Photomorphogenesis and Photosynthetic Trait Changes in Melon Seedlings Responding to Red and Blue Light
Abstract
:1. Introduction
2. Materials and Methods
2.1. Test Materials
2.2. Experimental Design
2.3. Measurement Items and Methods
2.3.1. Morphological Indicators
2.3.2. Stomatal Characteristics
2.3.3. Determination of Leaf Structure
2.3.4. Measurement of Photosynthetic Properties
2.3.5. Measurement of Rubisco Enzyme Activity
2.3.6. Determination of Chlorophyll Fluorescence
2.3.7. Determination of Carbohydrates and Soluble Protein
2.3.8. Real-Time Quantitative qRT-PCR Assay
3. Statistical Analysis
4. Results
4.1. Effect on Morphological Indexes of Melon Seedlings
4.2. Effects on Stomatal Characteristics of Melon Seedlings
4.3. Effect on Leaf Structure of Melon Seedlings
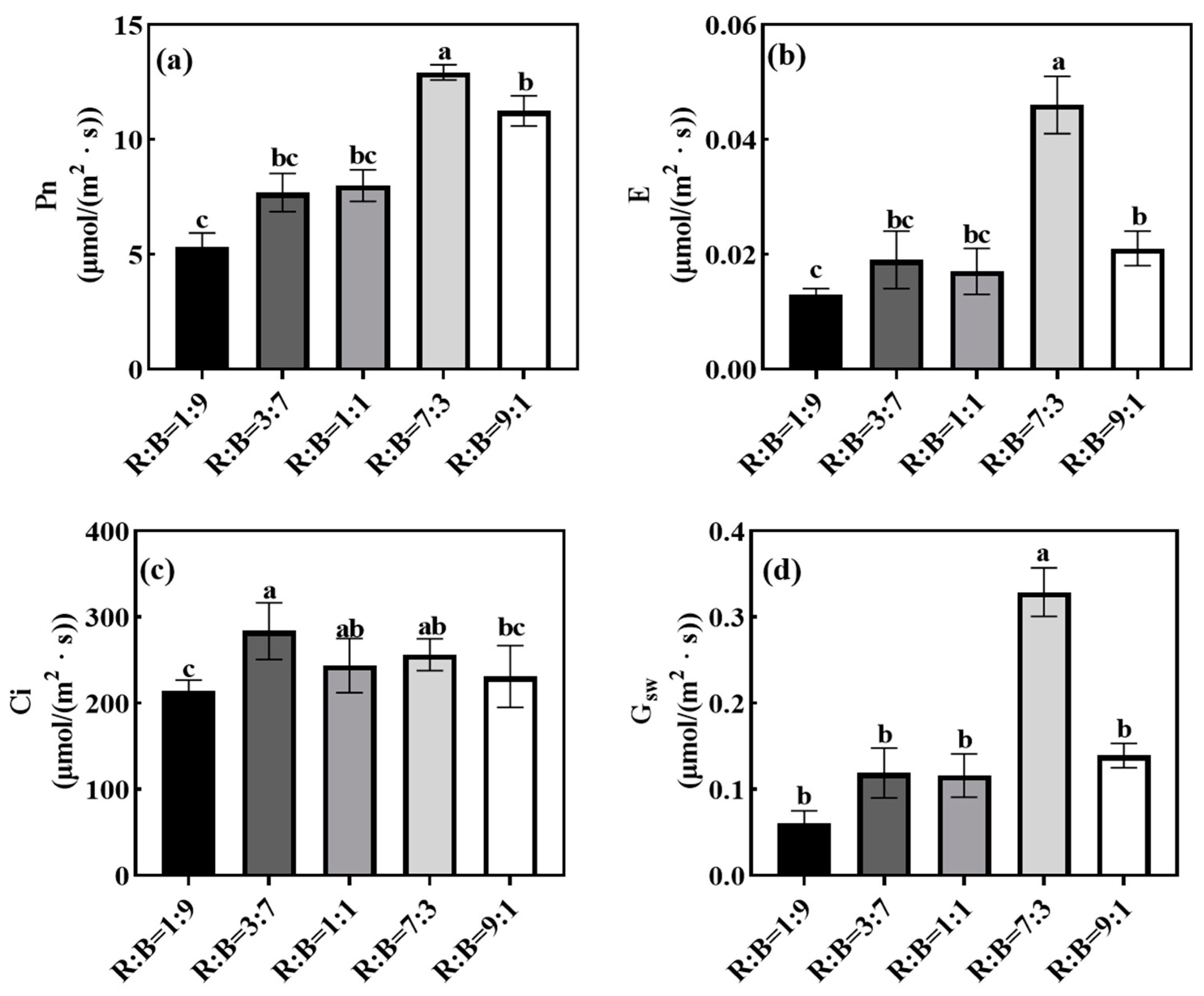
4.4. Effect on Photosynthetic Characteristics of Melon Seedlings
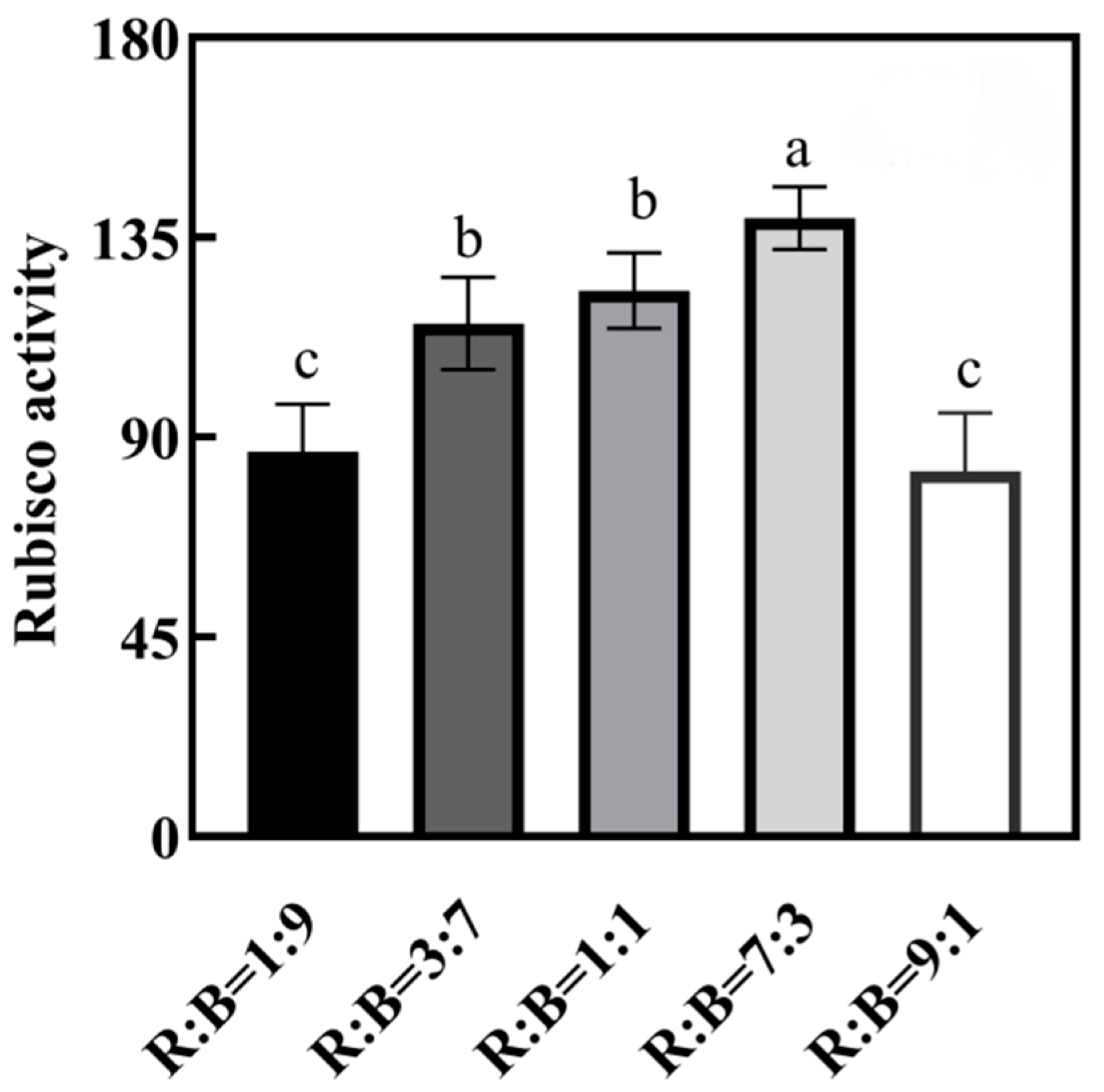
4.5. Effect on Rubisco Enzyme Activity in Melon Seedlings
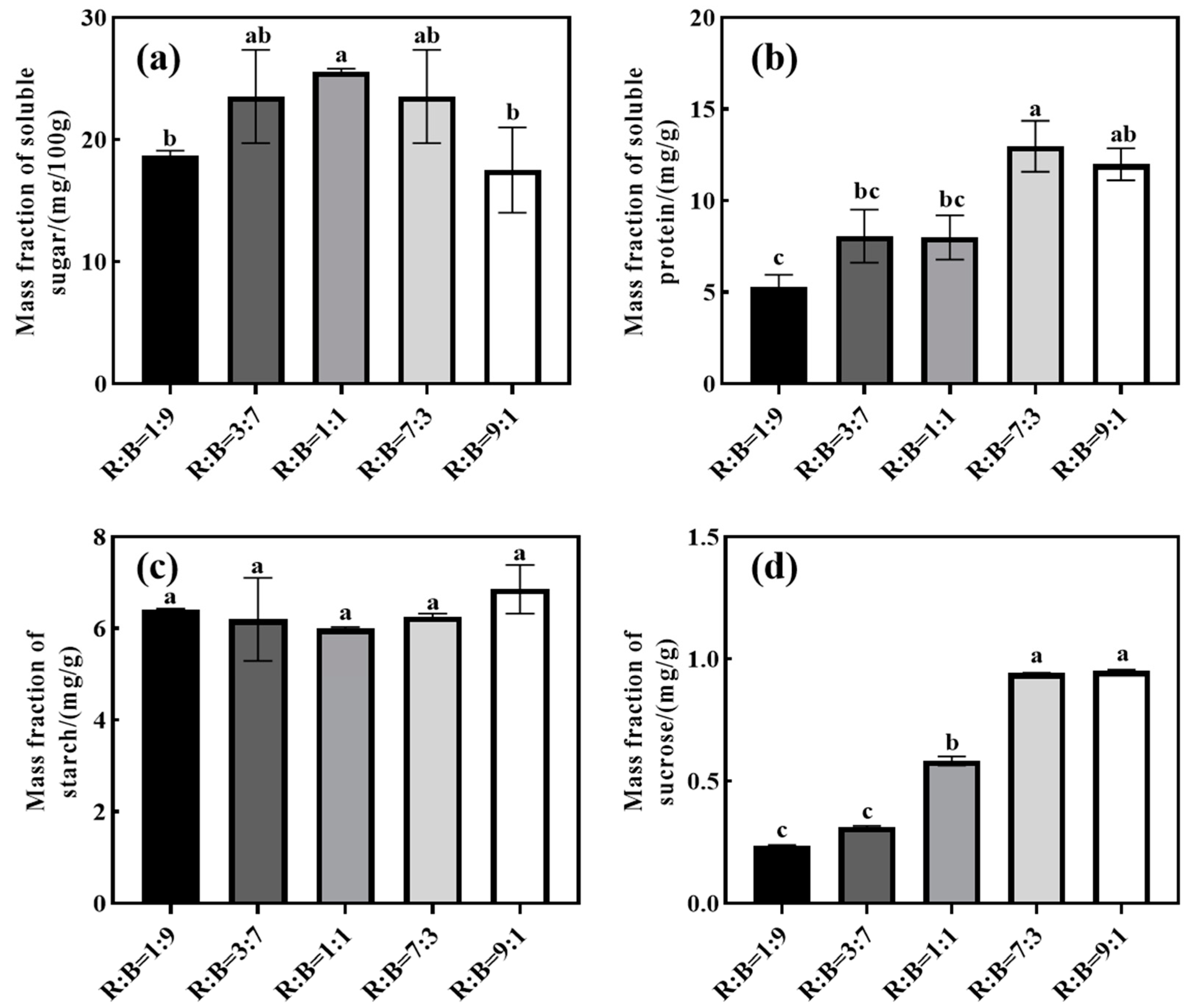
4.6. Effect on Carbohydrates and Soluble Protein of Melon Seedlings
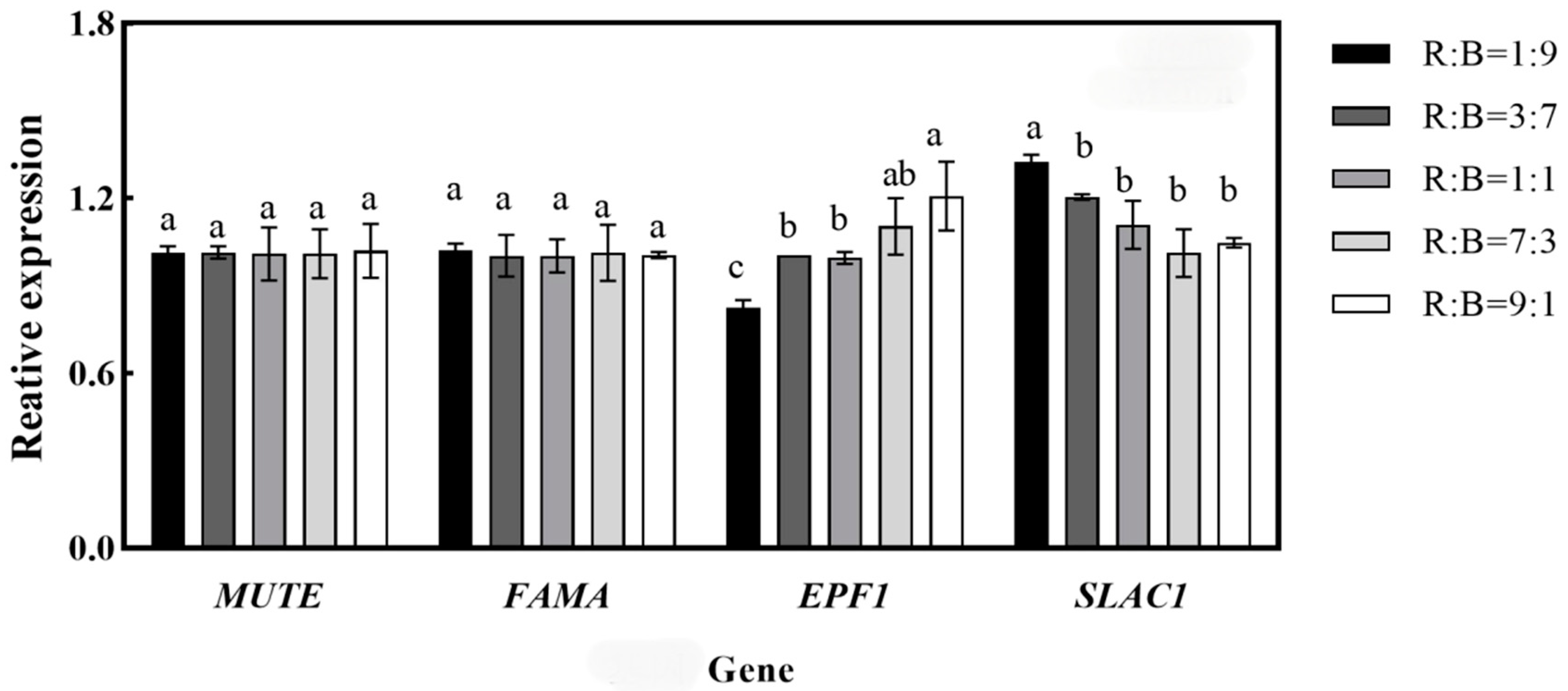
4.7. Effects on the Relative Expression of Key Genes for Stomatal Development in Melon Seedlings
5. Discussion
5.1. Effect on Morphological Indexes of Melon Seedlings
5.2. Effect on Cell Morphology of Melon Seedlings
5.3. Effect on Photosynthesis Related Parameters and Chlorophyll Fluorescence Parameters of Melon Seedlings
5.4. Effects on Carbon Assimilation Capacity and Carbohydrates of Cucumber and Melon Seedlings
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mei, Z.; Li, Z.; Lu, X.; Zhang, S.; Liu, W.; Zou, Q.; Yu, L.; Fang, H.; Zhang, Z.; Mao, Z.; et al. Supplementation of natural light duration promotes accumulation of sugar and anthocyanins in apple (Malus domestica Borkh.) fruit. Environ. Exp. Bot. 2023, 205, 105133. [Google Scholar] [CrossRef]
- Urbonavičiūtė, A.; Pinho, P.; Samuolienė, G.; Duchovskis, P.; Vitta, P.; Stonkus, A.; Tamulaitis, G.; Žukauskas, A.; Halonen, L. Effect of short-wavelength light on lettuce growth and nutritional quality. Sodininkystė Daržininkystė 2007, 26, 157–165. [Google Scholar]
- Lawson, T.; Oxborough, K.; Morison, J.I.; Baker, N.R. The responses of guard and mesophyll cell photosynthesis to CO2, O2, light, and water stress in a range of species are similar. J. Exp. Bot. 2003, 54, 1743–1752. [Google Scholar] [CrossRef]
- Kang, C.; Zhang, Y.; Cheng, R.; Kaiser, E.; Yang, Q.; Li, T. Acclimating cucumber plants to blue supplemental light promotes growth in full sunlight. Front. Plant Sci. 2021, 12, 782465. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, K.; Yanagi, T.; Takita, S.; Tanaka, M.; Higuchi, T.; Ushida, Y.; Watanabe, H. Development of plant growth apparatus using blue and red LED as artificial light source. In International Symposium on Plant Production in Closed Ecosystems; International Society for Horticultural Science: Leuven, Belgium, 1996; Volume 1. [Google Scholar]
- Hwang, H.; An, S.; Lee, B.; Chun, C. Improvement of growth and morphology of vegetable seedlings with supplemental far-red enriched led lights in a plant factory. Horticulturae 2020, 6, 109. [Google Scholar] [CrossRef]
- Hogewoning, S.W.; Trouwborst, G.; Maljaars, H.; Poorter, H.; Ieperen, W.V.; Harbinson, J. Blue light dose–responses of leaf photosynthesis, morphology, and chemical composition of Cucumis sativus grown under different combinations of red and blue light. J. Exp. Bot. 2010, 61, 3107–3117. [Google Scholar] [CrossRef]
- Wang, X.Y.; Xu, X.M.; Cui, J. The importance of blue light for leaf area expansion, development of photosynthetic apparatus, and chloroplast ultrastructure of Cucumis sativus grown under weak light. Photosynthetica 2015, 53, 213–222. [Google Scholar] [CrossRef]
- Trouwborst, G.; Hogewoning, S.W.; van Kooten, O.; Harbinson, J.; van Ieperen, W. Plasticity of photosynthesis after the ‘red light syndrome’ in cucumber. Environ. Exp. Bot. 2016, 121, 75–82. [Google Scholar] [CrossRef]
- Miao, Y.; Wang, X.; Gao, L.; Chen, Q.; Qu, M. Blue light is more essential than red light for maintaining the activities of photosystem II and I and photosynthetic electron transport capacity in cucumber leaves. J. Integr. Agric. 2016, 15, 87–100. [Google Scholar] [CrossRef]
- Miao, Y.; Chen, Q.; Qu, M.; Gao, L.; Hou, L. Blue light alleviates ‘red light syndrome’ by regulating chloroplast ultrastructure, photosynthetic traits and nutrient accumulation in cucumber plants. Sci. Hortic. 2019, 257, 108680. [Google Scholar] [CrossRef]
- Shimazaki, K.; Doi, M.; Assmann, S.M.; Kinoshita, T. Light regulation of stomatal movement. Annu. Rev. Plant Biol. 2007, 58, 219–247. [Google Scholar] [CrossRef]
- Li, X.; Zhao, S.; Lin, A.; Yang, Y.; Zhang, G.; Xu, P.; Wu, Y.; Yang, Z. Effect of different ratios of red and blue light on maximum stomatal conductance and response rate of cucumber seedling leaves. Agronomy 2023, 13, 1941. [Google Scholar] [CrossRef]
- Ren, M.; Liu, S.; Tang, C.; Mao, G.; Gai, P.; Guo, X.; Zheng, H.; Tang, Q. Photomorphogenesis and photosynthetic traits changes in rice seedlings responding to red and blue light. Int. J. Mol. Sci. 2023, 24, 11333. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.P.D.; Jang, D.C.; Tran, T.T.H.; Nguyen, Q.T.; Kim, I.S.; Hoang, T.L.H.; Vu, N.T. Influence of green light added with red and blue LEDs on the growth, leaf microstructure and quality of spinach (Spinacia oleracea L.). Agronomy 2021, 11, 1724. [Google Scholar] [CrossRef]
- Wang, T.; Sun, Q.; Zheng, Y.; Xu, Y.; Liu, B.; Li, Q. Effects of Red and Blue Light on the Growth, Photosynthesis, and Subsequent Growth under Fluctuating Light of Cucumber Seedlings. Plants 2024, 13, 1668. [Google Scholar] [CrossRef]
- Bourget, C.M. An introduction to light-emitting diodes. HortScience 2008, 43, 1944–1946. [Google Scholar] [CrossRef]
- Massa, G.D.; Kim, H.H.; Wheeler, R.M.; Mitchell, C.A. Plant productivity in response to LED lighting. HortScience 2008, 43, 1951–1956. [Google Scholar] [CrossRef]
- Morrow, R.C. LED lighting in horticulture. HortScience 2008, 43, 1947–1950. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef]
- Paponov, M.; Kechasov, D.; Lacek, J.; Verheul, M.J.; Paponov, I.A. Supplemental light-emitting diode inter-lighting increases tomato fruit growth through enhanced photosynthetic light use efficiency and modulated root activity. Front. Plant Sci. 2020, 10, 1656. [Google Scholar] [CrossRef]
- Zhang, Z.L.; Qu, W.J. Experimental Instruction of Plant Physiology; Higher Education Press: Beijing, China, 2003. (In Chinese) [Google Scholar]
- Darko, E.; Hamow, K.A.; Marček, T.; Dernovics, M.; Ahres, M.; Galiba, G. Modulated light dependence of growth, flowering, and the accumulation of secondary metabolites in chilli. Front. Plant Sci. 2022, 13, 801656. [Google Scholar] [CrossRef] [PubMed]
- Utasi, L.; Kovács, V.; Gulyás, Z.; Marcek, T.; Janda, T.; Darko, E. Threshold or not: Spectral composition and light-intensity dependence of growth and metabolism in tomato seedlings. Sci. Hortic. 2023, 313, 111946. [Google Scholar] [CrossRef]
- Kaiser, E.; Ouzounis, T.; Giday, H.; Schipper, R.; Heuvelink, E.; Marcelis, L.F.M. Adding blue to red supplemental light increases biomass and yield of greenhouse-grown tomatoes, but only to an optimum. Front. Plant Sci. 2019, 9, 2002. [Google Scholar] [CrossRef] [PubMed]
- Song, J.W.; Bhandari, S.R.; Shin, Y.K.; Lee, J.G. The influence of red and blue light ratios on growth performance, secondary metabolites, and antioxidant activities of Centella asiatica (L.) Urban. Horticulturae 2022, 8, 601. [Google Scholar] [CrossRef]
- Johkan, M.; Shoji, K.; Goto, F.; Hashida, S.; Yoshihara, T. Blue light-emitting diode light irradiation of seedlings improves seedling quality and growth after transplanting in red leaf lettuce. HortScience 2010, 45, 1809–1814. [Google Scholar] [CrossRef]
- Gonçalves, B.; Correia, C.M.; Silva, A.P.; Bacelar, E.A.; Santos, A.; Moutinho-Pereira, J.M. Leaf structure and function of sweet cherry tree (Prunus avium L.) cultivars with open and dense canopies. Sci. Hortic. 2008, 116, 381–387. [Google Scholar] [CrossRef]
- Tholen, D.; Boom, C.; Zhu, X.G. Opinion: Prospects for improving photosynthesis by altering leaf anatomy. Plant Sci. 2012, 197, 92–101. [Google Scholar] [CrossRef]
- Macedo, A.F.; Leal-Costa, M.V.; Tavares, E.S.; Lage, C.L.S.; Esquibel, M.A. The effect of light quality on leaf production and development of in vitro-cultured plants of Alternanthera brasiliana Kuntze. Environ. Exp. Bot. 2011, 70, 43–50. [Google Scholar] [CrossRef]
- Chang, S.; Li, C.; Yao, X.; Song, C.; Jiao, X.; Liu, X.; Xu, Z.; Guan, R. Morphological, photosynthetic, and physiological responses of rapeseed leaf to different combinations of red and blue lights at the rosette stage. Front. Plant Sci. 2016, 7, 1144. [Google Scholar]
- Gautier, H.; Varlet-Grancher, C.; Baudry, N. Effects of blue light on the vertical colonization of space by white clover and their consequences for dry matter distribution. Ann. Bot. 1997, 80, 665–671. [Google Scholar] [CrossRef]
- Ni, M.; Tepperman, J.M.; Quail, P.H. PIF3, a phytochrome-interacting factor necessary for normal photoinduced signal transduction, is a novel basic helix-loop-helix protein. Cell 1998, 95, 657–667. [Google Scholar] [CrossRef]
- Monte, E.; Tepperman, J.M.; Al-Sady, B.; Kaczorowski, K.A.; Alonso, J.M.; Ecker, J.R.; Li, X.; Zhang, Y.; Quail, P.H. The phytochrome-interacting transcription factor, PIF3, acts early, selectively, and positively in light-induced chloroplast development. Proc. Natl. Acad. Sci. USA 2004, 101, 16091–16098. [Google Scholar] [CrossRef] [PubMed]
- Hosotani, S.; Yamauchi, S.; Kobayashi, H.; Fuji, S.; Koya, S.; Shimazaki, K.I.; Takemiya, A. A BLUS1 kinase signal and a decrease in intercellular CO2 concentration are necessary for stomatal opening in response to blue light. Plant Cell 2021, 33, 1813–1827. [Google Scholar] [CrossRef] [PubMed]
- Barillot, R.; Frak, E.; Combes, D.; Durand, J.L.; Escobar-Gutiérrez, A.J. What determines the complex kinetics of stomatal conductance under blueless PAR in Festuca arundinacea? Subsequent effects on leaf transpiration. J. Exp. Bot. 2010, 61, 2795–2806. [Google Scholar] [CrossRef]
- Hara, K.; Kajita, R.; Torii, K.U.; Bergmann, D.C.; Kakimoto, T. The secretory peptide gene EPF1 enforces the stomatal one-cell-spacing rule. Genes Dev. 2007, 21, 1720–1725. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Lu, W.; Tong, Y.; Yang, Q. Leaf morphology, photosynthetic performance, chlorophyll fluorescence, stomatal development of lettuce (Lactuca sativa L.) exposed to different ratios of red light to blue light. Front. Plant Sci. 2016, 7, 250. [Google Scholar] [CrossRef]
- Inada, K. Action spectra for photosynthesis in higher plants. Plant Cell Physiol. 1976, 17, 355–365. [Google Scholar]
- Jin, D.; Su, X.; Li, Y.; Shi, M.; Yang, B.; Wan, W.; Wen, X.; Yang, S.; Ding, X.; Zou, J. Effect of red and blue light on cucumber seedlings grown in a plant factory. Horticulturae 2023, 9, 124. [Google Scholar] [CrossRef]
- Izzo, L.G.; Mele, B.H.; Vitale, L.; Vitale, E.; Arena, C. The role of monochromatic red and blue light in tomato early photomorphogenesis and photosynthetic traits. Environ. Exp. Bot. 2020, 179, 104195. [Google Scholar] [CrossRef]
- Sawbridge, T.I.; López-Juez, E.; Knight, M.R.; Jenkins, G.I. A blue-light photoreceptor mediates the fluence rate-dependant control of rbcS gene expression in light grown Phaseolus vulgaris primary leaves. Planta 1994, 192, 1–8. [Google Scholar]
- Dong, F.; Wang, C.; Sun, X.; Bao, Z.; Dong, C.; Sun, C.; Ren, Y.; Liu, S. Sugar metabolic changes in protein expression associated with different light quality combinations in tomato fruit. Plant Growth Regul. 2019, 88, 267–282. [Google Scholar] [CrossRef]
- Lin, K.H.; Huang, M.Y.; Huang, W.D.; Hsu, M.H.; Yang, Z.W.; Yang, C.M. The effects of red, blue, and white light-emitting diodes on the growth, development, and edible quality of hydroponically grown lettuce (Lactuca sativa L. var. capitata). Sci. Hortic. 2013, 150, 86–91. [Google Scholar] [CrossRef]
- Di, Q.; Li, J.; Du, Y.; Wei, M.; Shi, Q.; Li, Y.; Yang, F. Combination of red and blue lights improved the growth and development of eggplant (Solanum melongena L.) seedlings by regulating photosynthesis. J. Plant Growth Regul. 2021, 40, 1477–1492. [Google Scholar] [CrossRef]
- Li, H.; Tang, C.; Xu, Z.; Liu, X.; Han, X. Effects of different light sources on the growth of non-heading Chinese cabbage (Brassica campestris L.). J. Agric. Sci. 2012, 4, 262. [Google Scholar] [CrossRef]
| Treatment | Red Light | Blue Light | PAR |
|---|---|---|---|
| R:B = 1:9 | 20 | 180 | 200 |
| R:B = 3:7 | 60 | 140 | 200 |
| R:B = 1:1 | 100 | 100 | 200 |
| R:B = 7:3 | 140 | 60 | 200 |
| R:B = 9:1 | 180 | 20 | 200 |
| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| Action | GTGATGGTGTGAGTCACACTGTTC | ACGACCAGCAAGGTCCAAAC |
| EPF1 | GGAGTTATACCCTACAGGATCCAGC | CACATGCATCTGTAGACGGTTGGG |
| MUTE | GCTTTGGGGGTCCGATAAAAG | GAACCTCAAAGGACAGCCTCTC |
| SLAC1 | CAGTTGAGAAGCGGTCGAAG | CCTCCAAAACTTCTTCCACTCC |
| FAMA | CAGCTGAAAGCAAGTCGTG | GCAATGGCTTTGATGAGCTG |
| Treatment | Plant Height /μm | Stem Diameter /mm | Fresh Weight /g | Dry Weight /g | Total Leaf Area /cm2 |
|---|---|---|---|---|---|
| R:B = 1:9 | 5.187 | 2.980 | 5.800 | 0.445 | 138.497 |
| ±0.445 b | ±0.178 b | ±1.572 ab | ±0.120 b | ±19.258 b | |
| R:B = 3:7 | 5.880 | 3.427 | 5.047 | 0.425 | 128.605 |
| ±0.274 b | ±0.361 ab | ±1.489 b | ±0.156 b | ±21.588 b | |
| R:B = 1:1 | 5.997 | 3.48 | 8.285 | 0.669 | 149.691 |
| ±0.253 b | ±0.203 ab | ±1.377 a | ±0.196 a | ±13.398 b | |
| R:B = 7:3 | 9.707 | 4.103 | 8.429 | 0.767 | 178.770 |
| ±1.448 a | ±0.455 a | ±1.572 a | ±0.195 a | ±16.711 a | |
| R:B = 9:1 | 8.803 | 4.247 | 7.845 | 0.514 | 177.044 |
| ±1.989 a | ±0.989 a | ±1.783 ab | ±0.123 b | ±9.041 a |
| Treatment | Pore Width /μm | Pore Length /μm | Stomatal Width /μm | Stomatal Length /μm | Stomatal Density /(N/mm2) | Pore Area /μm2 |
|---|---|---|---|---|---|---|
| R:B = 1:9 | 9.171 | 11.171 | 22.623 | 26.714 | 947.164 | 25.978 |
| ±2.411 b | ±1.720 b | ±3.441 ab | ±2.601 a | ±120.022 a | ±5.631 b | |
| R:B = 3:7 | 9.479 | 10.845 | 22.782 | 26.289 | 819.576 | 25.664 |
| ±1.882 b | ±1.522 b | ±3.045 a | ±2.219 a | ±102.09 b | ±6.820 b | |
| R:B = 1:1 | 9.942 | 10.583 | 22.118 | 25.814 | 684.423 | 25.603 |
| ±1.233 b | ±1.587 b | ±3.174 b | ±2.724 a | ±80.561 c | ±5.543 b | |
| R:B = 7:3 | 10.958 | 10.745 | 22.447 | 26.480 | 667.107 | 29.662 |
| ±2.313 a | ±1.652 b | ±3.305 ab | ±2.076 a | ±120.587 c | ±8.543 a | |
| R:B = 9:1 | 10.560 | 14.923 | 22.97 | 26.505 | 536.920 | 29.191 |
| ± 2.797 a | ±1.644 a | ±3.289 ab | ±2.645 a | ±67.122 d | ±7.474 a |
| Treatment | Upper Epidermis | Palisade Tissue | Spongy Tissue | Inferior Epidermis | Leaf Thickness |
|---|---|---|---|---|---|
| R:B = 1:9 | 17.513 | 57.602 | 94.358 | 12.634 | 178.441 |
| ±4.311 a | ±3.885 a | ±11.416 a | ±3.468 a | ±13.606 a | |
| R:B = 3:7 | 15.710 | 58.446 | 94.963 | 12.121 | 177.210 |
| ±2.667 ab | ±5.919 a | ±7.741 a | ±1.376 a | ± 9.732 a | |
| R:B = 1:1 | 14.866 | 55.33 | 85.898 | 10.373 | 166.588 |
| ±3.048 ab | ±6.347 a | ±2.781 ab | ±2.465 a | ±4.447 a | |
| R:B = 7:3 | 13.569 | 48.752 | 76.696 | 12.510 | 155.090 |
| ±3.113 b | ±4.143 b | ±11.574 b | ±2.484 a | ±6.910 b | |
| R:B = 9:1 | 13.501 | 43.092 | 78.038 | 10.534 | 156.048 |
| ±3.025 b | ±12.559 b | ±14.062 b | ±2.555 a | ±6.268 b |
| Treatment | Fv/Fm (The Maximum Quantum Yield of PSII) | ΦPSII (PSII Actual Photochemical Quantum Yield) | ETR (PSII Photosynthetic Electron Transfer Rate) | ΦNO (PSII Quantum Yield of Unregulated Energy Dissipation) | ΦNPQ (Quantum Yield of PSII Regulatory Energy Dissipation) | NPQ (Non-Photochemical Quenching) |
|---|---|---|---|---|---|---|
| R:B = 1:9 | 0.862 | 0.557 | 216.567 | 0.132 | 0.311 | 2.357 |
| ±0.014 a | ±0.026 a | ±10.335 a | ±0.005 b | ±0.031 ab | ±0.319 a | |
| R:B = 3:7 | 0.836 | 0.534 | 207.767 | 0.204 | 0.261 | 1.254 |
| ±0.008 a | ±0.183 ab | ±71.163 ab | ±0.074 ab | ±0.110 b | ±0.118 b | |
| R:B = 1:1 | 0.861 | 0.505 | 196.500 | 0.198 | 0.304 | 1.583 |
| ±0.037 a | ±0.124 ab | ±48.417 ab | ±0.041 ab | ±0.086 ab | ±0.18 b | |
| R:B = 7:3 | 0.852 | 0.372 | 144.633 | 0.244 | 0.385 | 1.624 |
| ±0.049 a | ±0.086 ab | ±33.276 ab | ±0.049 a | ±0.066 ab | ±0.418 b | |
| R:B = 9:1 | 0.849 | 0.318 | 123.867 | 0.238 | 0.443 | 1.898 |
| ±0.027 a | ±0.102 b | ±39.691 b | ±0.04 a | ±0.094 a | ±0.543 ab |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, S.; Li, X.; Kang, Y.; Lin, Y.; Wu, Y.; Yang, Z. Photomorphogenesis and Photosynthetic Trait Changes in Melon Seedlings Responding to Red and Blue Light. Horticulturae 2024, 10, 961. https://doi.org/10.3390/horticulturae10090961
Zhao S, Li X, Kang Y, Lin Y, Wu Y, Yang Z. Photomorphogenesis and Photosynthetic Trait Changes in Melon Seedlings Responding to Red and Blue Light. Horticulturae. 2024; 10(9):961. https://doi.org/10.3390/horticulturae10090961
Chicago/Turabian StyleZhao, Shiwen, Xue Li, Yushi Kang, Yuqin Lin, Yongjun Wu, and Zhenchao Yang. 2024. "Photomorphogenesis and Photosynthetic Trait Changes in Melon Seedlings Responding to Red and Blue Light" Horticulturae 10, no. 9: 961. https://doi.org/10.3390/horticulturae10090961





