Projection of the Potential Global Geographic Distribution of the Solanum Fruit Fly Bactrocera latifrons (Hendel, 1912) (Diptera: Tephritidae) Based on CLIMEX Models
Abstract
1. Introduction
2. Materials and Methods
2.1. Research Model and Software
2.2. Data Collection
2.2.1. Climate Data
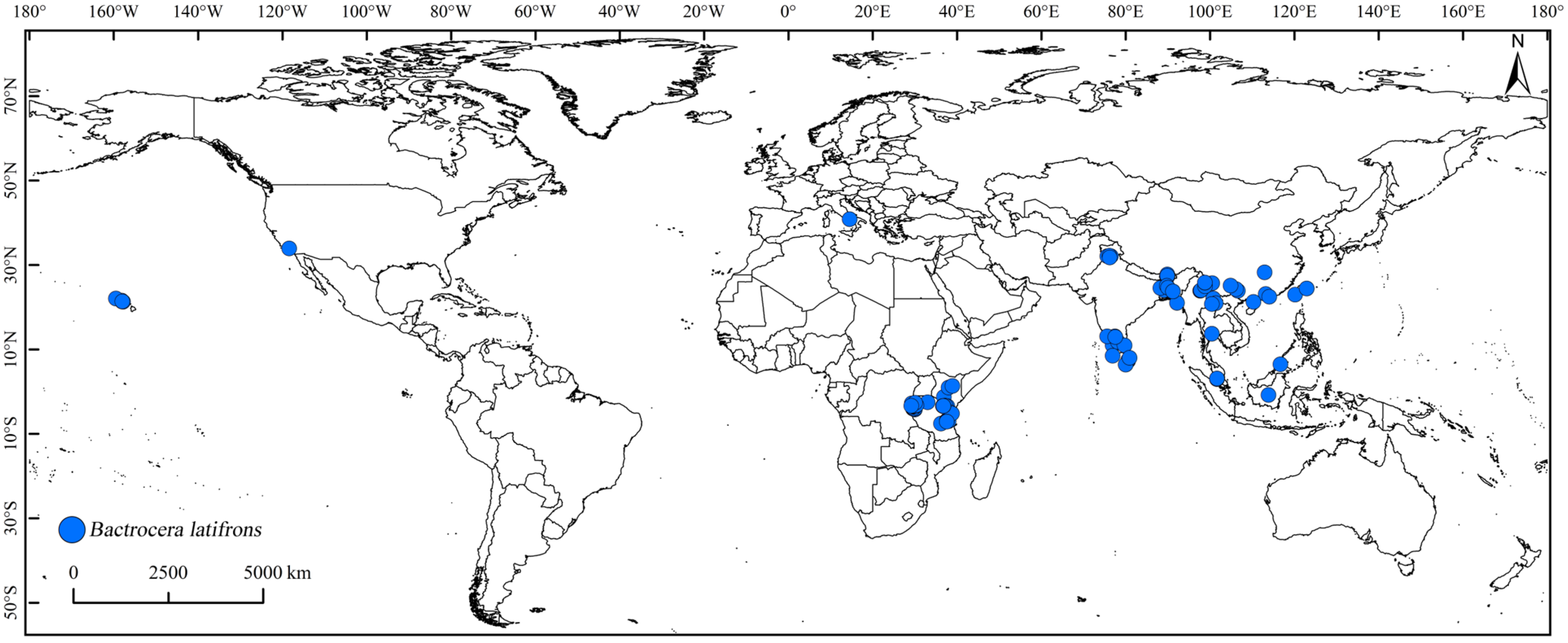
2.2.2. Global Distribution Records of B. latifrons
2.3. Research Methods
2.3.1. Fitting CLIMEX Parameters
2.3.2. Temperature Index
2.3.3. Moisture Index
2.3.4. Degree-Days per Generation (PDD)
2.3.5. Cold Stress
2.3.6. Heat Stress
2.3.7. Dry Stress
2.3.8. Wet Stress
2.4. Centroid Transfer and Threshold Chosen
3. Results
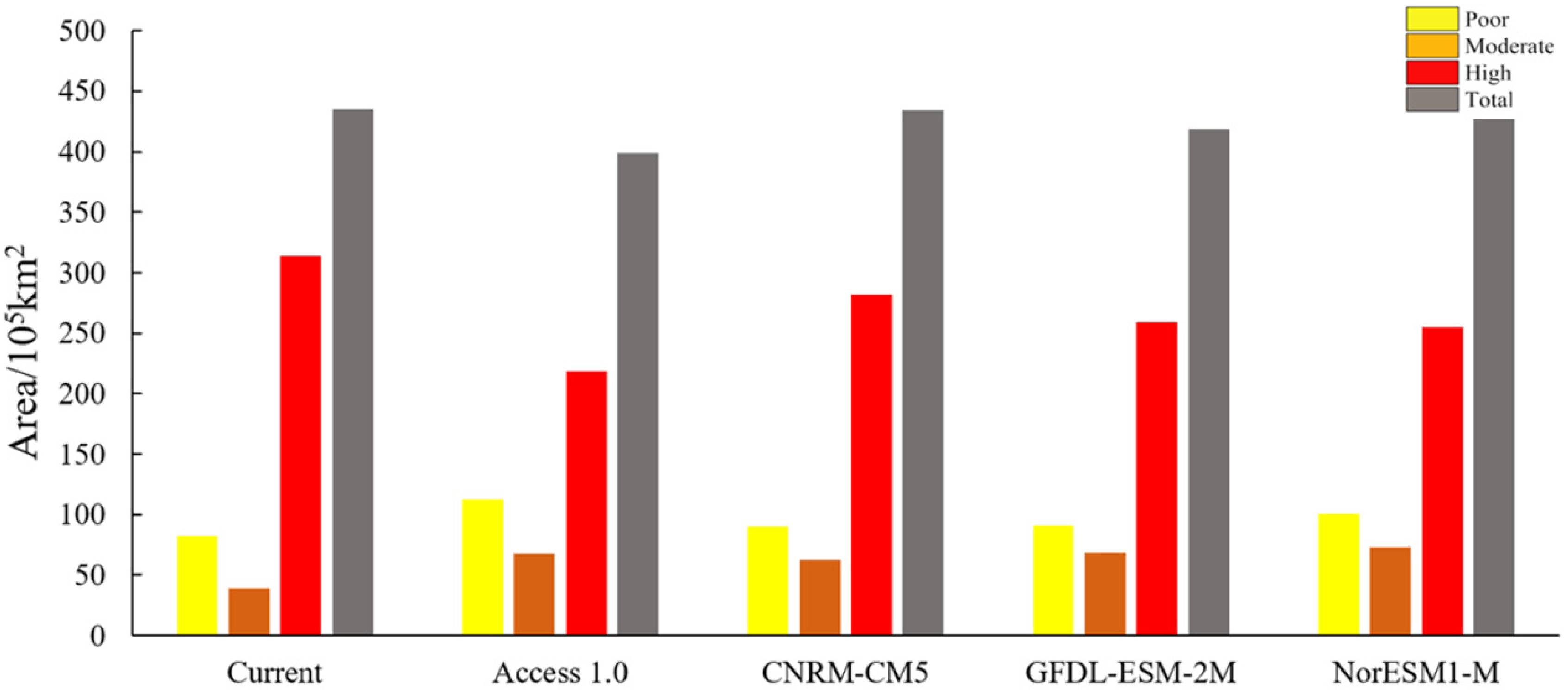
3.1. Potential Distribution of B. latifrons under the Current Climate Conditions
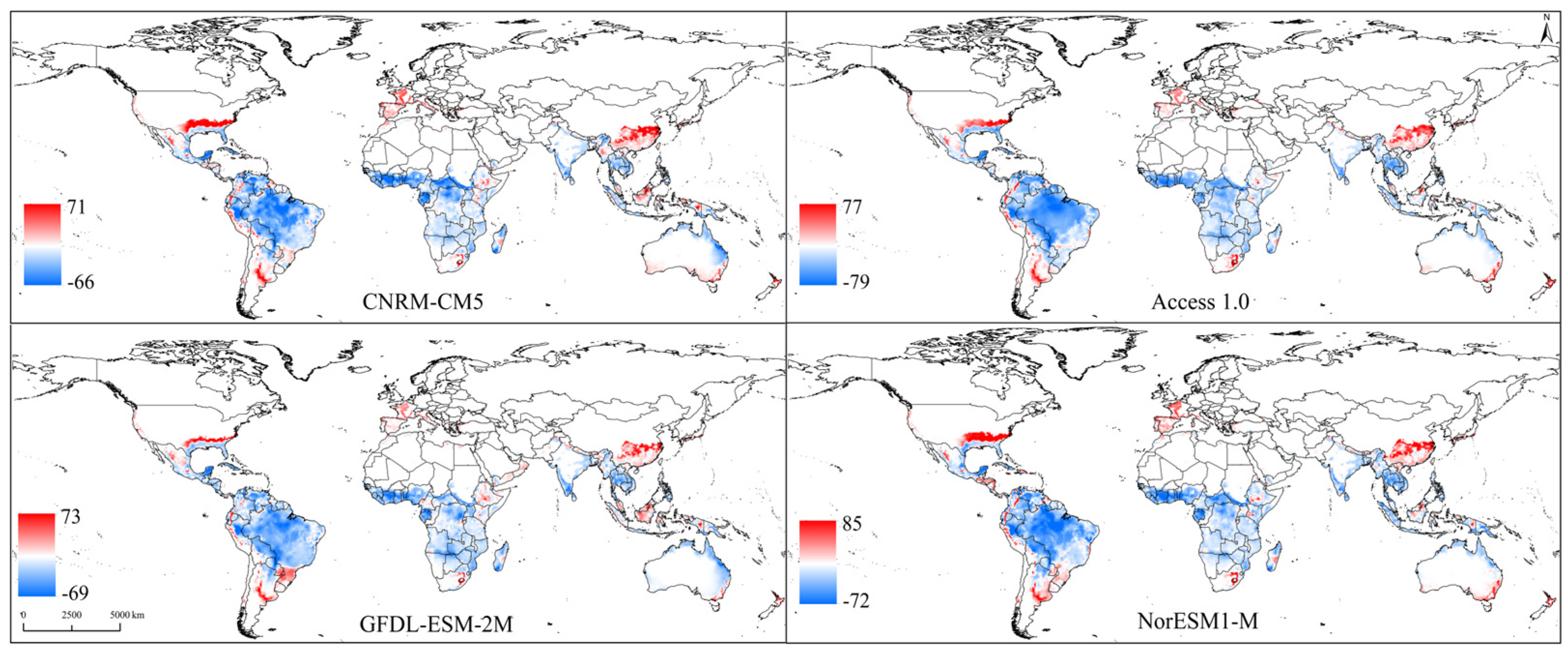
3.2. Changes in Potential Suitable Areas of B. latifrons under Future Climate Scenario Models
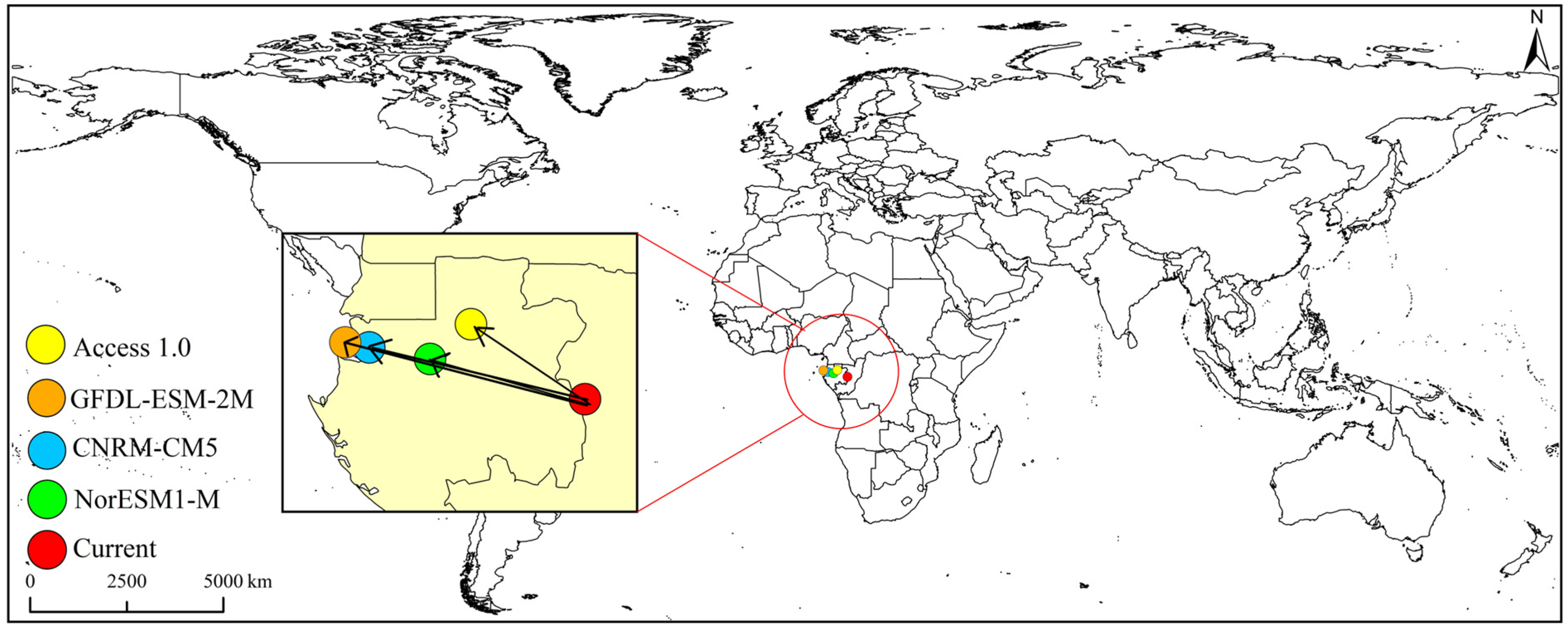
3.3. Centroid Shift of Potential Suitable Global Habitats
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rattanapun, W.; Tarasin, M.; Thitithanakul, S.; Sontikun, Y. Host Preference of Bactrocera latifrons (Hendel) (Diptera: Tephritidae) Among Fruits of Solanaceous Plants. Insects 2021, 12, 482. [Google Scholar] [CrossRef] [PubMed]
- Liquido, N.J.; Harris, E.J.; Dekker, L.A. Ecology of Bactrocera latifrons (Diptera: Tephritidae) populations: Host plants, natural enemies, distribution, and abundance. Ann. Entomol. Soc. Am. 1994, 87, 71–84. [Google Scholar] [CrossRef]
- Paulsen, M.J. Nomenclatural changes in the Nearctic Ochodaeinae and description of two new genera (Coleoptera: Scarabaeoidea: Ochodaeidae). Insecta Mundi 2007, 21, 1–13. [Google Scholar]
- Mziray, H.A.; Makundi, R.H.; Mwatawala, M.; Maerere, A.; Meyer, M. Host use of Bactrocera latifrons, a new invasive tephritid species in Tanzania. J. Econ. Entomol. 2010, 103, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Vijaysegaran, S.; Osman, M.S. Fruit flies in Peninsular Malaysia: Their economic importance and control strategies. In Proceedings of the International Symposium on the Biology and Control of Fruit Flies, Okinawa, Japan, 2–4 September 1991; Kawasaki, K., Iwahashi, O., Kaneshiro, K.Y., Eds.; Okinawa Prefectural Government: Naha, Japan, 1991; pp. 105–115. [Google Scholar]
- Kang, D.L.; Sun, H.Y.; Qin, Y.J.; Lu, G.C.; Lan, S.H.; Li, Z.H. The potential economic loss of chili industry in China caused by Bactrocera latifrons (Hendel) based on @RISK. Chin. J. Appl. Entomol. 2019, 56, 500–507. [Google Scholar] [CrossRef]
- Available online:. Available online: https://www.cabi.org/isc/datasheet/8719#todistribution (accessed on 8 February 2024).
- Steven, L.P.; Grant, T.M. Ecological Aspects of Bactrocera latifrons (Diptera: Tephritidae) on Maui, Hawaii: Movement and Host Preference. Environ. Entomol. 2004, 33, 1722–1731. [Google Scholar]
- Mwatawala, M.; Meyer, M.D.; White, I.M.; Maerere, A.; Makundi, R.H. Detection of the solanum fruit fly, Bactrocera latifrons (Hendel) in Tanzania (Dipt., Tephritidae). J. Appl. Entomol. 2007, 131, 501–503. [Google Scholar] [CrossRef]
- Available online:. Available online: https://gd.eppo.int/taxon/DACULA/distribution/KE (accessed on 5 February 2024).
- Ndayizeye, L.; Balangaliza, C.K. First report of Bactrocera latifrons Hendel in the Democratic Republic of Congo. EPPO Bull. 2021, 51, 311–313. [Google Scholar] [CrossRef]
- Ndayizeye, L.; Nzigidahera, B.; Gesmallah, A.E. Current distribution of Bactrocera latifrons Hendel in the different agro-ecological zones of Burundi. Int. J. Trop. Insect Sci. 2019, 39, 125–130. [Google Scholar] [CrossRef]
- NAPPO. Phytosanitary Alert System: Bactrocera latifrons (Malaysian Fruit Fly)—Removal of the Quarantine in the Westchester Area of Los Angeles County, California. 2016. Available online: http://www.pestalert.org/oprDetail.cfm?oprID=677 (accessed on 7 February 2024).
- Gargiulo, S.; Nugnes, F.; De Benedetta, F.; Bernardo, U. Bactrocera latifrons in Europe: The importance of the right attractant for detection. Bull. Insectology 2021, 74, 311–320. [Google Scholar]
- Balmès, V.; Germain, J.F. When fruit flies fly. Data on three years of Tephritidae interception by the French NPPO in Roissy Charles-de-Gaulle airport. In Proceedings of the 8th International Symposium on Fruit Flies of Economic Importance, Valencia, Spain, 26 September–1 October 2010. [Google Scholar]
- Sun, T.; Lu, L.H.; Teng, S.N.; Deng, Z.H.; Zhao, Y.; Liu, H.; Kong, D.Y. Identification of Fruit Fly Low Instar Larvae in Imported Red Pepper. Hubei Agric. Sci. 2013, 52, 4385–4387. [Google Scholar] [CrossRef]
- Available online:. Available online: https://gd.eppo.int/taxon/DACULA/distribution/IT (accessed on 11 February 2024).
- Liu, J.G.; Vanessa, H.; Mateus, B.; Ruth, S.D. Framing Sustainability in a Telecoupled World. Ecol. Soc. 2013, 36, 7870–7885. [Google Scholar] [CrossRef]
- Michael, I.W.; Michael, B.; Kathy, M.; Ian, N. The link between international trade and the global distribution of invasive alien species. Biol. Invasions 2008, 10, 391–398. [Google Scholar] [CrossRef]
- Seebens, B.; Blackburn, T.M.; Dyer, E.E.; Genovesi, P.; Hulme, P.E.; Jeschke, J.M.; Pagad, S.; Pyšek, P.; van Kleunen, M.; Winter, M.; et al. Global rise in emerging alien species results from increased accessibility of new source pools. Proc. Natl. Acad. Sci. USA 2018, 115, E2264–E2273. [Google Scholar] [CrossRef] [PubMed]
- Kenis, M.; Rabitsch, W.; Auger-Rozenberg, M.A.; Roques, A. How can alien species inventories and interception data help us prevent insect invasions? Bull. Entomol. Res. 2007, 97, 489–502. [Google Scholar] [CrossRef]
- Roques, A. Taxonomy, time and geographic patterns. Chapter 2. BioRisk 2010, 4, 11–26. [Google Scholar] [CrossRef][Green Version]
- Eschen, R.; Douma, J.C.; Grégoire, J.C.; Mayer, F.; Rigaux, L.; Potting, R.P.J. A risk categorisation and analysis of the geographic and temporal dynamics of the European import of plants for planting. Biol. Invasions 2017, 19, 3243–3257. [Google Scholar] [CrossRef]
- Roques, A.; Auger-Rozenberg, M.A.; Blackburn, T.M.; Garnas, J.; Pyšek, P.; Rabitsch, W.; Richardson, D.M.; Wingfield, M.J.; Liebhold, A.M.; Duncan, R.P. Temporal and interspecific variation in rates of spread for insect species invading Europe during the last 200 years. Biol. Invasions 2016, 18, 907–920. [Google Scholar] [CrossRef]
- Available online:. Available online: https://www.fao.org/faostat/zh/#data/TCL/visualize (accessed on 25 February 2024).
- Kituta, J.A.R.; Berkmans, M.B.J. Current status of the Solanum fruit fly Bactrocera latifrons (Hendel) in the eastern part of Democratic Republic of Congo. Insect Environ. 2021, 24, 370–380. [Google Scholar]
- Zhao, C.Y.; Li, J.S.; Xu, J.; Liu, X.Y. Disentangling Environmental and Anthropogenic Impacts on the Distribution of Unintentionally Introduced Invasive Alien Insects in Mainland China. J. Insect Sci. 2017, 17, 77. [Google Scholar] [CrossRef]
- Bebber, D.P. Range-Expanding Pests and Pathogens in a Warming World. Annu. Rev. Phytopathol. 2015, 53, 335–356. [Google Scholar] [CrossRef] [PubMed]
- Ge, F. Challenges facing entomologists in a changing global climate. Chin. J. Appl. Entomol. 2011, 48, 1117–1122. [Google Scholar]
- IPCC. Climate Change 2022: Impacts, Adaptation and Vulnerability; The Intergovernmental Panel on Climate Change: Geneva, Switzerland, 2022. [Google Scholar]
- Macfadyen, S.; Kriticos, D.J. Modelling the Geographical Range of a Species with Variable Life-History. PLoS ONE 2012, 7, e40313. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Li, Z.H.; Huang, G.S.; Wu, X.X.; Ni, W.L.; Qü, W.W. The current and future potential geographic range of West Indian fruit fly, Anastrepha obliqua (Diptera: Tephritidae). Insect Sci. 2014, 21, 234–244. [Google Scholar] [CrossRef]
- Sutherst, R.W.; Collyer, B.S.; Yonow, T. The vulnerability of Australian horticulture to the Queensland fruit fly, Bactrocera (Dacus) tryoni, under climate change. Aust. J. Agric. Res. 2000, 51, 467–480. [Google Scholar] [CrossRef]
- Stephens, A.E.A.; Kriticos, D.J.; Leriche, A. The current and future potential geographical distribution of the oriental fruit fly, Bactrocera dorsalis (Diptera: Tephritidae). Bull. Entomol. Res. 2007, 97, 369–378. [Google Scholar] [CrossRef]
- De Villiers, M.; Hattingh, V.; Kriticos, D.J.; Brunel, S.; Vayssieres, J.F.; Sinzogan, A.; Billah, M.K.; Mohamed, S.A.; Mwatawala, M.; Abdelgader, H.; et al. The potential distribution of Bactrocera dorsalis: Considering phenology and irrigation patterns. Bull. Entomol. Res. 2016, 106, 19–33. [Google Scholar] [CrossRef]
- Kriticos, D.J.; Maywald, G.F.; Yonow, T.; Zurcher, E.J.; Herrmann, N.I.; Sutherst, R.W. CLIMEX. Version 4. Exploring the Effects of Climate on Plants, Animals and Diseases; CSIRO: Canberra, Australia, 2015. [Google Scholar]
- Darren, J.K.; Bruce, L.W.; Agathe, L.; Noboru, O.; Ian, M.; Janice, B.; John, K.S. CliMond: Global high-resolution historical and future scenario climate surfaces for bioclimatic modelling. Methods Ecol. Evol. 2012, 3, 53–64. [Google Scholar] [CrossRef]
- Huang, Z. Morphological Identification, Artificial Diet, Suitability Analysis and Prediction, Qualitative and Quantitative Risk Analysis of Important Bactrocera Species. Master’s Thesis, Hainan University, Haikou, China, 2010. [Google Scholar]
- Syed, R.A. Studies on Trypetids and their natural enemies in West Pakistan. Dacus species of lesser importance. Pak. J. Zool. 1970, 2, 17–24. [Google Scholar]
- Deng, Y.P.; Qiu, Q. Survey on the occurrence and monitoring of Sitophilus in Guangxi. Guangxi Hortic. 2008, 19, 22–24. [Google Scholar]
- Boopathi, T.; Singh, S.B.; Manju, T.; Chowdhury, S.; Singh, A.R.; Duta, S.K.; Dayal, V.; Behere, G.T.; Ngachan, S.V.; Hazarika, S.; et al. First report of economic injury to tomato due to Zeugodacus tau (Diptera Tephritidae): Relative abundance and effects of cultivar and season on injury. Fla. Entomol. 2017, 100, 63–69. [Google Scholar] [CrossRef]
- Ma, X.L.; Li, Z.H.; Ni, W.L.; Qu, W.W.; Wu, J.J.; Wan, F.H.; Hu, X.N. The Current and Future Potential Geographical Distribution of the Solanum Fruit Fly, Bactrocera latifrons (Diptera: Tephritidae) in China. In Proceedings of the 5th Computer and Computing Technologies in Agriculture (CCTA), Beijing, China, 29–31 October 2011; pp. 236–246. [Google Scholar]
- Ayres, M.P.; Lombardero, M.J. Assessing the consequences of global change for forest disturbance from herbivores and pathogens. Total Env. 2000, 262, 263–286. [Google Scholar] [CrossRef] [PubMed]
- Lierop, P.; Lindquist, E.; Sathyapala, S.; Franceschini, G. Global forest area disturbance from fire, insect pests, diseases and severe weather events. For. Ecol. Manag. 2015, 352, 78–88. [Google Scholar] [CrossRef]
- Canelles, Q.; Aquilué, N.; James, P.M.A.; Lawler, J.; Brotons, L. Global review on interactions between insect pests and other forest disturbances. Landsc. Ecol. 2021, 36, 945–972. [Google Scholar] [CrossRef]
- da Silva Santana, G.; Ronchi-Teles, B.; Dos Santos, C.M.; Soares, M.A.; Souza, P.G.C.; Araújo, F.H.V.; de Aguiar, C.V.S.; da Silva, R.S. Climate suitability modeling for Anastrepha suspensa (Diptera: Tephritidae): Current and future invasion risk analysis. Int. J. Biometeorol. 2023, 67, 1185–1197. [Google Scholar] [CrossRef]
- Kumar, S.; Yee, W.L.; Neven, L.G. Mapping Global Potential Risk of Establishment of Rhagoletis pomonella (Diptera: Tephritidae) Using MaxEnt and CLIMEX Niche Models. J. Econ. Entomol. 2016, 109, 2043–2053. [Google Scholar] [CrossRef]
- Ni, W.L.; Li, Z.H.; Chen, H.J.; Wan, F.H.; Qu, W.W.; Zhang, Z.; Kriticos, D.J. Including climate change in pest risk assessment: The peach fruit fly, Bactrocera zonata (Diptera: Tephritidae). Bull. Entomol. Res. 2012, 102, 173–183. [Google Scholar] [CrossRef]
- Hawkins, E.; Sutton, R. The Potential to Narrow Uncertainty in Regional Climate Predictions. Bull. Am. Meteorol. Soc. 2009, 90, 1095–1107. [Google Scholar] [CrossRef]
- Seidl, R.; Thom, D.; Kautz, M.; Martin-Benito, D.; Peltoniemi, M.; Vacchiano, G.; Wild, J.; Ascoli, D.; Petr, M.; Honkaniemi, J.; et al. Forest disturbances under climate change. Nat. Clim. Change 2017, 7, 395–402. [Google Scholar] [CrossRef]
- Parmesan, C.; Yohe, G. A globally coherent fingerprint of climate change impacts across natural systems. Nature 2003, 421, 37–42. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2021: The Physical Science Basis; Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2021. [Google Scholar] [CrossRef]
- Bebber, D.P.; Holmes, T.; Gurr, S.J. The global spread of crop pests and pathogens. Glob. Ecol. Biogeogr. 2014, 23, 1398–1407. [Google Scholar] [CrossRef]
- Rao, J.; Zhang, Y.; Zhao, H.; Guo, J.; Wan, F.; Xian, X.; Yang, N.; Liu, W. Projecting the Global Potential Geographical Distribution of Ceratitis capitata (Diptera: Tephritidae) under Current and Future Climates. Biology 2024, 13, 177. [Google Scholar] [CrossRef]




| Parameter | Ma et al. (2012) [38] Parameter Values | Current Parameter Values |
|---|---|---|
| Lower threshold of soil moisture (SM0) | 0.10 | 0.10 |
| Lower limit of optimum soil moisture (SM1) | 0.50 | 0.50 |
| Upper limit of optimum soil moisture (SM2) | 1.00 | 1.00 |
| Upper threshold of soil moisture (SM3) | 1.80 | 1.80 |
| Lower threshold temperature (DV0) | 15.7 °C | 15.7 °C |
| Lower optimum temperature (DV1) | 18.0 °C | 18.0 °C |
| Upper optimum temperature (DV2) | 33.0 °C | 33.0 °C |
| Upper threshold temperature (DV3) | 36.0 °C | 36.0 °C |
| Cold stress temperature threshold (TTCS) | 2.00 °C | 2.00 °C |
| Cold stress accumulation rate (THCS) | −0.10/week | −0.10/week |
| Heat stress temperature threshold (TTHS) | 36.0 °C | 36.0 °C |
| Heat stress accumulation rate (THHS) | 0.005/week | 0.005/week |
| Dry stress soil moisture threshold (SMDS) | 0.10 | 0.10 |
| Dry stress accumulation rate (HDS) | −0.005/week | −0.0025/week |
| Wet stress soil moisture threshold (SMWS) | 1.80 | 1.80 |
| Wet stress accumulation rate (HWS) | 0.002/week | 0.001/week |
| Degree days taken to complete one generation (PDD) | 415.40 °C·d | 415.40 °C·d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, Y.; Xian, X.; Zhao, H.; Guo, J.; Yang, N.; Gong, Z.; Liu, W.; Peng, Z. Projection of the Potential Global Geographic Distribution of the Solanum Fruit Fly Bactrocera latifrons (Hendel, 1912) (Diptera: Tephritidae) Based on CLIMEX Models. Horticulturae 2024, 10, 977. https://doi.org/10.3390/horticulturae10090977
Wei Y, Xian X, Zhao H, Guo J, Yang N, Gong Z, Liu W, Peng Z. Projection of the Potential Global Geographic Distribution of the Solanum Fruit Fly Bactrocera latifrons (Hendel, 1912) (Diptera: Tephritidae) Based on CLIMEX Models. Horticulturae. 2024; 10(9):977. https://doi.org/10.3390/horticulturae10090977
Chicago/Turabian StyleWei, Yajie, Xiaoqing Xian, Haoxiang Zhao, Jianyang Guo, Nianwan Yang, Zhi Gong, Wanxue Liu, and Zhengqiang Peng. 2024. "Projection of the Potential Global Geographic Distribution of the Solanum Fruit Fly Bactrocera latifrons (Hendel, 1912) (Diptera: Tephritidae) Based on CLIMEX Models" Horticulturae 10, no. 9: 977. https://doi.org/10.3390/horticulturae10090977
APA StyleWei, Y., Xian, X., Zhao, H., Guo, J., Yang, N., Gong, Z., Liu, W., & Peng, Z. (2024). Projection of the Potential Global Geographic Distribution of the Solanum Fruit Fly Bactrocera latifrons (Hendel, 1912) (Diptera: Tephritidae) Based on CLIMEX Models. Horticulturae, 10(9), 977. https://doi.org/10.3390/horticulturae10090977








