Extracellular Fragmented Self-DNA Displays Biostimulation of Lettuce in Soilless Culture
Abstract
:1. Introduction
2. Materials and Methods
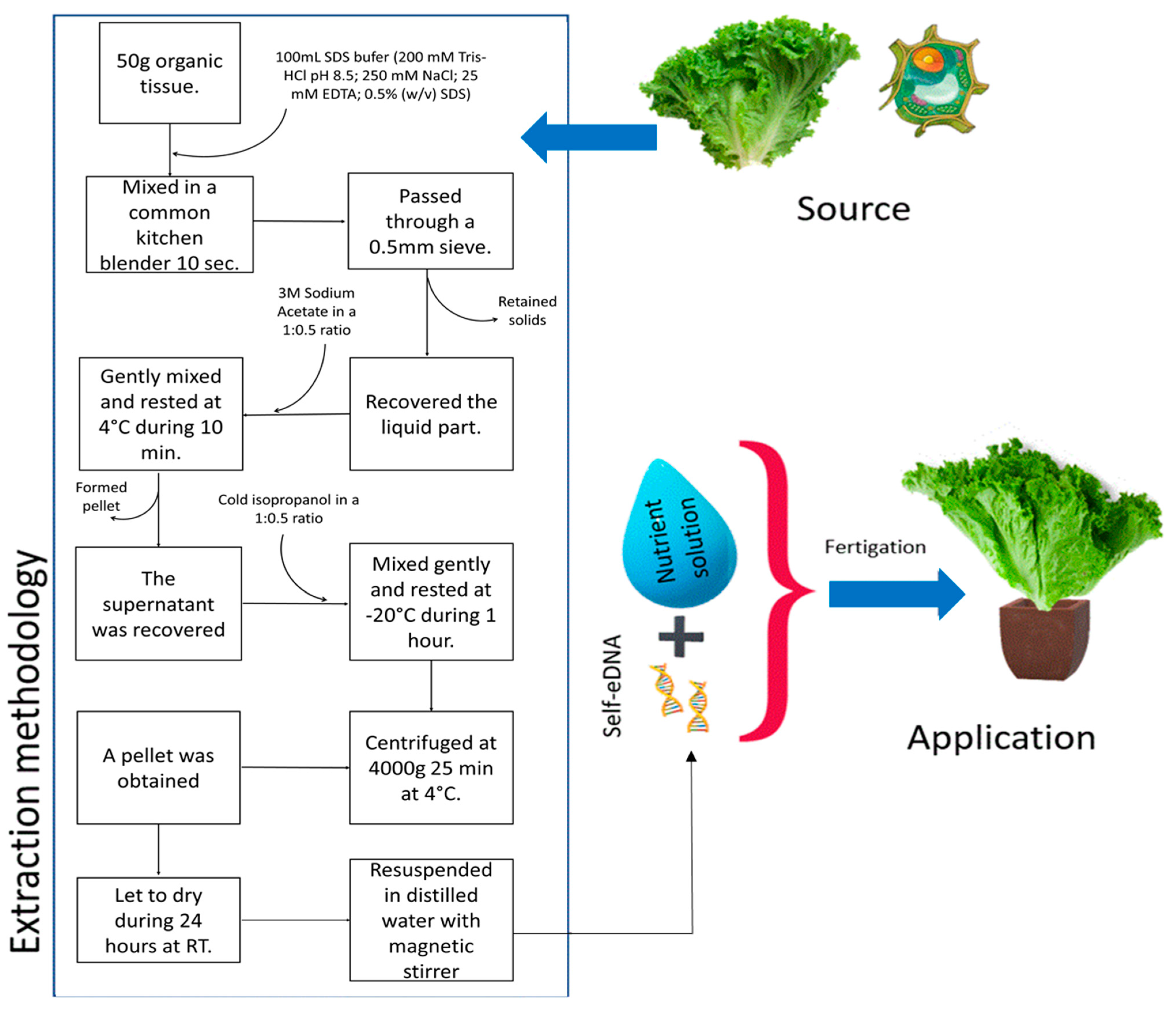
2.1. Biological Material and DNA Extraction
2.2. Treatment and Plant-Growing Conditions
2.3. Fertigation Parameters
2.4. Growth Parameters
2.5. Statistical Analysis and Experimental Design
3. Results and Discussion
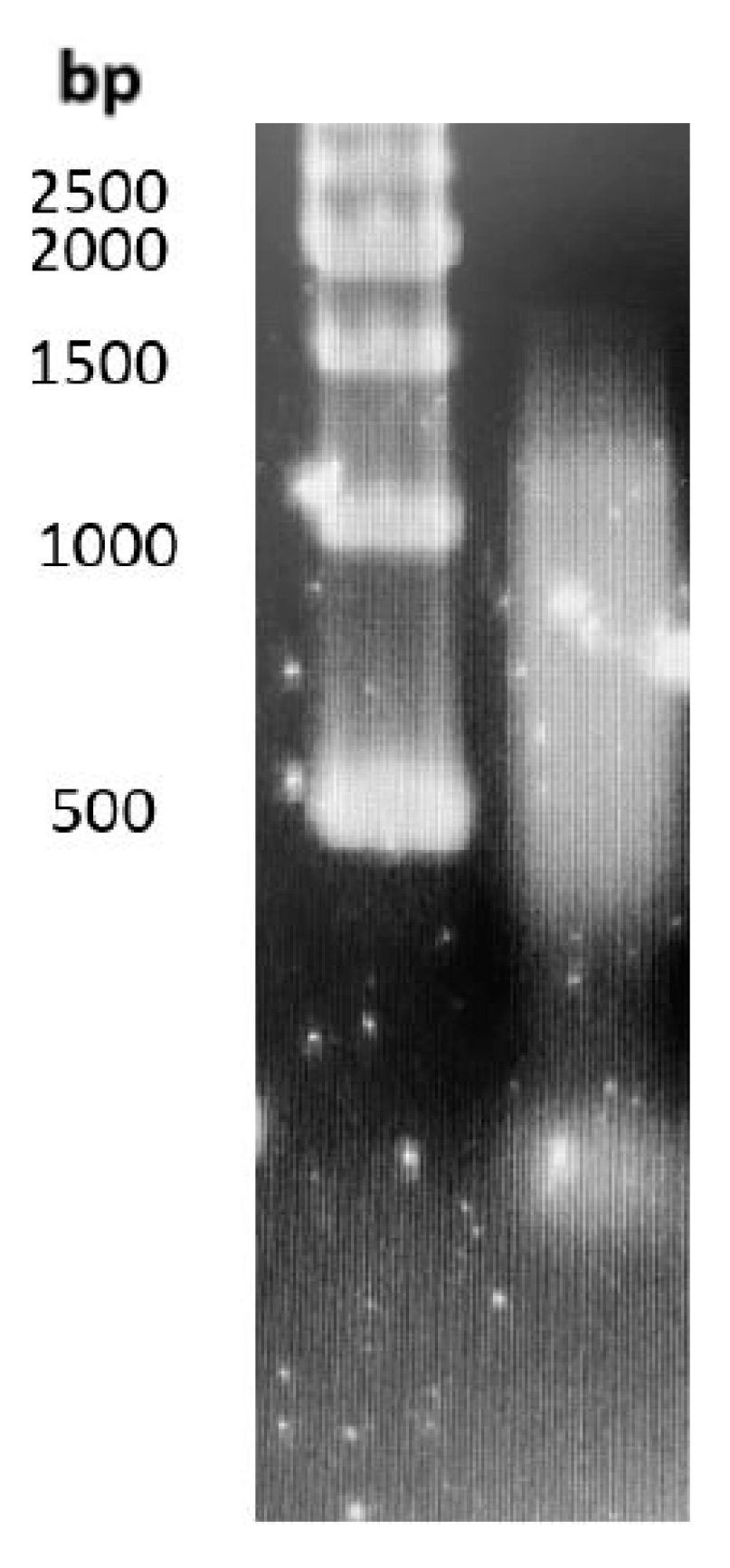
3.1. Yield from Self-eDNA Extraction
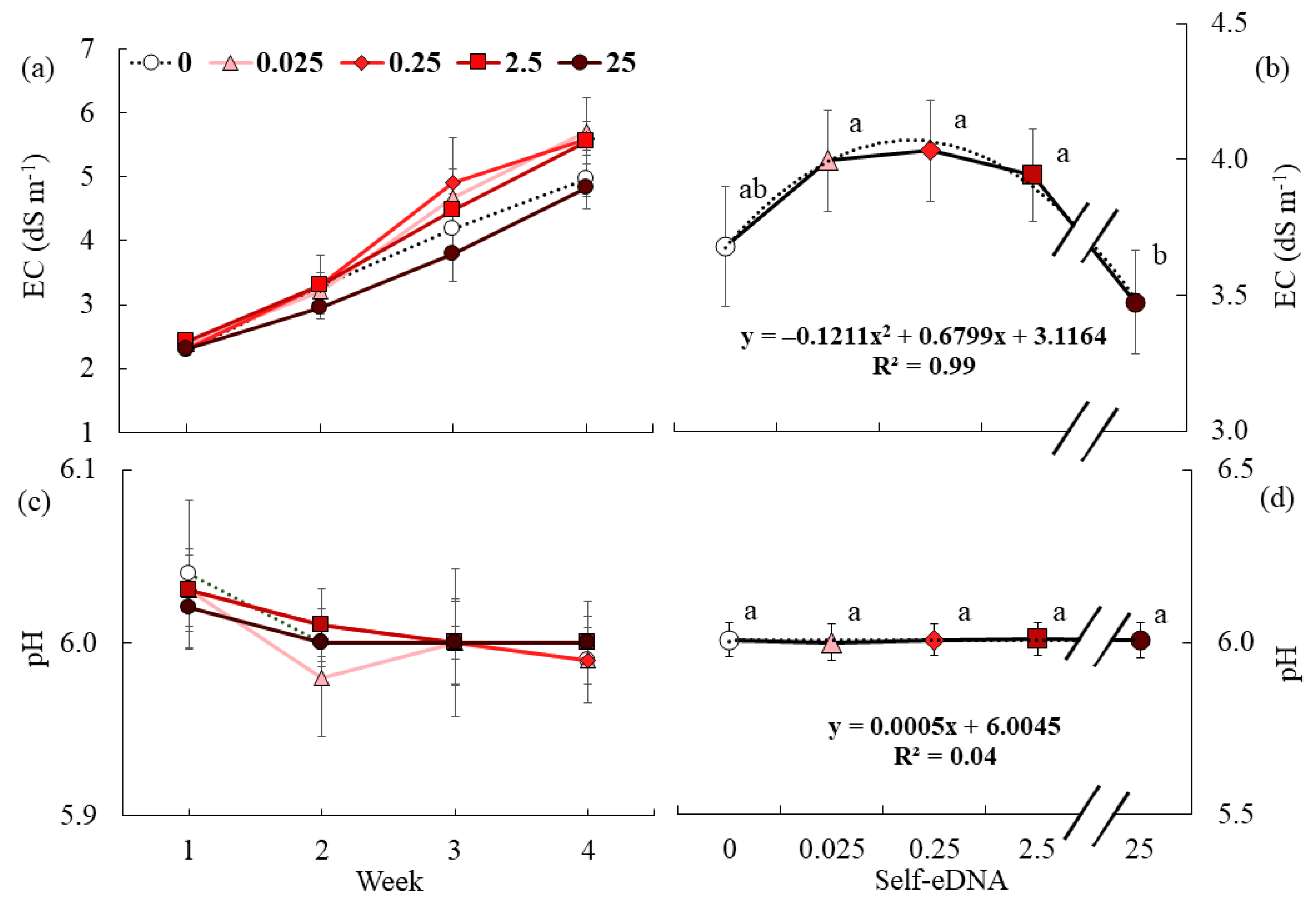
3.2. Effect of Self-eDNA Biostimulation on Fertigation Uptake Parameters of Lettuce
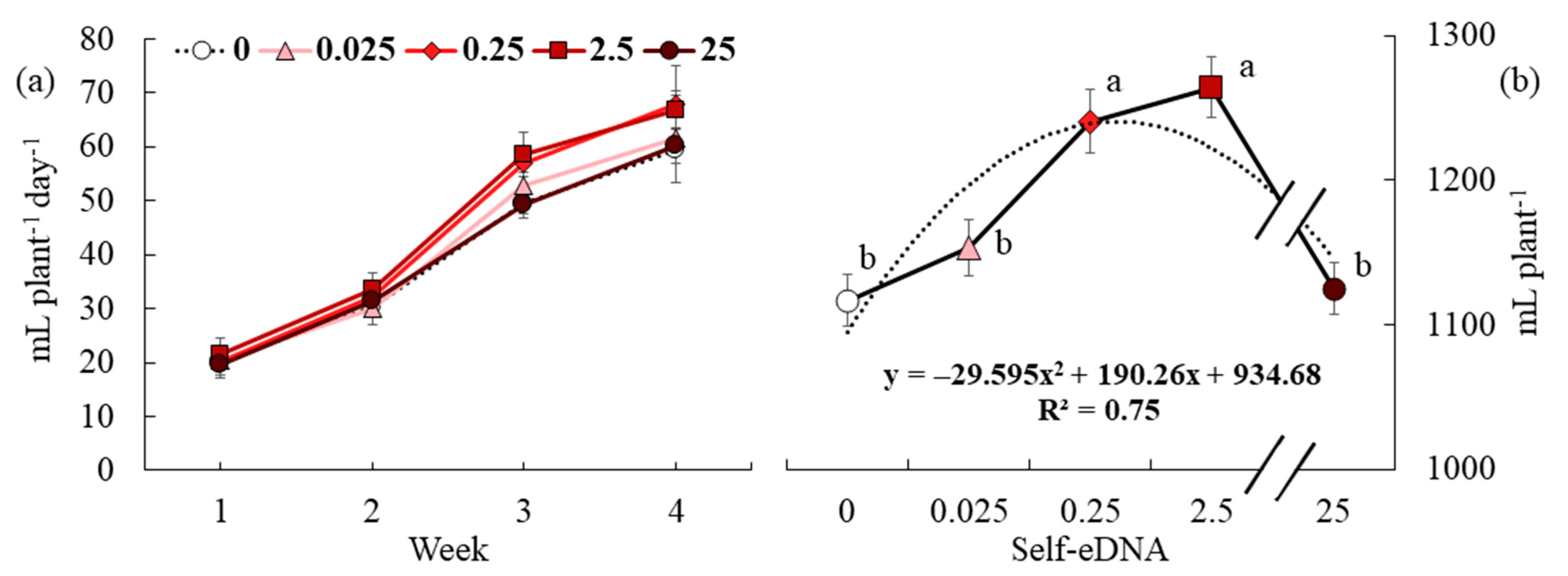
3.3. Effect of Self-eDNA Biostimulation on Water Uptake Parameters of Lettuce
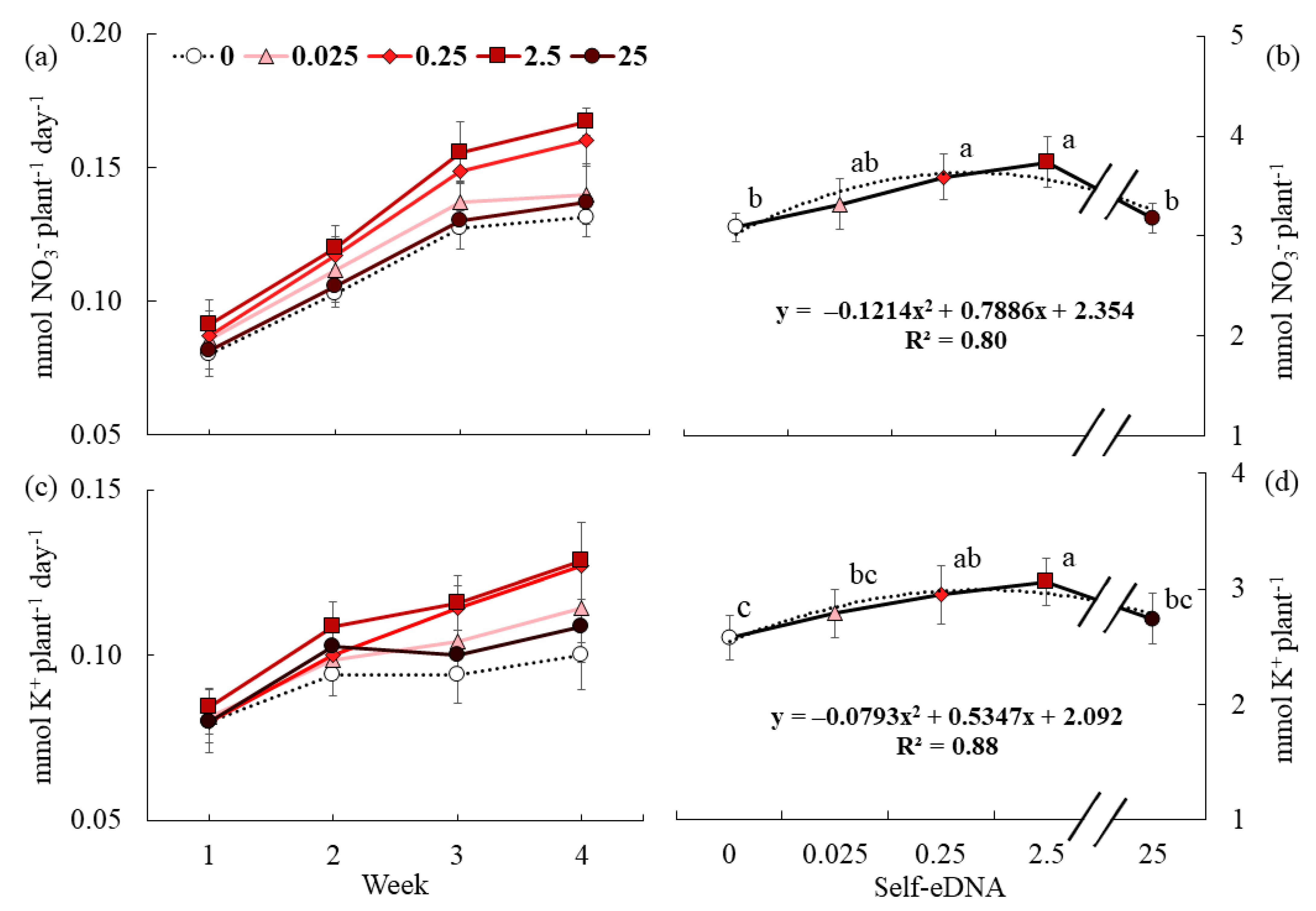
3.4. Effect of Self-eDNA Biostimulation on Nutrient Uptake Parameters of Lettuce
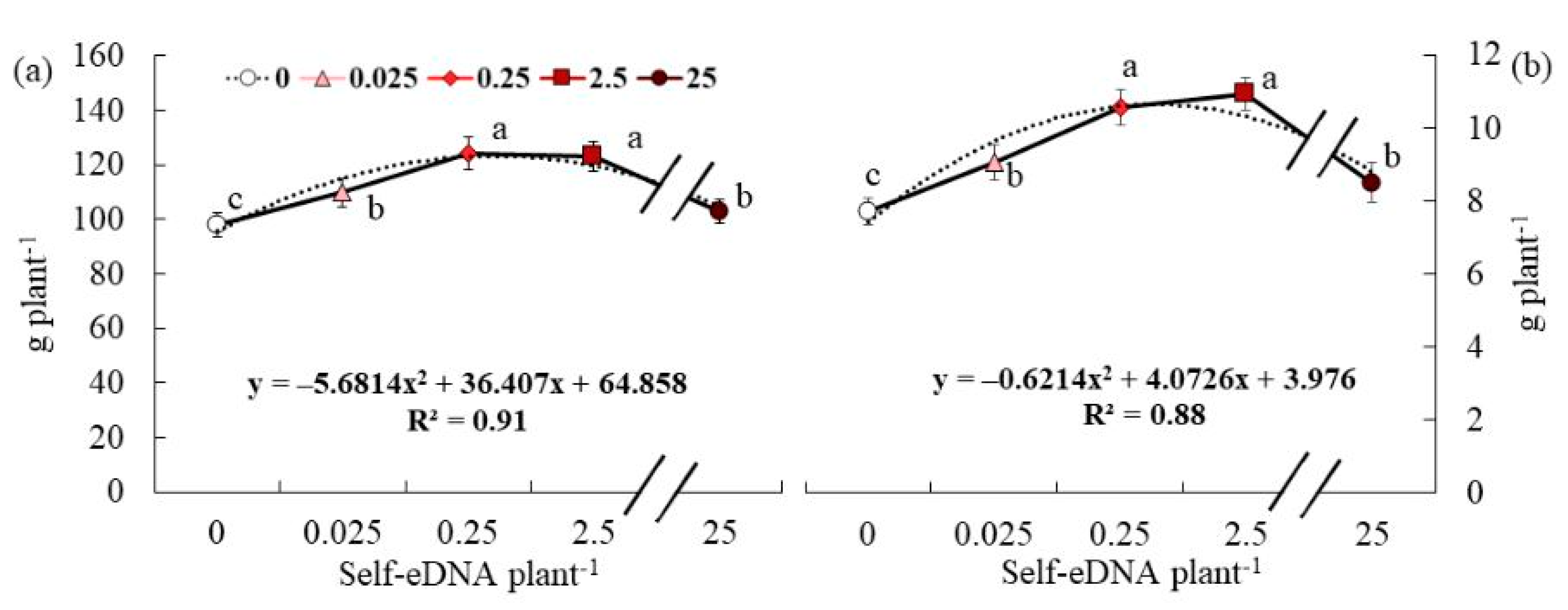
3.5. Effect of Self-eDNA Biostimulation on Growth Parameters of Lettuce
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Duran-Flores, D.; Heil, M. Extracellular self-DNA as a damage-associated molecular pattern (DAMP) that triggers self-specific immunity induction in plants. Brain Behav. Immun. 2018, 72, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Barbero, F.; Guglielmotto, M.; Capuzzo, A.; Maffei, M.E. Extracellular self-DNA (esDNA), but not heterologous plant or insect DNA (etDNA), induces plasma membrane depolarization and calcium signaling in lima bean (Phaseolus lunatus) and maize (Zea mays). Int. J. Mol. Sci. 2016, 17, 1659. [Google Scholar] [CrossRef] [PubMed]
- Vega-Muñoz, I.; Feregrino-Pérez, A.A.; Torres-Pacheco, I.; Guevara-González, R.G. Exogenous fragmented DNA acts as a damage-Associated molecular pattern (DAMP) inducing changes in CpG DNA methylation and defence-related responses in Lactuca sativa. Funct. Plant Biol. 2018, 45, 1065–1072. [Google Scholar] [CrossRef] [PubMed]
- Barbero, F.; Guglielmotto, M.; Islam, M.; Maffei, M.E. Extracellular fragmented self-DNA is involved in plant responses to biotic stress. Front. Plant Sci. 2021, 12, 686121. [Google Scholar] [CrossRef] [PubMed]
- Chiusano, M.L.; Incerti, G.; Colantuono, C.; Termolino, P.; Palomba, E.; Monticolo, F.; Benvenuto, G.; Foscari, A.; Esposito, A.; Marti, L.; et al. Arabidopsis thaliana response to extracellular DNA:Self versus nonself exposure. Plants 2021, 10, 1744. [Google Scholar] [CrossRef] [PubMed]
- Carbajal-Valenzuela, I.A.; Guzmán-Cruz, R.; González-Chavira, M.M.; Medina-Ramos, G.; Serrano-Jamaica, L.M.; Torres-Pacheco, I.; Vázquez, L.; Feregrino-Pérez, A.A.; Rico-García, E.; Guevara-González, R.G. Response of plant immunity markers to early and late application of extracellular DNA from different sources in tomato (Solanum lycopersicum). Agriculture 2022, 12, 1587. [Google Scholar] [CrossRef]
- Zhou, X.; Gao, H.; Zhang, X.; Khashi, U.; Rahman, M.; Mazzoleni, S.; Du, M.; Wu, F. Plant extracellular self-DNA inhibits growth and induces immunity via the jasmonate signaling pathway. Plant Physiol. 2023, 192, 2475–2491. [Google Scholar] [CrossRef]
- Serrano-Jamaica, L.M.; Villordo-Pineda, E.; González-Chavira, M.M.; Guevara-González, R.G.; Medina-Ramos, G. Effect of fragmented DNA from plant pathogens on the protection against wilt and root rot of Capsicum annuum L. plants. Front. Plant Sci. 2021, 11, 581891. [Google Scholar] [CrossRef]
- Ferrusquía-Jiménez, N.I.; Chandrakasan, G.; Torres-Pacheco, I.; Rico-Garcia, E.; Feregrino-Perez, A.A.; Guevara-González, R.G. Extracellular DNA: A relevant plant damage-associated molecular pattern (DAMP) for crop protection against pests—A review. J. Plant Growth Regul. 2020, 40, 451–463. [Google Scholar] [CrossRef]
- Cedillo-Jiménez, C.A.; Guevara-González, R.G.; Hernández, A.C. Exogenous dsRNA sequence based on miR1917 downregulates its target gene related to ethylene signaling in tomato seedlings and fruit. Sci. Hortic. 2024, 331, 113090. [Google Scholar] [CrossRef]
- Carbajal-Valenzuela, I.A.; Medina-Ramos, G.; Caicedo-Lopez, L.H.; Jim, A.; Ortega-Torres, A.E.; Contreras-Medina, L.M.; Torres-Pacheco, I. Extracellular DNA: Insight of a signal molecule in crop protection. Biology 2021, 10, 1022. [Google Scholar] [CrossRef] [PubMed]
- Dipak-Kumar, H.; Aloke, P. Role of biostimulant formulations in crop production: An overview. Int. J. Agric. Sci. Vet. Med. 2020, 8, 38–46. [Google Scholar]
- Baltazar, M.; Correia, S.; Guinan, K.J.; Sujeeth, N.; Bragança, R.; Gonçalves, B. Recent advances in the molecular effects of biostimulants in plants: An overview. Biomolecules 2021, 11, 1096. [Google Scholar] [CrossRef] [PubMed]
- Jardin, P.D. Plant biostimulants: Definition, concept, main categories and regulation. Sci. Hortic. 2015, 30, 3–14. [Google Scholar] [CrossRef]
- Sible, C.N.; Seebauer, J.R.; Below, F.E. Plant biostimulants: A categorical review, their implications for row crop production, and relation to soil health indicators. Agronomy 2021, 11, 1297. [Google Scholar] [CrossRef]
- Godinez-Mendoza, P.L.; Rico-Chavez, A.K.; Ferrusquia-Jimenez, N.I.; Carbajal-Valenzuela, I.A.; Villagomez-Aranda, A.L.; Torres-Pacheco, I.; Guevara-González, R.G. Plant hormesis: Revising of the concepts of biostimulation, elicitation and their application in a sustainable agricultural production. Sci. Total Environ. 2023, 894, 164883. [Google Scholar] [CrossRef]
- Sonneveld, C.; Voogt, W. Plant Nutrition of Greenhouse Crops, 1st ed.; Springer: Dordrecht, The Netherlands; Heidelberg, Germany; London, UK; New York, NY, USA, 2009; 431p. [Google Scholar]
- Camejo, D.; Frutos, A.; Mestre, T.C.; Piñero, M.C.; Rivero, R.M.; Martínez, V. Artificial light impacts the physical and nutritional quality of lettuce plants. Hortic. Environ. Biotechnol. 2020, 61, 69–82. [Google Scholar] [CrossRef]
- FAO Annual Production Volume of Lettuce in the World between 2012 and 2021. Available online: https://es.statista.com/estadisticas/529438/producciones-de-lechugas-en-el-mundo/ (accessed on 4 August 2024).
- Gruda, N.S. Increasing sustainability of growing media constituents and stand-alone substrates in soilless culture systems. Agronomy 2020, 9, 298. [Google Scholar] [CrossRef]
- Choudhary, D.K.; Prakash, A.; Johri, B.N. Induced systemic resistance (ISR) in plants: Mechanism of action. Indian J. Microbiol. 2007, 47, 289–297. [Google Scholar] [CrossRef]
- Rodríguez, D.; Reca, J.; Martínez, J.; Lao, M.T.; Urrestarazu, M. Effect of controlling the leaching fraction on the fertigation and production of a tomato crop under soilless culture. Sci. Hortic. 2014, 179, 153–157. [Google Scholar] [CrossRef]
- Urrestarazu, M.; Carrasco, G. Soilless Culture and Hydroponics, 1st ed.; Mundi-Prensa: Madrid, Spain, 2023; 279p. [Google Scholar]
- Rodríguez, D.; Reca, J.; Martínez, J.; López-Luque, R.; Urrestarazu, M. Development of a new control algorithm for automatic irrigation scheduling in soilless culture. Appl. Math Inf. Sci. 2015, 9, 47–56. [Google Scholar] [CrossRef]
- Peçanha, D.A.; Cunha-Chiamolera, T.P.L.; Chourak, Y.; Martínez-Rivera, E.Y.; Urrestarazu, M. Effect of the matric potential on growth and water, nitrate and potassium absorption of vegetables under soilless culture. J. Soil Sci. Plant Nutr. 2021, 21, 3493–3501. [Google Scholar] [CrossRef]
- Sonneveld, C.; Straver, N.B. Nutrient Solution for Vegetables and Flowers Grown in Water or Substrates, 10th ed.; Glasshouse Crops Research Station: Naaldwijk, The Netherlands, 1994; pp. 1–33. [Google Scholar]
- Petersen, R.G. Agricultural Field Experiments: Design and Analysis, 1st ed.; Marcel Dekker: New York, NY, USA, 1994; 409p. [Google Scholar]
- Gorbe, E.; Calatayud, A. Optimization of nutrition in soilless systems: A review. Adv. Bot. Res. 2010, 53, 193–245. [Google Scholar] [CrossRef]
- Moya, C.; Oyanedel, E.; Verdugo, G.; Flores, M.F.; Urrestarazu, M.; Álvaro, J.E. Increased electrical conductivity in nutrient solution management enhances dietary and organoleptic qualities in soilless culture tomato. HortScience 2017, 52, 868–872. [Google Scholar] [CrossRef]
- Cunha-Chiamolera, T.P.L.; Urrestarazu, M.; Cecílio-Filho, A.B.; Morales, I. Agronomic and economic feasibility of tomato and lettuce intercropping in a soilless system as a function of the electrical conductivity of the nutrient solution. HortScience 2017, 52, 1195–1200. [Google Scholar] [CrossRef]
- Carvalho, R.S.C.; Bastos, R.G.; Souza, C.F. Influence of the use of wastewater on nutrient absorption and production of lettuce grown in a hydroponic system. Agric. Water Manag. 2018, 203, 311–321. [Google Scholar] [CrossRef]
- Cecílio-Filho, A.B.; Coelho, R.L.; Cunha, T.P.L.; Urrestarazu, M. Nitrogen efficiency in hydroponic chicory. J. Plant Nutr. 2017, 40, 2532–2539. [Google Scholar] [CrossRef]
- Wamser, A.F.; Cecílio Filho, A.B.; Nowaki, R.H.D.; Mendoza-Cortez, J.W.; Urrestarazu, M. Influence of drainage and nutrient-solution nitrogen and potassium concentrations on the agronomic behavior of bell-pepper plants cultivated in a substrate. PloS ONE 2017, 12, e0180529. [Google Scholar] [CrossRef]
- Zhang, G.; Yi, H.Y.B.; Gong, J.M. The Arabidopsis Ethylene/Jasmonic acid-NRT signaling module coordinates nitrate reallocation and the trade-off between growth and environmental adaptation. Plant Cell 2014, 26, 3984–3998. [Google Scholar] [CrossRef]
- Armengaud, P.; Breitling, R.; Amtmann, A. The potassium-dependent transcriptome of arabidopsis reveals a prominent role of jasmonic acid in nutrient signaling. Plant Physiol. 2004, 136, 2556–2576. [Google Scholar] [CrossRef]
- Singh, D.A. Expression dynamics indicate the role of Jasmonic acid biosynthesis pathway in regulating macronutrient (N, P and K+) deficiency tolerance in rice (Oryza sativa L.). Plant Cell Rep. 2021, 40, 1495–1512. [Google Scholar] [CrossRef]
- Cardarelli, M.; Rouphael, Y.; Coppa, E.; Hoagland, L. Using microgranular-based biostimulant in vegetable transplant production to enhance growth and nitrogen uptake. Agronomy 2020, 10, 842. [Google Scholar] [CrossRef]
- Vargas-Hernandez, M.; Macias-Bobadilla, I.; Guevara-Gonzalez, R.G.; Romero-Gomez, S.J.; Rico-Garcia, E.; Ocampo-Velazquez, R.V.; Alvarez-Arquieta, L.L.; Torres-Pacheco, I. Plant hormesis management with biostimulants of biotic origin in agriculture. Front. Plant Sci. 2017, 8, 1762. [Google Scholar] [CrossRef] [PubMed]
- García-Mier, L.; Guevara-González, R.G.; Mondragón-Olguín, V.M.; Verduzco-Cuellar, B.R.; Torres-Pacheco, I. Agriculture and bioactives: Achieving both crop yield and phytochemicals. Int. J. Mol. Sci. 2013, 14, 4203–4222. [Google Scholar] [CrossRef]
| Drainage | Absorption | Growth | |||||
|---|---|---|---|---|---|---|---|
| EC | pH | Water | NO3− | K+ | FW | DW | |
| pH | −0.0352 ns | - | - | - | - | - | - |
| Water | 0.7025 *** | 0.0168 ns | - | - | - | - | - |
| NO3− | 0.6904 *** | −0.0111 ns | 0.9714 *** | - | - | - | - |
| K+ | 0.6012 ** | 0.0442 ns | 0.9256 *** | 0.9421 *** | - | - | - |
| FW | 0.7463 *** | 0.0682 ns | 0.9665 *** | 0.9434 *** | 0.9284 *** | - | - |
| DW | 0.7012 *** | −0.0052 ns | 0.9802 *** | 0.9778 *** | 0.9600 *** | 0.9710 *** | - |
| DNA | −0.7911 *** | −0.0192 ns | −0.3903 ns | −0.3431 ns | −0.1598 ns | −0.3258 ns | −0.3016 ns |
| pH | 0.00 | - | - | - | - | - | - |
| Water | 0.49 | 0.00 | - | - | - | - | - |
| NO3− | 0.47 | 0.00 | 0.94 | - | - | - | - |
| K+ | 0.36 | 0.00 | 0.86 | 0.89 | - | - | - |
| FW | 0.56 | 0.01 | 0.93 | 0.89 | 0.86 | - | - |
| DW | 0.49 | 0.00 | 0.96 | 0.96 | 0.92 | 0.94 | - |
| DNA | 0.63 | 0.00 | 0.15 | 0.12 | 0.02 | 0.11 | 0.09 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cunha-Chiamolera, T.P.L.; Urrestarazu, M.; Carbajal-Valenzuela, I.A.; Ramos, J.B.; Ortega, R.; Miralles, I.; Guevara-González, R.G. Extracellular Fragmented Self-DNA Displays Biostimulation of Lettuce in Soilless Culture. Horticulturae 2024, 10, 964. https://doi.org/10.3390/horticulturae10090964
Cunha-Chiamolera TPL, Urrestarazu M, Carbajal-Valenzuela IA, Ramos JB, Ortega R, Miralles I, Guevara-González RG. Extracellular Fragmented Self-DNA Displays Biostimulation of Lettuce in Soilless Culture. Horticulturae. 2024; 10(9):964. https://doi.org/10.3390/horticulturae10090964
Chicago/Turabian StyleCunha-Chiamolera, Tatiana P. L., Miguel Urrestarazu, Ireri A. Carbajal-Valenzuela, José Barroso Ramos, Raúl Ortega, Isabel Miralles, and Ramón Gerardo Guevara-González. 2024. "Extracellular Fragmented Self-DNA Displays Biostimulation of Lettuce in Soilless Culture" Horticulturae 10, no. 9: 964. https://doi.org/10.3390/horticulturae10090964
APA StyleCunha-Chiamolera, T. P. L., Urrestarazu, M., Carbajal-Valenzuela, I. A., Ramos, J. B., Ortega, R., Miralles, I., & Guevara-González, R. G. (2024). Extracellular Fragmented Self-DNA Displays Biostimulation of Lettuce in Soilless Culture. Horticulturae, 10(9), 964. https://doi.org/10.3390/horticulturae10090964






