Chlorine Dioxide Delays Enzymatic Browning in Postharvest Cherimoya and Enables Establishment of Kinetics Substrate Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Treatments
2.2. Appearance
2.3. Weight Loss and Firmness
2.4. Starch and Total Sugar Content Measurements
2.5. Titratable Acid (TA) Content Measurements
2.6. Phenolic Compound Content Measurements
2.7. PPO Activity Assay
2.8. Morphological Observation and FTIR
2.9. Kinetic Model Statistical Analyses
2.10. Statistical Analysis
3. Results
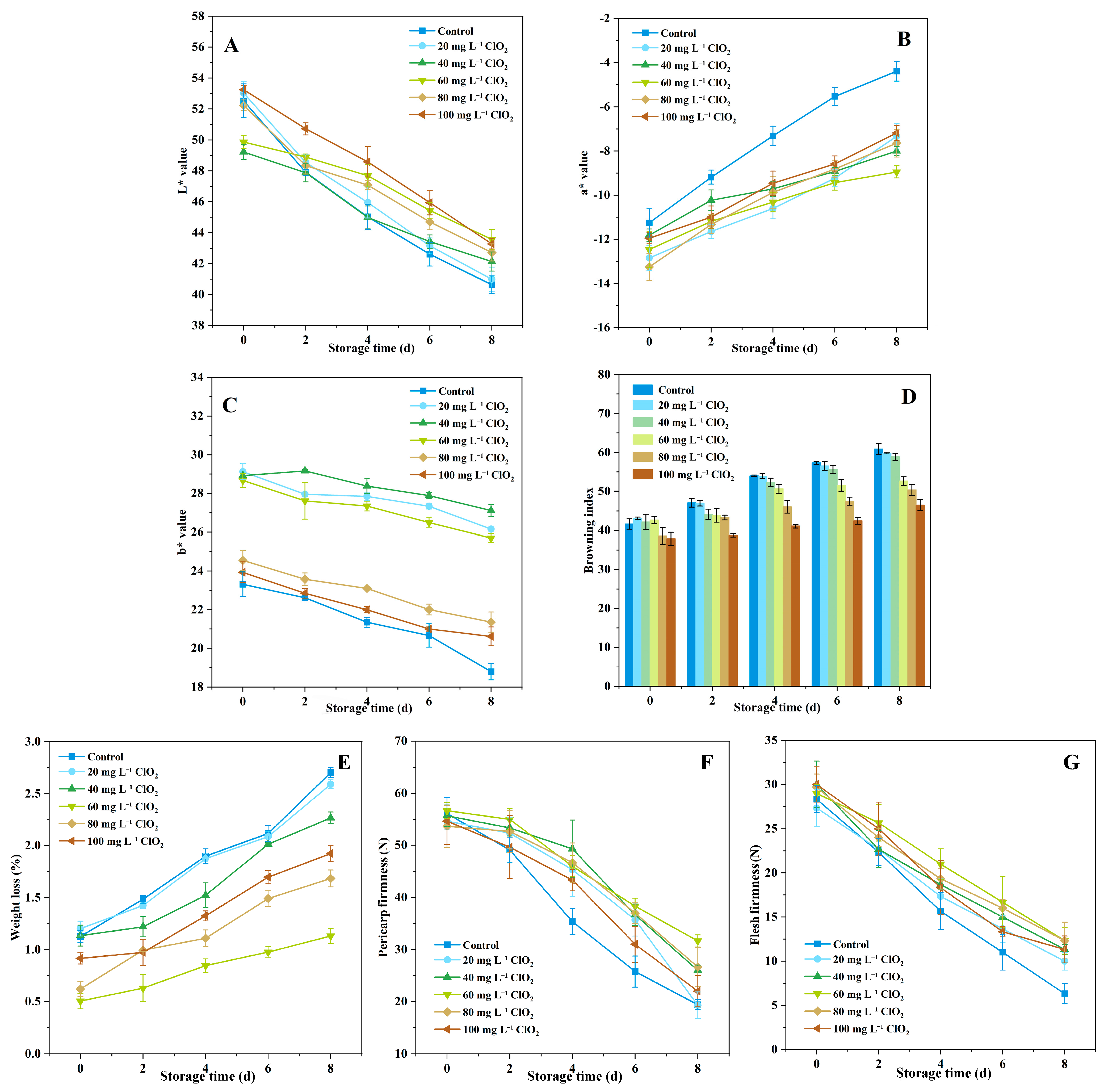
3.1. Cherimoya Appearance
3.2. Weight Loss and Firmness Changes
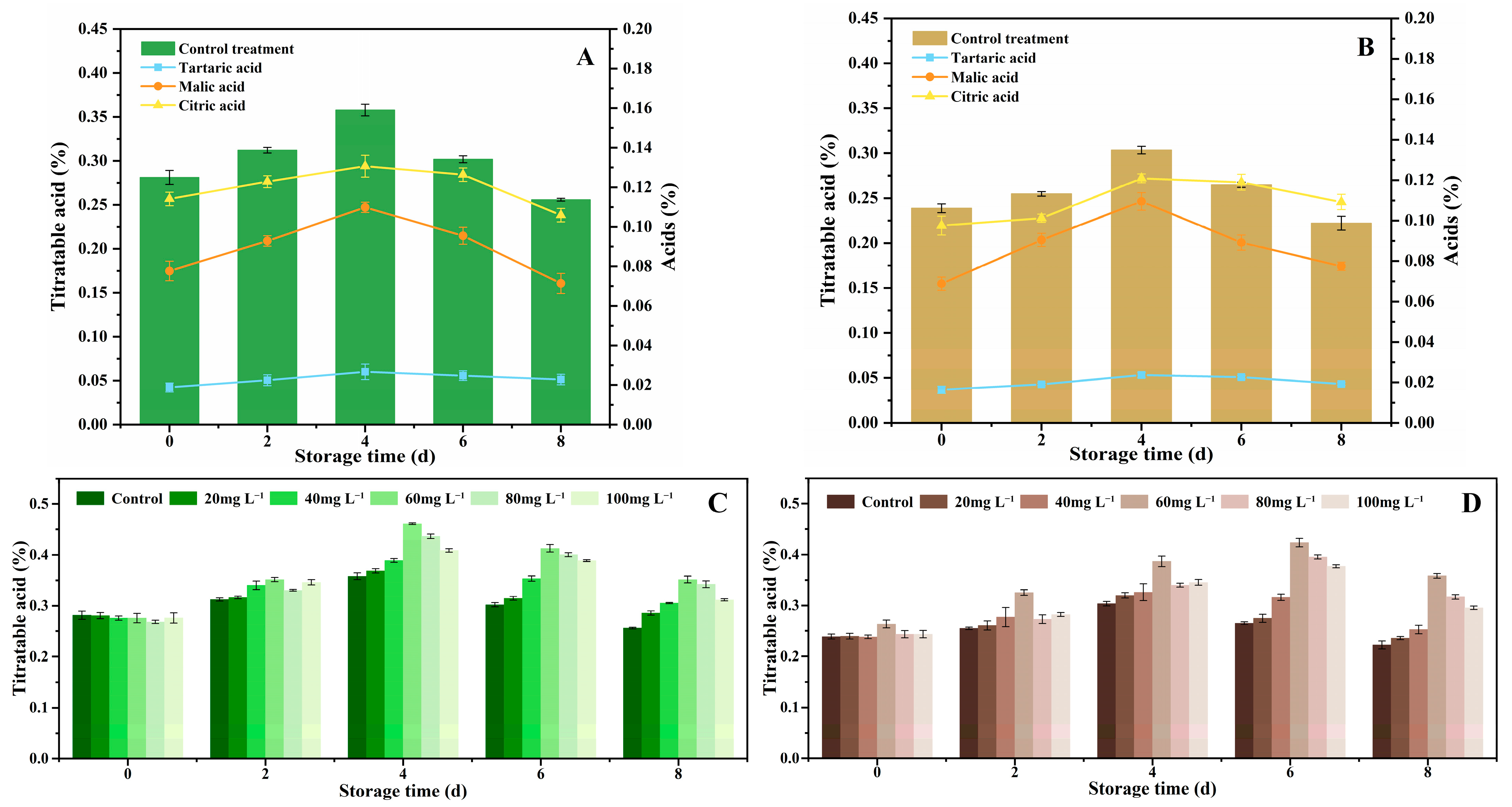
3.3. TA Content, Starch, and Total Sugar Changes
3.4. Phenolic Compounds Content Changes
3.5. PPO Activity Change
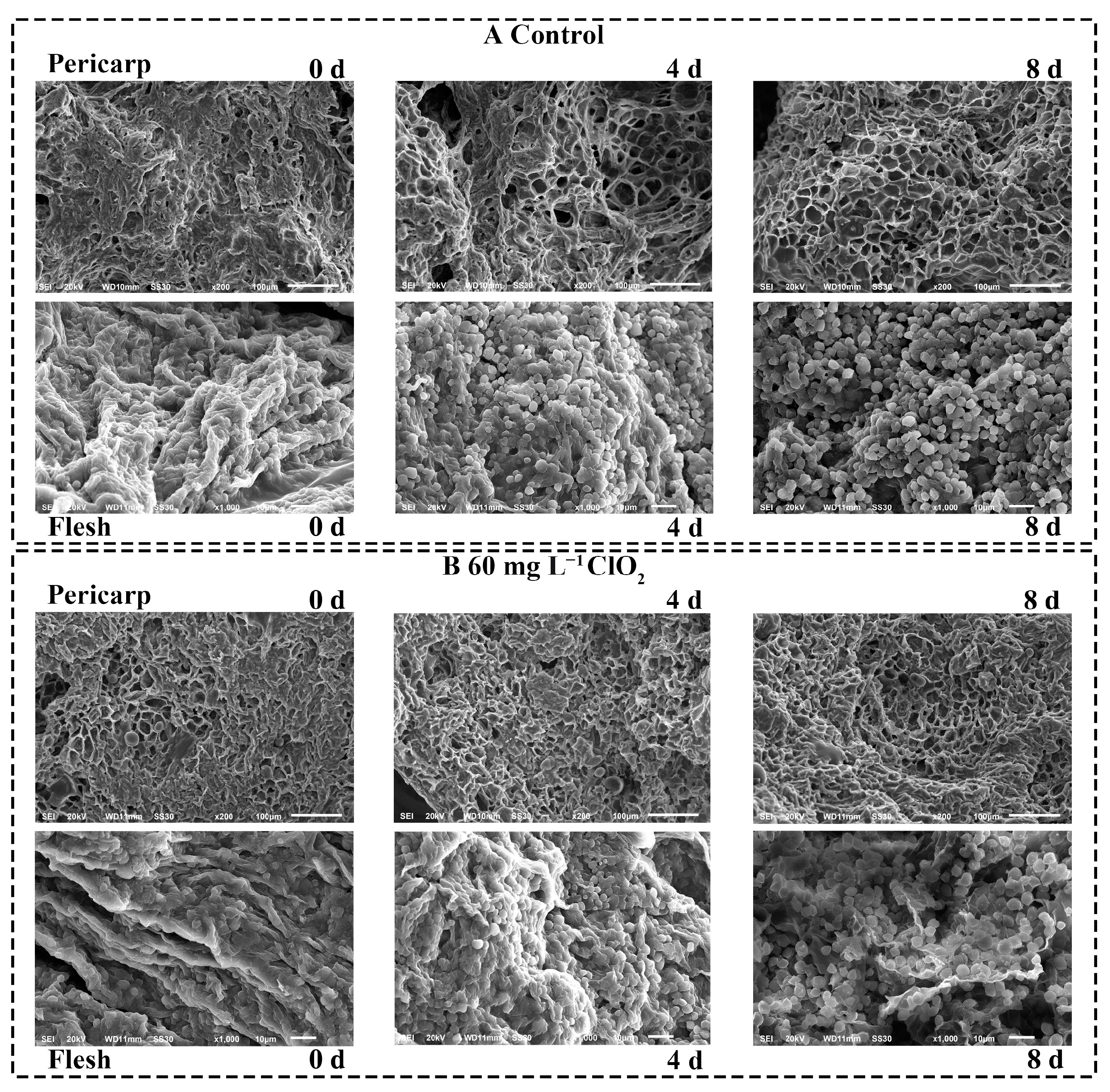
3.6. Morphological Observation of Cherimoya
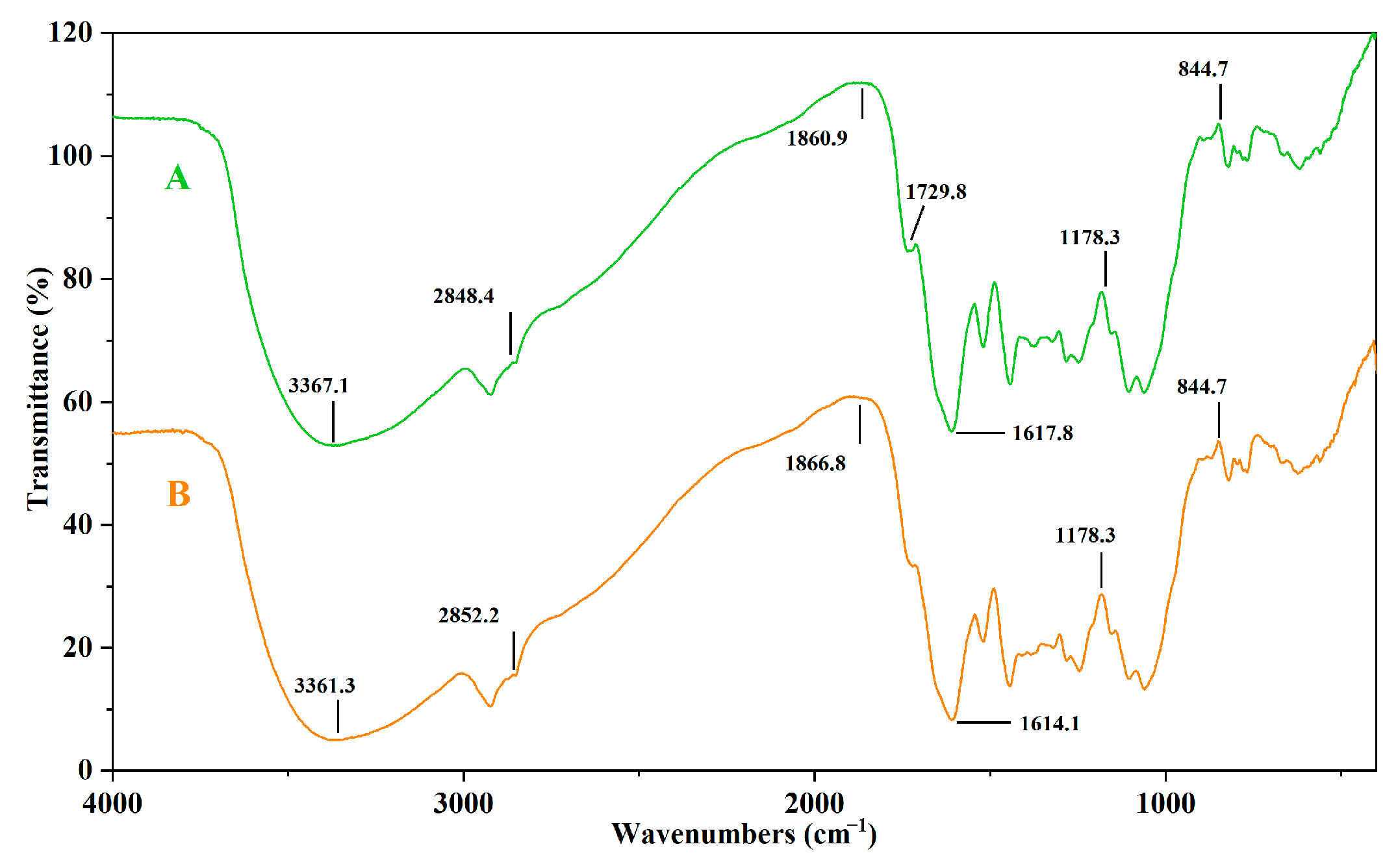
3.7. FTIR Analysis
3.8. Kinetics of Enzymatic Browning
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Perrone, A.; Yousefi, S.; Salami, A.; Papini, A.; Martinelli, F. Botanical, genetic, phytochemical and pharmaceutical aspects of Annona cherimola Mill. Sci. Hortic. 2022, 296, 110896. [Google Scholar] [CrossRef]
- Manríquez, D.; Muñoz-Robredo, P.; Gudenschwager, O.; Robledo, P.; Defilippi, B. Development of flavor-related metabolites in cherimoya (Annona cherimola Mill.) fruit and their relationship with ripening physiology. Postharvest Biol. Technol. 2014, 94, 58–65. [Google Scholar] [CrossRef]
- Barry, C.S. The stay-green revolution: Recent progress in deciphering the mechanisms of chlorophyll degradation in higher plants. Plant Sci. 2009, 176, 325–333. [Google Scholar] [CrossRef]
- Sui, X.; Meng, Z.; Dong, T.; Fan, X.; Wang, Q. Enzymatic browning and polyphenol oxidase control strategies. Curr. Opin. Biotechnol. 2023, 81, 102921. [Google Scholar] [CrossRef]
- Iqbal, A.; Murtaza, A.; Hu, W.; Ahmad, I.; Ahmed, A.; Xu, X. Activation and inactivation mechanisms of polyphenol oxidase during thermal and non-thermal methods of food processing. Food Bioprod. Process. 2019, 117, 170–182. [Google Scholar] [CrossRef]
- Chen, Z.; Gong, X.; Liu, Y.; Zhou, W.; Wei, X.; Li, J.; Lin, M. Review of Biological Characteristics and Research Progress of Annona squamosa. Farm Prod. Process. 2018, 15, 85–88+93. [Google Scholar]
- Wang, L.; Peng, H.; Liu, C.; Li, C.; Qu, J.; Geng, X.; Zhu, Z. Effect of chitosan-ascorbic acid composite coating on postharvest quality of Custard apple (Annona squamosa). Process Biochem. 2023, 129, 76–85. [Google Scholar] [CrossRef]
- Zhang, Y.; Qiu, J.; Yang, K.; Lu, Y.; Xu, Z.; Yang, H.; Zhong, T. Generation, mechanisms, kinetics, and effects of gaseous chlorine dioxide in food preservation. Compr. Rev. Food Sci. Food Saf. 2023, 22, 3105–3129. [Google Scholar] [CrossRef]
- Liu, S.; Zheng, Y.; Wang, R.; Shi, Y.; Cai, W.; Xu, T.; Zuo, J. Chlorine dioxide affects metabolism of harvested sweet corn. Postharvest Biol. Technol. 2024, 211, 112834. [Google Scholar] [CrossRef]
- Zhu, X.; Hui, S.; Huang, H.; Liu, R.; Wang, S.; Huang, C. Antimicrobial mechanism of chlorine dioxide and its impacts on postharvest management in horticultural produce: A review. Postharvest Biol. Technol. 2024, 213, 112921. [Google Scholar] [CrossRef]
- World Health Organization (WHO); Food and Agriculture Organization of the United Nations (FAO). Benefits and risks of the use of chlorine-containing disinfectants in food production and food processing. In Proceedings of the FAO/WHO Expert Meeting, Ann Arbor, MI, USA, 27–30 May 2008. [Google Scholar]
- Chen, Z.; Zhu, C.; Han, Z. Effects of aqueous chlorine dioxide treatment on nutritional components and shelf-life of mulberry fruit (Morus alba L.). J. Biosci. Bioeng. 2011, 111, 675–681. [Google Scholar] [CrossRef]
- Chen, Z.; Zhu, C.; Zhang, Y.; Niu, D.; Du, J. Effects of aqueous chlorine dioxide treatment on enzymatic browning and shelf-life of fresh-cut asparagus lettuce (Lactuca sativa L.). Postharvest Biol. Technol. 2010, 58, 232–238. [Google Scholar] [CrossRef]
- Chen, S.; Wang, H.; Wang, R.; Fu, Q.; Zhang, W. Effect of gaseous chlorine dioxide (ClO2) with different concentrations and numbers of treatments on controlling berry decay and rachis browning of table grape. J. Food Proces. Preservat. 2018, 42, 13662. [Google Scholar] [CrossRef]
- Chiabrando, V.; Giuggioli, N.; Maghenzani, M.; Peano, C.; Giacalone, G. Improving Storability of Strawberries with Gaseous Chlorine Dioxide in Perforated Clamshell Packaging. Pol. J. Food Nutr. Sci. 2018, 68, 141–148. [Google Scholar] [CrossRef]
- Jangir, B.; Pilania, S.; Saharan, V.; Lakhawat, S.S.; Prajapat, D.; Meena, M.; Kumar Tak, J. A Comprehensive Study of Hexanal Formulation on Extending Shelf Life of Custard Apple (Annona squamosa L.). Curr. J. Appl. Sci. Technol. 2021, 40, 30–37. [Google Scholar] [CrossRef]
- Lin, X.; Zhang, H.; Guo, X.; Qin, Y.; Shen, P.; Peng, Q. A Novel Sodium Alginate-Carnauba Wax Film Containing Calcium Ascorbate: Structural Properties and Preservative Effect on Fresh-Cut Apples. Molecules 2023, 28, 367. [Google Scholar] [CrossRef]
- Feumba Dibanda, R.; Panyoo Akdowa, E.; Rani, P.A.; Metsatedem Tongwa, Q.; Mbofung, F.C. Effect of microwave blanching on antioxidant activity, phenolic compounds and browning behaviour of some fruit peelings. Food Chem. 2020, 302, 125308. [Google Scholar] [CrossRef]
- Batista, K.; Batista, G.; Alves, G.; Fernandes, K. Extraction, partial purification and characterization of polyphenol oxidase from Solanum lycocarpum fruits. J. Mol. Catal. B Enzym. 2014, 102, 211–217. [Google Scholar] [CrossRef]
- Remini, H.; Mertz, C.; Belbahi, A.; Achir, N.; Dornier, M.; Madani, K. Degradation kinetic modelling of ascorbic acid and colour intensity in pasteurised blood orange juice during storage. Food Chem. 2015, 173, 665–673. [Google Scholar] [CrossRef]
- Medina-Santamarina, J.; Guillén, F.; Ilea, M.I.M.; Ruiz-Aracil, M.C.; Valero, D.; Castillo, S.; Serrano, M. Melatonin Treatments Reduce Chilling Injury and Delay Ripening, Leading to Maintenance of Quality in Cherimoya Fruit. Int. J. Mol. Sci. 2023, 24, 3787. [Google Scholar] [CrossRef]
- Chen, Z.; Zhu, C. Combined effects of aqueous chlorine dioxide and ultrasonic treatments on postharvest storage quality of plum fruit (Prunus salicina L.). Postharvest Biol. Technol. 2011, 61, 117–123. [Google Scholar] [CrossRef]
- Malka, S.K.; Park, M.-H. Fresh Produce Safety and Quality: Chlorine Dioxide’s Role. Front. Plant Sci. 2022, 12, 775629. [Google Scholar] [CrossRef]
- Jaeger, S.; Lund, C.; Lau, K.; Harker, F. In Search of the “Ideal” Pear (Pyrus spp.): Results of a Multidisciplinary Exploration. J. Food Sci. 2003, 68, 1108–1117. [Google Scholar] [CrossRef]
- Öz, A.; Ali, A. Retaining overall quality of fresh figs by postharvest hexanal vapor treatment during cold storage. Postharvest Biol. Technol. 2023, 205, 112539. [Google Scholar] [CrossRef]
- Saengnil, K.; Chumyam, A.; Faiyue, B.; Uthaibutra, J. Use of chlorine dioxide fumigation to alleviate enzymatic browning of harvested ‘Daw’ longan pericarp during storage under ambient conditions. Postharvest Biol. Technol. 2014, 91, 49–56. [Google Scholar] [CrossRef]
- Ayranci, E.; Tunc, S. A method for the measurement of the oxygen permeability and the development of edible films to reduce the rate of oxidative reactions in fresh foods. Food Chem. 2003, 80, 423–431. [Google Scholar] [CrossRef]
- Wang, Z.; Narciso, J.; Biotteau, A.; Plotto, A.; Baldwin, E.; Bai, J. Improving Storability of Fresh Strawberries with Controlled Release Chlorine Dioxide in Perforated Clamshell Packaging. Food Bioprocess Technol. 2014, 7, 3516–3524. [Google Scholar] [CrossRef]
- Zhang, L.; Huang, C.; Zhao, Y.; Zheng, C.; Hu, C. Post-ripening and senescence behavior of atemoya (Annona cherimola × A. squamosa) under two typical storage temperatures. Postharvest Biol. Technol. 2023, 200, 112336. [Google Scholar] [CrossRef]
- Hou, J.; Sun, Y.; Chen, F.; Yu, L.; Mao, Q.; Wang, L.; Guo, X.; Liu, C. Analysis of microstructures and macrotextures for different apple cultivars based on parenchyma morphology. Microsc. Res. Tech. 2016, 79, 304–312. [Google Scholar] [CrossRef]
- Li, Y.; Ren, D.; Xu, D. Preparation of Coated Corrugated Box for Controlled-Release of Chlorine Dioxide and Its Application in Strawberry Preservation. Coatings 2020, 10, 242. [Google Scholar] [CrossRef]
- Yi, Y.; Sun, J.; Xie, J.; Min, T.; Wang, L.; Wang, H. Phenolic Profiles and Antioxidant Activity of Lotus Root Varieties. Molecules 2016, 21, 863. [Google Scholar] [CrossRef]
- Panadare, D.; Rathod, V.K. Extraction and purification of polyphenol oxidase: A review. Biocat. Agric. Biotech. 2018, 14, 431–437. [Google Scholar] [CrossRef]
- Arslan, O.; Temur, A.; Tozlu, İ. Polyphenol Oxidase from Allium sp. Agric. Food Chem. 1997, 45, 2861–2863. [Google Scholar] [CrossRef]
- Radi, M.; Mahrouz, M.; Jaouad, A.; Tacchini, M.; Aubert, S.; Hugues, M.; Amiot, M. Phenolic composition, browning susceptibility, and carotenoid content of several apricot cultivars at maturity. HortScience 1997, 32, 1087–1091. [Google Scholar] [CrossRef]
- Chomkitichai, W.; Chumyam, A.; Rachtanapun, P.; Uthaibutra, J.; Saengnil, K. Reduction of reactive oxygen species production and membrane damage during storage of ‘Daw’ longan fruit by chlorine dioxide. Sci. Hortic. 2014, 170, 143–149. [Google Scholar] [CrossRef]
- Fu, Y.; Zhang, K.; Wang, N.; Du, J. Effects of aqueous chlorine dioxide treatment on polyphenol oxidases from Golden Delicious apple. LWT-Food Sci. Technol. 2007, 40, 1362–1368. [Google Scholar] [CrossRef]
- Remorini, D.; Landi, M.; Tardelli, F.; Lugani, A.; Massai, R.; Graziani, G.; Fogliano, V.; Guidi, L. Effect of Chlorine Dioxide and Ascorbic Acid on Enzymatic Browning and Shelf Life of Fresh-Cut Red Delicious and Granny Smith Apples. J. Food Process. Preserv. 2015, 39, 2925–2934. [Google Scholar] [CrossRef]
- Toivonen, P.; Brummell, D. Biochemical bases of appearance and texture changes in fresh-cut fruit and vegetables. Postharvest Biol. Technol. 2008, 48, 1–14. [Google Scholar] [CrossRef]
- Méndez-Galarraga, M.; Van de Velde, F.; Piagentini, A.; Pirovani, M. Kinetic modeling of the changes in bioactive compounds and quality attributes of fresh-cut strawberries stored in controlled atmospheres with high oxygen alone or with carbon dioxide. Postharvest Biol. Technol. 2022, 190, 111947. [Google Scholar] [CrossRef]
- Quevedo, R.; Díaz, O.; Caqueo, A.; Ronceros, B.; Aguilera, J. Quantification of enzymatic browning kinetics in pear slices using non-homogenous L* color information from digital images. LWT-Food Sci. Technol. 2009, 42, 1367–1373. [Google Scholar] [CrossRef]



| Specific Organic Acid | Tartaric Acid | Malic Acid | Citric Acid |
|---|---|---|---|
| Coefficient | 0.075 | 0.067 | 0.064 |
| Phenolic Compound | Standard Curve | R2 |
|---|---|---|
| Neohesperidin | y = 24366x + 2.6414 | 0.9999 |
| Epicatechin | y = 26474x + 4.0669 | 0.9979 |
| p-Hydroxybenzoic acid | y = 32669x + 21.324 | 0.9984 |
| Catechol | y = 45826x + 19.523 | 0.9999 |
| Fruit Part | ClO2 Concentration | Phenolic Compound | Content (mg kg−1 FW) | ||||
|---|---|---|---|---|---|---|---|
| 0 d | 2 d | 4 d | 6 d | 8 d | |||
| Pericarp | 0 mg L−1 | Neohesperidin | 45.97 ± 2.94 ab | 46.97 ± 1.87 ab | 50.84 ± 4 a | 46.83 ± 1.95 ab | 43.38 ± 2.68 b |
| Epicatechin | 84.38 ± 4.35 b | 88.16 ± 5.47 b | 96.68 ± 6.12 a | 88.2 ± 1.56 b | 81.17 ± 2.06 b | ||
| p-Hydroxybenzoic acid | 119.6 ± 2.08 b | 128.19 ± 2.91 b | 148.66 ± 5.77 a | 119.43 ± 6.03 b | 95.9 ± 6.03 c | ||
| Catechol | 93.28 ± 5.17 b | 101.02 ± 8.51 b | 117.5 ± 4.85 a | 94.77 ± 6.66 b | 73.81 ± 5.13 c | ||
| 20 mg L−1 | Neohesperidin | 41.31 ± 5.36 a | 42.06 ± 5.76 a | 45.13 ± 8.79 a | 42.35 ± 4.32 a | 40.27 ± 5.51 a | |
| Epicatechin | 86.42 ± 5.16 b | 89.71 ± 0.55 ab | 96.03 ± 4.78 a | 90.16 ± 0.84 ab | 85.73 ± 5.94 b | ||
| p-Hydroxybenzoic acid | 122.78 ± 5.51 b | 132.8 ± 9.56 b | 149.5 ± 2.14 a | 123.67 ± 7.3 b | 100.74 ± 5.68 c | ||
| Catechol | 107.63 ± 3.68 c | 116.72 ± 4.3 b | 133.06 ± 2.55 a | 109.06 ± 3.85 c | 84.84 ± 3.94 d | ||
| 40 mg L−1 | Neohesperidin | 41.31 ± 1.77 b | 41.94 ± 3.25 ab | 44.89 ± 1.81 ab | 46.3 ± 1.22 a | 42.83 ± 3.29 ab | |
| Epicatechin | 86.42 ± 5.16 b | 88.28 ± 6.71 ab | 92.8 ± 4.52 ab | 98.99 ± 2.09 a | 90.16 ± 8.94 ab | ||
| p-Hydroxybenzoic acid | 122.78 ± 5.51 c | 128.49 ± 5.27 c | 145.95 ± 4.59 b | 168.27 ± 1.93 a | 140.99 ± 2.62 b | ||
| Catechol | 97.57 ± 5.36 c | 102.81 ± 4.2 c | 118.3 ± 1.51 b | 136.25 ± 3.36 a | 116.69 ± 4.48 b | ||
| 60 mg L−1 | Neohesperidin | 40.64 ± 2.82 a | 41.27 ± 1.32 a | 44.88 ± 5.1 a | 47.39 ± 3.77 a | 45.87 ± 4.12 a | |
| Epicatechin | 83.13 ± 2.67 c | 85.46 ± 4.56 bc | 90.72 ± 4.52 ab | 95.73 ± 2.44 a | 92.34 ± 3.56 ab | ||
| p-Hydroxybenzoic acid | 124.39 ± 2.54 d | 131.05 ± 1.15 c | 149.5 ± 5.53 b | 171.06 ± 4.09 a | 149.95 ± 3.13 b | ||
| Catechol | 104.36 ± 4.35 c | 109.76 ± 2.64 c | 129.83 ± 1.45 b | 152.48 ± 4.34 a | 133.54 ± 3.47 b | ||
| 80 mg L−1 | Neohesperidin | 41.31 ± 1.77 c | 42.09 ± 2.25 bc | 45.38 ± 0.85 ab | 47.38 ± 2.56 a | 44.1 ± 1.97 abc | |
| Epicatechin | 73.09 ± 2.05 d | 75.85 ± 1.1 cd | 80.95 ± 6.08 bc | 87.6 ± 2.99 a | 82.93 ± 0.55 ab | ||
| p-Hydroxybenzoic acid | 122.78 ± 3.51 d | 129.73 ± 1.62 c | 145.52 ± 3.62 b | 169.21 ± 1.03 a | 143.26 ± 4.45 b | ||
| Catechol | 107.63 ± 4.85 c | 114.86 ± 2.03 c | 131.58 ± 2.21 b | 153.8 ± 6.31 a | 127.67 ± 2.57 b | ||
| 100 mg L−1 | Neohesperidin | 38.97 ± 1.48 c | 39.6 ± 0.45 c | 42.09 ± 2.24 c | 45.08 ± 1.49 a | 42.74 ± 1.44 b | |
| Epicatechin | 79.76 ± 1.51 b | 83.16 ± 4.59 b | 88.19 ± 3.80 a | 93.68 ± 3.47 a | 87.04 ± 4.58 a | ||
| p-Hydroxybenzoic acid | 122.78 ± 3.51 c | 130.91 ± 4.81 c | 151.76 ± 5.02 b | 173.75 ± 2.35 a | 149.84 ± 3.56 b | ||
| Catechol | 107.63 ± 0.73 d | 113.71 ± 2.88 c | 130.65 ± 4.09 b | 149.37 ± 1.53 a | 127.06 ± 1.49 b | ||
| Fruit Part | ClO2 Concentration | Phenolic Compound | Content (mg kg−1 FW) | ||||
|---|---|---|---|---|---|---|---|
| 0 d | 2 d | 4 d | 6 d | 8 d | |||
| Flesh | 0 mg L−1 | Neohesperidin | 20.88 ± 3 a | 22.65 ± 4.57 a | 25.21 ± 4.9 a | 23.16 ± 1.66 a | 20.69 ± 1.4 a |
| Epicatechin | 21.17 ± 1.77 a | 22.97 ± 5.26 a | 26.19 ± 6.98 a | 24.55 ± 3.86 a | 22.09 ± 4.72 a | ||
| p-Hydroxybenzoic acid | 29.17 ± 1.05 b | 32.34 ± 5.8 ab | 37.24 ± 3.66 a | 29.82 ± 1.69 b | 22.65 ± 2.79 c | ||
| Catechol | 30.04 ± 2.66 b | 33.34 ± 4.09 ab | 39.07 ± 2.6 a | 31.3 ± 4.09 b | 22.01 ± 2.95 c | ||
| 20 mg L−1 | Neohesperidin | 17.71 ± 1.52 a | 19.08 ± 4.61 a | 20.88 ± 3.86 a | 19.3 ± 2.62 a | 18.01 ± 1.57 a | |
| Epicatechin | 22.41 ± 2.95 a | 24.23 ± 3.72 a | 26.41 ± 2.9 a | 24.97 ± 3.57 a | 22.75 ± 1.43 a | ||
| p-Hydroxybenzoic acid | 24.59 ± 2.34 b | 28.43 ± 5.63 ab | 32.73 ± 5.71 a | 27.93 ± 1.71 ab | 23.28 ± 2.51 b | ||
| Catechol | 32.17 ± 3.39 ab | 36.66 ± 6.57 ab | 41.36 ± 3.2 a | 34.87 ± 4.18 ab | 27.3 ± 5.9 b | ||
| 40 mg L−1 | Neohesperidin | 17.71 ± 1.52 a | 18.38 ± 3.06 a | 19.48 ± 2.22 a | 21.88 ± 2.48 a | 20.79 ± 2.12 a | |
| Epicatechin | 22.41 ± 2.95 a | 23.09 ± 1.88 a | 24.5 ± 3.14 a | 26.24 ± 4.02 a | 24.32 ± 4.13 a | ||
| p-Hydroxybenzoic acid | 24.59 ± 1.74 b | 26.46 ± 4.51 b | 30.05 ± 4.57 ab | 33.39 ± 2.08 a | 28.83 ± 1.94 ab | ||
| Catechol | 32.17 ± 3.39 d | 35.55 ± 1.42 cd | 40.76 ± 1.54 b | 45.71 ± 3.51 a | 39.37 ± 2.52 bc | ||
| 60 mg L−1 | Neohesperidin | 21.1 ± 1.91 b | 22.05 ± 0.55 ab | 23.86 ± 1.41 ab | 25.43 ± 3.66 a | 24.25 ± 0.34 ab | |
| Epicatechin | 24.01 ± 6.36 a | 25.05 ± 2.69 a | 27.18 ± 1.23 a | 28.92 ± 2.64 a | 26.96 ± 3.26 a | ||
| p-Hydroxybenzoic acid | 32.74 ± 1.51 c | 36.01 ± 2.99 bc | 40.77 ± 3.11 b | 47.35 ± 2.45 a | 40.7 ± 2.3 b | ||
| Catechol | 33.84 ± 5.42 c | 36.5 ± 3.81 bc | 40.88 ± 3.2 ab | 46.33 ± 2.63 a | 39.36 ± 1.16 bc | ||
| 80 mg L−1 | Neohesperidin | 17.71 ± 1.52 b | 18.36 ± 0.98 b | 20.1 ± 2.3 ab | 22.19 ± 2.72 a | 20.9 ± 1.81 ab | |
| Epicatechin | 15.74 ± 1.47 b | 16.58 ± 1.48 ab | 17.91 ± 2.21 ab | 19.76 ± 0.73 a | 18.73 ± 3.15 ab | ||
| p-Hydroxybenzoic acid | 24.59 ± 1.74 d | 27.16 ± 1.41 cd | 30.24 ± 1.56 bc | 36.8 ± 3.1 a | 30.95 ± 1.34 b | ||
| Catechol | 32.17 ± 3.39 c | 35.47 ± 2.4 bc | 39.93 ± 2.07 b | 46.63 ± 2.51 a | 39.53 ± 4.5 b | ||
| 100 mg L−1 | Neohesperidin | 17.71 ± 1.52 a | 18.94 ± 3.7 a | 20.66 ± 1.45 a | 22.97 ± 4 a | 22.08 ± 2.82 a | |
| Epicatechin | 22.41 ± 2.95 a | 23.01 ± 1.01 a | 24.21 ± 2.03 a | 25.37 ± 2.07 a | 24.53 ± 0.88 a | ||
| p-Hydroxybenzoic acid | 24.59 ± 1.74 c | 28.16 ± 3.46 c | 32.63 ± 1.95 b | 37.94 ± 2.67 a | 33.05 ± 1.74 b | ||
| Catechol | 32.17 ± 1.96 d | 36.37 ± 1.52 c | 41.33 ± 2.06 b | 48.81 ± 3.03 a | 42.42 ± 1.46 b | ||
| Kinetic Model | Reaction Substrate | |
|---|---|---|
| Zero-order | P-p-hydroxybenzoic acid | 0.97692 |
| F- p-hydroxybenzoic acid | 0.96164 | |
| P- catechol | 0.97506 | |
| F- catechol | 0.96177 | |
| First-order | P-p-hydroxybenzoic acid | 0.98241 |
| F-p-hydroxybenzoic acid | 0.97276 | |
| P-catechol | 0.9876 | |
| F-catechol | 0.98227 |
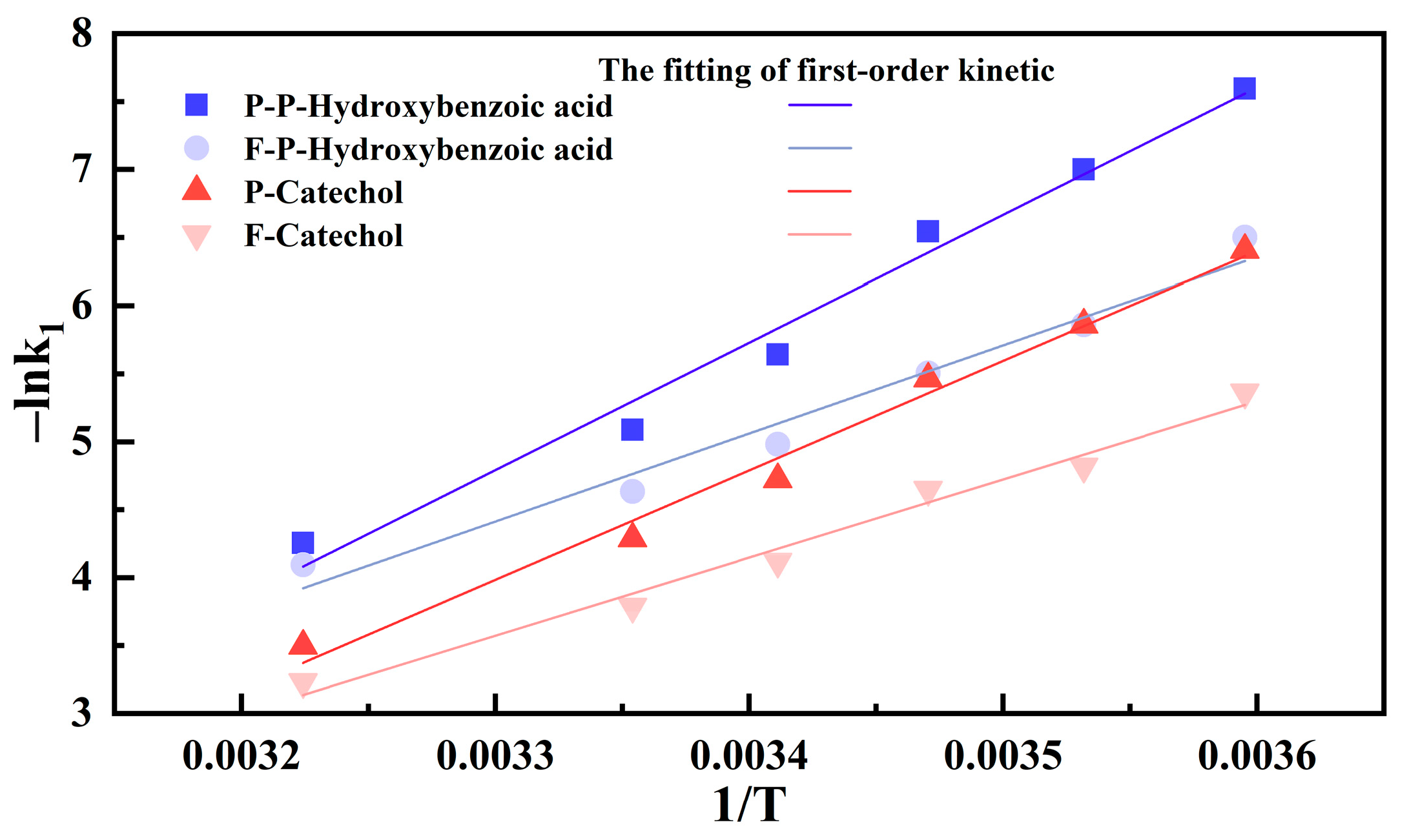
| Phenolic Compound | First-Order Kinetic Equation | k1 (min−1) | Ea (kJ mol−1) |
|---|---|---|---|
| P-p-hydroxybenzoic acid | y = 0.3982e(−0.00446x) | 0.00445809 | 77.91 |
| F-p-hydroxybenzoic acid | y = 0.3982e(−0.00694x) | 0.00693669 | 53.90 |
| P-catechol | y = 0.2495e(−0.01026x) | 0.0102588 | 67.00 |
| F-catechol | y = 0.2495e(−0.0168x) | 0.01680211 | 47.83 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, T.; Huang, C.; Jing, X.; Huang, H.; Deng, R.; Fang, J. Chlorine Dioxide Delays Enzymatic Browning in Postharvest Cherimoya and Enables Establishment of Kinetics Substrate Model. Horticulturae 2024, 10, 901. https://doi.org/10.3390/horticulturae10090901
Zheng T, Huang C, Jing X, Huang H, Deng R, Fang J. Chlorine Dioxide Delays Enzymatic Browning in Postharvest Cherimoya and Enables Establishment of Kinetics Substrate Model. Horticulturae. 2024; 10(9):901. https://doi.org/10.3390/horticulturae10090901
Chicago/Turabian StyleZheng, Tianchang, Chongxing Huang, Xianghai Jing, Huijie Huang, Rongrong Deng, and Jingjing Fang. 2024. "Chlorine Dioxide Delays Enzymatic Browning in Postharvest Cherimoya and Enables Establishment of Kinetics Substrate Model" Horticulturae 10, no. 9: 901. https://doi.org/10.3390/horticulturae10090901






