Physio-Morphological Traits Contributing to Genotypic Differences in Nitrogen Use Efficiency of Leafy Vegetable Species under Low N Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material, Treatments, and Experimental Design
2.2. Plant Growth and Biomass Partitioning
2.3. Leaf Physiological Measurements
- (A)
- Total chlorophyll (a + b) (µmol g−1) = [(5.24 WL664.2 + 22.24 WL648.6) × 8.1]/sample (g).
- (B)
- Chlorophyll a (µmol g−1) = [(13.36 WL664.2 − 5.19 WL648.6) × 8.1]/sample (g).
- (C)
- Chlorophyll b (µmol g−1) = [(27.43 WL648.6 − 8.12 WL664.2) × 8.1]/sample (g).
- (D)
- Total carotenoids (µmol g−1) = [(4.785 WL470 + 3.657 WL664.2 − 12.76 WL648.6) × 8.1]/sample (g).
2.4. Root Morphological Measurements
2.5. Leaf Nitrate Reductase Enzyme Activity (NRA) Measurement
2.6. Data Analysis
3. Results
3.1. Biomass Production and Partitioning
3.2. Total Leaf Number, Total Leaf Area, Photosynthetic Activity, and Leaf Chlorophyll Index (SPAD)
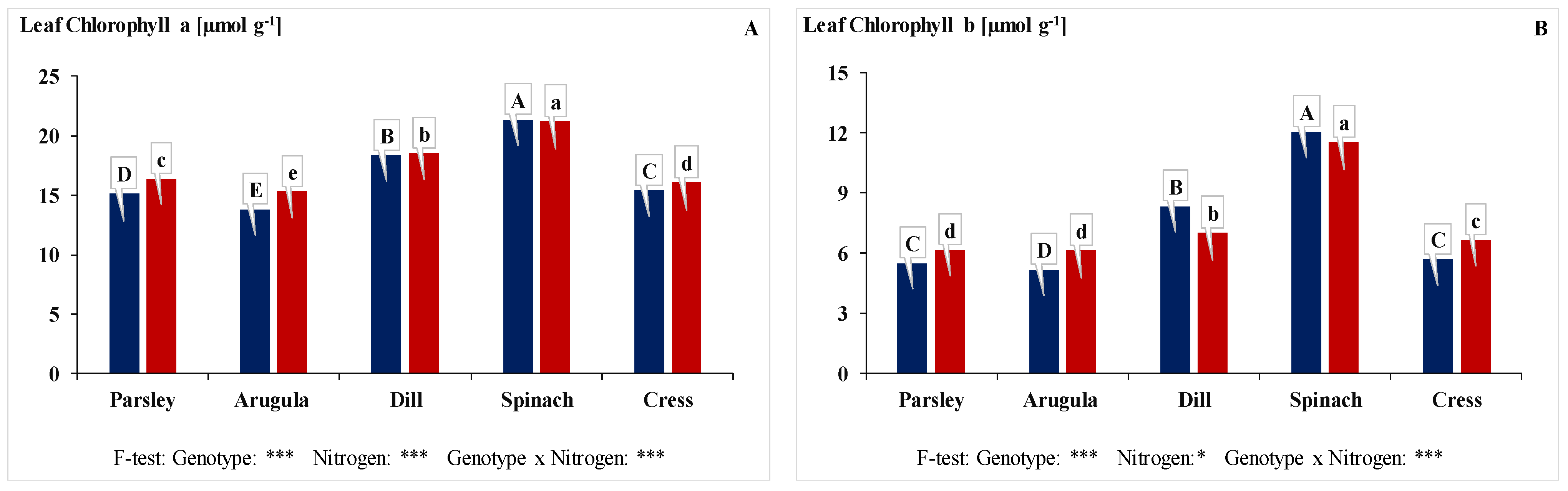
3.3. Leaf Chlorophyll (a + b) Content, Leaf Carotenoid Content, and Leaf Nitrate Reductase Enzyme Activity
3.4. Root Morphology
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zeifman, L.; Hertog, S.; Kantorova, V.; Wilmoth, J. A World of 8 Billion. 5 August 2023. Available online: https://www.un.org/development/desa/pd/sites/www.un.org.development.desa.pd/files/undesa_pd_2022_pb_140.pdf (accessed on 12 July 2024).
- Adam, M.B.; Ulas, A. Vigorous rootstocks improve nitrogen efficiency of tomato by inducing morphological, physiological and biochemical responses. Gesunde Pflanz. 2023, 75, 565–575. [Google Scholar] [CrossRef]
- Liu, C.W.; Sung, Y.; Chen, B.C.; Lai, H.Y. Effects of nitrogen fertilizers on the growth and nitrate content of lettuce (Lactuca sativa L.). Int. J. Environ. Res. Public Health 2014, 11, 4427–4440. [Google Scholar] [CrossRef] [PubMed]
- Grindlay, D.J.C. Towards an explanation of crop nitrogen demand based on the optimization of leaf nitrogen per unit leaf area. J. Agric. Sci. 1997, 128, 377–396. [Google Scholar] [CrossRef]
- Brégard, A.; Bélanger, G.; Michaud, R. Nitrogen use efficiency and morphological characteristics of timothy populations selected for low and high forage nitrogen concentrations. Crop Sci. 2000, 40, 422–429. [Google Scholar] [CrossRef]
- Schroder, J.L.; Zhang, H.; Girma, K.; Raun, W.R.; Penn, C.J.; Payton, M.E. Soil acidification from long-term use of nitrogen fertilizers on winter wheat. Soil Sci. Soc. Am. J. 2011, 75, 957–964. [Google Scholar] [CrossRef]
- Slavin, J.L.; Lloyd, B. Health benefits of fruits and vegetables. Adv. Nutr. 2012, 3, 506–516. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Gupta, A.K.; Gaur, V.S.; Kumar, A. Relationship of nitrogen use efficiency with the activities of enzymes involved in nitrogen uptake and assimilation of finger millet genotypes grown under different nitrogen inputs. Sci. World J. 2012, 2012, 625731. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Khan, M.M.A.; Naeem, M. Effect of nitrogen on growth, nutrient assimilation, essential oil content, yield and quality attributes in Zingiber officinale Rosc. J. Saudi Soc. Agric. Sci. 2016, 15, 171–178. [Google Scholar] [CrossRef]
- Raun, W.R.; Johnson, G.V. Improving nitrogen use efficiency for cereal production. Agron. J. 1999, 91, 357–363. [Google Scholar] [CrossRef]
- Byrnes, B.H.; Bumb, B.L. Population growth, food production and nutrient requirements. J. Crop Prod. 1998, 1, 1–27. [Google Scholar] [CrossRef]
- Laegreid, M.; Bockman, O.C.; Kaarstad, O. Agriculture, Fertilisers and the Environment; CABI Publishing: Wallingford, UK, 1999; p. 294. [Google Scholar]
- Watanabe, N.S.; Yamasaki, H. Dynamics of Nitrite Content in Fresh Spinach Leaves: Evidence for Nitrite Formation Caused by Microbial Nitrate Reductase Activity. J. Nutr. Food Sci. 2016, 7, 572. [Google Scholar] [CrossRef]
- Yamasaki, H.; Watanabe, N.S.; Fukuto, J.; Cohen, M.F. Nitrite-Dependent Nitric Oxide Production Pathway: Diversity of NO Production Systems. In Studies on Pediatric Disorders; Oxidative Stress in Applied Basic Research and Clinical Practice; Tsukahara, H., Kaneko, K., Eds.; Springer: New York, NY, USA, 2014. [Google Scholar]
- Schroeder, J.I.; Delhaize, E.; Frommer, W.B.; Guerinot, M.L.; Harrison, M.J.; Herrera-Estrella, L.; Horie, T.; Kochian, L.V.; Munns, R.; Nishizawa, N.K.; et al. Using membrane transporters to improve crops for sustainable food production. Nature 2013, 497, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Hanif, M.N. Factors affecting nitrogen use efficiency (NUE): Meta-analysis. Turk. J. Agric. Res. 2023, 10, 231–242. [Google Scholar] [CrossRef]
- Hoffer, G.N. Some differences in the functioning of selfed lines of corn under varying nutritional conditions. Agron. J. 1926, 18, 322–334. [Google Scholar] [CrossRef]
- Ulas, A.; Doganci, E.; Ulas, F.; Yetisir, H. Root-growth characteristics contributing to genotypic variation in nitrogen efficiency of bottle gourd and rootstock potential for watermelon. Plants 2019, 8, 77. [Google Scholar] [CrossRef]
- Graham, R.D. Breeding characteristics in cereals. Adv. Plant Nutr. 1984, 1, 57–102. [Google Scholar]
- Sattelmacher, B.; Horst, W.J.; Becker, H.C. Factors that contribute to genetic variation for nutrient efficiency of crop plants. Z. Pflanzenernährung Bodenkd. 1994, 157, 215–224. [Google Scholar] [CrossRef]
- Tennessen, D.J.; Singsaas, E.L.; Sharkey, T.D. Light-emitting diodes as a light source of photosynthesis research. Photosynth. Res. 1994, 39, 85–92. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar]
- Harley, S. Use of a simple colorimetric assay to determine conditions for induction of nitrate reductase in plants. Am. Biol. Teach. 1993, 55, 161–164. [Google Scholar] [CrossRef]
- Goltsev, V.N.; Kalaji, H.M.; Paunov, M.; Bąba, W.; Horaczek, T.; Mojski, J.; Kociel, H.; Allakhverdiev, S.I. Variable chlorophyll fluorescence and its use for assessing physiological condition of plant photosynthetic apparatus. Russ. J. Plant Physiol. 2016, 63, 869–893. [Google Scholar] [CrossRef]
- SAS Institute Inc. Base SASVR 9.4 Procedures Guide: Stat. Procedures, 2nd ed.; SAS Institute, Inc.: Cary, NC, USA, 2013. [Google Scholar]
- Ulas, A.; Erley, G.S.A.; Kamh, M.; Wiesler, F.; Horst, W.J. Root-growth characteristics 448 contributing to genotypic variation in nitrogen efficiency of oilseed rape. J. Plant Nutr. Soil Sci. 2012, 175, 489–498. [Google Scholar] [CrossRef]
- Ulas, F.; Erdogdu, S.; Yetisir, H.; Ulas, A. Investigation on morphology and physiology of Nitrogen efficiency in different pepper (Capsicum annuum L.) inbred lines. Genetika 2021, 53, 3. [Google Scholar] [CrossRef]
- Ulas, F.; Yetisir, H.; Ulas, A. Root-growth characteristics contributing to nitrogen efficiency of reciprocally grafted potatoes (Solanum tuberosum L.) under Hydroponic conditions. Gesunde Pflanz. 2021, 73, 417–425. [Google Scholar] [CrossRef]
- Clarkson, D.T. Factors affecting mineral nutrient acquisition by plants. Annu. Rev. Plant Physiol. 1985, 36, 77–115. [Google Scholar] [CrossRef]
- Jackson, W.A.; Pan, W.L.; Moll, R.H.; Kamprath, E.J. Uptake, translocation, and reduction of nitrate. Biochem. Basis Plant Breed. 1986, 2, 73–108. [Google Scholar]
- Lewin, S.A.; Mooney, H.A.; Field, C. The dependence of plant root: Shoot ratios on internal nitrogen concentration. Ann. Bot. 1989, 64, 71–75. [Google Scholar]
- Merrill, S.D.; Tanaka, D.L.; Hanson, J.D. Root length growth of eight crop species in haplustoll soils. Soil Sci. Soc. Am. J. 2002, 66, 913–923. [Google Scholar] [CrossRef]
- Hirasawa, T.; Hsiao, T.C. Some characteristics of reduced leaf photosynthesis at midday in maize growing in the field. Field Crops Res. 1999, 62, 53–62. [Google Scholar] [CrossRef]
- Zheng, Z.L. Carbon and nitrogen nutrient balance signaling in plants. J. Plant Signal Behav. 2009, 4, 584–591. [Google Scholar] [CrossRef]
- Mae, T. Physiological nitrogen efficiency in rice: Nitrogen utilization, photosynthesis and yield potential. Plant Soil 1997, 196, 201–210. [Google Scholar] [CrossRef]
- Marschner, H. Mineral Nutrition of Higher Plants, 2nd ed.; Academic Press: London, UK, 1995. [Google Scholar]
- de González, M.T.N.; Osburn, W.N.; Hardin, M.D.; Longnecker, M.; Garg, H.K. A Survey of nitrate and nitrite concentrations in conventional and organic-labeled raw vegetables at retail. J. Food Sci. 2015, 80, 942–949. [Google Scholar] [CrossRef]
- Ndukwe, O.K.; Edeoga, H.O.; Okwulehie, I.C.; Omosun, G. Variability in the chlorophyll and carotene composition of ten maize (Zea mays) varieties. Eur. J. Phys. Agric. Sci. 2016, 4, 1–6. [Google Scholar]
- Chen, T.W.; Kahlen, K.; Stützel, H. Disentangling the contributions of osmotic and ionic effects of salinity on stomatal, mesophyll, biochemical and light limitations to photosynthesis. Plant Cell Environ. 2015, 38, 1528–1542. [Google Scholar] [CrossRef]
- Hristov, A.; Bannink, A.; Crompton, L.; Huhtanen, P.; Kreuzer, M.; McGee, M.; Nozière, P.; Reynolds, C.; Bayat, A.; Yáñez-Ruiz, D.; et al. Invited review: Nitrogen in ruminant nutrition: A review of measurement techniques. J. Dairy Sci. 2019, 102, 5811–5852. [Google Scholar] [CrossRef]

| Shoot Fresh Weight [g Plant−1] | Root Fresh Weight [g Plant−1] | Plant Height [cm Plant−1] | ||||
|---|---|---|---|---|---|---|
| Genotypes | Low N | High N | Low N | High N | Low N | High N |
| Parsley | 4.10 d | 4.93 D | 2.34 e | 2.84 D | 13 c | 14 BC |
| Arugula | 19.10 a | 15.10 A | 5.57 a | 4.71 A | 15 b | 14 B |
| Dill | 7.50 c | 9.00 B | 3.14 d | 4.28 B | 19 a | 20 A |
| Spinach | 11.09 b | 8.70 B | 3.31 c | 3.65 C | 13 c | 12 CD |
| Cress | 11.11 b | 7.30 C | 3.38 b | 2.87 D | 13 c | 12 D |
| F-test | ||||||
| Genotype | *** | *** | *** | |||
| Nitrogen | *** | *** | n.s. | |||
| Genotype × Nitrogen | *** | *** | n.s. | |||
| Shoot Dry Weight [g Plant−1] | Root Dry Weight [g Plant−1] | Root–Shoot Ratio [g g−1] | ||||
|---|---|---|---|---|---|---|
| Genotypes | Low N | High N | Low N | High N | Low N | High N |
| Parsley | 0.42 c | 0.50 C | 0.09 d | 0.10 C | 0.21 a | 0.20 AB |
| Arugula | 1.60 a | 1.26 A | 0.25 a | 0.24 A | 0.16 c | 0.19 BC |
| Dill | 0.60 c | 0.67 BC | 0.11 d | 0.13 BC | 0.18 bc | 0.19 BC |
| Spinach | 1.10 b | 0.90 B | 0.15 c | 0.17 B | 0.13 d | 0.18 C |
| Cress | 1.00 b | 0.70 BC | 0.19 b | 0.17 B | 0.20 ab | 0.24 A |
| F-test | ||||||
| Genotype | *** | *** | ** | |||
| Nitrogen | *** | n.s. | ** | |||
| Genotype × Nitrogen | *** | n.s. | n.s. | |||
| Total Leaf Chlorophyll (a + b) [µmol g−1] | Total Leaf Carotenoids [µmol g−1] | Leaf Nitrate Reductase Enzyme Activity [µmol s−1 g−1] | ||||
|---|---|---|---|---|---|---|
| Genotypes | Low N | High N | Low N | High N | Low N | High N |
| Parsley | 22.53 c | 20.66 D | 3.47 c | 3.38 C | 3.274 a | 2.385 A |
| Arugula | 21.46 d | 19.00 E | 2.78 d | 2.62 E | 0.459 c | 0.370 D |
| Dill | 25.54 b | 26.69 B | 3.85 b | 3.51 B | 2.257 b | 0.627 C |
| Spinach | 32.77 a | 33.38 A | 4.15 a | 4.27 A | 0.433 d | 0.771 B |
| Cress | 22.73 c | 21.11 C | 2.76 d | 2.66 D | 0.250 e | 0.285 E |
| F test | ||||||
| Genotype | *** | *** | *** | |||
| Nitrogen | *** | * | *** | |||
| Genotype × Nitrogen | *** | *** | *** | |||
| Total Root Length [cm Plant−1] | Total Root Volume [cm3 Plant−1] | Av. Root Diameter [mm Plant−1] | ||||
|---|---|---|---|---|---|---|
| Genotypes | Low N | High N | Low N | High N | Low N | High N |
| Parsley | 1563 e | 1732 E | 0.683 e | 0.797 E | 0.237 c | 0.244 C |
| Arugula | 5840 a | 5990 A | 1.867 a | 2.353 A | 0.203 d | 0.216 E |
| Dill | 1952 d | 2959 C | 0.982 d | 1.514 C | 0.264 a | 0.264 A |
| Spinach | 2052 c | 2585 D | 1.462 c | 1.558 B | 0.251 b | 0.256 B |
| Cress | 3717 b | 3544 B | 1.665 b | 1.432 D | 0.236 c | 0.227 D |
| F test | ||||||
| Genotype | *** | *** | *** | |||
| Nitrogen | *** | *** | ** | |||
| Genotype × Nitrogen | *** | *** | *** | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ulas, F.; Yücel, Y.C.; Ulas, A. Physio-Morphological Traits Contributing to Genotypic Differences in Nitrogen Use Efficiency of Leafy Vegetable Species under Low N Stress. Horticulturae 2024, 10, 984. https://doi.org/10.3390/horticulturae10090984
Ulas F, Yücel YC, Ulas A. Physio-Morphological Traits Contributing to Genotypic Differences in Nitrogen Use Efficiency of Leafy Vegetable Species under Low N Stress. Horticulturae. 2024; 10(9):984. https://doi.org/10.3390/horticulturae10090984
Chicago/Turabian StyleUlas, Firdes, Yusuf Cem Yücel, and Abdullah Ulas. 2024. "Physio-Morphological Traits Contributing to Genotypic Differences in Nitrogen Use Efficiency of Leafy Vegetable Species under Low N Stress" Horticulturae 10, no. 9: 984. https://doi.org/10.3390/horticulturae10090984
APA StyleUlas, F., Yücel, Y. C., & Ulas, A. (2024). Physio-Morphological Traits Contributing to Genotypic Differences in Nitrogen Use Efficiency of Leafy Vegetable Species under Low N Stress. Horticulturae, 10(9), 984. https://doi.org/10.3390/horticulturae10090984









