Mitigating High-Temperature Stress in Peppers: The Role of Exogenous NO in Antioxidant Enzyme Activities and Nitrogen Metabolism
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Treatments
2.2. Determination of Growth and Electrolyte Leakage
2.3. Determination of Physiological Indexes
2.4. Sample Preparation and Extraction
2.5. UPLC and ESI-MS/MS Conditions
2.6. Principal Component Analysis (PCA), Hierarchical Cluster Analysis (HCA), and Pearson Correlation Coefficients (PCC)
2.7. Differential Metabolite Selection, KEGG Annotation, and Enrichment Analysis
2.8. Statistical Analysis
3. Results
3.1. Growth Parameters
3.2. Physiological Parameters
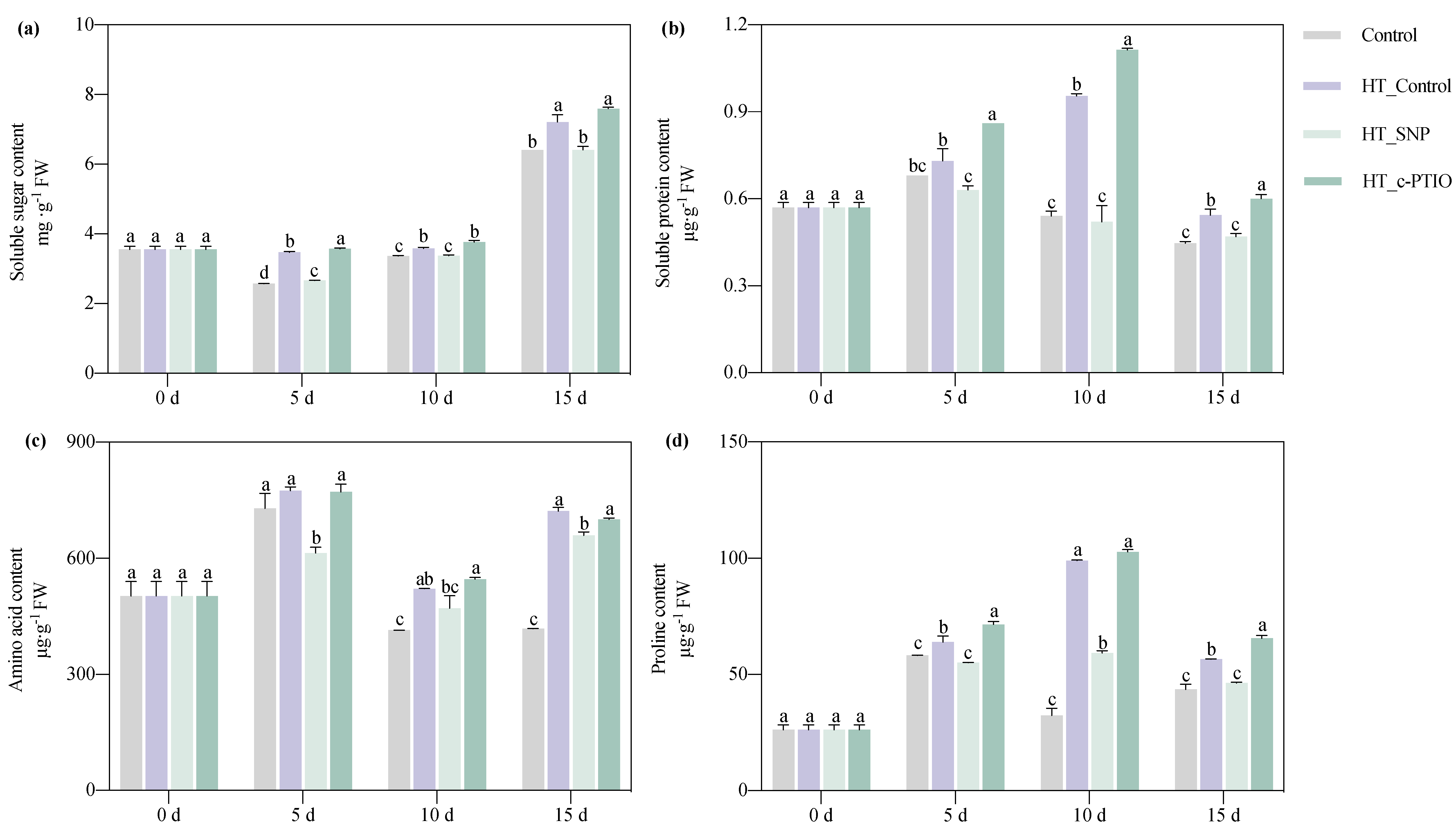
3.2.1. Osmotic Adjustment Substances
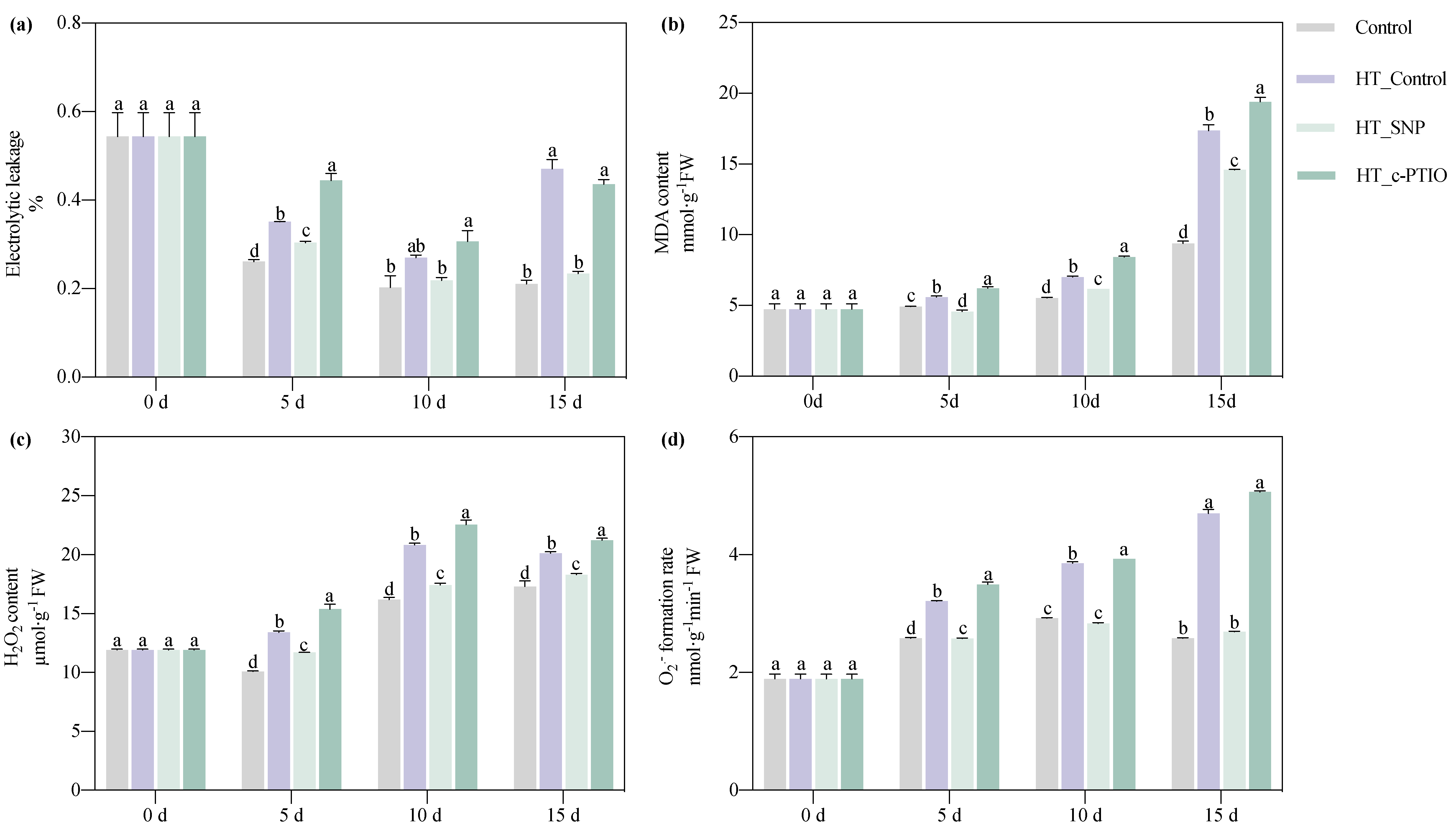
3.2.2. Lipid Peroxidation
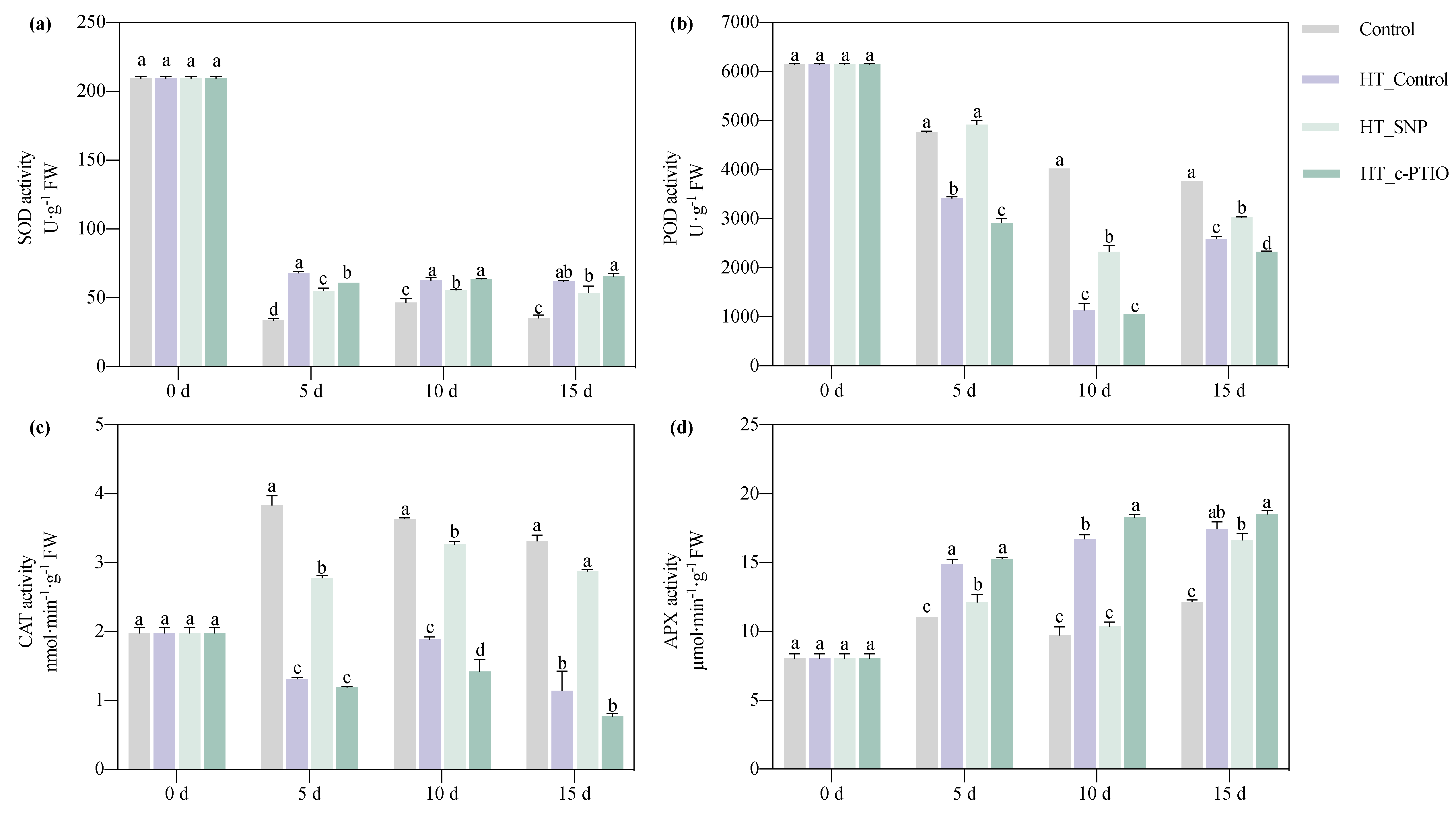
3.2.3. Antioxidant Enzyme Activities
3.2.4. Nitrogen Synthesis and Metabolism-Related Parameters
3.3. Quality Control (QC) of LC-MS Data
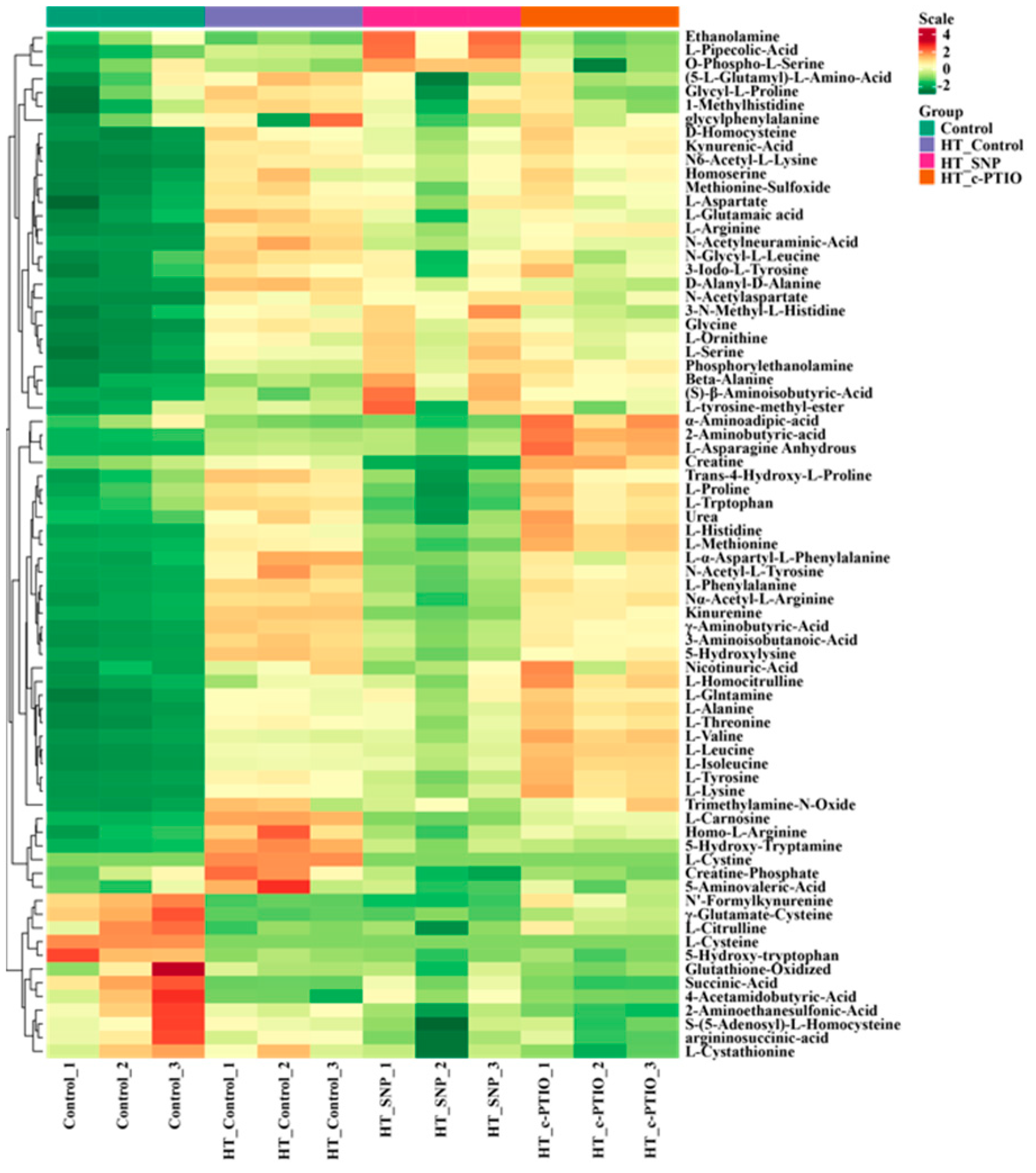
3.4. Quantification and Classification of Identified Metabolites
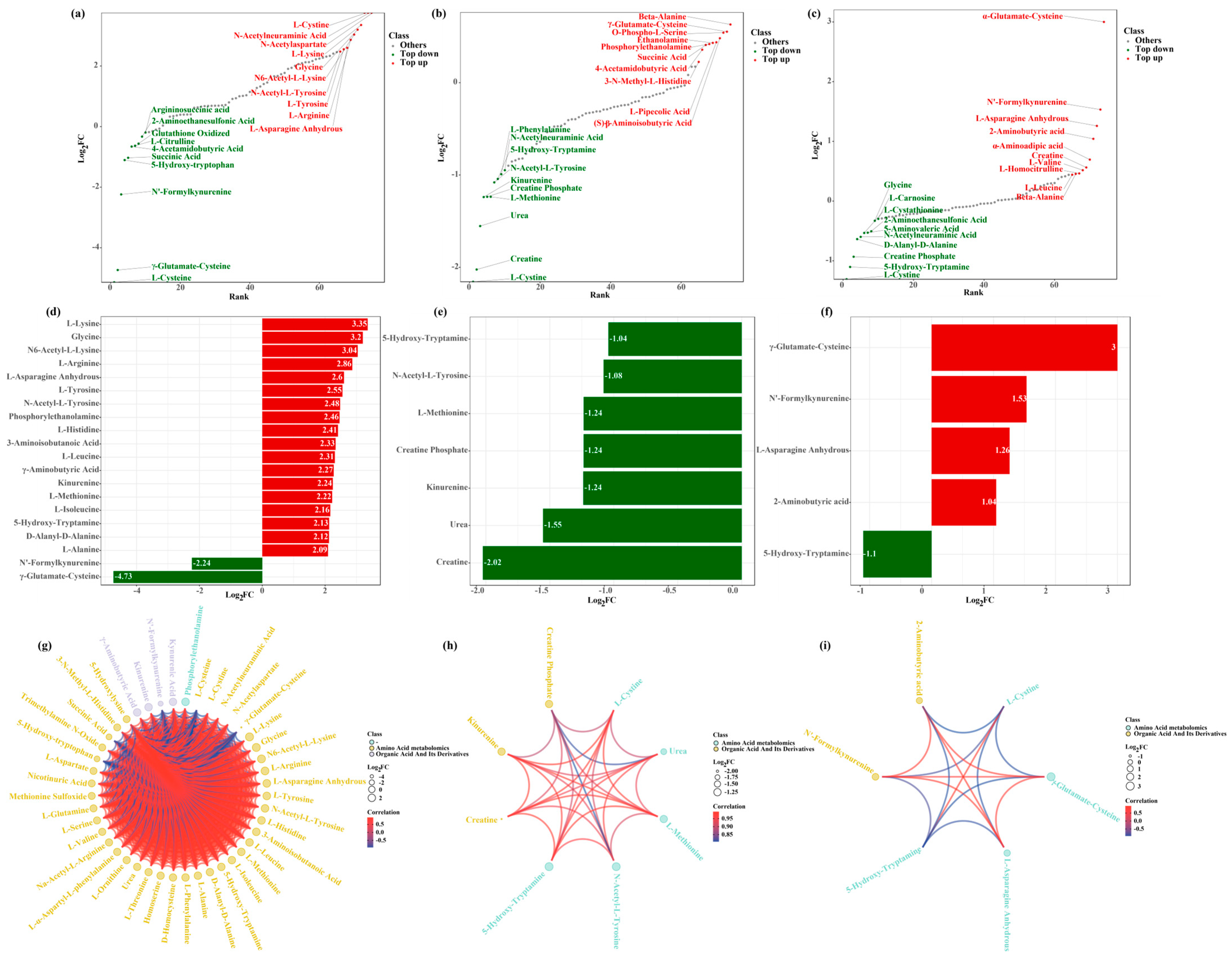
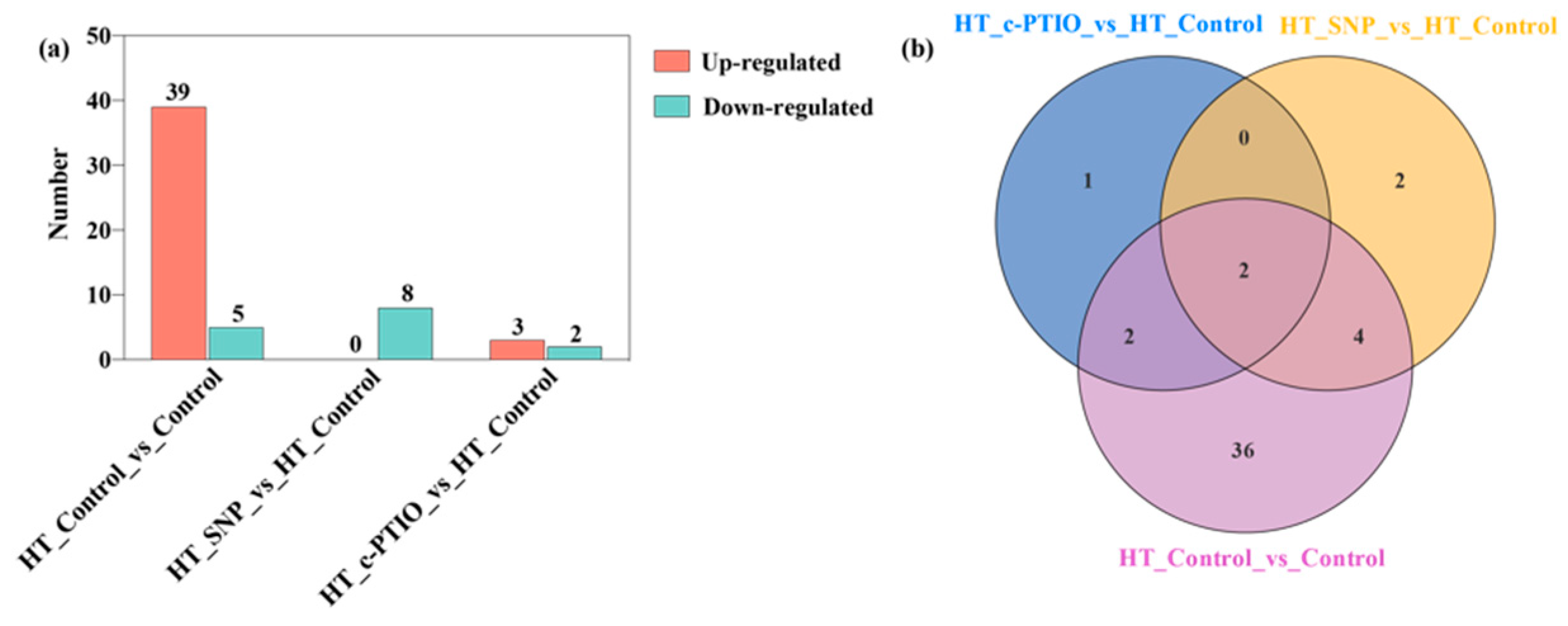
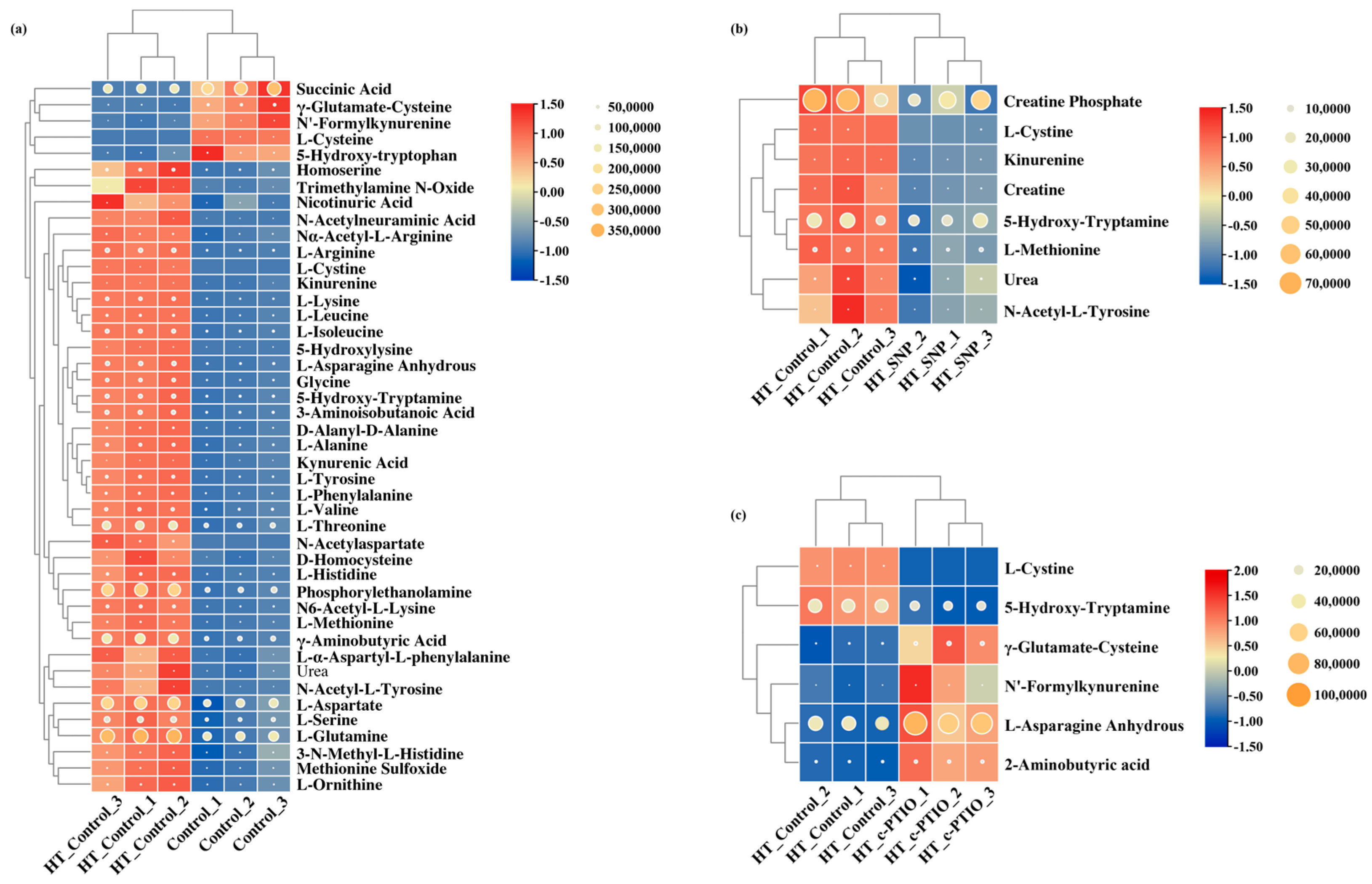
3.5. Differential Metabolite Screening, Categorization, and Correlation
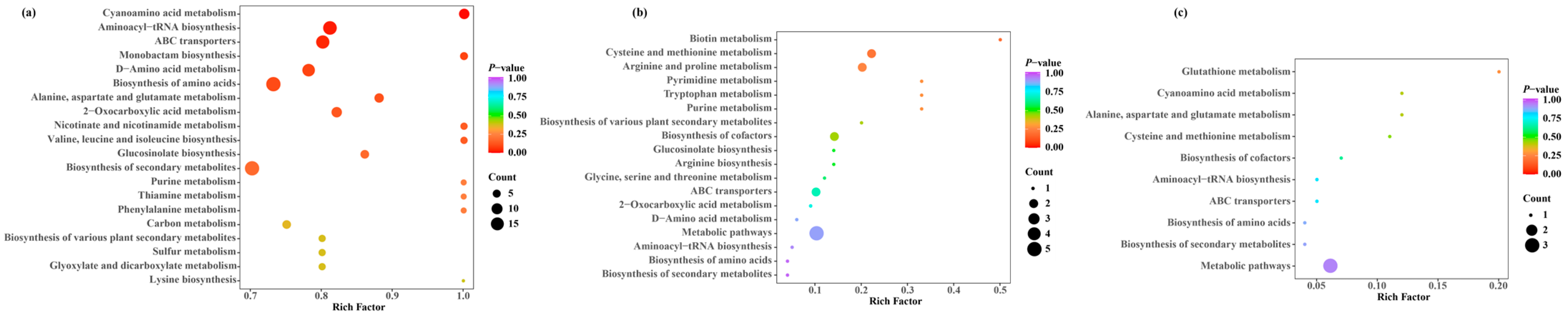
3.6. KEGG Analysis of Differentially Accumulated Metabolites
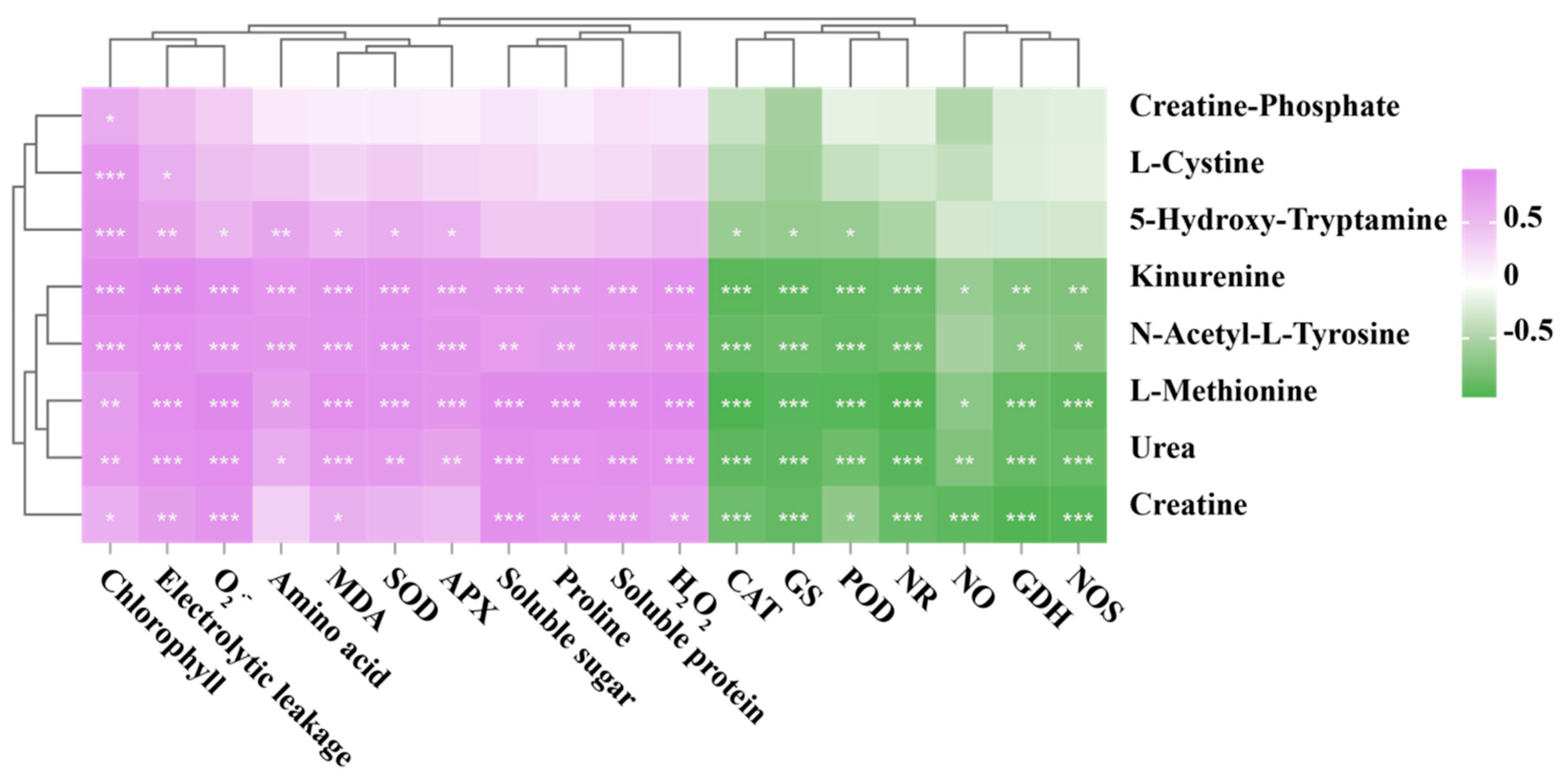
3.7. Correlation Analysis of Biochemical Indicators and Metabolomic Data
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hatfield, J.L.; Prueger, J.H. Temperature Extremes: Effect on Plant Growth and Development. Weather. Clim. Extrem. 2015, 10, 4–10. [Google Scholar] [CrossRef]
- Rosmaina; Utami, D.; Aryanti, E. Zulfahmi Impact of Heat Stress on Germination and Seedling Growth of Chili Pepper (Capsicum annuum L.). IOP Conf. Ser. Earth Environ. Sci. 2021, 637, 012032. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Nahar, K.; Alam, M.; Roychowdhury, R.; Fujita, M. Physiological, Biochemical, and Molecular Mechanisms of Heat Stress Tolerance in Plants. Int. J. Mol. Sci. 2013, 14, 9643–9684. [Google Scholar] [CrossRef] [PubMed]
- Swan, C.L.; Sistonen, L. Cellular Stress Response Cross Talk Maintains Protein and Energy Homeostasis. EMBO J. 2015, 34, 267–269. [Google Scholar] [CrossRef] [PubMed]
- Yuce, M.; Ekinci, M.; Turan, M.; Agar, G.; Aydin, M.; Ilhan, E.; Yildirim, E. Chrysin Mitigates Copper Stress by Regulating Antioxidant Enzymes Activity, Plant Nutrient and Phytohormones Content in Pepper. Sci. Hortic. 2024, 328, 112887. [Google Scholar] [CrossRef]
- Rajput, V.D.; Harish; Singh, R.K.; Verma, K.K.; Sharma, L.; Quiroz-Figueroa, F.R.; Meena, M.; Gour, V.S.; Minkina, T.; Sushkova, S.; et al. Recent Developments in Enzymatic Antioxidant Defence Mechanism in Plants with Special Reference to Abiotic Stress. Biology 2021, 10, 267. [Google Scholar] [CrossRef]
- Abdulfatah, H. Non-Enzymatic Antioxidants in Stressed Plants: A Review. J. Univ. Anbar Pure Sci. 2022, 16, 25–37. [Google Scholar] [CrossRef]
- Planchet, E.; Limami, A.M. Amino Acid Synthesis under Abiotic Stress. In Amino Acids in Higher Plants; D’Mello, J.P.F., Ed.; CAB International: Wallingford, UK, 2015; pp. 262–276. ISBN 978-1-78064-263-5. [Google Scholar]
- Kesawat, M.S.; Satheesh, N.; Kherawat, B.S.; Kumar, A.; Kim, H.-U.; Chung, S.-M.; Kumar, M. Regulation of Reactive Oxygen Species during Salt Stress in Plants and Their Crosstalk with Other Signaling Molecules—Current Perspectives and Future Directions. Plants 2023, 12, 864. [Google Scholar] [CrossRef] [PubMed]
- Hildebrandt, T.M. Synthesis versus Degradation: Directions of Amino Acid Metabolism during Arabidopsis Abiotic Stress Response. Plant Mol. Biol. 2018, 98, 121–135. [Google Scholar] [CrossRef]
- Xiong, Q.; Cao, C.; Shen, T.; Zhong, L.; He, H.; Chen, X. Comprehensive Metabolomic and Proteomic Analysis in Biochemical Metabolic Pathways of Rice Spikes under Drought and Submergence Stress. Biochim. Biophys. Acta (BBA)-Proteins Proteom. 2019, 1867, 237–247. [Google Scholar] [CrossRef]
- Airaki, M.; Leterrier, M.; Mateos, R.M.; Valderrama, R.; Chaki, M.; Barroso, J.B.; Del Río, L.A.; Palma, J.M.; Corpas, F.J. Metabolism of Reactive Oxygen Species and Reactive Nitrogen Species in Pepper (Capsicum annuum L.) Plants under Low Temperature Stress. Plant Cell Environ. 2012, 35, 281–295. [Google Scholar] [CrossRef] [PubMed]
- Gou, W.; Zheng, P.; Tian, L.; Gao, M.; Zhang, L.; Akram, N.A.; Ashraf, M. Exogenous Application of Urea and a Urease Inhibitor Improves Drought Stress Tolerance in Maize (Zea mays L.). J Plant Res 2017, 130, 599–609. [Google Scholar] [CrossRef]
- Lin, Y.; Liu, Z.; Shi, Q.; Wang, X.; Wei, M.; Yang, F. Exogenous Nitric Oxide (NO) Increased Antioxidant Capacity of Cucumber Hypocotyl and Radicle under Salt Stress. Sci. Hortic. 2012, 142, 118–127. [Google Scholar] [CrossRef]
- Ahmad, P.; Abass Ahanger, M.; Nasser Alyemeni, M.; Wijaya, L.; Alam, P.; Ashraf, M. Mitigation of Sodium Chloride Toxicity in Solanum lycopersicum L. by Supplementation of Jasmonic Acid and Nitric Oxide. J. Plant Interact. 2018, 13, 64–72. [Google Scholar] [CrossRef]
- Wu, P.; Xiao, C.; Cui, J.; Hao, B.; Zhang, W.; Yang, Z.; Ahammed, G.J.; Liu, H.; Cui, H. Nitric Oxide and Its Interaction with Hydrogen Peroxide Enhance Plant Tolerance to Low Temperatures by Improving the Efficiency of the Calvin Cycle and the Ascorbate–Glutathione Cycle in Cucumber Seedlings. J. Plant Growth Regul. 2021, 40, 2390–2408. [Google Scholar] [CrossRef]
- Shi, H.; Ye, T.; Zhu, J.-K.; Chan, Z. Constitutive Production of Nitric Oxide Leads to Enhanced Drought Stress Resistance and Extensive Transcriptional Reprogramming in Arabidopsis. J. Exp. Bot. 2014, 65, 4119–4131. [Google Scholar] [CrossRef]
- Majeed, S.; Nawaz, F.; Naeem, M.; Ashraf, M.Y.; Ejaz, S.; Ahmad, K.S.; Tauseef, S.; Farid, G.; Khalid, I.; Mehmood, K. Nitric Oxide Regulates Water Status and Associated Enzymatic Pathways to Inhibit Nutrients Imbalance in Maize (Zea mays L.) under Drought Stress. Plant Physiol. Biochem. 2020, 155, 147–160. [Google Scholar] [CrossRef]
- Majeed, S.; Nawaz, F.; Naeem, M.; Ashraf, M.Y. Effect of Exogenous Nitric Oxide on Sulfur and Nitrate Assimilation Pathway Enzymes in Maize (Zea mays L.) under Drought Stress. Acta Physiol. Plant 2018, 40, 206. [Google Scholar] [CrossRef]
- Huang, J.; Zhu, C.; Hussain, S.; Huang, J.; Liang, Q.; Zhu, L.; Cao, X.; Kong, Y.; Li, Y.; Wang, L.; et al. Effects of Nitric Oxide on Nitrogen Metabolism and the Salt Resistance of Rice (Oryza sativa L.) Seedlings with Different Salt Tolerances. Plant Physiol. Biochem. 2020, 155, 374–383. [Google Scholar] [CrossRef] [PubMed]
- Gulyas, Z.; Simon-Sarkadi, L.; Badics, E.; Novak, A.; Mednyanszky, Z.; Szalai, G.; Galiba, G.; Kocsy, G. Redox Regulation of Free Amino Acid Levels in Arabidopsis Thaliana. Physiol. Plant 2017, 159, 264–276. [Google Scholar] [CrossRef]
- Lel, Y.; Wen, L.; Liao, L.; Lin, S.; Zheng, E.; Li, Y.; Zhang, Y. Comparative Transcriptome Analysis Unveiling Reactive Oxygen Species Scavenging System of Sonneratia Caseolaris under Salinity Stress. Front. Plant Sci. 2022, 13, 953450. [Google Scholar] [CrossRef]
- Zhou, Y.; Huang, X.; Li, R.; Lin, H.; Huang, Y.; Zhang, T.; Mo, Y.; Liu, K. Transcriptome and Biochemical Analyses of Glutathione-Dependent Regulation of Tomato Fruit Ripening. J. Plant Interact. 2022, 17, 537–547. [Google Scholar] [CrossRef]
- Zhou, Y.; Hu, L.; Chen, Y.; Liao, L.; Li, R.; Wang, H.; Mo, Y.; Lin, L.; Liu, K. The Combined Effect of Ascorbic Acid and Chitosan Coating on Postharvest Quality and Cell Wall Metabolism of Papaya Fruits. LWT 2022, 171, 114134. [Google Scholar] [CrossRef]
- Zhou, Y.; Huang, L.; Liu, S.; Zhao, M.; Liu, J.; Lin, L.; Liu, K. Physiological and Transcriptomic Analysis of IAA-Induced Antioxidant Defense and Cell Wall Metabolism in Postharvest Mango Fruit. Food Res. Int. 2023, 174, 113504. [Google Scholar] [CrossRef]
- Gautam, H.; Sehar, Z.; Rehman, M.T.; Hussain, A.; AlAjmi, M.F.; Khan, N.A. Nitric Oxide Enhances Photosynthetic Nitrogen and Sulfur-Use Efficiency and Activity of Ascorbate-Glutathione Cycle to Reduce High Temperature Stress-Induced Oxidative Stress in Rice (Oryza sativa L.) Plants. Biomolecules 2021, 11, 305. [Google Scholar] [CrossRef]
- Sharma, S.; Singh, V.; Tanwar, H.; Mor, V.S.; Kumar, M.; Punia, R.C.; Dalal, M.S.; Khan, M.; Sangwan, S.; Bhuker, A.; et al. Impact of High Temperature on Germination, Seedling Growth and Enzymatic Activity of Wheat. Agriculture 2022, 12, 1500. [Google Scholar] [CrossRef]
- Marchi, L.; Degola, F.; Baruffini, E.; Restivo, F.M. How to Easily Detect Plant NADH-Glutamate Dehydrogenase (GDH) Activity? A Simple and Reliable in Planta Procedure Suitable for Tissues, Extracts and Heterologous Microbial Systems. Plant Sci. 2021, 304, 110714. [Google Scholar] [CrossRef]
- Liu, J.; Hasanuzzaman, M.; Wen, H.; Zhang, J.; Peng, T.; Sun, H.; Zhao, Q. High Temperature and Drought Stress Cause Abscisic Acid and Reactive Oxygen Species Accumulation and Suppress Seed Germination Growth in Rice. Protoplasma 2019, 256, 1217–1227. [Google Scholar] [CrossRef]
- Yu, X.; Hao, D.; Yang, J.; Ran, L.; Zang, Y.; Xiong, F. Effects of Low Temperature at Stem Elongation Stage on the Development, Morphology, and Physicochemical Properties of Wheat Starch. PeerJ 2020, 8, e9672. [Google Scholar] [CrossRef]
- Commuri, P.D.; Jones, R.J. High Temperatures during Endosperm Cell Division in Maize: A Genotypic Comparison under In Vitro and Field Conditions. Crop Sci. 2001, 41, 1122–1130. [Google Scholar] [CrossRef]
- Collins, N.C.; Parent, B. Diverging Temperature Responses of CO2 Assimilation and Plant Development Explain the Overall Effect of Temperature on Biomass Accumulation in Wheat Leaves and Grains. AoB Plants 2017, 9, plw092. [Google Scholar] [CrossRef] [PubMed]
- Afzal, I.; Akram, M.W.; Rehman, H.U.; Rashid, S.; Basra, S.M.A. Moringa Leaf and Sorghum Water Extracts and Salicylic Acid to Alleviate Impacts of Heat Stress in Wheat. S. Afr. J. Bot. 2020, 129, 169–174. [Google Scholar] [CrossRef]
- Djukic, N.; Markovic, S.; Mastilovic, J.; Simovic, P. Differences in Proline Accumulation between Wheat Varieties in Response to Heat Stress. Bot. Serbica 2021, 45, 61–69. [Google Scholar] [CrossRef]
- El-kereamy, A.; Bi, Y.-M.; Ranathunge, K.; Beatty, P.H.; Good, A.G.; Rothstein, S.J. The Rice R2R3-MYB Transcription Factor OsMYB55 Is Involved in the Tolerance to High Temperature and Modulates Amino Acid Metabolism. PLoS ONE 2012, 7, e52030. [Google Scholar] [CrossRef]
- Kumar, N.; Suyal, D.C.; Sharma, I.P.; Verma, A.; Singh, H. Elucidating Stress Proteins in Rice (Oryza sativa L.) Genotype under Elevated Temperature: A Proteomic Approach to Understand Heat Stress Response. 3 Biotech 2017, 7, 205. [Google Scholar] [CrossRef]
- Rahim, W.; Khan, M.; Al Azzawi, T.N.I.; Pande, A.; Methela, N.J.; Ali, S.; Imran, M.; Lee, D.-S.; Lee, G.-M.; Mun, B.-G.; et al. Exogenously Applied Sodium Nitroprusside Mitigates Lead Toxicity in Rice by Regulating Antioxidants and Metal Stress-Related Transcripts. Int. J. Mol. Sci. 2022, 23, 9729. [Google Scholar] [CrossRef] [PubMed]
- Nabi, R.B.S.; Tayade, R.; Hussain, A.; Kulkarni, K.P.; Imran, Q.M.; Mun, B.-G.; Yun, B.-W. Nitric Oxide Regulates Plant Responses to Drought, Salinity, and Heavy Metal Stress. Environ. Exp. Bot. 2019, 161, 120–133. [Google Scholar] [CrossRef]
- Bajguz, A. Nitric Oxide: Role in Plants Under Abiotic Stress. In Physiological Mechanisms and Adaptation Strategies in Plants under Changing Environment; Ahmad, P., Wani, M.R., Eds.; Springer: New York, NY, USA, 2014; pp. 137–159. ISBN 978-1-4614-8599-5. [Google Scholar]
- Hryvusevich, P.V.; Samokhina, V.V.; Demidchik, V.V. Stress-Induced Electrolyte Leakage from Root Cells of Higher Plants: Background, Mechanism and Physiological Role. Exp. Biol. Biotechnol. 2022, 2, 4–18. [Google Scholar] [CrossRef]
- Tsikas, D. Assessment of Lipid Peroxidation by Measuring Malondialdehyde (MDA) and Relatives in Biological Samples: Analytical and Biological Challenges. Anal. Biochem. 2017, 524, 13–30. [Google Scholar] [CrossRef]
- Saxena, I.; Srikanth, S.; Chen, Z. Cross Talk between H2O2 and Interacting Signal Molecules under Plant Stress Response. Front. Plant Sci. 2016, 7, 570. [Google Scholar] [CrossRef]
- Huang, D.; Tian, C.; Sun, Z.; Niu, J.; Wang, G. Potential Synergistic Regulation of Hsp70 and Antioxidant Enzyme Genes in Pyropia yezoensis under High Temperature Stress. Algal Res. 2024, 78, 103375. [Google Scholar] [CrossRef]
- Sang, Q.Q.; Shu, S.; Shan, X.; Guo, S.R.; Sun, J. Effects of Exogenous Spermidine on Antioxidant System of Tomato Seedlings Exposed to High Temperature Stress. Russ. J. Plant Physiol. 2016, 63, 645–655. [Google Scholar] [CrossRef]
- Tan, J.; Zhao, H.; Hong, J.; Han, Y.; Li, H.; Zhao, W. Effects of Exogenous Nitric Oxide on Photosynthesis, Antioxidant Capacity and Proline Accumulation in Wheat Seedlings Subjected to Osmotic Stress. World J. Agric. Sci 2008, 4, 307–313. [Google Scholar]
- Begara-Morales, J.C.; Sánchez-Calvo, B.; Chaki, M.; Valderrama, R.; Mata-Pérez, C.; Padilla, M.N.; Corpas, F.J.; Barroso, J.B. Modulation of the Ascorbate–Glutathione Cycle Antioxidant Capacity by Posttranslational Modifications Mediated by Nitric Oxide in Abiotic Stress Situations. In Reactive Oxygen Species and Oxidative Damage in Plants Under Stress; Gupta, D.K., Palma, J.M., Corpas, F.J., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 305–320. ISBN 978-3-319-20420-8. [Google Scholar]
- Liang, C.; Chen, L.; Wang, Y.; Liu, J.; Xu, G.; Li, T. High Temperature at Grain-Filling Stage Affects Nitrogen Metabolism Enzyme Activities in Grains and Grain Nutritional Quality in Rice. Rice Sci. 2011, 18, 210–216. [Google Scholar] [CrossRef]
- Parankusam, S.; Adimulam, S.S.; Bhatnagar-Mathur, P.; Sharma, K.K. Nitric Oxide (NO) in Plant Heat Stress Tolerance: Current Knowledge and Perspectives. Front. Plant Sci. 2017, 8, 1582. [Google Scholar] [CrossRef]
- Joshi, J.; Hasnain, G.; Logue, T.; Lynch, M.; Wu, S.; Guan, J.-C.; Alseekh, S.; Fernie, A.R.; Hanson, A.D.; McCarty, D.R. A Core Metabolome Response of Maize Leaves Subjected to Long-Duration Abiotic Stresses. Metabolites 2021, 11, 797. [Google Scholar] [CrossRef]
- Rizwan, M.; Mostofa, M.G.; Ahmad, M.Z.; Imtiaz, M.; Mehmood, S.; Adeel, M.; Dai, Z.; Li, Z.; Aziz, O.; Zhang, Y.; et al. Nitric Oxide Induces Rice Tolerance to Excessive Nickel by Regulating Nickel Uptake, Reactive Oxygen Species Detoxification and Defense-Related Gene Expression. Chemosphere 2018, 191, 23–35. [Google Scholar] [CrossRef]
- Tahjib-Ul-Arif, M.; Wei, X.; Jahan, I.; Hasanuzzaman, M.; Sabuj, Z.H.; Zulfiqar, F.; Chen, J.; Iqbal, R.; Dastogeer, K.M.G.; Sohag, A.A.M.; et al. Exogenous Nitric Oxide Promotes Salinity Tolerance in Plants: A Meta-Analysis. Front. Plant Sci. 2022, 13, 957735. [Google Scholar] [CrossRef]
- Amahisa, M.; Tsukagoshi, M.; Kadooka, C.; Masuo, S.; Takeshita, N.; Doi, Y.; Takagi, H.; Takaya, N. The Metabolic Regulation of Amino Acid Synthesis Counteracts Reactive Nitrogen Stress via Aspergillus Nidulans Cross-Pathway Control. JoF 2024, 10, 58. [Google Scholar] [CrossRef]
- Monreal, J.A.; Arias-Baldrich, C.; Tossi, V.; Feria, A.B.; Rubio-Casal, A.; García-Mata, C.; Lamattina, L.; García-Mauriño, S. Nitric Oxide Regulation of Leaf Phosphoenolpyruvate Carboxylase-Kinase Activity: Implication in Sorghum Responses to Salinity. Planta 2013, 238, 859–869. [Google Scholar] [CrossRef]
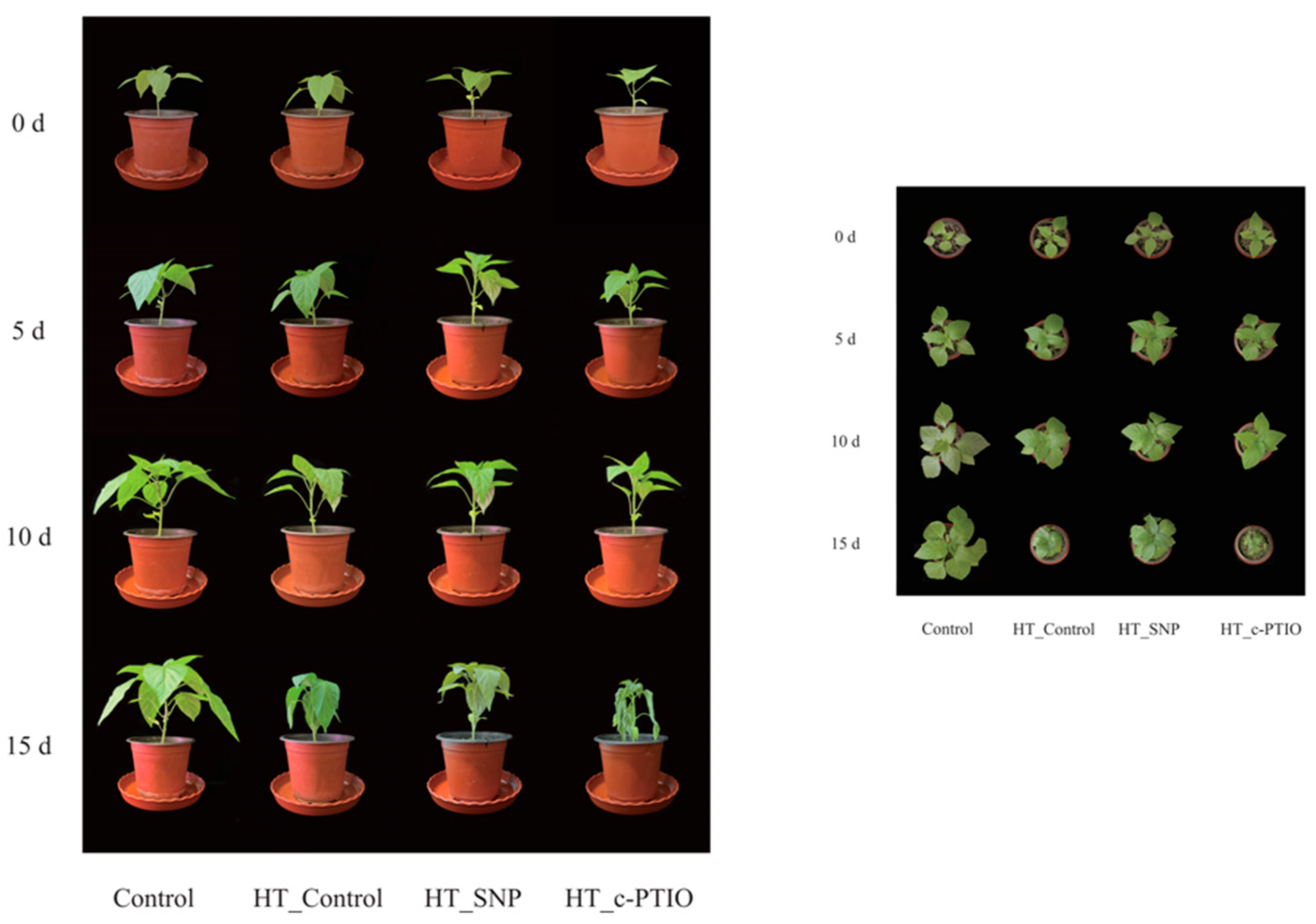


| Treatment | Time | Plant Height Increment (cm) | Stem Diameter Increment (mm) | Leaf Length Increment (cm) | Leaf Width Increment (cm) | Leaf Fresh Weight (g) | Leaf Dry Weight (g) | Root Fresh Weight (g) | Root Dry Weight (g) |
|---|---|---|---|---|---|---|---|---|---|
| Control | 5 d | 2.65 ± 0.07 a | 0.73 ± 0.02 a | 2.00 ± 0.10 a | 1.10 ± 0.10 a | 12.67 ± 0.61 a | 1.09 ± 0.05 a | 2.78 ± 0.02 a | 0.30 ± 0.00 a |
| HT_Control | 1.47 ± 0.12 c | 0.25 ± 0.01 c | 1.08 ± 0.10 c | 0.65 ± 0.07 bc | 7.20 ± 0.10 c | 0.62 ± 0.01 c | 1.59 ± 0.01 c | 0.17 ± 0.00 c | |
| HT_SNP | 1.90 ± 0.10 b | 0.60 ± 0.06 b | 1.40 ± 0.10 b | 0.85 ± 0.07 ab | 10.20 ± 0.10 b | 0.88 ± 0.01 b | 2.30 ± 0.10 b | 0.25 ± 0.01 b | |
| HT_c-PTIO | 1.10 ± 0.00 d | 0.26 ± 0.01 c | 0.93 ± 0.15 c | 0.45 ± 0.07 c | 5.37 ± 0.15 d | 0.46 ± 0.01 d | 1.10 ± 0.10 d | 0.12 ± 0.01 d | |
| Control | 10 d | 2.20 ± 0.00 a | 0.77 ± 0.04 a | 1.37 ± 0.12 a | 0.55 ± 0.07 a | 20.67 ± 0.61 a | 1.41 ± 0.02 a | 4.78 ± 0.02 a | 0.39 ± 0.01 a |
| HT_Control | 0.80 ± 0.00 c | 0.36 ± 0.03 c | 0.73 ± 0.06 c | 0.30 ± 0.00 b | 15.20 ± 0.10 c | 1.07 ± 0.01 c | 3.59 ± 0.01 c | 0.29 ± 0.02 c | |
| HT_SNP | 1.35 ± 0.07 b | 0.63 ± 0.05 b | 0.90 ± 0.00 b | 0.40 ± 0.00 b | 18.20 ± 0.10 b | 1.33 ± 0.01 b | 4.30 ± 0.10 b | 0.36 ± 0.01 b | |
| HT_c-PTIO | 0.67 ± 0.06 c | 0.18 ± 0.01 d | 0.75 ± 0.06 bc | 0.27 ± 0.06 b | 13.37 ± 0.15 d | 0.94 ± 0.03 d | 3.10 ± 0.10 d | 0.24 ± 0.02 d | |
| Control | 15 d | 0.90 ± 0.00 a | 0.26 ± 0.00 a | 0.07 ± 0.06 a | 0.30 ± 0.00 a | 25.67 ± 0.61 a | 1.53 ± 0.02 a | 9.78 ± 0.02 a | 0.50 ± 0.01 a |
| HT_Control | 0.40 ± 0.00 b | 0.14 ± 0.01 b | 0.00 ± 0.00 a | 0.00 ± 0.00 b | 18.20 ± 0.10 c | 1.19 ± 0.01 c | 8.59 ± 0.01 c | 0.40 ± 0.02 c | |
| HT_SNP | 1.00 ± 0.00 a | 0.23 ± 0.04 a | 0.08 ± 0.10 a | 0.00 ± 0.00 b | 23.20 ± 0.10 b | 1.45 ± 0.01 b | 9.30 ± 0.10 b | 0.47 ± 0.01 b | |
| HT_c-PTIO | 0.15 ± 0.07 c | 0.14 ± 0.01 b | 0.02 ± 0.05 a | 0.00 ± 0.00 b | 15.37 ± 0.15 d | 1.06 ± 0.03 d | 8.10 ± 0.10 d | 0.34 ± 0.02 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Y.; Li, Q.; Yang, X.; Wang, L.; Li, X.; Liu, K. Mitigating High-Temperature Stress in Peppers: The Role of Exogenous NO in Antioxidant Enzyme Activities and Nitrogen Metabolism. Horticulturae 2024, 10, 906. https://doi.org/10.3390/horticulturae10090906
Zhou Y, Li Q, Yang X, Wang L, Li X, Liu K. Mitigating High-Temperature Stress in Peppers: The Role of Exogenous NO in Antioxidant Enzyme Activities and Nitrogen Metabolism. Horticulturae. 2024; 10(9):906. https://doi.org/10.3390/horticulturae10090906
Chicago/Turabian StyleZhou, Yan, Qiqi Li, Xiuchan Yang, Lulu Wang, Xiaofeng Li, and Kaidong Liu. 2024. "Mitigating High-Temperature Stress in Peppers: The Role of Exogenous NO in Antioxidant Enzyme Activities and Nitrogen Metabolism" Horticulturae 10, no. 9: 906. https://doi.org/10.3390/horticulturae10090906
APA StyleZhou, Y., Li, Q., Yang, X., Wang, L., Li, X., & Liu, K. (2024). Mitigating High-Temperature Stress in Peppers: The Role of Exogenous NO in Antioxidant Enzyme Activities and Nitrogen Metabolism. Horticulturae, 10(9), 906. https://doi.org/10.3390/horticulturae10090906





