Climate Influence on Leaf Appearance and Ligustroflavone and Rhoifolin Compounds of Turpinia arguta (Lindl.) Seem. from Different Chinese Habitats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Instruments and Reagents
2.2. Sampling of Plant Material
2.3. HPLC Quantification of Ligustroflavone and Rhoifolin
2.3.1. Chromatographic Conditions
2.3.2. Preparation of the Standards and Working Solutions
2.3.3. Linearity of the Standard Solution
2.3.4. Methodology
2.4. Climatic Factors
2.5. Statistical Analysis
3. Results
3.1. Leaf Appearance and Ligustroflavone and Rhoifolin Compounds across Different Habitats
3.1.1. Leaf Appearance
3.1.2. Ligustroflavone and Rhoifolin Compounds
3.1.3. Comprehensive Ranking
3.2. Correlation between Leaf Appearance and Ligustroflavone and Rhoifolin Compounds
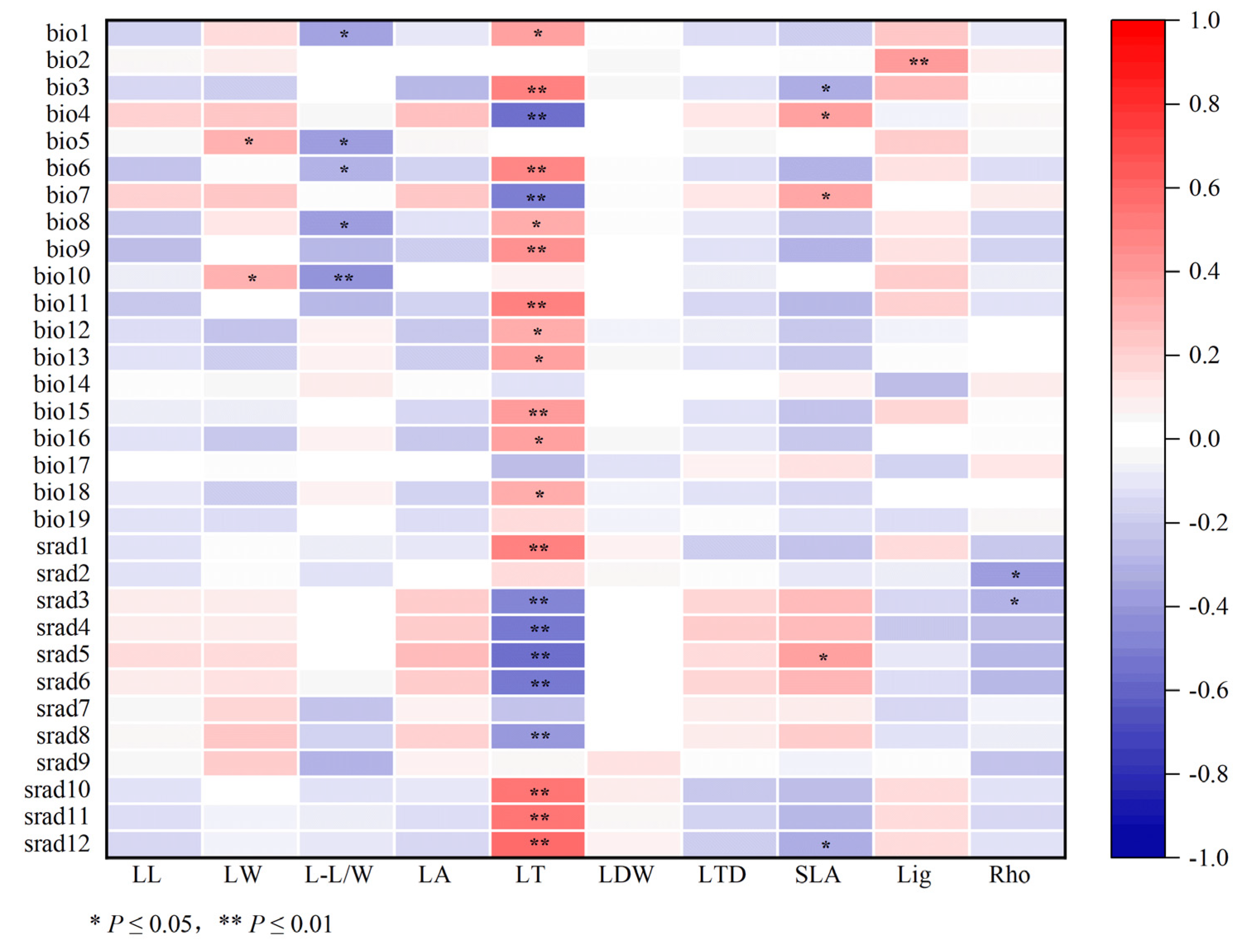
3.3. Correlation among Leaf Appearance, Ligustroflavone and Rhoifolin Compounds, and Climatic Factors
3.4. Redundancy Analysis of Climatic Factors as Determinants of T. arguta Leaf Appearance and Ligustroflavone and Rhoifolin Compounds
4. Discussion
4.1. Variability of Leaf Appearance and Quality Indices in T. arguta across Chinese Regions
4.2. Link between Leaf Appearance and Quality in T. arguta
4.3. Incidence of Climate Factors on Leaf Appearance and Quality in T. arguta
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, Z.; Raven, P.H.; Hong, D. Flora of China, Volume 7: Menispermaceae through Capparaceae; Science Press: Beijing, China, 2008. [Google Scholar]
- Tao, X.; Jiang, J.; Luo, H.; Li, D.; Luo, Y. Study on the Biological Characteristics and Container Seeding Raising Technology of Turpinia arguta fresh leaves. Jiangxi Sci. 2020, 38, 188–190. [Google Scholar] [CrossRef]
- Commission, N.P. Part 1. In Pharmacopoeia of the People’s Republic of CHINA (Part 1); China Medical Science and Technology Press: Beijing, China, 2020; p. 29. [Google Scholar]
- Li, Y.; Lei, X.; Feng, Y.; Xu, Q.; Xu, L.; Yang, S. Study on the Chemical Constituents of Terpinia arguta. Chin. Pharm. J. 2012, 47, 261–264. [Google Scholar]
- Ma, S.-G.; Yuan, S.-P.; Hou, Q.; Li, Y.; Chen, X.; Yu, S.-S. Flavonoid Glycosides from Leaves of Turpinia arguta and Their Anti-inflammatory Activity. China J. Chin. Mater. Medica 2013, 38, 1747–1750. [Google Scholar]
- Xiao, C.R.; Tu, L.F.; Zhang, R.Z.; Liu, D.P.; Luo, Y.M. Research Progress on Chemical Constituents and Biological Activities from Turpinia species. China J. Chin. Mater. Medica 2019, 44, 1295–1304. [Google Scholar] [CrossRef]
- Guo, X.; Xia, Z.; Song, M.; Li, C.; Wang, J.; Kang, W. Dynamic Changes of Secondary Metabolites and Antioxidant Activity of Ligustrum lucidum During Fruit Growth. Open Chem. 2018, 16, 99–107. [Google Scholar] [CrossRef]
- Zhang, L.; Li, J.; Yu, S.-C.; Jin, Y.; Lv, X.-W.; Zou, Y.-H.; Li, Y. Therapeutic Effects and Mechanisms of Total Flavonoids of Turpinia Arguta Seen on Adjuvant Arthritis in Rats. J. Ethnopharmacol. 2008, 116, 167–172. [Google Scholar] [CrossRef]
- Wu, Y.; Dong, G.; Sheng, C. Targeting Necroptosis in Anticancer Therapy: Mechanisms and Modulators. Acta Pharm. Sin. B 2020, 10, 1601–1618. [Google Scholar] [CrossRef]
- Yu, X.X.; Liu, Q.D.; Wu, J.W.; Liang, Z.K.; Zhao, M.Q.; Xu, X.J. Simultaneous Determination of Four Major Constituents in Citri Grandis Exocarpium by HPLC-DAD. Acta Chromatogr. 2016, 28, 129–143. [Google Scholar] [CrossRef]
- Li, L.; Zhao, Y.; Liu, W.; Feng, F.; Xie, N. HPLC with Quadrupole TOF-MS and Chemometrics Analysis for the Characterization of Folium Turpiniae from Different Regions. J. Sep. Sci. 2013, 36, 2552–2561. [Google Scholar] [CrossRef] [PubMed]
- Ou, B.; Liu, R. A Numerical Study on Variation of Vegetaeive Ovgan’s Characeers of Turpinia arguta. J.-Fujian Coll. For. 2003, 23, 371–374. [Google Scholar]
- Liu, D.; Li, Z.; Cheng, F.; Wen, H.; Liao, G.; Yang, X. Quality Evaluation of Turpinia arguta from Different Origins. Lishizhen Med. Mater. Medica Res. 2012, 23, 476–477. [Google Scholar]
- Pant, P.; Pandey, S.; Dall’Acqua, S. The Influence of Environmental Conditions on Secondary Metabolites in Medicinal Plants: A Literature Review. Chem. Biodivers. 2021, 18, e2100345. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Ran, J.; Liu, H.; Song, J.; Xie, C. Geographic Variation of Functional Components and the Climatic Response Characteristics of Rheum tanguticum Maxim. Acta Ecol. Sin. 2021, 41, 3645–3655. [Google Scholar]
- Wei, M.; Zhang, Y.J.; Wang, T.; Guo, Q.S.; Zou, Q.J.; Chen, F.R.; Kang, Z.X.; Han, H.J.; Han, Z.Z.; Liu, H.H. Correlations between Content of Linarin in Chrysanthemum indicum and Climatic Factors in Habitats. China J. Chin. Mater. Medica 2021, 46, 2167–2172. [Google Scholar] [CrossRef]
- Huang, L.; Xie, C.; Duan, B.; Chen, S. Mapping the Potential Distribution of High Artemisinin-yielding Artemisia annua L. (Qinghao) in China with a Geographic Information System. Chin. Med. 2010, 5, 18. [Google Scholar] [CrossRef]
- Singh, S.P.; Misra, A.; Kumar, B.; Adhikari, D.; Srivastava, S.; Barik, S.K. Identification of Potential Cultivation Areas for Centelloside-specific Elite Chemotypes of Centella asiatica (L.) using Ecological Niche Modeling. Ind. Crops Prod. 2022, 188, 115657. [Google Scholar] [CrossRef]
- Liang, H.; Kong, Y.; Chen, W.; Wang, X.; Jia, Z.; Dai, Y.; Yang, X. The Quality of Wild Salvia miltiorrhiza from Dao Di Area in China and Its Correlation with Soil Parameters and Climate Factors. Phytochem. Anal. 2021, 32, 318–325. [Google Scholar] [CrossRef]
- Li, L.; Zheng, S.; Brinckmann, J.A.; Fu, J.; Zeng, R.; Huang, L.; Chen, S. Chemical and Genetic Diversity of Astragalus mongholicus Grown in Different Eco-climatic Regions. PLoS ONE 2017, 12, e0184791. [Google Scholar] [CrossRef]
- Sun, J.; Liu, X.; Wu, H.; Liu, K. Progress on Chemical Composition and Pharmacological Activity of Turpinia arguta Seem. Food Drug 2011, 13, 441–444. [Google Scholar]
- Hu, J.; Cai, J.; Hu, X.; Wang, L.; Cheng, Q.; Tao, X. Efficient In Vitro Propagation of Turpinia arguta and Quantitative Analysis of Its Ligustroflavone and Rhoifolin Content. Horticulturae 2024, 10, 587. [Google Scholar] [CrossRef]
- Song, W.J.; Song, Q.L.; Zou, Z.H.; Chen, X.L.; Liu, L.X.; Tan, J.; Wei, L.M.; Xiong, P.W.; Tao, X.H.; Sun, R.P. Effects of Turpiniae folium Extract on Growth Performance, Serum Immune and Antioxidant Function and Intestinal Microflora of Wenchang Chickens. Chin. J. Anim. Nutr. 2022, 34, 4380–4393. [Google Scholar] [CrossRef]
- Zhang, J.H.; Tao, X.H.; Wu, Y.Z.; Wang, J. A Chinese Herbal Microecological Compound Preparation for the Prevention and Treatment of Enteritis in Newborn Piglets, Its Preparation Method and Application. CN202011350102.8. 24 May 2022. [Google Scholar]
- Yang, X.L.; Tao, X.H.; Huang, J.; Xia, S.Q. A Ointment of Turpinia arguta and Its Preparation Method. CN202311216922.1. 10 November 2023. [Google Scholar]
- Zhou, J.Y.; Hu, S.; Tao, X.H.; Luo, J.; Ou, L.H.; Zhu, S.L.; Feng, J.X. The Application of Turpinia arguta and Its Extract in Mosquito Repellent Products, the Extraction Method and the Mosquito Repellent Products. CN202011159327.5. 18 October 2022. [Google Scholar]
- Xia, S.Q.; Tao, X.H.; Lin, H.; Yang, X.L. The Application of the Extract from Turpinia arguta in the Preparation of Hands-free Disinfectant. CN202410343494.7. 26 April 2024. [Google Scholar]
- Wang, X.Y.; Zhang, L.; Cao, L.; Liang, F. ISSR Analysis of Genetic Relationship and Population Structure of Turpinia arguta Fresh Leaves in Jiangxi Province. Chin. J. Exp. Tradit. Med. Formulae 2020, 24, 150–155. [Google Scholar]
- Flatscher, R.; Frajman, B.; Schnswetter, P.; Paun, O. Environmental Heterogeneity and Phenotypic Divergence: Can Heritable Epigenetic Variation Aid Speciation. Genet. Res. Int. 2012, 3, 698421. [Google Scholar] [CrossRef]
- Tang, X.Q.; Wang, K.C.; Chen, X.; Wu, J.; Yu, B.Y. Clustering Analysis on Contents of Lipophilic Components from Four Cultivars of Salvia miltiorrhiza Bge. Nat. Prod. Res. Dev. 2007, 5, 748–752. [Google Scholar] [CrossRef]
- Su, W.H.; Lu, J.; Zhang, G.F. Ecological and Biological Analysis of Total Flavonoids in Erigeron breviscapus. Chin. Tradit. Herb. Drugs 2001, 32, 1119–1121. [Google Scholar]
- Cheng, G.Y.; Gao, F.; Ge, C. Variation Regularity of Taxol Content in Natural Populations of Taxus cuspidata Sieb.et Zucc. in China. J. Beijing For. Univ. 2005, 27, 7–11. [Google Scholar]
- Yamazaki, M.; Sato, A.; Saito, K. Molecular Phylogeny Based on RFLP and Its Relation with Alkaloid Patterns in Lupinus plants. Biol. Pharm. Bull. 1993, 16, 1182–1184. [Google Scholar] [CrossRef] [PubMed]
- Xie, D.M.; Yu, N.J.; Huang, L.Q.; Peng, D.Y.; Liu, C.B.; Zhu, Y.J.; Huang, H. Next Generation Sequencing and Transcriptome Analysis of Root Bark from Paeonia suffruticosa cv. Feng Dan. China J. Chin. Mater. Medica 2017, 15, 2954–2961. [Google Scholar] [CrossRef]
- Huang, L.; Zhang, X.; Chen, S. Research Progress in Quality Ecology of Genuine Regional Drugs. Mod. Tradit. Chin. Med. Mater. Med.-World Sci. Technol. 2019, 21, 844–853. [Google Scholar]
- Rao, Z.; Zhang, F.; Dong, Y.; Wei, Y. Research Progress on “Quality Evaluation through Morphological Identification” and Cause of Quality Formation in Gardeniae Fructus. Chin. Tradit. Herb. Drugs 2023, 54, 1998–2004. [Google Scholar]
- Wang, W.X.; Du, X.W.; Hou, B.Y.; Zhu, H.R.; Yu, D. Correlations between Appearance Traits and Internal Quality of Bupleurum scorzonerifolium Roots based on Theory of "Quality Evaluation through Morphological Identification”. China J. Chin. Mater. Medica 2023, 48, 1815–1823. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, D.; Huang, L. Analysis of the Correlation between Commodity Grade and Quality of Angelica sinensis by Determination of Active Compounds Using Ultraperformance Liquid Chromatography Coupled with Chemometrics. Evid.-Based Complement. Altern. Med. 2014, 1, 143286. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yang, B.; Wang, J.; Zhang, G.; Yan, Y.; Gao, J.; Yang, X.; Hu, B.; Peng, L. Content Determination and Quality Analysis of Five Main Components in Polygala tenuifolia from Different Habitats. Chin. Tradit. Herb. Drugs 2020, 51, 1609–1616. [Google Scholar]
- Zhang, K.; Zhang, S.; Ebihara, A.; Zhou, X.; Fan, L.; Li, P.; Zhang, Z.; Wang, Y.; Shen, Y. The Current Research Progress of Ginseng Species: The cultivation and application. Cogent Food Agric. 2023, 9, 2216483. [Google Scholar] [CrossRef]
- Traiser, C.; Klotz, S.; Uhl, D.; Mosbrugger, V. Environmental Signals from Leaves—A Physiognomic Analysis of European Vegetation. New Phytol. 2005, 166, 465–484. [Google Scholar] [CrossRef] [PubMed]
- Moles, A.T.; Perkins, S.E.; Laffan, S.W.; Flores-Moreno, H.; Awasthy, M.; Tindall, M.L.; Sack, L.; Pitman, A.; Kattge, J.; Aarssen, L.W.; et al. Which is A Better Predictor of Plant Traits: Temperature or precipitation? J. Veg. Sci. 2014, 25, 1167–1180. [Google Scholar] [CrossRef]
- Givnish, T.J. Leaf and Canopy Adaptations in Tropical Forests. In Physiological Ecology of Plants of the Wet Tropics: Proceedings of an International Symposium Held in Oxatepec and Los Tuxtlas, Mexico, June 29 to July 6, 1983; Medina, E., Mooney, H.A., Vázquez-Yánes, C., Eds.; Springer: Dordrecht, The Netherlands, 1984; pp. 51–84. [Google Scholar]
- Peppe, D.J.; Royer, D.L.; Cariglino, B.; Oliver, S.Y.; Newman, S.; Leight, E.; Enikolopov, G.; Fernandez-Burgos, M.; Herrera, F.; Adams, J.M.; et al. Sensitivity of Leaf Size and Shape to Climate: Global Patterns and Paleoclimatic Applications. New Phytol. 2011, 190, 724–739. [Google Scholar] [CrossRef]
- Tian, L. The Effect of Microorganism and Climate Factors on the Quality of Panax quinquefolium L. Ph.D.thesis, Peking Union Medical College, Beijing, China, 2023. [Google Scholar]
- Liu, Y.; Ren, X.; Jeong, B.R. Manipulating the Difference between the Day and Night Temperatures Can Enhance the Quality of Astragalus membranaceus and Codonopsis lanceolata Plug Seedlings. Agronomy 2019, 9, 654. [Google Scholar] [CrossRef]
- Devkota, A.; Dall’Acqua, S.; Comai, S.; Innocenti, G.; Jha, P.K. Centella asiatica (L.) urban from Nepal: Quali-quantitative analysis of samples from several sites, and selection of high terpene containing populations for cultivation. Biochem. Syst. Ecol. 2010, 38, 12–22. [Google Scholar] [CrossRef]
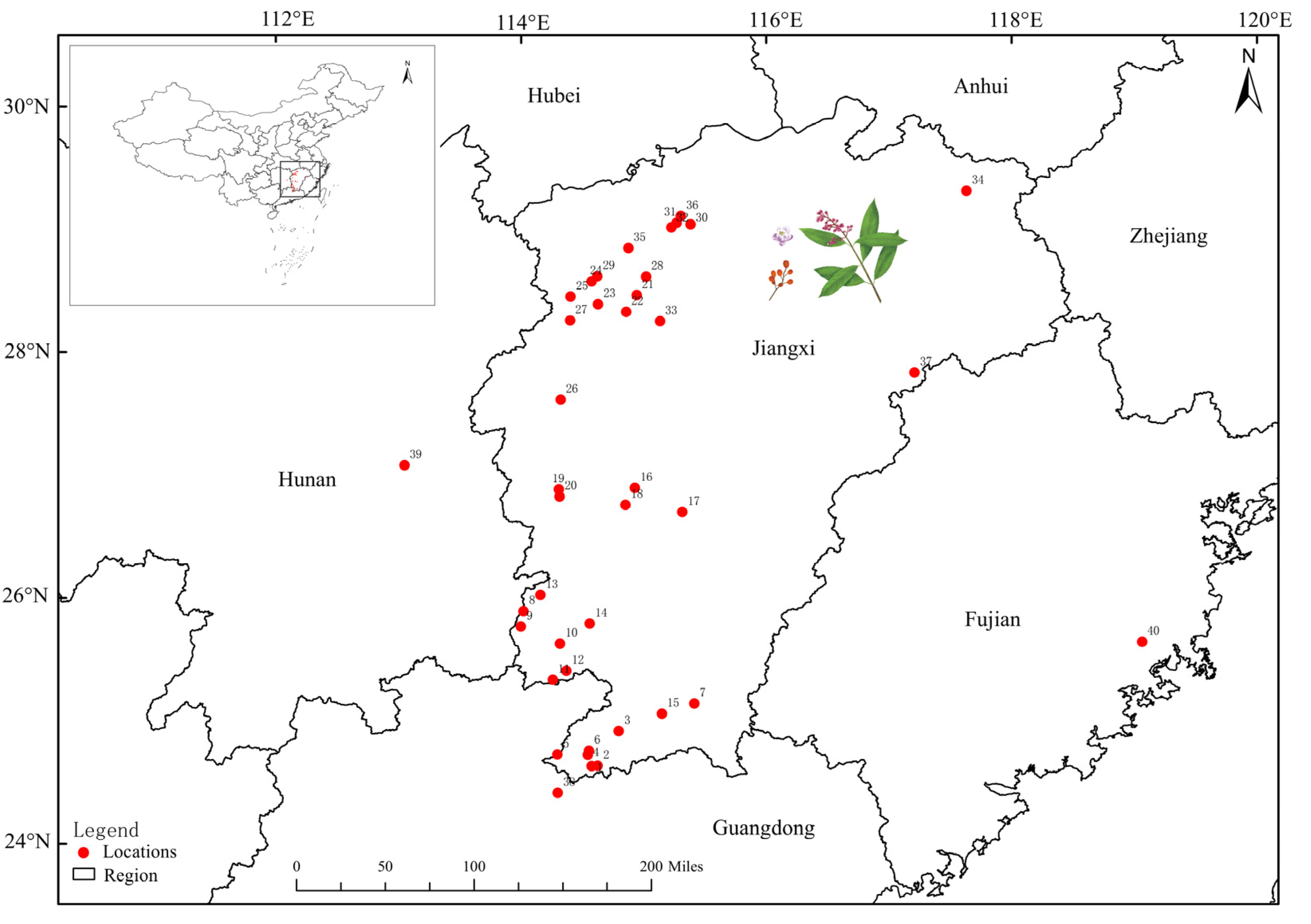





| Id. Code | Origin | Longitude | Latitude | No. of Plants Sampled | Date of Collection |
|---|---|---|---|---|---|
| 1 | L(J-mt) | 114°34′28″ | 24°37′51″ | 33 | 2020.09.30 |
| 2 | L(Y-vg) | 114°37′28″ | 24°37′53″ | 3 | 2020.10.09 |
| 3 | L(A-mt) | 114°47′48″ | 24°54′60″ | 3 | 2020.10.11 |
| 4 | Q(T-wl) | 114°32′38″ | 24°43′20″ | 10 | 2020.09.30 |
| 5 | Q(F-vg) | 114°17′45″ | 24°43′29″ | 20 | 2020.10.08 |
| 6 | Q(J-t) | 114°33′12″ | 24°45′19″ | 7 | 2020.10.16 |
| 7 | A(X-t) | 115°24′46″ | 25°08′18″ | 3 | 2020.11.05 |
| 8 | C(Q-mt) | 114°01′12″ | 25°53′26″ | 14 | 2020.09.16 |
| 9 | C(C-vg) | 113°59′53″ | 25°45′59″ | 8 | 2020.09.18 |
| 10 | C(Y-mt) | 114°18′60″ | 25°37′34″ | 19 | 2020.09.20 |
| 11 | D(M-r) | 114°15′27″ | 25°20′02″ | 14 | 2020.11.06 |
| 12 | D(Z-t) | 114°22′06″ | 25°24′27″ | 7 | 2020.11.16 |
| 13 | S(F-mt) | 114°09′30″ | 26°01′23″ | 9 | 2020.11.20 |
| 14 | S(G-mt) | 114°33′31″ | 25°47′26″ | 3 | 2020.11.21 |
| 15 | X(A-ff) | 115°08′51″ | 25°03′26″ | 3 | 2020.09.15 |
| 16 | T(B-t) | 114°55′40″ | 26°53′38″ | 3 | 2020.09.21 |
| 17 | T(B-mt) | 115°18′54″ | 26°41′58″ | 6 | 2020.09.22 |
| 18 | T(Y-vg) | 114°51′01″ | 26°45′29″ | 3 | 2020.09.22 |
| 19 | Y(B-mt) | 114°18′21″ | 26°53′04″ | 7 | 2020.10.06 |
| 20 | Y(Q-t) | 114°18′46″ | 26°49′32″ | 5 | 2020.10.07 |
| 21 | Yf(Y-vg) | 114°56′32″ | 28°27′52″ | 8 | 2020.09.18 |
| 22 | Yf(S-ff) | 114°51′22″ | 28°19′42″ | 3 | 2020.09.18 |
| 23 | Yf(J-vg) | 114°37′35″ | 28°23′23″ | 5 | 2020.09.19 |
| 24 | Yf(G-mt) | 114°34′29″ | 28°34′42″ | 16 | 2020.09.10 |
| 25 | Yf(S-vg) | 114°24′10″ | 28°27′05″ | 3 | 2020.11.14 |
| 26 | Yc(M-mt) | 114°19′17″ | 27°36′50″ | 23 | 2020.09.02 |
| 27 | W(J-t) | 114°23′55″ | 28°15′35″ | 3 | 2020.11.13 |
| 28 | F(S-vg) | 115°01′08″ | 28°36′50″ | 3 | 2020.11.25 |
| 29 | Tg(H-vg) | 114°37′12″ | 28°37′03″ | 10 | 2020.10.12 |
| 30 | J(P-vg) | 115°22′49″ | 29°02′24″ | 3 | 2020.11.02 |
| 31 | J(H-cy) | 115°16′04″ | 29°03′17″ | 3 | 2020.11.02 |
| 32 | J(G-vg) | 115°13′26″ | 29°01′02″ | 8 | 2020.11.03 |
| 33 | G(X-t) | 115°07′56″ | 28°15′10″ | 10 | 2020.10.30 |
| 34 | Wy(F-t) | 117°37′54″ | 29°18′53″ | 3 | 2020.11.12 |
| 35 | Xs(H-t) | 114°52′27″ | 28°50′49″ | 5 | 2020.10.20 |
| 36 | Wn(S-mt) | 115°18′11″ | 29°06′32″ | 6 | 2020.11.16 |
| 37 | Z(M-mt) | 117°12′31″ | 27°50′01″ | 5 | 2020.11.19 |
| 38 | Lp(P-t) | 114°17′55″ | 24°24′46″ | 3 | 2020.10.06 |
| 39 | H(G-t) | 113°02′52″ | 27°04′48″ | 3 | 2020.11.01 |
| 40 | P(S-mt) | 119°03′52″ | 25°38′33″ | 3 | 2020.10.28 |
| Id. Code | Subordinate Function Values | Average Membership Function Value | Ranking Position | ||||
|---|---|---|---|---|---|---|---|
| LA | LT | LDW | Lig | Rho | |||
| 1 | 0.218 | 0.415 | 0.237 | 0.412 | 0.394 | 0.335 | 30 |
| 2 | 0.231 | 0.382 | 0.145 | 0.699 | 0.424 | 0.376 | 27 |
| 3 | 0.363 | 0.490 | 0.303 | 0.525 | 0.275 | 0.391 | 22 |
| 4 | 0.581 | 0.345 | 0.286 | 0.485 | 0.605 | 0.460 | 9 |
| 5 | 0.335 | 0.337 | 0.268 | 0.480 | 0.540 | 0.392 | 21 |
| 6 | 0.841 | 0.383 | 0.910 | 0.457 | 0.604 | 0.639 | 2 |
| 7 | 0.870 | 1.000 | 0.763 | 1.000 | 0.316 | 0.790 | 1 |
| 8 | 0.526 | 0.479 | 0.602 | 0.066 | 0.375 | 0.410 | 17 |
| 9 | 0.289 | 0.427 | 0.531 | 0.113 | 0.098 | 0.292 | 33 |
| 10 | 0.388 | 0.501 | 0.458 | 0.326 | 0.353 | 0.405 | 19 |
| 11 | 0.412 | 0.304 | 0.222 | 0.489 | 0.520 | 0.389 | 23 |
| 12 | 0.785 | 0.405 | 0.323 | 0.241 | 0.523 | 0.456 | 11 |
| 13 | 0.733 | 0.408 | 0.596 | 0.328 | 0.610 | 0.535 | 5 |
| 14 | 0.864 | 0.331 | 1.000 | 0.134 | 0.255 | 0.517 | 6 |
| 15 | 0.000 | 0.363 | 0.000 | 0.148 | 0.327 | 0.167 | 40 |
| 16 | 0.411 | 0.344 | 0.342 | 0.469 | 0.693 | 0.452 | 13 |
| 17 | 0.659 | 0.382 | 0.263 | 0.396 | 0.449 | 0.430 | 15 |
| 18 | 0.639 | 0.414 | 0.329 | 0.322 | 0.435 | 0.428 | 16 |
| 19 | 0.562 | 0.397 | 0.453 | 0.290 | 1.000 | 0.540 | 4 |
| 20 | 0.235 | 0.304 | 0.029 | 0.225 | 0.404 | 0.240 | 37 |
| 21 | 0.618 | 0.324 | 0.398 | 0.267 | 0.381 | 0.397 | 20 |
| 22 | 0.772 | 0.382 | 0.645 | 0.502 | 0.845 | 0.629 | 3 |
| 23 | 0.283 | 0.274 | 0.061 | 0.332 | 0.466 | 0.283 | 34 |
| 24 | 0.346 | 0.176 | 0.240 | 0.484 | 0.551 | 0.359 | 29 |
| 25 | 0.722 | 0.293 | 0.197 | 0.402 | 0.271 | 0.377 | 26 |
| 26 | 0.549 | 0.378 | 0.428 | 0.387 | 0.523 | 0.453 | 12 |
| 27 | 0.163 | 0.217 | 0.329 | 0.260 | 0.680 | 0.330 | 31 |
| 28 | 0.638 | 0.236 | 0.684 | 0.482 | 0.444 | 0.497 | 7 |
| 29 | 0.270 | 0.159 | 0.266 | 0.247 | 0.468 | 0.282 | 35 |
| 30 | 0.830 | 0.019 | 0.303 | 0.486 | 0.260 | 0.380 | 24 |
| 31 | 0.358 | 0.000 | 0.039 | 0.359 | 0.100 | 0.171 | 39 |
| 32 | 0.711 | 0.181 | 0.319 | 0.342 | 0.295 | 0.370 | 28 |
| 33 | 0.707 | 0.154 | 0.562 | 0.288 | 0.457 | 0.433 | 14 |
| 34 | 1.000 | 0.140 | 0.526 | 0.373 | 0.000 | 0.408 | 18 |
| 35 | 0.676 | 0.373 | 0.400 | 0.177 | 0.663 | 0.458 | 10 |
| 36 | 0.591 | 0.131 | 0.388 | 0.378 | 0.915 | 0.481 | 8 |
| 37 | 0.398 | 0.167 | 0.179 | 0.036 | 0.402 | 0.236 | 38 |
| 38 | 0.089 | 0.732 | 0.079 | 0.478 | 0.512 | 0.378 | 25 |
| 39 | 0.386 | 0.446 | 0.408 | 0.235 | 0.124 | 0.320 | 32 |
| 40 | 0.312 | 0.395 | 0.421 | 0.000 | 0.074 | 0.240 | 36 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ji, H.; Cai, J.; Chen, C.; Song, X.; Luo, Y.; Yu, J.; Zhang, Y.; Tao, X. Climate Influence on Leaf Appearance and Ligustroflavone and Rhoifolin Compounds of Turpinia arguta (Lindl.) Seem. from Different Chinese Habitats. Horticulturae 2024, 10, 935. https://doi.org/10.3390/horticulturae10090935
Ji H, Cai J, Chen C, Song X, Luo Y, Yu J, Zhang Y, Tao X. Climate Influence on Leaf Appearance and Ligustroflavone and Rhoifolin Compounds of Turpinia arguta (Lindl.) Seem. from Different Chinese Habitats. Horticulturae. 2024; 10(9):935. https://doi.org/10.3390/horticulturae10090935
Chicago/Turabian StyleJi, Hongli, Junhuo Cai, Chao Chen, Xiaomin Song, Yun Luo, Jinbao Yu, Yang Zhang, and Xiuhua Tao. 2024. "Climate Influence on Leaf Appearance and Ligustroflavone and Rhoifolin Compounds of Turpinia arguta (Lindl.) Seem. from Different Chinese Habitats" Horticulturae 10, no. 9: 935. https://doi.org/10.3390/horticulturae10090935






