Serendipita indica: A Promising Biostimulant for Improving Growth, Nutrient Uptake, and Sugar Accumulation in Camellia oleifera
Abstract
1. Introduction
2. Materials and Methods
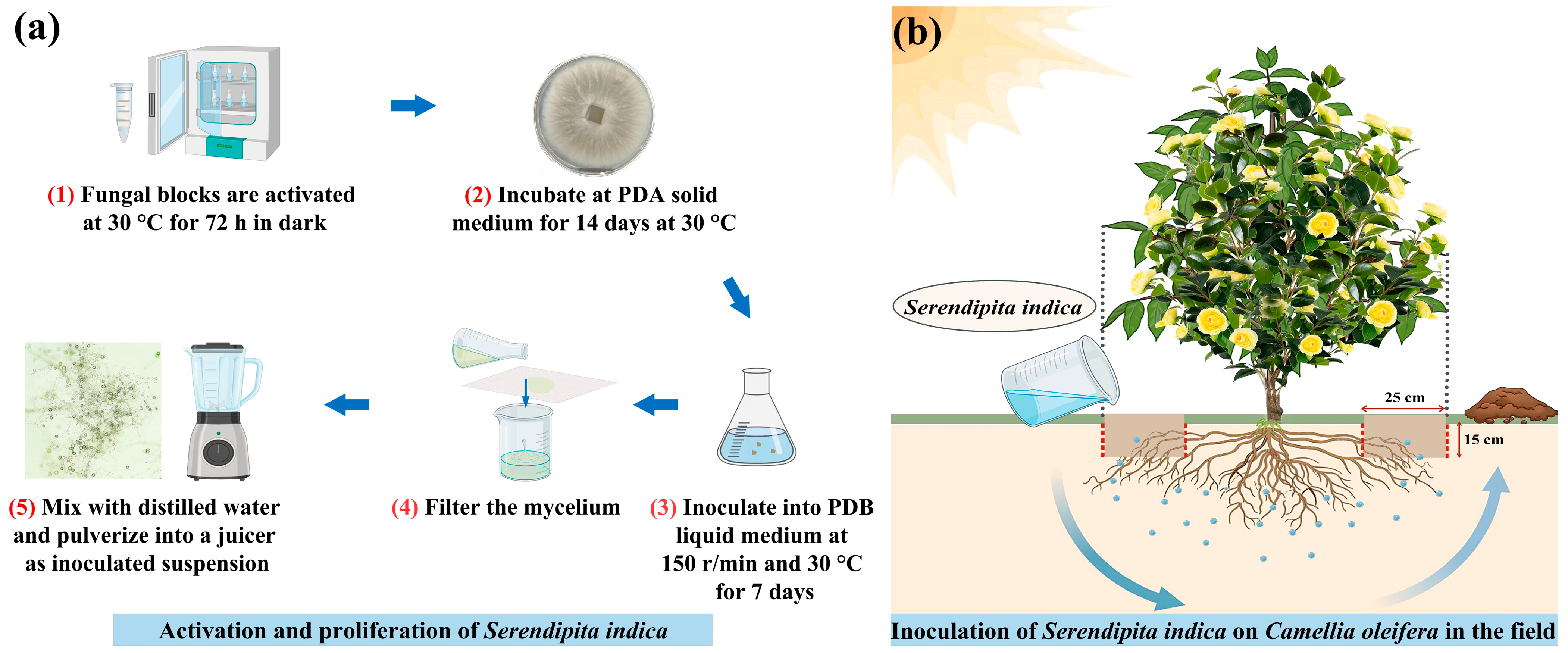
2.1. Preparation of S. indica
2.2. Experimental Set-Up and Design
2.3. Determination of Growth Behavior and Root Fungal Colonization Rate
2.4. Measurement of Photosynthetic Characteristics
2.5. Determination of P, K, Ca, and Sugar Concentrations in Leaves and Roots
2.6. Determination of Soil Properties
2.7. Transporter Genes Expression
2.8. Statistical Analysis
3. Results
3.1. Effect of Inoculation with S. indica on the Growth and Root Colonization Rate of Field C. oleifera
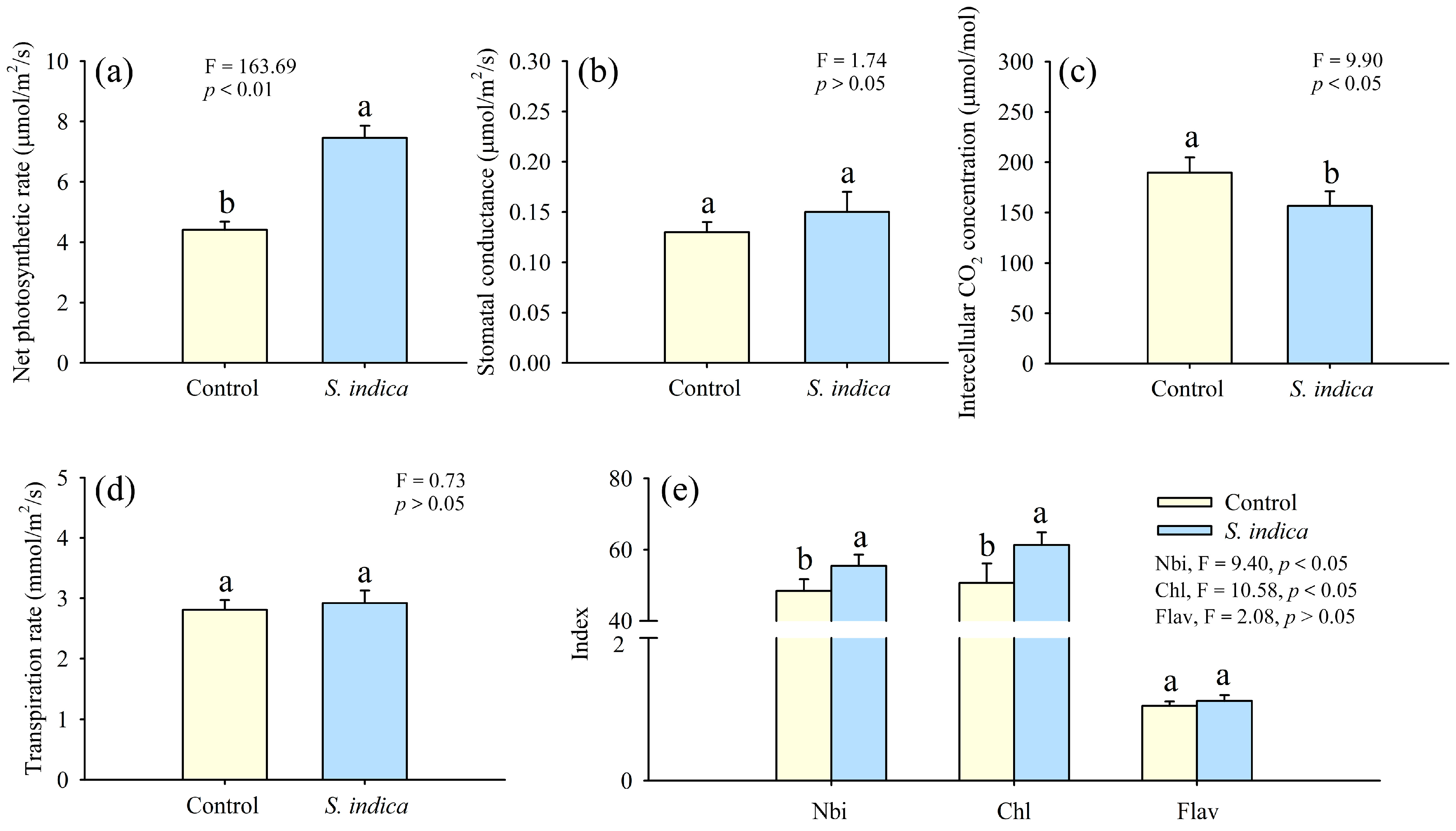
3.2. Effect of Inoculation with S. indica on the Leaf Photosynthetic Characteristics of Field C. oleifera
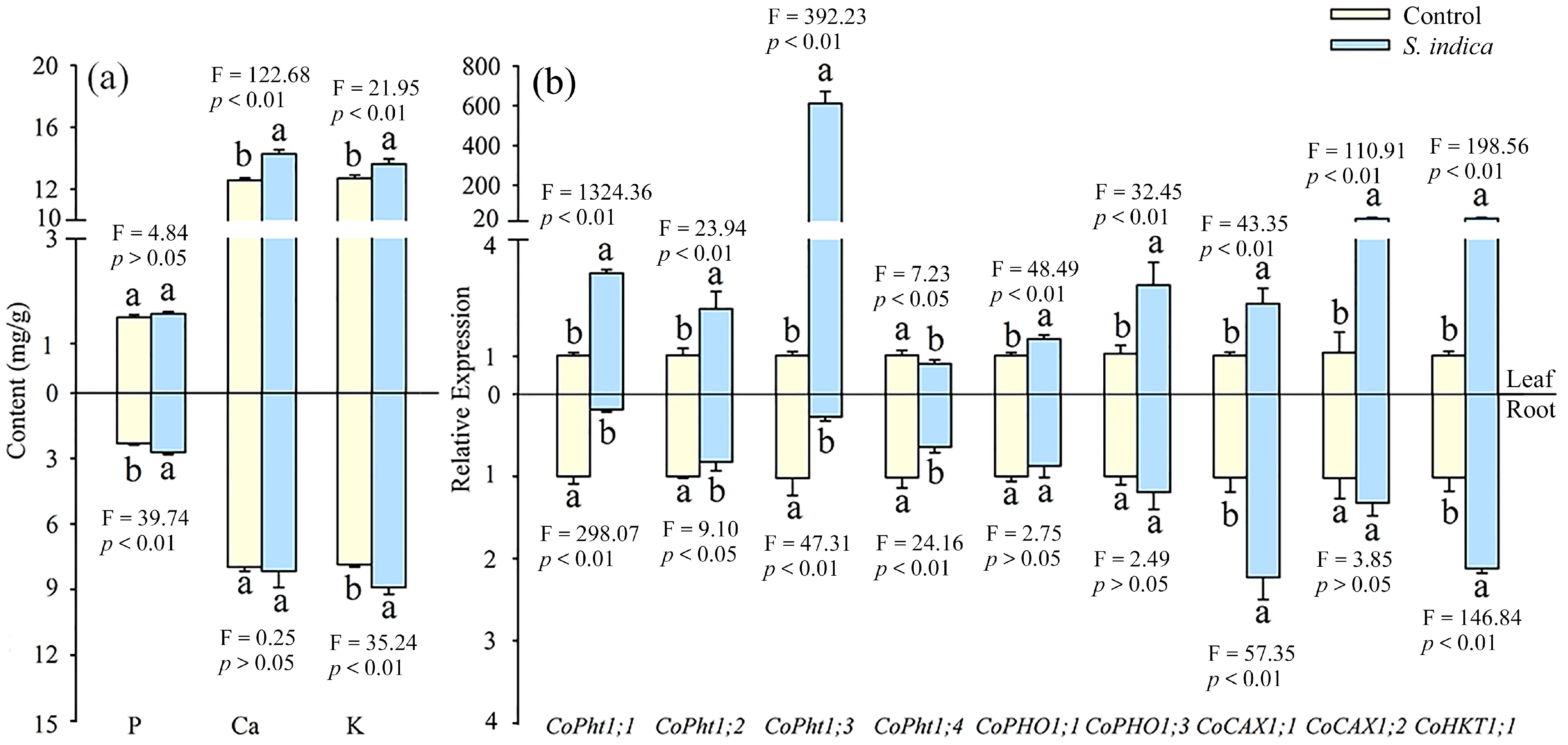
3.3. Effect of Inoculation with S. indica on P, K, and Ca Concentrations and Their Transporter Gene Expression in Field C. oleifera
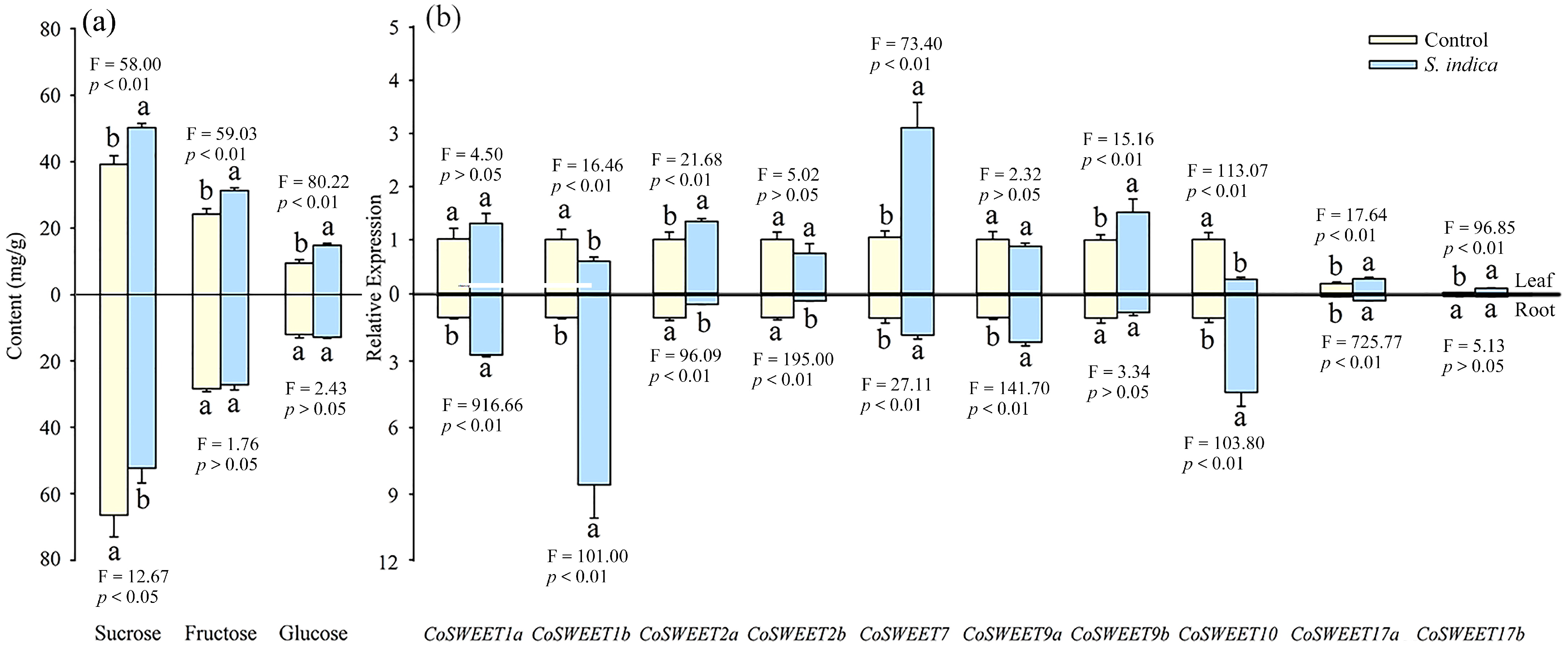
3.4. Effect of Inoculation with S. indica on Sugar Concentrations and CsSWEET Gene Expression in Field C. oleifera
3.5. Effect of Inoculation with S. indica on the Soil Properties of Field C. oleifera
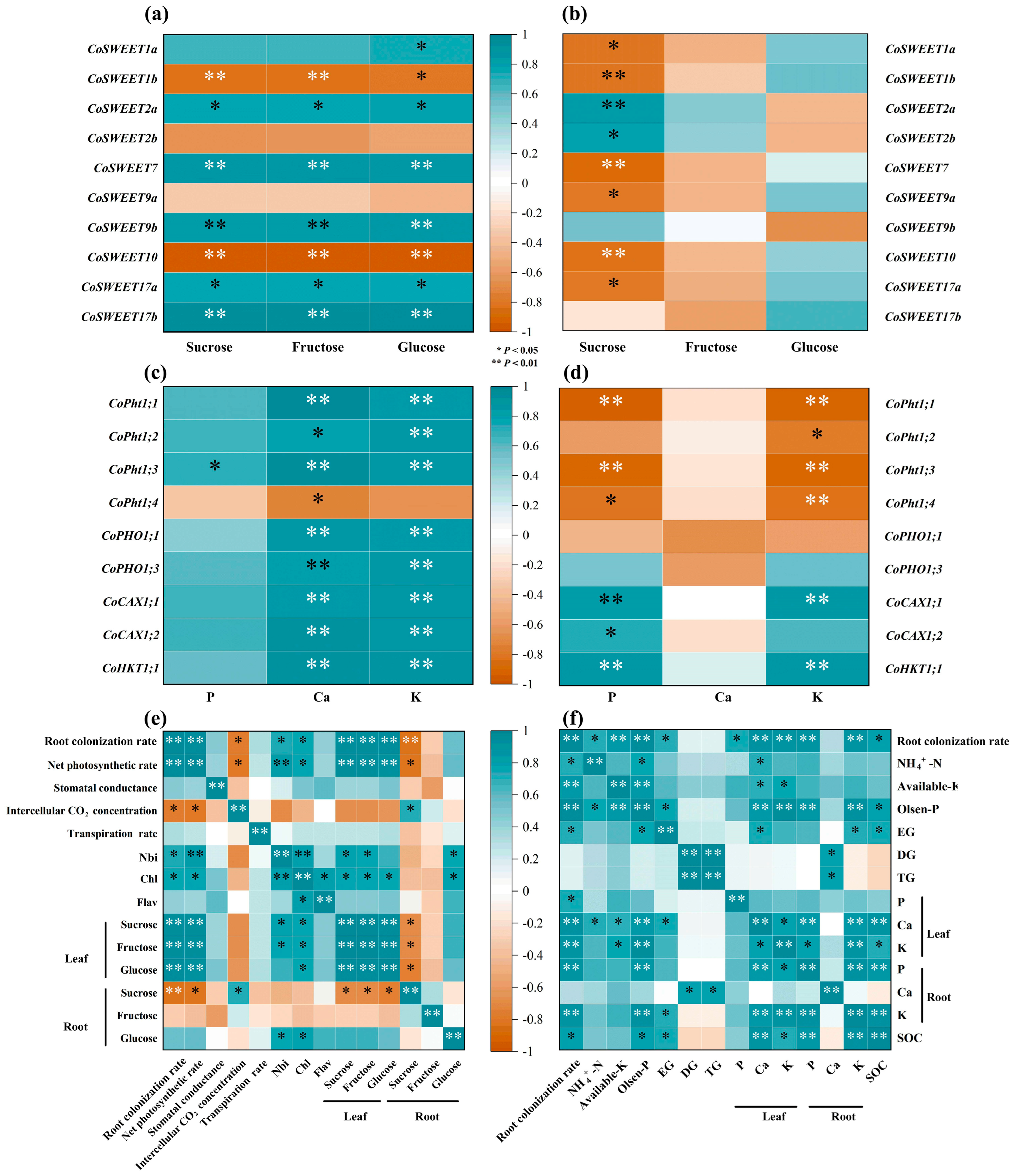
3.6. Correlation Studies
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xu, G.; Li, C.T.; Gui, W.; Xu, M.Q.; Lu, J.; Qian, M.S.; Zhang, Y.Y.; Yang, G.Q. Colonization of Piriformospora indica enhances rice resistance against the brown planthopper Nilaparvata lugens. Pest Manag. Sci. 2024, 80, 4386–4398. [Google Scholar] [CrossRef]
- Yang, L.; Cao, J.L.; Zou, Y.N.; Wu, Q.S.; Kuča, K. Piriformospora indica: A root endophytic fungus and its roles in plants. Not. Bot. Horti Agrobo. 2020, 48, 1–13. [Google Scholar] [CrossRef]
- Sun, C.; Johnson, J.M.; Cai, D.G.; Sherameti, I.; Oelmüller, R.; Lou, B.G. Piriformospora indica confers drought tolerance in Chinese cabbage leaves by stimulating antioxidant enzymes, the expression of drought related genes and the plastid-localized CAS protein. J. Plant Physiol. 2010, 167, 1009–1017. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.M.; Hashem, A.; Abd-Allah, E.F.; Wu, Q.S. Root-associated symbiotic fungi enhance waterlogging tolerance of peach seedlings by increasing flavonoids and activities and gene expression of antioxidant enzymes. Chem. Biol. Technol. Agric. 2023, 10, 124. [Google Scholar] [CrossRef]
- Singh, M.; Sharma, J.G.; Giri, B. Augmentative role of arbuscular mycorrhizal fungi, Piriformospora indica, and plant growth-promoting bacteria in mitigating salinity stress in maize (Zea mays L.). J. Growth Regul. 2024, 43, 1195–1215. [Google Scholar] [CrossRef]
- Rahman, S.U.; Khalid, M.; Hui, N.; Rehman, A.; Kayani, S.I.; Fu, X.Q.; Zheng, H.; Shao, J.; Khan, A.A.; Ali, M.; et al. Piriformospora indica alter root-associated microbiome structure to enhance Artemisia annua L. tolerance to arsenic. J. Hazard. Mater. 2023, 457, 131752. [Google Scholar] [CrossRef]
- Kundu, A.; Vadassery, J. Molecular mechanisms of Piriformospora indica mediated growth promotion in plants. Plant Signal. Behav. 2022, 17, 2096785. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.J.; Wang, F.L.; Han, M.Z.; Liu, Y.Y.; Liu, R.C.; Zou, Y.N.; Wu, Q.S. Effects of root endophytic fungi on growth, gas exchange, and soil properties of field Camellia oleifera plants. Non-Wood For. Res. 2023, 41, 163–169+190. [Google Scholar] [CrossRef]
- Wen, Y.; Su, S.C.; Ma, L.Y.; Yang, S.Y.; Wang, Y.W.; Wang, X.N. Effects of canopy microclimate on fruit yield and quality of Camellia oleifera. Sci. Hortic. 2018, 235, 132–141. [Google Scholar] [CrossRef]
- Chen, Y.; Zheng, J.J.; Yang, Z.J.; Xu, C.H.; Liao, P.H.; Pu, S.S.; EI-Kassaby, Y.A.; Feng, J.L. Role of soil nutrient elements transport on Camellia oleifera yield under different soil types. BMC Plant Biol. 2023, 23, 378. [Google Scholar] [CrossRef]
- Liu, J.Y.; Zhang, M.X.; Fan, J.; Ding, W.N.; Chen, L.S.; Luo, J.; Liu, Y.Z.; Mei, L. The synergistic effects of AMF inoculation and boron deficiency on the growth and physiology of Camellia oleifera seedlings. Forests 2023, 14, 1126. [Google Scholar] [CrossRef]
- Alexis, M.A.; Rasse, D.P.; Knicker, H.; Anquetil, C.; Rumpel, C. Evolution of soil organic matter after prescribed fire: A 20-year chronosequence. Geoderma 2012, 189, 98–107. [Google Scholar] [CrossRef]
- Wu, W.J.; Zou, Y.N.; Hashem, A.; Avila-Quezada, G.D.; Abd_Allah, E.F.; Wu, Q.S. Rhizoglomus intraradices is more prominent in improving soil aggregate distribution and stability than in improving plant physiological activities. Agronomy 2023, 13, 1427. [Google Scholar] [CrossRef]
- Wu, W.J.; Zou, Y.N.; Xiao, Z.Y.; Wang, F.L.; Hashem, A.; Abd_Allah, E.F.; Wu, Q.S. Changes in fatty acid profiles in seeds of Camellia oleifera treated by mycorrhizal fungi and glomalin. Horticulturae 2024, 10, 580. [Google Scholar] [CrossRef]
- Liu, R.C.; Xiao, Z.Y.; Hashem, A.; Abd_Allah, E.F.; Wu, Q.S. Mycorrhizal fungal diversity and its relationship with soil properties in Camellia oleifera. Agriculture 2021, 11, 470. [Google Scholar] [CrossRef]
- Cao, M.A.; Liu, R.C.; Xiao, Z.Y.; Hashem, A.; Abd_Allah, E.F.; Alsayed, M.F.; Harsonowati, W.; Wu, Q.S. Symbiotic fungi alter the acquisition of phosphorus in Camellia oleifera through regulating root architecture, plant phosphate transporter gene expressions and soil phosphatase activities. J. Fungi 2022, 8, 800. [Google Scholar] [CrossRef]
- Pedersen, B.P.; Kumar, H.; Waight, A.B.; Risenmay, A.J.; Roe-Zurz, Z.; Chau, B.H.; Schlessinger, A.; Bonomi, M.; Harries, W.; Sali, A.; et al. Crystal structure of a eukaryotic phosphate transporter. Nature 2013, 496, 533–536. [Google Scholar] [CrossRef]
- Moreira, B.C.; Mendes, F.C.; Mendes, I.R.; Paula, T.A.; Junior, P.P.; Salomão, L.C.C.; Stürmer, S.L.; Otoni, W.C.; Guarçoni M., A.; Kasuya, M.C.M. The interaction between arbuscular mycorrhizal fungi and Piriformospora indica improves the growth and nutrient uptake in micropropagation-derived pineapple plantlets. Sci. Hortic. 2015, 197, 183–192. [Google Scholar] [CrossRef]
- Zhang, T.Z.; Liang, K.; Chen, H.; Zhu, Y.Y.; Xu, J. Identification and analysis of AUX/IAA gene family in Camellia oleifera. Mol. Plant Breed. 2024, 22, 2847–2855. [Google Scholar] [CrossRef]
- Xing, K.F.; Yao, X.Z.; Zhou, J.; Xie, H.X.; Chen, R.P.; Zhao, Y.; Rong, J.; Zhang, J. Identification and alaysis of CCCH type zinc finger protein gene family in Camellia oleifera under cold stress. Non-Wood For. Res. 2024, 42, 11–19. [Google Scholar] [CrossRef]
- Du, B.S.; Zou, X.H.; Wang, Z.H.; Zhang, X.Y.; Cao, Y.B.; Zhang, L.Y. Genome-wide identification and expression analysis of the SWEET gene family in Camellia oleifera. Biotechnol. Bull. 2024, 40, 179–190. [Google Scholar] [CrossRef]
- Liu, R.C.; Yang, L.; Zou, Y.N.; Wu, Q.S. Root-associated endophytic fungi modulate endogenous auxin and cytokinin levels to improve plant biomass and root morphology of trifoliate orange. Hortic. Plant J. 2023, 9, 463–472. [Google Scholar] [CrossRef]
- Phillips, J.M.; Hayman, D.S. Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans. Br. Mycol. Soc. 1970, 55, 158–160. [Google Scholar] [CrossRef]
- He, W.X.; Wu, Q.S.; Hashem, A.; Abd_Allah, E.F.; Muthuramalingam, P.; Al-Arjani, A.-B.F.; Zou, Y.N. Effects of symbiotic fungi on sugars and soil fertility and structure-mediated changes in plant growth of Vicia villosa. Agriculture 2022, 12, 1523. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Walkley, A.; Black, I.A. An examination of the Degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Du, B.S.; Cao, Y.B.; Zhou, J.; Chen, Y.Q.; Ye, Z.H.; Huang, Y.M.; Zhao, X.Y.; Zou, X.H.; Zhang, L.Y. Sugar import mediated by sugar transporters and cell wall invertases for seed development in Camellia oleifera. Hortic. Res. 2024, 11, uhae133. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and 2−∆∆Ct method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Shukla, J.; Narayan, S.; Mishra, A.; Shirke, P.A.; Kumar, M. Reduction of arsenic accumulation in rice grain by endophytic fungus Serendipita indica. Rhizosphere 2023, 26, 100680. [Google Scholar] [CrossRef]
- Zhang, K.L.; Zhang, H.X.; Xie, C.; Zhu, Z.; Lin, L.; An, Q.; Li, D. Piriformospora indica colonization enhances remediation of cadmium and chromium co-contaminated soils by king grass through plant growth promotion and rhizosphere microecological regulation. J. Hazard. Mater. 2023, 46, 132728. [Google Scholar] [CrossRef]
- Li, H.; Fu, S.; Zhu, J.W.; Gao, W.; Chen, L.; Li, X.; Liu, Y. Nitric oxide generated by Piriformospora indica-induced nitrate reductase promotes tobacco growth by regulating root architecture and ammonium and nitrate transporter gene expression. J. Plant Interact. 2022, 17, 861–872. [Google Scholar] [CrossRef]
- Xu, T.Y.; Niu, X.; Wang, B.; Qiu, X.H.; Shou, Y.; Luo, J.N.; Guo, Y.J. Variations in leaf functional traits and photosynthetic parameters of Cunninghamia lanceolata provenances. Forests 2023, 14, 1708. [Google Scholar] [CrossRef]
- Abdolmaleki, A.K.; Pirdashti, H.; Yaghoubian, Y.; Abbasian, A.; Ghadirnezhad Shiade, S.R. Endophytic fungi improve growth and yield of wheat (Triticum aestivum L.) under limited light conditions. Gesunde Pflanz. 2023, 75, 1517–1529. [Google Scholar] [CrossRef]
- Göbel, M.; Fichtner, F. Functions of sucrose and trehalose 6-phosphate in controlling plant development. J. Plant Physiol. 2023, 291, 154140. [Google Scholar] [CrossRef]
- Thompson, M.; Gamage, D.; Hirotsu, N.; Martin, A.; Seneweera, S. Effects of elevated carbon dioxide on photosynthesis and carbon partitioning: A perspective on root sugar sensing and hormonal crosstalk. Front. Physiol. 2017, 8, 253220. [Google Scholar] [CrossRef]
- Ye, Z.H.; Du, B.S.; Zhou, J.; Cao, Y.B.; Zhang, L.Y. Camellia oleifera CoSWEET10 is crucial for seed development and drought resistance by mediating sugar transport in transgenic Arabidopsis. Plants 2023, 12, 2818. [Google Scholar] [CrossRef]
- Manck-Götzenberger, J.; Requena, N. Arbuscular mycorrhiza symbiosis induces a major transcriptional reprogramming of the potato SWEET sugar transporter family. Front. Plant Sci. 2016, 7, 191086. [Google Scholar] [CrossRef]
- De Rocchis, V.; Jammer, A.; Camehl, I.; Franken, P.; Roitsch, T. Tomato growth promotion by the fungal endophytes Serendipita indica and Serendipita herbamans is associated with sucrose de-novo synthesis in roots and differential local and systemic effects on carbohydrate metabolisms and gene expression. J. Plant Physiol. 2022, 276, 153755. [Google Scholar] [CrossRef]
- Waqar, S.; Bhat, A.A.; Khan, A.A. Endophytic fungi: Unravelling plant-endophyte interaction and the multifaceted role of fungal endophytes in stress amelioration. Plant Physiol. Biochem. 2023, 206, 108174. [Google Scholar] [CrossRef]
- Li, Q.S.; Srivastava, A.K.; Zou, Y.N.; Wu, Q.S. Field inoculation responses of arbuscular mycorrhizal fungi versus endophytic fungi on sugar metabolism associated changes in fruit quality of Lane late navel orange. Sci. Hortic. 2023, 308, 111587. [Google Scholar] [CrossRef]
- Liu, X.X.; Jiang, X.X.; He, X.Y.; Zhao, W.R.; Cao, Y.Y.; Guo, T.T.; Li, T.; Ni, H.T.; Tang, X.Y. Phosphate-solubilizing Pseudomonas sp. strain P34-L promotes wheat growth by colonizing the wheat rhizosphere and improving the wheat root system and soil phosphorus nutritional status. J. Plant Growth Regul. 2019, 38, 1314–1324. [Google Scholar] [CrossRef]
- Bouzouina, M.; Kouadria, R.; Lotmani, B. Fungal endophytes alleviate salt stress in wheat in terms of growth, ion homeostasis and osmoregulation. J. Appl. Microbiol. 2021, 130, 913–925. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zou, Y.N.; Tian, Z.H.; Wu, Q.S.; Kuča, K. Effects of beneficial endophytic fungal inoculants on plant growth and nutrient absorption of trifoliate orange seedlings. Sci. Hortic. 2021, 277, 109815. [Google Scholar] [CrossRef]
- Bandyopadhyay, P.; Yadav, B.G.; Kumar, S.G.; Kumar, R.; Kogel, K.; Kumar, S. Piriformospora indica and Azotobacter chroococcum consortium facilitates higher acquisition of N, P with improved carbon allocation and enhanced plant growth in Oryza sativa. J. Fungi 2022, 8, 453. [Google Scholar] [CrossRef]
- Zhou, J.Q.; Lu, M.Q.; Zhang, C.H.; Qu, X.J.; Liu, Y.Y.; Yang, J.; Yuan, J. Isolation and functional characterisation of the PHT1 gene encoding a high-affinity phosphate transporter in Camellia oleifera. J. Hortic. Sci. Biotechnol. 2020, 95, 553–564. [Google Scholar] [CrossRef]
- Solanki, S.; Gupta, S.; Kapoor, R.; Varma, A. Chemically synthesized AgNPs and Piriformospora indica synergistically augment nutritional quality in black rice. J. Fungi 2023, 9, 611. [Google Scholar] [CrossRef]
- Vadassery, J.; Ranf, S.; Drzewiecki, C.; Mithöfer, A.; Mazars, C.; Scheel, D.; Lee, J.; Oelmüller, R. A cell wall extract from the endophytic fungus Piriformospora indica promotes growth of Arabidopsis seedlings and induces intracellular calcium elevation in roots. Plant J. 2009, 59, 193–206. [Google Scholar] [CrossRef]
- Catalá, R.; Santos, E.; Alonso, J.M.; Ecker, J.R.; Martínez-Zapater, J.M.; Salinas, J. Mutations in the Ca2+/H+ transporter CAX1 increase CBF/DREB1 expression and the cold-acclimation response in Arabidopsis. Plant Cell 2003, 15, 2940–2951. [Google Scholar] [CrossRef]
- Pittman, J.K.; Hirschi, K.D. CAX-ing a wide net: Cation/H+ transporters in metal remediation and abiotic stress signalling. Plant Biol. 2016, 18, 741–749. [Google Scholar] [CrossRef]
- Wang, Y.; Kang, Y.; Ma, C.L.; Miao, R.Y.; Wu, C.L.; Long, Y.; Ge, T.; Wu, Z.N.; Hou, X.Y.; Zhang, J.X.; et al. CNGC2 is a Ca2+ influx channel that prevents accumulation of apoplastic Ca2+ in the leaf. Plant Physiol. 2017, 173, 1342–1354. [Google Scholar] [CrossRef]
- Paola, A.; Pierre, B.; Vincenza, C.; Bruce, V. Short term clay mineral release and re-capture of potassium in a Zea mays field experiment. Geoderma 2016, 264, 54–60. [Google Scholar] [CrossRef]
- Gao, R.; Jia, Y.T.; Xu, X.; Fu, P.; Zhou, J.Q.; Yang, G.H. Structural insights into the Oryza sativa cation transporters HKTs in salt tolerance. J. Integr. Plant Biol. 2024, 66, 700–708. [Google Scholar] [CrossRef] [PubMed]
- Boorboori, M.R.; Zhang, H.Y. The role of Serendipita indica (Piriformospora indica) in improving plant resistance to drought and salinity stresses. Biology 2022, 11, 952. [Google Scholar] [CrossRef]
- Zhou, L.J.; Wang, Y.; Alqahtani, M.D.; Wu, Q.S. Positive changes in fruit quality, leaf antioxidant defense system, and soil fertility of Beni-Madonna Tangor citrus (Citrus nanko × C. amakusa) after field AMF inoculation. Horticulturae 2023, 9, 1324. [Google Scholar] [CrossRef]
- Cheng, X.F.; Xie, M.M.; Li, Y.; Liu, B.Y.; Liu, C.Y.; Wu, Q.S.; Kuča, K. Effects of field inoculation with arbuscular mycorrhizal fungi and endophytic fungi on fruit quality and soil properties of Newhall navel orange. Appl. Soil Ecol. 2022, 17, 104308. [Google Scholar] [CrossRef]


| Genes | Forward Sequence (5′ → 3′) | Reverse Sequence (5′ → 3′) |
|---|---|---|
| CoPHT1;1 | GTTCTTGGCGGAGTCAATTTC | CATCCTCATCTTCCTCGTTCTC |
| CoPHT1;2 | TCCCTTTGCTTCTTCCGATTT | TCCCTTTGCTTCTTCCGATTT |
| CoPHT1;3 | GAGTCAGAGCAGCAGAAAGTAG | TGTAGTCCCAAGCAAGTGAAG |
| CoPHT1;4 | CCGTTACACCGCCCTTATC | CTGGGTTCTTCAGCCATCTT |
| CoPHO1-1 | AGCAGCCCTTGAAGTCATTAG | GAACTTGCCCGCATTGTTTAG |
| CoPHO1-3 | GAGCTTTCAGTGGCCTAACA | GTCGCCTCACCGAGTTTATC |
| CoCAX1;1 | ATCAGCGATGGCTCACCTGTTATC | GGAGGAAGTGGGAGAGAGGGAAAT |
| CoCAX1;2 | CCGCCGCCAACATCTCTTCT | CCGTAATTGGAAGTGAGGGAGCAA |
| CoHKT1;1 | TGGGTTACTTGGCTTTGAAGGTTT | GCAGACACAGAGGTGAAGAACAA |
| EF-1α | AGACTGTGGCTGTTGGTGTT | ATCCAAACCCGCACAGTTCA |
| CoSWEET1a | GGTGAAGCCAAGAAACCTCCT | CTACTTGCTCGATCGCTTCTCT |
| CoSWEET1b | TTGGAGGAAGAGAAGCTATCTG | ATGATTTCTTGACATGCGATTGAG |
| CoSWEET2a | GCAGAGAACGCGAAAAAGGT | ATATCCAACGAAGATCTGTCGATT |
| CoSWEET2b | ATGGTTTCTTTGGCTTTGTCCC | AGATATGAGTGGCGTGCCGT |
| CoSWEET7 | CCAAGCTCCGGTCACTCATT | ATTAGCACAGGAAGCCAGGG |
| CoSWEET9a | AGGTTCTACCAGAAGTCAAGCTG | TGGATTCGCTCGGTTTGATA |
| CoSWEET9b | AAGAGCGTGGAGTACATGCC | GCAAATCCCGAGAGACGACA |
| CoSWEET10 | TGGAGGAAGAGAAGCTATCTGAAT | TGAGGCATGACTGGAATGATTTCT |
| CoSWEET17a | ATGTTGGGTTTCTTGGGGCA | GACAGCTAGAGGTGCTGCAT |
| CoSWEET17b | GCAAGGCCTCGATGACCAC | TTTGTCGATGCTGTATTGCCG |
| CoGAPDH | GGTGCCAAGAAGGTGGTAATA | GTTGTGCAGCTTGCATTAGAG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, W.-L.; Wu, W.-J.; Xiao, Z.-Y.; Wang, F.-L.; Cheng, J.-Y.; Zou, Y.-N.; Hashem, A.; Abd_Allah, E.F.; Wu, Q.-S. Serendipita indica: A Promising Biostimulant for Improving Growth, Nutrient Uptake, and Sugar Accumulation in Camellia oleifera. Horticulturae 2024, 10, 936. https://doi.org/10.3390/horticulturae10090936
Fu W-L, Wu W-J, Xiao Z-Y, Wang F-L, Cheng J-Y, Zou Y-N, Hashem A, Abd_Allah EF, Wu Q-S. Serendipita indica: A Promising Biostimulant for Improving Growth, Nutrient Uptake, and Sugar Accumulation in Camellia oleifera. Horticulturae. 2024; 10(9):936. https://doi.org/10.3390/horticulturae10090936
Chicago/Turabian StyleFu, Wan-Lin, Wei-Jia Wu, Zhi-Yan Xiao, Fang-Ling Wang, Jun-Yong Cheng, Ying-Ning Zou, Abeer Hashem, Elsayed Fathi Abd_Allah, and Qiang-Sheng Wu. 2024. "Serendipita indica: A Promising Biostimulant for Improving Growth, Nutrient Uptake, and Sugar Accumulation in Camellia oleifera" Horticulturae 10, no. 9: 936. https://doi.org/10.3390/horticulturae10090936
APA StyleFu, W.-L., Wu, W.-J., Xiao, Z.-Y., Wang, F.-L., Cheng, J.-Y., Zou, Y.-N., Hashem, A., Abd_Allah, E. F., & Wu, Q.-S. (2024). Serendipita indica: A Promising Biostimulant for Improving Growth, Nutrient Uptake, and Sugar Accumulation in Camellia oleifera. Horticulturae, 10(9), 936. https://doi.org/10.3390/horticulturae10090936








