contributing to its vulnerability. The absence of organized conservation plans and limited efforts in in vitro conservation further exacerbate the species’ precarious status [6,17]. In vitro conservation methods, being rapid, cost-effective, and season-independent, are invaluable for preserving species diversity, especially for threatened species [18,19]. To enhance genetic diversity and PTOX content, favoring breeding, selection, and biotechnological approaches is crucial for both ex situ and in situ conservation measures of P. hexandrum [15,20].
The in vitro culture of P. hexandrum, an endangered medicinal plant from Pakistan, is a challenging task. The in vitro culture of P. hexandrum faces several challenges, such as establishing initial cultures from explants, contamination, low germination rates, and a low frequency of explant regeneration. Inducing roots in regenerated shoots and acclimatizing them to ex vitro conditions requires finely tuned protocols to ensure high survival rates [5]. The plant also experiences somatic mutations or chromosomal abnormalities during successive subcultures, which can negatively affect plant quality. Scaling up plantlet production to meet conservation or commercial demands is resource intensive and requires efficient multiplication protocols [11]. Cell necrosis, browning, and eventual death also occur due to the release of secondary metabolites from the explants of P. hexandrum. Establishing and maintaining a tissue culture facility is costly and demands specialized equipment and skilled personnel. Additionally, P. hexandrum seeds do not germinate easily in laboratory conditions, complicating tissue culture initiation [15]. Overcoming seed dormancy is critical for successful propagation, as many plants exhibit poor responses to tested medium formulations, making it essential to find the right combination of growth regulators and nutrients [17,19].
Only a few studies have reported tissue culture with limited success for P. hexandrum [6,7,15,19,20,21,22]. The issues and limitations identified in these studies included high contamination rates, which made it difficult to maintain sterile cultures [13,15]. Additionally, there were challenges with low regeneration efficiency and slow growth rates, resulting in the inconsistent development of explants. Ensuring genetic stability was another significant concern, as somaclonal variations could affect the medicinal properties of the plant [20]. Optimizing the culture medium composition, including the balance of hormones and nutrients, proved complex and critical for successful propagation. Furthermore, developing a robust rooting system and acclimatizing plantlets to external conditions posed significant difficulties, often leading to high mortality rates during the transition from in vitro to ex vitro environments [21]. To address the urgent need for conservation and propagation, efficient methods, both conventional and in vitro, such as rooting of rhizome segments, seed germination, somatic embryogenesis, and micropropagation, must be developed. Successful tissue culture studies for P. hexandrum are limited, and protocols for callus cultures from mature explants are particularly lacking. Overexploitation and climate change necessitate immediate action, making plant tissue culture a crucial tool for achieving conservation goals. In view of the preceding discussion, the current study was designed, aiming to develop a cost-effective and efficient protocol for in vitro conservation and micropropagation of P. hexandrum.
2. Materials and Methods
2.1. Plant Material
The plant materials of P. hexandrum were collected from Saifullah Lake and Koz Lalko in the Swat district of Pakistan, using a random sampling approach. A total of 15–20 P. hexandrum plants were collected as representative samples for in vitro culture between May and September of 2021 and 2022 (Figure 1). The rationale for collecting plant samples of P. hexandrum between May and September 2021 and 2022 likely stems from the plant’s growth cycle and seasonal variations. During these months, the availability of all plant parts such as stems, roots, leaves, flowers, and seeds is at its peak. This period typically coincides with the active growing season for many plants in temperate regions. During this time, plants are more vigorous and have higher metabolic activity, making it easier to obtain healthy and viable explants for in vitro culture [5,6].

Figure 1.
P. hexandrum at flowering and fruiting stage, and seeds (photo taken at Saif Ullah Lake, 2021).
2.2. Sterilization of Plant Materials
Surface sterilization of seeds, leaf, stem, rhizome, radicle, and cotyledon was carried out by washing with running tap water to remove any external contaminants. The explants were then submerged in a 20% Tween-20 solution for 5 min, effectively eliminating the surface contaminants present. Following this, aqueous solutions of mercuric chloride (HgCl2) at concentrations of 0.05%, 0.1%, and 0.2% were used, with each concentration applied for 5 min to the explants. After each sterilization treatment, the explants were rinsed three times with double-distilled water (ddH2O) and gently dried by blotting before inoculation in an airflow chamber. These concentrations of mercuric chloride were tested to evaluate their effectiveness in preventing fungal and bacterial contamination, following a partially modified protocol based on Boruah et al. [22]. Mercuric chloride was utilized for seed germination and contamination percentage of P. hexandrum.
2.3. Isolation and Aseptic Transfer of Explants
Explants were inoculated inside a laminar flow chamber that was thoroughly cleaned with 70% ethanol. All the tissue culture tools and the prepared MS medium in flasks were autoclaved and were UV sterilized for 30 min. Following sterilization, explants were prepared and inoculated on the growth medium in 250–500 mL flasks and Petri dishes, following a partially modified protocol based on the work of Nadeem et al. [13].
2.4. In Vitro Conservation and Propagation of P. hexandrum
MS medium was used for in vitro propagation and conservation of P. hexandrum explants. Callus induction, indirect organogenesis (shooting and rooting) and direct organogenesis (shooting) were carried out using the respective growth substances as described below. Three replications were used for each combination with four explants per Petri dish and flask.
2.5. Callus Induction in Explants of P. hexandrum
Leaf, stem, rhizome, radicle, and cotyledon explants were used for callus induction with various growth hormones. The explants were cultured on MS media supplemented with different hormone combinations:
- BA (0.5, 1.5, and 2.0 mg L−1) + 2,4-D (1.0, 2.5, and 3.0 mg L−1)
- KIN (0.2, 0.5, and 1.0 mg L−1) + 2,4-D (1.0, 2.5, and 3.0 mg L−1)
- BA (1.0, 1.5, and 2.5 mg L−1) + NAA (0.5 and 1.0 mg L−1)
- TDZ (1.0, 1.5, and 2.0 mg L−1) + NAA (0.5 and 1.0 mg L−1)
The synergistic and antagonistic effects of these combinations on callus induction were examined. Ascorbic acid (50, 100, and 150 mg L−1) and activated charcoal (3 mg L−1) were added to the MS media to prevent callus necrosis and browning. Cultured explants were incubated in a growth room at 26 ± 2 °C under dark conditions for 45 days. After callus initiation, the cultures were maintained at the same temperature and relative humidity (54–65%) but exposed to a 16 h photoperiod using cool fluorescent tubes. Callus induction percentage was recorded for each combination, and to prevent browning and necrosis, explants were subcultured on fresh media every 28 days following a partially modified protocol based on Sharma et al. [6] and Nadeem et al. [13].
2.6. Indirect Shoots and Root Induction
The embryogenic calluses at the maturity stage were placed in culture flasks containing shoot-inducing media supplemented with various combinations and concentrations of auxins and cytokinins. Plant growth regulators (PGRs) used included the following:
- BA (0.1–0.5 mg L−1)
- KIN (0.1–1.0 mg L−1)
- TDZ (1.0–2.0 mg L−1)
- BA (0.1–0.5 mg L−1) + IAA (0.1–0.5 mg L−1)
The percentage of shoot induction, the number of shoots per explant, and the shoot length per callus clump were recorded. For rooting, the shoots, approximately 1–2 cm in length, were excised from callus clusters and transferred to MS media supplemented with different auxins:
- NAA (0.5–1.5 mg L−1)
- IAA (0.5–1.5 mg L−1)
- 2,4-D (1.0–2.0 mg L−1)
The cultures were incubated at 26 ± 2 °C with 54–65% relative humidity and a 16 h photoperiod for four weeks. The explants on MS media were monitored daily, and data on root induction, root number per explant, and root length were noted.
2.7. Direct Shoot Induction
Healthy rhizome explants were utilized for direct organogenesis and cultured on a medium supplemented with activated charcoal, cytokinins, and auxins, either individually or in combinations. The hormone combinations included the following:
- BA (1.5–3.5 mg L−1)
- TDZ (2.0–4.5 mg L−1)
- KIN (2.5–4.5 mg L−1)
- BA (3.5–4.5 mg L−1) + IAA (0.1–0.5 mg L−1)
- BA (2.0–4.5 mg L−1) + NAA (0.1–0.5 mg L−1)
The inoculated cultures were maintained at 25 °C with 54–65% relative humidity and a 16 h photoperiod for four weeks. The explants were observed daily, and data regarding shoot induction, the number of shoots per explant, shoot length, the number of roots per explant, and root length were collected, following a partially modified protocol of Chakraborty et al. [23].
2.8. Hardening off and Acclimatization of In Vitro Plantlet
The plantlets developed in vitro were removed from flasks and rinsed with tap water to remove the nutrient medium and were planted in pots with a mixture of vermiculite and sterilized soil (1:1). To maintain high humidity, pots were covered with transparent polyethylene bags. The plantlets were placed in a growth chamber with a photoperiod of 16 h with a relative humidity of 65–70%. The temperature was set at 25 °C during the day and 18 °C during the night. The plantlets were watered every two days with half-strength Hoagland’s solution for 3–4 weeks. The survival and growth of the plants were recorded every week. After a month, the successfully hardened off plantlets were transplanted to bigger pots with garden soil and kept in a glasshouse.
2.9. Statistical Analysis and Data Visualization
Parameters related to callus, shoot, and root development were evaluated approximately 2–8 weeks after culture initiation. Each parameter related to callus formation, shoot development, and root growth was measured in three repetitions per experimental condition, ensuring reliable results and robust statistical analysis. Four explants were placed in each flask and Petri dish to maintain uniformity and enable accurate comparisons. The data collected were analyzed statistically via MS Excel 2010, Statistix 8.1 and visualized via Sigma Plot 12.0. An analysis of variance (ANOVA) was performed to assess the overall significance of the data. The significance of differences among different treatments was assessed using the least significant difference (LSD) test at 1 and 5% levels of significance.
3. Results
3.1. Sterilization of Plant Materials
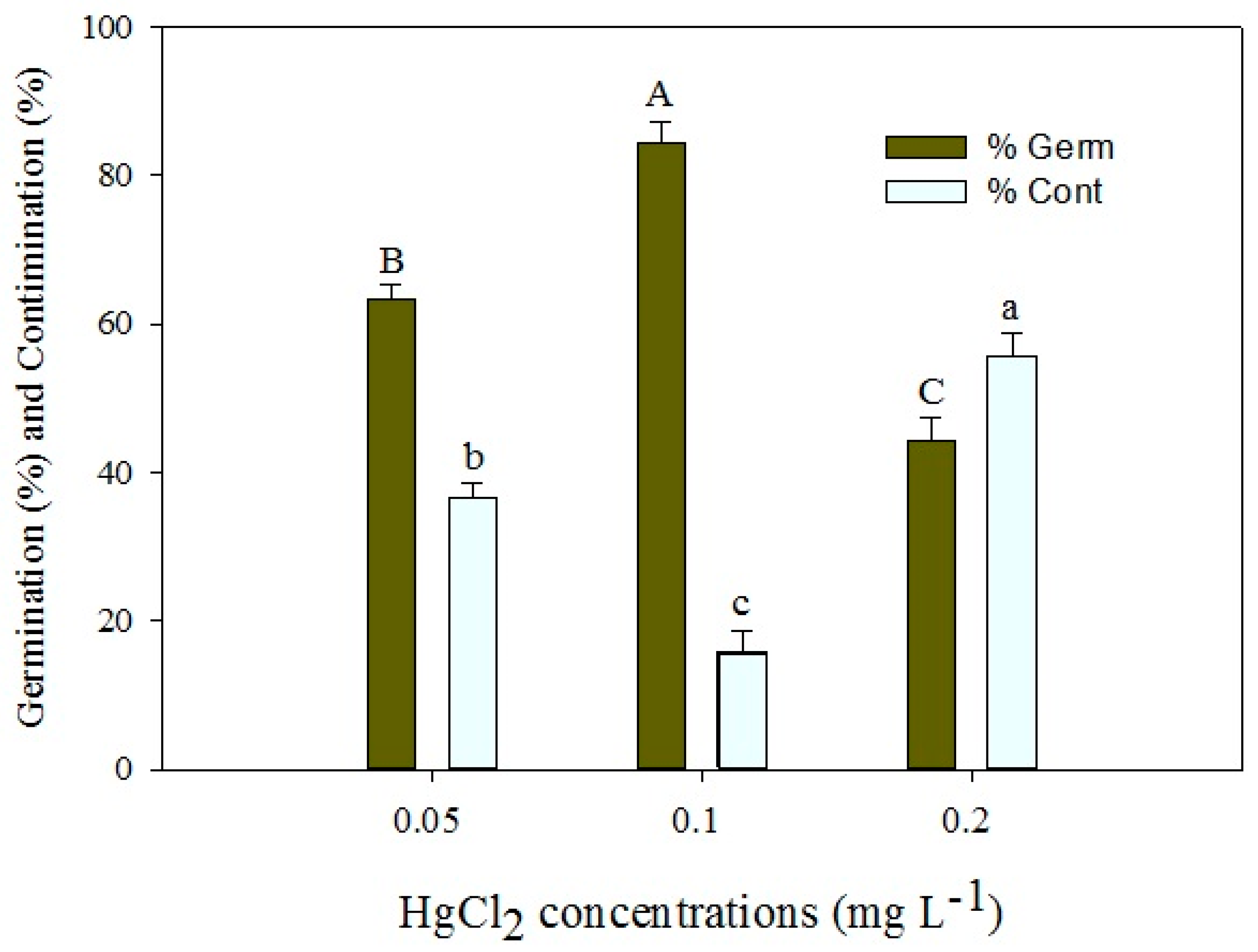
In this experiment, P. hexandrum seeds underwent sterilization using different concentrations of HgCl2 for 5 min at a consistent temperature (25 ± 2 °C) to assess their impact on seed decontamination and germination. Results indicated that the 0.1% HgCl2 treatment was the most successful, yielding significantly higher percentage germination (% germ) and lower percentage contamination (% cont) compared to other treatments. However, the 0.2% HgCl2 treatment negatively affected germination. Thus, 0.1% HgCl2 emerged as the optimal concentration for effective decontamination and germination of P. hexandrum seeds (Figure 2).
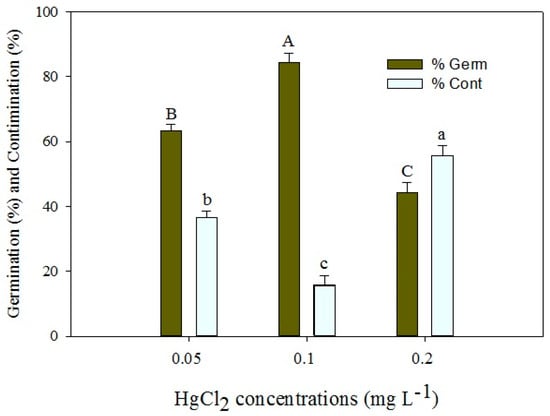
Figure 2.
Effects of different concentrations of HgCl2 on seed germination percentage (% germ) and contamination percentage (% cont) of P. hexandrum. Error bars represent the standard error of the respective means for three replicates. Capital and small letters represent mean comparisons at p ≤ 0.01% and p ≤ 0.05%.
3.2. Callus Induction from the Explants of P. hexandrum
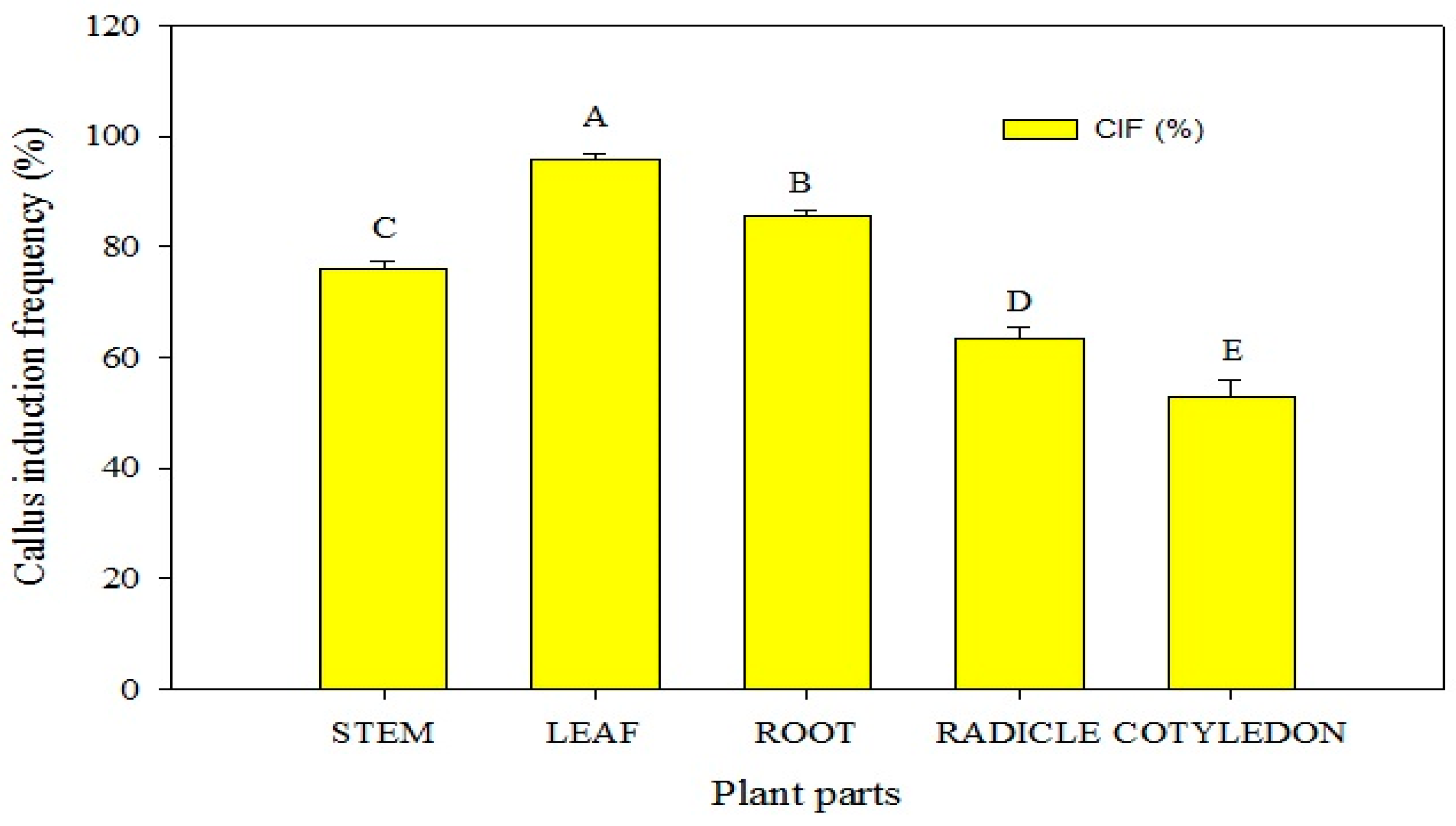
In this study, various combinations of plant growth regulators (PGRs) were assessed to induce callogenesis in P. hexandrum explants, aiming at germplasm conservation and micropropagation. Callogenesis was observed in stem, root, radicle, and cotyledon explants. These explants were successfully induced to form calluses on the MS medium supplemented with PGRs within 3 to 5 weeks. (Figure 3). The results indicated that the maximum callus induction PGR combinations induced callogenesis in P. hexandrum explants for conservation and micropropagation. MS basal media and supplements optimized the callus-inducing medium. Stem, root, radicle, and cotyledon explants formed calluses in 3 to 5 weeks on MS media with PGRs. The results indicated that the highest callus induction occurred in leaf explants (95.67%), followed by radicle explants (85.67%). Stem and cotyledon explants showed moderate frequencies (78% and 63%, respectively) on MS media with BA (1 mg L−1) and 2,4-D (3 mg L−1). However, the callus induction frequency (53%) of root explants was relatively lower (Figure 3). Within 10 days, primary callus formation extended across all sides of the cuttings, swelling along the cut edges. The addition of BA (1 mg L−1) and 2,4-D (3 mg L−1) to MS media was most effective in callogenesis (p ≤ 0.05), yielding the highest callus weight (215 mg), induction percentage (100%), and growth rate (13.620 mg/day) (Table 1; Figure 4A,B).
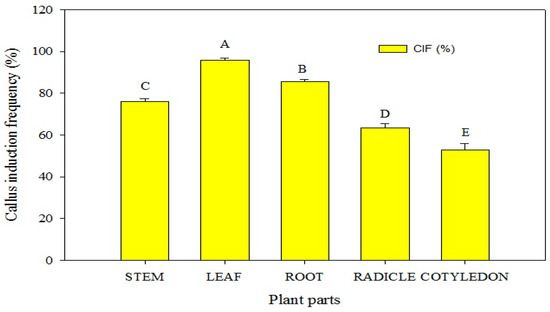
Figure 3.
Callus induction frequency (CIF) of various explants of P. hexandrum under different combinations of cytokinins and auxins. Capital letters represent data mean comparisons at p ≤ 0.01%. Error bars show standard errors of respective means.

Table 1.
Effects of different combinations and concentrations of plant growth regulators on callogenesis for in vitro conservation of P. hexandrum.
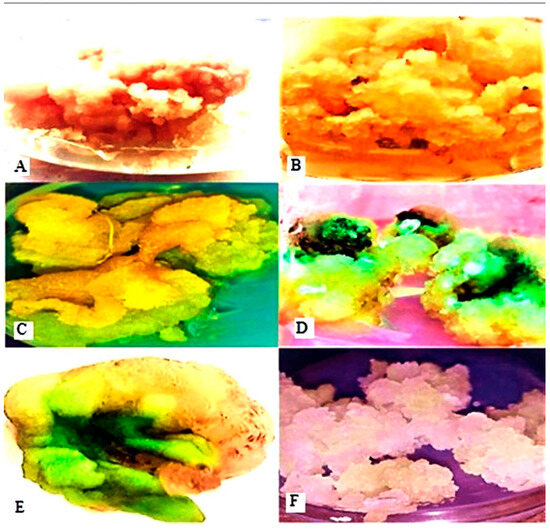
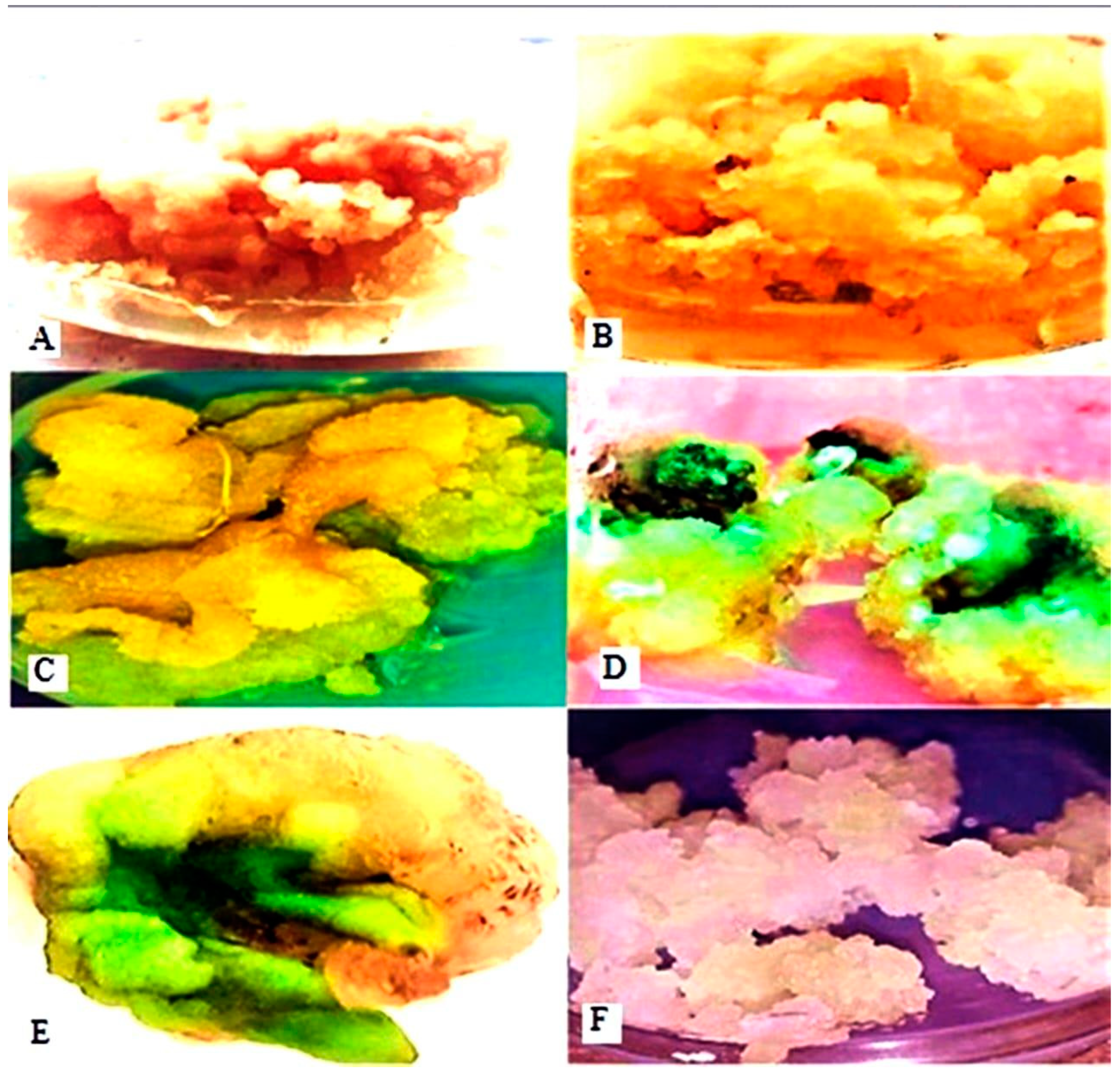
Figure 4.
Callus induction on MS media supplemented with different PGRs. Callus culture induced from explants on MS medium supplemented with 1 mg L−1 BA and 3 mg L−1 2,4-D (A,B). Callus cultured on MS medium supplemented with 1 mg L−1 KIN and 3 mg L−1 2,4-D (C). Callus cultured on MS medium supplemented with 2.5 mg L−1 BA and 1 mg L−1 NAA (D). Callus cultured on MS medium supplemented with 2 mg L−1 TDZ and 1 mg L−1 NAA (E). Callus cultured on MS medium supplemented with 3 mg L−1 BA and 1.0 mg L−1 IAA (F).
In contrast, the lowest callus weight (209.37 mg), callus induction percentage (69%), and growth rate (8.0833 mg/day) were observed with BA and IAA supplementation in MS culture media (Table 1) (Figure 4F). The addition of 100 mg L−1 ascorbic acid and 3 mg L−1 activated charcoal to the MS media prevented callus browning in in vitro cultures. Various PGRs resulted in calluses of different sizes, colors, and textures. Leaf explants produced compact, friable, and soft calluses in colors ranging from dark yellow to white and green (Table 1 and Figure 4). The induced callus was subcultured on MS media with various PGR concentrations and combinations, including BA + 2,4-D and TDZ + NAA, which led to the successful transformation of the proliferative callus into highly nodular callus formations in all tested combinations.
3.3. Shoot Induction of P. hexandrum from Calluses via Indirect Organogenesis
The present study successfully developed an in vitro protocol for P. hexandrum shoot multiplication from calluses, influenced by different PGRs and activated charcoal. The effects of various plant growth regulators (PGRs) on shoot frequency, the number of shoots, and shoot length per callus clump of P. hexandrum were evaluated to promote indirect organogenesis (Table 2 and Figure 5). Shoot regeneration from callus clumps was significantly induced by four different cytokinin PGRs, either alone or in combination with auxins. Statistical analysis of the data indicated that the highest shoot length (5.50 ± 0.10 cm), number of shoot regenerations (6.50 ± 0.10), and shoot regeneration percentage (85.00 ± 0.00%) were observed in calluses cultured on the MS growth medium fortified with BA (0.5 mg L−1) (Table 2 and Figure 5G–I), indicating its effectiveness in promoting shoot growth and regeneration. The second-highest shoot regeneration percentage (78.00 ± 1.66%), number of shoots per callus clump (4.80 ± 0.08), and shoot length (4.00 ± 0.11 cm) were obtained on the MS growth medium containing TDZ (0.5 mg L−1) (Table 1 and Figure 5C,D).

Table 2.
Effects of various plant growth hormones on shoot induction of P. hexandrum from calluses via indirect organogenesis.

Figure 5.
Indirect organogenesis on MS media. Shoot induction/growth from callus clump on MS medium supplemented with the following: 2.0 mg L−1 BA and 0.5 mg L−1 IAA (A,B); 2.0 mg L−1 TDZ (C,D); 1.05 mg L−1 KIN (E,F); and 0.5 mg L−1 BA (G–I).
Conversely, the lowest number of shoot regenerations was observed on the MS medium containing a combination of BA (2.0 mg L−1) and indole-3-acetic acid (IAA) (0.5 mg L−1) (Table 2) (Figure 5A,B). However, when IAA and BA were used in combination, it also resulted in a significant improvement in the number of shoots per callus clump, shoot length, and shoot regeneration percentage. The use of BA alone or in combination with other PGRs can significantly impact shoot regeneration percentage, the number of shoots, shoot length, and shoot induction (as shown in Table 2 and Figure 5).
3.4. Root Induction in Calluses of P. hexandrum
Root induction plays a crucial role in the in vitro reproduction of plants that are challenging to root. Auxins, crucial hormones for initiating and fostering root growth, necessitate specific types and concentrations tailored to individual plant species and the conditions of their cultivation. In the present study, the effect of three different auxins, namely 2,4-D, IAA, and NAA, on root induction in the calluses of P. hexandrum via indirect organogenesis was investigated. These results were based on different concentrations of auxins and their corresponding effects on the number and length of roots, as well as the rooting percentage through indirect organogenesis.
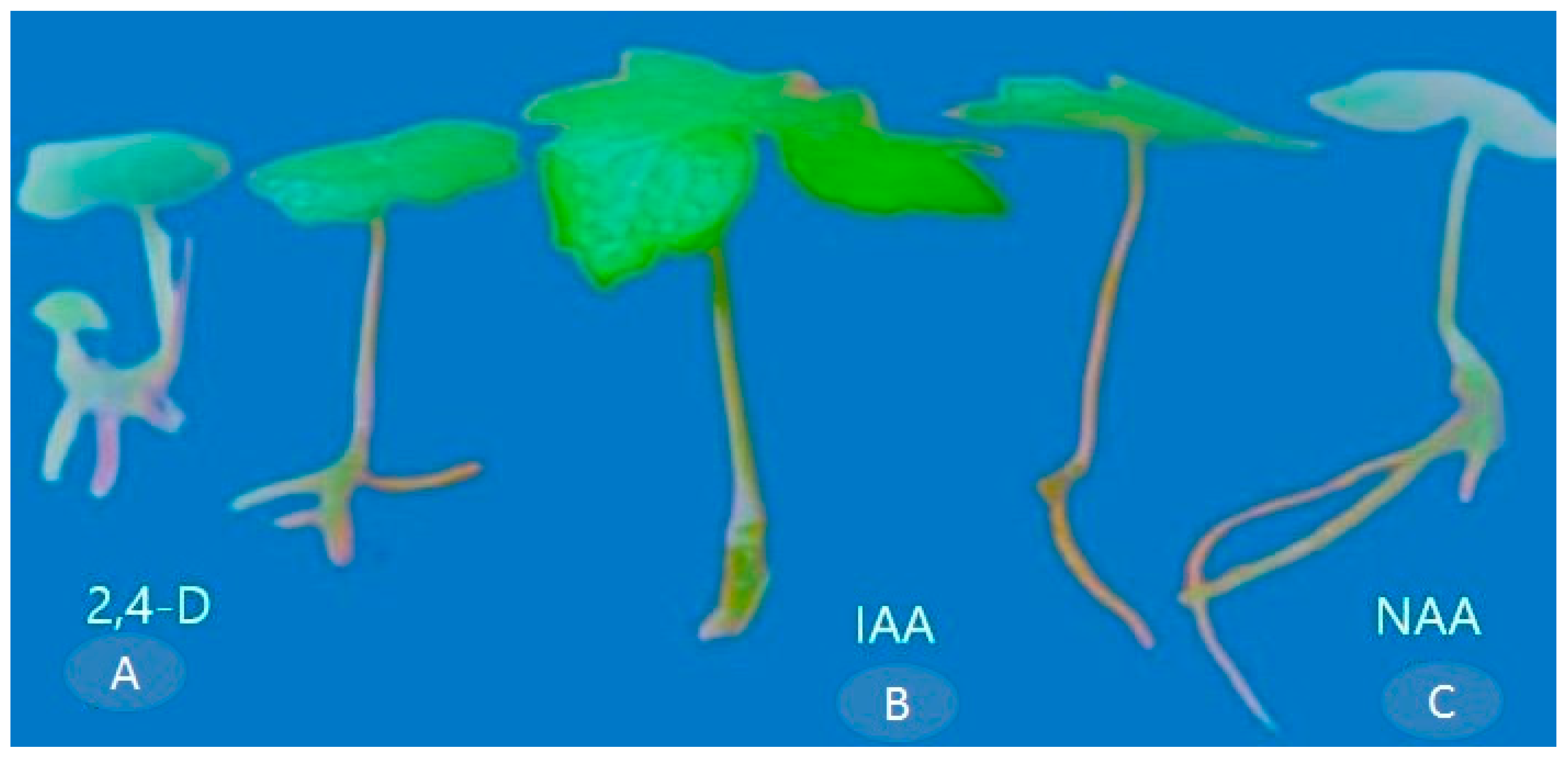
The maximum rooting, characterized by the number of roots per explant (4.08 ± 0.08), root length (5.40 ± 1.56 cm), and rooting percentage (87.00% ± 1.66), was observed in the MS medium supplemented with NAA (1.5 mg L−1) (Table 3 and Figure 6C). However, the lowest response in terms of the number of roots per explant (2.33 ± 0.08) and root length (2.85 ± 0.06 cm) was observed in MS media IAA (Table 3) (Figure 6A). Among the various auxins tested, the response of the explants was more favorable when MS media were fortified with NAA. These results suggested that the supplementation of 1.5 mg L−1 of NAA, IAA, and 2,4-D to the MS medium rapidly promotes root induction in P. hexandrum calluses through indirect organogenesis (Table 3 and Figure 6).

Table 3.
Effect of various auxins on root induction in calluses of P. hexandrum during organogenesis.
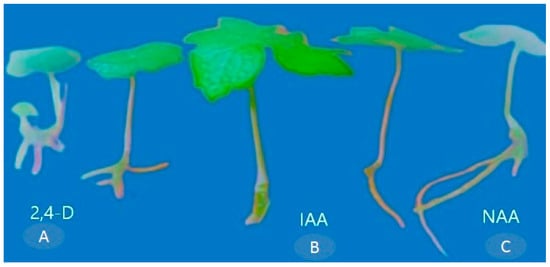
Figure 6.
Root induction on MS medium supplemented with (A) 2,4-D (0.5 mg L−1), (B) IAA (0.5 mg L−1), and (C) NAA (1.5 mg L−1).
3.5. In Vitro Shoot Proliferation from the P. hexandrum Explants through Direct Organogenesis
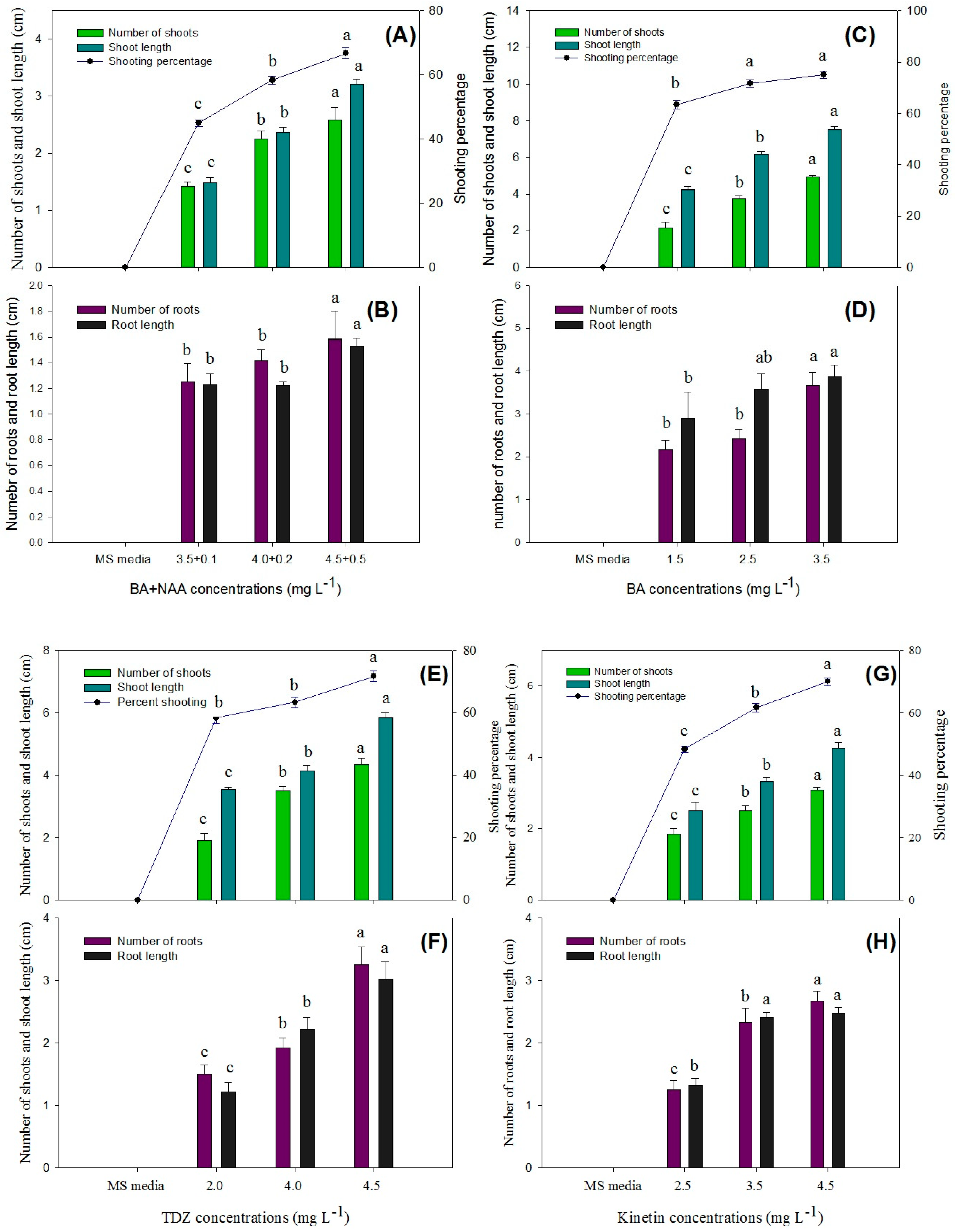
In this study, an efficient method for the in vitro regeneration of P. hexandrum has been successfully developed, using rhizome explants freshly collected from the plant through direct organogenesis for the micropropagation of P. hexandrum. The statistical analysis showed that the highest response in terms of the number of shoots per explant (5.30 ± 0.08), shoot length (12.40 ± 0.61), and shooting percentage (86.67 ± 1.66%) was obtained when the MS medium was augmented with BA (4.5 mg L−1) + NAA (0.5 mg L−1) and 3 mg L−1 of activated charcoal (Figure 7A,B and Figure 8A,F).
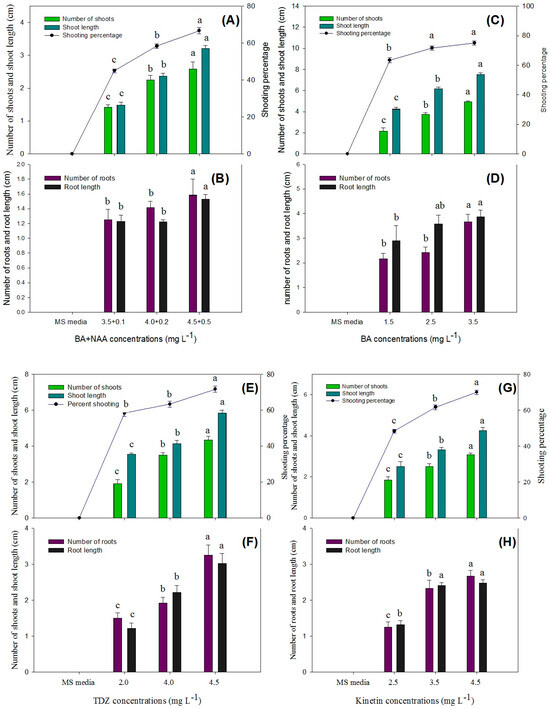
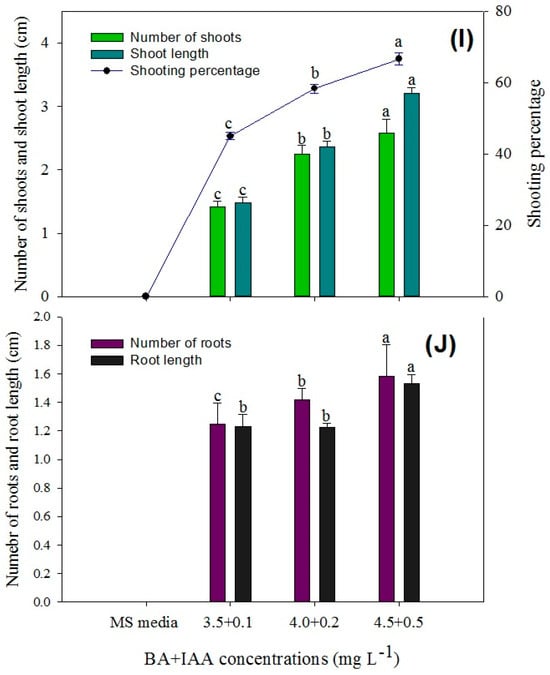
Figure 7.
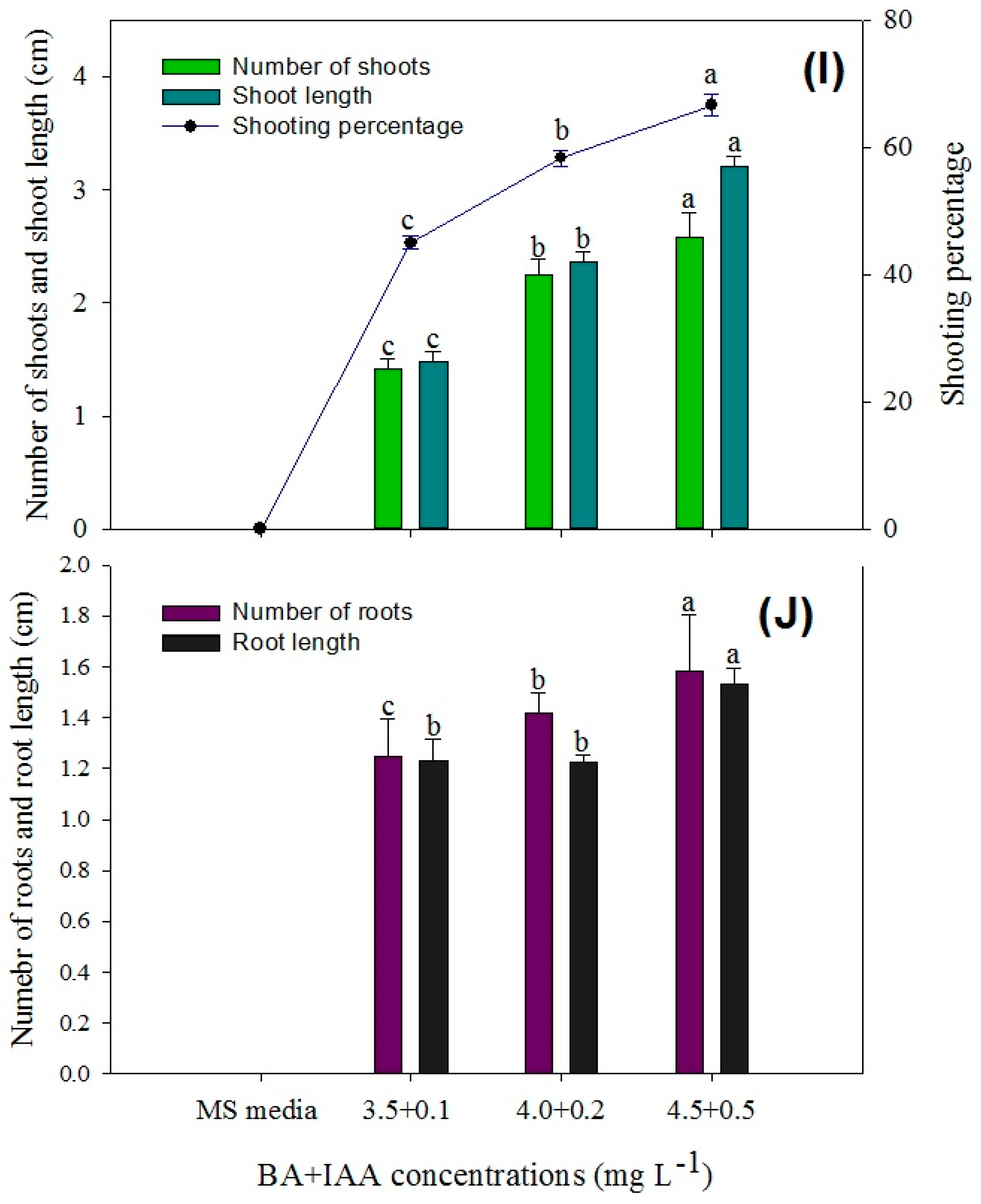
Comparison of the number of shoots, shoot length, shoot induction percentage, number of roots, and root length in various explants of P. hexandrum during indirect organogenesis under different concentrations of BA + NAA (A,B), BA (C,D), TDZ (E,F), KIN (G,H), and BA + IAA (I,J). Small letters represent data mean comparisons at p ≤ 0.05%. Error bars represent the standard error.

Figure 8.
Direct organogenesis from rhizomes having active buds on MS medium. (A,F) Supplemented with 4 mg L−1 BA + 0.5 mg L−1 NAA. (B,G) Shoot propagation from rhizomes on MS medium supplemented with 3.5 mg L−1 BA. (C,H) Shoot propagation from rhizomes on MS medium supplemented with 4.5 mg L−1 TDZ. (D,I) Shoot propagation from rhizomes on MS medium supplemented with 4.5 mg L−1 KIN. (E,I,J) Shoot propagation from rhizomes on MS medium supplemented with 4 mg L−1 BA + 0.5 mg L−1 IAA. (K) Shoot propagation from rhizomes on MS medium supplemented with BA + NAA, BA, TDZ, KIN, and BA + NAA.
The second-highest number of shoots per explant (4.92 ± 0.08), shoot length (7.52 ± 0.14), and shooting percentage (76.67% ± 1.60) was achieved with a medium containing BA (2.5 mg L−1) (Figure 7C,D and Figure 8B,G). TDZ treatment resulted in an average of 4.50 ± 0.14 shoots per explant, with a shoot length of 5.90 ± 0.11 cm and a shooting percentage of 73.33 ± 1.66%. (Figure 7E,F and Figure 8C,H). Similarly, KIN treatment also yielded 3.70 ± 0.08 shoots per explant, with a shoot length of 3.40 ± 0.07 cm and a shooting percentage of 70.00% ± 0.00 (Figure 7G,H and Figure 8D,I).
Explants cultured on media augmented with cytokinins alone and in combination with auxins (TDZ, BA + NAA, BA, KIN, and BA + NAA) exhibited varying levels of organogenesis induction with distinct responses (Figure 7 and Figure 8). In conclusion, the BA + NAA treatment demonstrated the most favorable outcomes in terms of shoot induction in this experiment, leading to high shoot multiplication and longer shoot lengths. The combination of auxin (NAA) and cytokinin (BA), along with activated charcoal in the growth medium, proved to be effective in promoting shoot formation from the explants through direct organogenesis.
3.6. Hardening off and Acclimatization
In this study, after the completion of the four-week culture period on the rooting medium, plantlets were carefully selected that exhibited not only robust and well-established root systems but also optimal physiological parameters indicative of their readiness for acclimatization. These carefully chosen plantlets underwent a gradual acclimatization process, which aimed to facilitate their transition from the synthetic media to soil and from the controlled environment of the growth chamber to the natural environment of the greenhouse. This acclimatization phase commenced with the methodical removal of the polyethylene bags, thereby exposing the plantlets to the fluctuating light and temperature patterns characteristic of outdoor settings.
Furthermore, as part of the acclimatization regimen, ambient humidity levels were gradually introduced to the plantlets, thereby simulating the moisture conditions they would encounter in their natural habitat. Subsequently, the plantlets were transplanted into larger pots filled with a suitable substrate, ensuring adequate space for root expansion and nutrient uptake. These pots were then placed within a greenhouse facility where environmental parameters such as temperature, humidity, and light intensity could be carefully regulated to facilitate the plantlets’ adjustment to their new surroundings.
Over time, under the nurturing care provided within the greenhouse, the plantlets continued to develop and thrive, exhibiting signs of healthy growth and adaptation to ex vivo conditions. Notably, the culmination of this acclimatization process was reflected in the remarkable achievement of an impressive survival rate of 87% among the transplanted plantlets, underscoring their successful transition and establishment in the natural environment (Figure 9).

Figure 9.
P. hexandrum successfully acclimatized to outdoor conditions after a hardening off period of 4 to 6 weeks under ex vivo conditions.
4. Discussion
4.1. Seed Sterilization and Growth Response
Seed sterilization is crucial to prevent microbial damage and enhance germination [23]. Aseptic conditions are essential in tissue culture to prevent contamination. Various disinfectants, including HgCl2, can improve germination rates, but microbial infestation can hinder growth. Surface sterilization with mercuric chloride is important but may affect biological characteristics [24]. HgCl2 is an effective disinfectant for reducing bacterial and fungal contamination in tissue culture [25].
In this study, treating P. hexandrum seeds with 0.1% HgCl2 for 5 min resulted in an 85.43% germination rate, suggesting its efficacy in seed sterilization and the enhancement of germination [26,27,28]. However, concentrations beyond 0.1% cased browning and the eventual death of seeds and explants. Sterilization outcomes varied significantly based on HgCl2 concentration and exposure duration, with higher concentrations and longer exposures reducing bacterial contamination but leading to rapid explant necrosis [29,30]. Previous research supports HgCl2 and Bavistin’s effectiveness as a disinfectant for various plant species. Its efficacy depends on factors such as concentration, contact time, explant type, and microbial species [31,32]. Optimal concentrations and exposure times range from 0.1% for 3–15 min for some species. However, HgCl2 treatment can induce issues like browning and toxicity, which can be mitigated with certain agents [33]. Comparatively, HgCl2 is more effective than NaOCl in combating microbial contamination. Therefore, optimizing HgCl2 concentration and duration is crucial for effective decontamination without compromising seed viability [22,34].
4.2. Callus Induction
Callus induction varies with explant type and forms undifferentiated callus tissue due to the hormone’s effects [35,36]. The balance of auxin and cytokinin influences de-differentiation and re-differentiation, inducing callus formation in specific medium ratios. This study found that leaf explants were the most efficient in callus formation, while root explants were the least responsive, reflecting the role of auxins and cytokinins in cell division and differentiation [37].
Previous studies have reported P. hexandrum callus induction and proliferation using different explants and PGRs [15,17,38]. Optimal PGR combinations (BA + 2,4-D, BA + NAA) resulted in varied callus textures at different concentrations, aligning with findings on callus formation and micropropagation in P. hexandrum [39]. Similar callogenesis results were observed by Sharma et al [6] and Chawla et al [36,40,41,42] in P. hexandrum and Roylea elegans using the same PGRs. However, studies on hypocotyl and root explants showed different outcomes in callus induction and podophyllotoxin production [38], possibly due to variations in sources, genotypes, explant treatments, and extraction methods [16,33,34].
In this study, shoot and leaf explants of P. hexandrum produced yellow, white, and greenish compact calluses in all treatments. However, leaf and stem explants displayed different colors on similar media with 2,4-D and BA; BA and NAA; and TDZ and NAA. Leaf explants produced brown-white, greenish, and friable calluses, while stem explants produced yellow-white, greenish, and friable calluses. Different colors in calluses result from variations in hormone types, concentrations, and conditions for different explants [43]. Callus morphology and color also depend on explant type, plant growth regulators, and pigment accumulation influenced by light, temperature, pH, and stress [34,44]. The induced calluses are capable of conservation, subculture, and further development into embryos or organs with the use of different PGRs [45].
4.3. Shoot Induction of P. hexandrum from Calluses via Indirect Organogenesis
In vitro plant regeneration, involving callus and direct explant methods, is influenced by various factors [13,20,34]. The agar-based culture medium initially caused shoot browning, impeding growth and proliferation. This issue was addressed by augmenting the MS medium with activated charcoal supplementation. This study successfully established a protocol for P. hexandrum shoot multiplication from calluses, incorporating different PGRs and activated charcoal. Initially facing shoot browning on the agar-based culture medium, the addition of 3 mg L−1 activated charcoal to the MS medium effectively controlled necrosis, resulting in robust and healthy shoot formations. The most effective shoot induction in P. hexandrum was achieved with the MS medium containing BA (4 mg L−1) (Figure 4D) + NAA (0.5 mg L−1) and 3 mg L−1 activated charcoal, resulting in the highest shoot formation and length. BA in the shooting medium favored in vitro shoot multiplication, consistent with previous studies [6,41,46].
The study investigated the effects of BA and TDZ, both individually and in combination with auxins, on indirect organogenesis and shoot regeneration. Notably, BA at a concentration of 2.0 mg L−1 demonstrated high efficacy in promoting shoot formation. This finding contrasts with the work of Chen et al. [35], who reported that IAA exhibited optimal efficiency compared to other plant growth regulators (PGRs). However, our study revealed that IAA combinations had limitations in terms of shoot multiplication. Specifically, lower concentrations of BA resulted in fewer shoots, leading us to expect that higher BA concentrations would increase shoot numbers. Interestingly, in Vitex negundo L., higher BA concentrations actually reduced shoot production, emphasizing the need for species-specific optimization in tissue culture protocols [39,40]. In contrast, our study on P. hexandrum showed a significant increase in shoot multiplication with BA concentrations exceeding 0.5 mg L−1. BA’s impact on natural hormone metabolism, tissue penetration, and cell uptake enhanced shoot formation and quality in P. hexandrum in vitro. The type and concentration of PGR also influenced shoot quality and quantity in the explants, with a significant amount of readily accessible BA contributing to the observed effects [39,40]. Geng et al. [45] found BA to be more effective than thidiazuron (TDZ) for increasing shoot number and elongation at concentrations of 0 to 2.0 mg L−1 [47,48,49]. In summary, our study highlights BA as the most effective cytokinin for indirect organogenesis among those tested.
4.4. In Vitro Root Induction in Calluses of P. hexandrum
Root induction is vital for in vitro propagation of hard-to-root plants. Auxins are key hormones for root initiation and development, but their optimal type and concentration depend on the plant and the culture conditions [50]. NAA’s effectiveness is attributed to its influence on cell division, enlargement, meristematic activity, and nucleic acid and protein synthesis [51].
The results align with previous studies emphasizing NAA’s role in successful root induction in P. hexandrum and other plant species [52]. NAA’s stability and mobility further facilitate its uptake and translocation within plant tissues. The study aligns with previous research highlighting the importance of NAA supplementation for successful root induction in the in vitro culture of different plant species [53]. The findings contribute to the optimization of in vitro propagation techniques for P. hexandrum and underline the importance of selecting the right type and concentration of plant growth regulators for enhancing roots. IAA resulted in the lowest rooting percentage due to its rapid degradation and potential inhibition of root elongation. The findings contributed to the optimization of in vitro propagation techniques for P. hexandrum and demonstrate the importance of selecting the right PGR types and concentrations for enhancing shoot and root organogenesis and propagation.
4.5. In Vitro Shoot Proliferation from Explants via Direct Organogenesis
Direct organogenesis is crucial for genetic manipulations and the micropropagation of plants [15]. However, some plant species, including P. hexandrum, face challenges in shoot organogenesis due to the slow growth rate and overexploitation, as the plant contains secondary metabolites. This study successfully established the in vitro regeneration and proliferation of P. hexandrum from rhizome explants through direct organogenesis. These findings are in line with prior research that has achieved regeneration from various explants, including seeds, cotyledons, hypocotyls, and leaves, using different PGRs such as BA, TDZ, NAA, and IAA [6,23].
In overcoming challenges in shoot organogenesis, antioxidants and charcoal were employed, aligning with prior research achieving regeneration from different explants using various PGRs [45]. Different cytokinins (BA with NAA, TDZ, and KIN) in the media induced distinct responses, enhancing in vitro regeneration of P. hexandrum [15,16]. The results contrast with studies reporting low or no shoot regeneration from rhizome explants of P. hexandrum, potentially influenced by varied plant growth regulators (IBA, IAA, 2iP, BA, and NAA) and the absence of activated charcoal [6,21,27]. This study underlines the need for suitable PGR combinations; BA and NAA with activated charcoal proves most promising for efficient shoot multiplication and longer shoot lengths in P. hexandrum.
In conclusion, the BA + NAA treatment demonstrated the most favorable outcomes in terms of shoot induction in this experiment, leading to high shoot multiplication and longer shoot lengths. The study emphasized P. hexandrum’s responsiveness to organogenesis, highlighting the role of plant growth regulators (PGRs) in regulating shoot induction through cell division and elongation.
5. Conclusions
P. hexandrum is an endangered but valuable medicinal plant native to the temperate and cold climates of the Himalayan region. It provides socioeconomic, medicinal, ecological, and traditional benefits, but faces threats from overexploitation, habitat loss, and environmental adversities. This study developed an efficient in vitro propagation and conservation protocol using plant growth regulators (PGRs) and various explant sources. We optimized conditions for seed germination, sterilization, callus formation, direct and indirect organogenesis, and acclimatization of P. hexandrum. Among the PGR combinations tested, 1 mg L−1 BA combined with 2 mg L−1 (2,4-D) yielded the highest callus induction and growth. For direct organogenesis, BA was the most effective in promoting shoot growth and regeneration, while for indirect organogenesis, BA and NAA produced the maximum number of shoots from rhizomes. The plantlets were successfully acclimatized and transferred to ex vitro conditions with a high survival rate. This protocol can facilitate the mass propagation and preservation of P. hexandrum, providing large numbers of uniform and disease-free plants in a short time. Future research should focus on field trials, quality assessment, genetic diversity analysis, and biotechnological production of podophyllotoxin. This study contributes to plant biotechnology, sustainable utilization, and conservation biology for endangered or economically important plant species.
Author Contributions
Conceptualization, Z.K. and B.K.; methodology, S.T.S.; software, J.I.; validation, A.J., S.T.S. and A.B.; formal analysis, Z.K. and S.T.S.; investigation, Z.K., M.S.K. and W.I.; resources, M.F.E.; data curation, A.J., S.T.S., M.A.A. and S.T.S.; writing—original draft preparation, Z.K., B.K., W.I. and M.S.K.; writing—review and editing, A.J., S.T.S., M.F.E., M.A.A. and D.P.; visualization, A.J.; supervision, D.P.; project administration, A.J.; funding acquisition, D.P. and M.A.A. All authors have read and agreed to the published version of the manuscript.
Funding
The authors extend their appreciation to the researchers supporting project number (RSP2024R306), King Saud University, Riyadh, Saudi Arabia.
Data Availability Statement
Data are contained within the article.
Acknowledgments
The authors extend their appreciation to the researchers supporting project number (RSP2024R306), King Saud University, Riyadh, Saudi Arabia. We also express our sincere gratitude to Mian Afaq Ahmad for his invaluable assistance and unwavering support during the experimental work conducted at the Plant Tissue Culture and Germplasm Conservation Division, Institute of Biotechnology and Genetic Engineering (IBGE), The University of Agriculture, Peshawar.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- Kour, J.; Balgotra, S.; Rajput, P.; Kour, H.; Verma, P.K.; Sawant, S.D. Medicinal value of high-altitude plants of Indian Himalaya. In Botanical Leads for Drug Discovery; Springer: Berlin/Heidelberg, Germany, 2020; pp. 295–324. [Google Scholar]
- El Merzougui, S.; Benelli, C.; El Boullani, R.; Serghini, M.A. The Cryopreservation of Medicinal and Ornamental Geophytes: Application and Challenges. Plants 2023, 12, 2143. [Google Scholar] [CrossRef] [PubMed]
- Ionkova, I. Podophyllotoxin and related lignans: Biotechnological production by in vitro plant cell cultures. Med. Plant Biotechnol. 2010, 8, 138–155. [Google Scholar]
- Chhetri, D.R. Medicinal Plants of the Himalaya: Production Technology and Utilization; Agrobios: Nepal, 2014. [Google Scholar]
- Hamayun, M.; Khan, S.A.; Lee, I.J.; Khan, M.A. Conservation assessment of Hindu-Kush Mountain Region of Pakistan: A case study of Utror and Gabral Valleys, District Swat, Pakistan. Asian J. Plant Sci. 2006, 4, 34–39. [Google Scholar]
- Sharma, N.; Thakur, M.; Sharma, P.; Sharma, Y.P.; Dutt, B. In vitro propagation from rhizomes, molecular evaluation and podophyllotoxin production in Himalayan May Apple (Sinopodophyllum hexandrum Royle TS Ying): An endangered medicinal plant. Plant Cell Tissue Organ Cult. (PCTOC) 2022, 149, 159–173. [Google Scholar] [CrossRef]
- Singh, J.; Shah, N.C. Podophyllum: A review. Curr. Res. Med. 1994, 16, 53–83. [Google Scholar]
- Wong, S.-K.; Tsui, S.-K.; Kwan, S.-Y.; Su, X.-L.; Lin, R.-C. Identification and characterization of Podophyllum emodi by API-LC/MS/MS. J. Mass Spectrom. 2000, 35, 1246–1251. [Google Scholar] [CrossRef]
- Regassa, H.; Sourirajan, A.; Kumar, V.; Pandey, S.; Kumar, D.; Dev, K. A review of medicinal plants of the himalaya with anti-proliferative activity for the treatment of various cancers. Cancers 2022, 14, 3898. [Google Scholar] [CrossRef] [PubMed]
- Haq, F. Conservation status of the critically endangered and endangered species in the Nandiar Khuwar catchment District Battagram, Pakistan. Int. J. Biodivers. Conserv. 2011, 3, 27–35. [Google Scholar]
- Qazi, P.H.; Rashid, A.; Shawl, S.A. Podophyllum hexandrum: A versatile medicinal plant. Int. J. Pharm. Sci. 2011, 3, 261–268. [Google Scholar]
- Shah, Z.; Gohar, U.F.; Jamshed, I.; Mushtaq, A.; Mukhtar, H.; Zia-UI-Haq, M.; Toma, S.I.; Manea, R.; Moga, M.; Popovici, B. Podophyllotoxin: History, recent advances and future prospects. Biomolecules 2021, 11, 603. [Google Scholar] [CrossRef]
- Nadeem, M.; Palni, L.M.S.; Purohit, A.N.; Pandey, H.; Nandi, S.K. Propagation and conservation of Podophyllum hexandrum Royle: An important medicinal herb. Biol. Conserv. 2009, 92, 121–129. [Google Scholar] [CrossRef]
- Setyawati, A.; Samanhudi, S.; Prameswari, W.; Syukri, D.; Ramadhani, D.F.; Talitha, O. In vitro Propagation and Secondary Metabolite Production of Medicinal Plant of Euchresta horsfieldii (Lesch) Benn. Plant Breed. Biotechnol. 2023, 11, 34–48. [Google Scholar] [CrossRef]
- Zuhra, Z.; Saleem, D.; Akhtar, W.; Mahmood, T. Tissue culture optimization of Podophyllum hexandrum L., an endangered medicinal plant. J. Ani. Plant Sci. 2021, 31, 2. [Google Scholar]
- Kumar, J.; Sandal, P.; Singh, A.; Kumar, A.; Arya, V.; Devi, R.; Sharma, B.P.; Verma, R. Conservation status, anticancer compounds and pharmacological aspects of Podophyllum hexandrum Royle: A review. Indian J. Ecol. 2022, 49, 1096–1102. [Google Scholar]
- Tariq, A.S.; Naz, K.; Shahzadi, S.; Ilyas, S.; Javed, S. Study of genetic stability in in vitro conserved Podophyllum hexandrum using RAPD markers. J. Anim. Plant. Sci. 2015, 4, 1114–1120. [Google Scholar]
- Kuldeep, Y.; Narender, S.; Sharuti, V.; Gupta, R.; Sethi, K.L. Conservation of medicinal plant resources in Himalayan region. Conserve. Trop. Plant Res. 1983, 1, 101–107. [Google Scholar]
- Rizwan, A.; Saurabh, S.; Vishal, K.; Chhavi, V. Invitro Propagation of Podophyllum hexandrum. Int. J. Pharm. Res. Appl. 2021, 6, 169–172. [Google Scholar]
- Chakraborty, A.; Bhattacharya, D.; Ghanta, S.; Chattopadhyay, S.; Steinitz, B.; Tabib, Y.; Gaba, V.; Gefen, T.; Vaknin, Y. Vegetative micro-cloning to sustain biodiversity of threatened Moringa species. In Vitro Cell. Dev. Biol. Plant 2009, 45, 65–71. [Google Scholar]
- Nazir, K.; Hassan, S.W.; Khan, M.I.; Elamin, K.M.; Niyazi, H.A. The use of ZnO NPs and Ag NPs along with sterilizing agents for managing contamination in banana tissue culture. Biomass Convers. Biorefin. 2023, 17, 1–8. [Google Scholar] [CrossRef]
- Boruah, J. Effect of 0.1% HgCl2 on surface sterilization of som (Persea bombycina King) explant during tissue culture-a major host plant of muga silkworm. Int. J. Curr. Microbiol. Appl. Sci. 2020, 9, 954–958. [Google Scholar] [CrossRef]
- Chakraborty, A.; Bhattacharya, D.; Ghanta, S.; Chattopadhyay, S. An efficient protocol for in vitro regeneration of Podophyllum hexandrum, a critically endangered medicinal plant. Indian J. Biotechnol. 2010, 9, 217–220. [Google Scholar]
- Padhi, M.; Singh, S.P. Surface sterilization for reducing microbial contamination in in vitro propagation of lasora (Cordia myxa Roxb.) using nodal segments. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 836–842. [Google Scholar] [CrossRef]
- Li, M.F.; Li, W.; Yang, D.L.; Sun, P. The dormancy mechanism and bioactivity of hydroquinone extracted from Podophyllum hexandrum Royle seed. Electr. J. Biol. 2009, 5, 11–16. [Google Scholar]
- Gu, M.; Li, Y.; Jiang, H.; Zhang, S.; Que, Q.; Chen, X.; Zhou, W. Efficient in vitro sterilization and propagation from stem segment explants of Cnidoscolus aconitifolius (Mill.) IM Johnst, a multipurpose woody plant. Plants 2022, 11, 1937. [Google Scholar] [CrossRef]
- Madhale, S.V. Effect of HgCl2 on Surface Sterilization of Explants of Momordica Cymbalaria Hook. F. J. Sci. Res. Int. 2016, 2, 1. [Google Scholar]
- Babu, G.A.; Mosa Christas, K.; Kowsalya, E.; Ramesh, M.; Sohn, S.I.; Pandian, S. Improved sterilization techniques for successful in vitro micropropagation. In Commercial Scale Tissue Culture for Horticulture and Plantation Crops; Springer Nature: Singapore, 2022; pp. 1–21. [Google Scholar]
- Sharma, R.K.; Sharma, S.; Sharma, S.S. Seed germination behavior of some medicinal plants of Lahaul and Spiti cold desert (Himachal Pradesh): Implications for conservation and cultivation. Curr. Sci. 2006, 11, 1118. [Google Scholar]
- Mng’omba, S.A.; du Toit, E.S.; Akinnifesi, F.K.; Sileshi, G. Efficacy and Utilization of Fungicides and Other Antibiotics for Aseptic Plant Cultures; INTECH Open Access Publisher: London, UK, 2012. [Google Scholar]
- Bhojwani, S.S.; Dantu, P.K.; Bhojwani, S.S.; Dantu, P.K. Micropropagation. In Plant Tissue Culture: An Introductory Text; Springer: Berlin/Heidelberg, Germany, 2013; pp. 245–274. [Google Scholar]
- Karunaratne, M.L.; Peries, S.E.; Egodawatta, W.C. Callus induction and organogenesis from leaf explants of Tectona grandis. Ann. Biol. Res. 2014, 5, 74–82. [Google Scholar]
- Shin, J.; Bae, S.; Seo, P.J. De novo shoot organogenesis during plant regeneration. J. Exp. Bot. 2020, 1, 63–72. [Google Scholar] [CrossRef]
- Kharkwal, A.C.; Tellez, M.; Lata, H.; Khan, I.; Cushman, K.E.; Moraes, R.M. Post-harvest and scale-up extraction of American Mayapple leaves for podophyllotoxin production. Ind. Crops Prod. 2006, 24, 3–7. [Google Scholar]
- Chen, X.; Ye, C.; Yang, H.; Ji, W.; Xu, Z.; Ye, S.; Wang, H.; Jin, S.; Yu, C.; Zhu, X. Callogenesis and plant regeneration in peony (Paeonia suffruticosa) using flower petal explants. Horticulturae 2022, 8, 357. [Google Scholar] [CrossRef]
- Chawla, S.U.; Kumar, A.S.; Kajla, S.U.; Goyal, S.C.; Choudhary, P.O. In vitro development of plantlets from axillary buds of Nyctanthes arbortristis Linn. A medicinal plant. Haryana J. Hortic. Sci. 2010, 39, 295–297. [Google Scholar]
- Waoo, A.A.; Khare, S.; Ganguly, S. In vitro culture of Latana camera from nodal and shoot-tip explants in phytoremediation studies. Curr. Trends Tech. Sci. 2013, 2, 183–186. [Google Scholar]
- Steephen, M.; Nagarajan, S.; Ganesh, D. Phloroglucinol and silver nitrate enhance axillary shoot proliferation in nodal explants of Vitex negundo L.—An aromatic medicinal plant. Iran. J. Biotechnol. 2010, 8, 82–89. [Google Scholar]
- Klimek-Chodacka, M.; Kadluczka, D.; Lukasiewicz, A.; Malec-Pala, A.; Baranski, R.; Grzebelus, E. Effective callus induction and plant regeneration in callus and protoplast cultures of Nigella damascena L. Plant Cell Tissue Organ Cult. (PCTOC) 2020, 143, 693–707. [Google Scholar] [CrossRef]
- Keutgen, A.J.; Tomaszewska-Sowa, M.; Bomberski, A.; Keutgen, N. The Influence of Phytohormones on the Efficiency of Callus Formation, Its Morphologically Properties and Content of Bioactive Compounds in in vitro Cultures of Daucus carota L. Horticulturae 2022, 8, 100. [Google Scholar] [CrossRef]
- Sati, P.; Chauhan, M.; Trivedi, V.L.; Nautiyal, M.C.; Semwal, P. Challenges and prospects for the in vitro conservation of plants having anticarcinogenic potential in the Western Himalaya, India. Plant Cell Tissue Organ Cult. (PCTOC) 2023, 152, 237–252. [Google Scholar] [CrossRef]
- Rajesh, M.; Sivanandhan, G.; Jeyaraj, M.; Chackravarthy, R.; Manickavasagam, M.; Selvaraj, N.; Ganapathi, A. An efficient in vitro system for somatic embryogenesis and podophyllotoxin production in Podophyllum hexandrum Royle. Protoplasma 2014, 251, 1231–1243. [Google Scholar] [CrossRef] [PubMed]
- Güner, B. In Vitro Culture, Biological Activity and Essential oil Analysis of Yellow Loosestrife (Lysimachia vulgaris L.). Master’s Thesis, Fen Bilimleri Enstitüsü, Ankara, Turkey, 2011. [Google Scholar]
- Parveen, S.; Kamili, A.N.; Shah, A.M. Impact of BAP and different auxins on in vitro shoot proliferation of Rheum emodi Wall. J. Pharm. Biol. Sci. 2012, 4, 47–52. [Google Scholar]
- Geng, F.; Moran, R.; Day, M.; Halteman, W.; Zhang, D. Increasing in vitro Shoot Elongation and Proliferation of ‘G.30’ and ‘G.41’ Apple by Chilling Explants and Plant Growth Regulators. Hort. Sci. 2016, 51, 899–904. [Google Scholar] [CrossRef]
- Alizadeh, S.; Dumanoğlu, H. The effects of zinc oxide nanoparticles loaded with IAA and IBA on in vitro rooting of apple microcuttings. Turk. J. Agric. For. 2022, 46, 306–317. [Google Scholar] [CrossRef]
- Trunjaruen, A.P.; Taratima, W. The Optimization of Medium Conditions and Auxins in the Induction of Adventitious Roots of Pokeweed (Phytolacca americana L.) and Their Phytochemical Constituents. Scientifica 2023, 2023, 2983812. [Google Scholar] [CrossRef] [PubMed]
- Sagar, B.P.S.; Zafar, R. In vitro. Enhanced Production of Podophyllotoxin in Phytohormonal-Induced and Regenerated Roots of Podophyllum hexandrum. Pharm. Biol. 2005, 43, 404–410. [Google Scholar] [CrossRef]
- Jemal, K. In Vitro Regeneration of Allophylus serratus. Roxb (Kurz), an Important Medicinal Plant. 2022. Available online: https://www.researchsquare.com/article/rs-1989679/v1 (accessed on 25 July 2024).
- Özkul, M.; Özel, Ç.A.; Yüzbaşıoğlu, D.; Ünal, F. Does 2,4-dichlorophenoxyacetic acid (2,4-D) induce genotoxic effects in tissue cultured Allium roots. Cytotechnology 2016, 68, 2395–2405. [Google Scholar] [CrossRef] [PubMed]
- Sosnowski, J.; Truba, M.; Vasileva, V. The Impact of Auxin and Cytokinin on the Growth and Development of Selected Crops. Agriculture 2023, 13, 724. [Google Scholar] [CrossRef]
- Hesami, M.; Daneshvar, M.H. In vitro adventitious shoot regeneration through direct and indirect organogenesis from seedling-derived hypocotyl segments of Ficus religiosa L.: An important medicinal plant. Hort. Sci. 2018, 53, 55–61. [Google Scholar] [CrossRef]
- Shukla, A.; Mishra, P.; Raturi, A. In vitro regeneration of Podophyllum hexandrum from rhizome explants. Asian Pac. J. Trop. Biomed. 2012, 2, 455–458. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).