Protocol for Pre-Selection of Dwarf Garden Rose Varieties
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Site Conditions
2.2. Morphological, Anatomical, and Histological Assessment
2.3. Statistical Analyses
3. Results
3.1. Morphology and Anatomy of the Investigated Rose Cultivars
3.2. Cambial Plasticity and the Formation of Growth Rings
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Adhikary, K.; Sarkar, M.M. Varietal evaluation of miniature rose cultivars under the plains of West Bengal, India. J. Pharmacogn. Phytochem. 2019, 8, 1618–1621. [Google Scholar]
- De Vries, D.P.; Dubois, L.A.M. On the inheritance of the dwarf character in polyantha × Rosa chinensis minima (Sims) Voss F1 populations. Euphytica 1987, 36, 535–539. [Google Scholar]
- Shepherd, R.E. History of the Rose; Mackmillan: New York, NY, USA, 1954. [Google Scholar]
- Michonneau, P.; Roblin, G.; Béré, E.; Fleurat-Lessard, P.; Atanassova, R. Adaptive responses of miniature rose to cultivation modes and abiotic stresses. Trees 2021, 35, 809–829. [Google Scholar] [CrossRef]
- Zlesak, D.C. Rose. In Flower Breeding and Genetics: Issues, Challenges and Opportunities for the 21st Century; Anderson, N.O., Ed.; Springer: Dordrecht, The Netherlands, 2006; pp. 695–738. [Google Scholar]
- Borrell, J.S.; Dodsworth, S.; Forest, F.; Pérez-Escobar, O.A.; Lee, M.A.; Mattana, E.; Stevenson, P.C.; Howes, M.-J.R.; Pritchard, H.W.; Ballesteros, D.; et al. The climatic challenge: Which plants will people use in the next century? Environ. Exp. Bot. 2020, 170, 103872. [Google Scholar] [CrossRef]
- Ouyang, L.; Leus, L.; Van Labeke, M.C. Three-year screening for cold hardiness of garden roses. Sci. Hortic. 2019, 245, 12–18. [Google Scholar] [CrossRef]
- Urban, L. Influences of Abiotic Factors in Growth and Development. In Encyclopedia of Rose Science; Roberts, A.V., Debener, T., Gudin, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2003; Volume 1, pp. 369–374. [Google Scholar]
- Amoroso, G.; Piatti, R.; Frangi, P. Evaluation of ground-cover roses in Northern Italy. Acta Hortic. 2010, 885, 47–53. [Google Scholar] [CrossRef]
- Simin, N.; Lesjak, M.; Živanović, N.; Božanić Tanjga, B.; Orčić, D.; Ljubojević, M. Morphological Characters, Phytochemical Profile and Biological Activities of Novel Garden Roses Edible Cultivars. Horticulturae 2023, 9, 1082. [Google Scholar] [CrossRef]
- UPOV. Guidelines for the Conduct of Tests Distinctness, Uniformity and Stability—Rosa L.; International Union for the Protection of New Varieties of Plants: Geneva, Switzerland, 2010. [Google Scholar]
- Jones, A.; Reed, R.; Weyers, J. Practical Skills in Biology; Pearson Education Limited: London, UK, 2003. [Google Scholar]
- Morales-Orellana, R.J.; Winkelmann, T.; Bettin, A.; Rath, T. Stimulation of adventitious root formation by laser wounding in rose cuttings: A matter of energy and pattern. Front. Plant Sci. 2022, 13, 1009085. [Google Scholar] [CrossRef]
- Toft, B.D.; Alam, M.M.; Topp, B.L. Anatomical structure associated with vegetative growth variation in macadamia. Plant Soil 2019, 444, 343–350. [Google Scholar] [CrossRef]
- Cohen, G.; Mascarini, L.; Xifreda, C.C. Anatomía y micromorfología de hojas y tallos de dos cultivares de Rosa hybrida L. para flor de corte. Rev. Int. Botánica Exp. 2012, 81, 199–204. [Google Scholar]
- Monder, M.J.; Bąbelewski, P.; Sołtan, S. Diversity in anatomical features of rose rootstock root necks: Rosa canina ‘Inermis’, ‘Pfänder’, ‘Schmid’s Ideal’, Rosa laxa Retz. and Rosa multiflora Thunb. Sci. Hortic. 2023, 316, 112004. [Google Scholar] [CrossRef]
- Kurian, R.M.; Iyer, C.P.A. Stem anatomical characters in relation to tree vigour in mango (Mangifera indica L.). Sci. Hortic. 1992, 50, 245–253. [Google Scholar] [CrossRef]
- Chen, B.; Wang, C.; Tian, Y.; Chu, Q.; Hu, C. Anatomical characteristics of young stems and mature leaves of dwarf pear. Sci. Hortic. 2015, 186, 172–179. [Google Scholar] [CrossRef]
- Zorić, L.; Ljubojević, M.; Merkulov, L.; Luković, J.; Ognjanov, V. Anatomical Characteristics of Cherry Rootstocks as Possible Preselecting Tools for Prediction of Tree Vigor. J. Plant. Growth. Regul. 2012, 31, 320–331. [Google Scholar] [CrossRef]
- Kim, K.S.; Beard, J.B. Comparative turfgrass evapotranspiration rates and associated plant morphological characteristics. Crop Sci. 1988, 28, 328–331. [Google Scholar] [CrossRef]
- Tyree, M.T.; Sperry, J.S. Vulnerability of xylem to cavitation and embolism. Annu. Rev. Plant Phys. 1989, 40, 19–36. [Google Scholar] [CrossRef]
- Williams, M.H.; Rosenqvist, E.; Buchhave, M. Response of potted miniature roses (Rosa × hybrida) to reduced water availability during production. J. Hortic. Sci. Biotechnol. 1999, 74, 301–308. [Google Scholar] [CrossRef]
- Riseman, A.; Jensen, C.; Williams, M. Stomatal conductivity and osmotic adjustment during acclimation to multiple cycles of drought stress in potted miniature rose (Rosa × hybrida). J. Hortic. Sci. Biotechnol. 2001, 76, 138–144. [Google Scholar] [CrossRef]
- Vaganov, E.A.; Hughes, M.K.; Shashkin, A.V. Environmental control of xylem differentiation. In Growth Dynamics of Conifer Tree Rings: Images of Past and Future Environments; Caldwell, M.M., Heldmaier, G., Jackson, R.B., Lange, O.L., Mooney, H.A., Schulze, E.-D., Sommer, U., Eds.; Ecological Studies; Springer: Berlin/Heidelberg, Germany, 2006; Volume 183, pp. 151–187. [Google Scholar] [CrossRef]
- Türker, M.; Yörük, I.; Battal, P.; Kazankaya, A.; Tileklioğlu, B. Seasonal changes in cambial activity in Rosa canina. Acta Hort. 2005, 690, 217–222. [Google Scholar] [CrossRef]
- Prislan, P.; Gričar, J.; de Luis, M.; Smith, K.T.; Čufar, K. Phenological variation in xylem and phloem formation in Fagus sylvatica from two contrasting sites. Agric. For. Meteorol. 2013, 180, 142–151. [Google Scholar] [CrossRef]
- Zhu, L.; Cooper, D.J.; Yuan, D.; Li, Z.; Zhang, Y.; Liang, H.; Wang, X. Regional scale temperature rather than precipitation determines vessel features in earlywood of Manchurian ash in temperate forests. J. Geophys. Res. Biogeosci. 2020, 125, e2020JG005955. [Google Scholar] [CrossRef]
- Gričar, J.; Prislan, P.; de Luis, M.; Gryc, V.; Hacurová, J.; Vavrčík, H.; Čufar, K. Plasticity in variation of xylem and phloem cell characteristics of Norway spruce under different local conditions. Front. Plant Sci. 2015, 6, 730. [Google Scholar] [CrossRef] [PubMed]
- Mäkinen, H.; Nöjd, P.; Kahle, H.-P.; Neumann, U.; Tveite, B.; Mielikäinen, K.; Röhle, H.; Spiecker, H. Large-scale climatic variability and radial increment variation of Picea abies (L.) Karst. in central and northern Europe. Trees 2003, 17, 173–184. [Google Scholar] [CrossRef]
- Ren, P.; Rossi, S.; Gričar, J.; Liang, E.; Čufar, K. Is precipitation a trigger for the onset of xylogenesis in Juniperus przewalskii on the north-eastern Tibetan Plateau? Ann. Bot. 2015, 115, 629–639. [Google Scholar] [CrossRef] [PubMed]
- Ljubojević, M.; Narandžić, T. Roots Before Branches: Evidence of the Prunus Root Cambial Responses to the Environmental Stimuli. J. Plant Growth Regul. 2023, 42, 4240–4252. [Google Scholar] [CrossRef]
- Dinella, A.; Giammarchi, F.; Prendin, A.L.; Carrer, M.; Tonon, G. Xylem traits of peatland Scots pines reveal a complex climatic signal: A study in the Eastern Italian Alps. Dendrochronologia 2021, 67, 125824. [Google Scholar] [CrossRef]
- Wang, W.; Huang, J.G.; Zhang, T.; Qin, L.; Jiang, S.; Zhou, P.; Zhang, Y.; Peñuelas, J. Precipitation regulates the responses of xylem phenology of two dominant tree species to temperature in arid and semi-arid forest of the southern Altai Mountains. Sci. Total Environ. 2023, 886, 163951. [Google Scholar] [CrossRef]

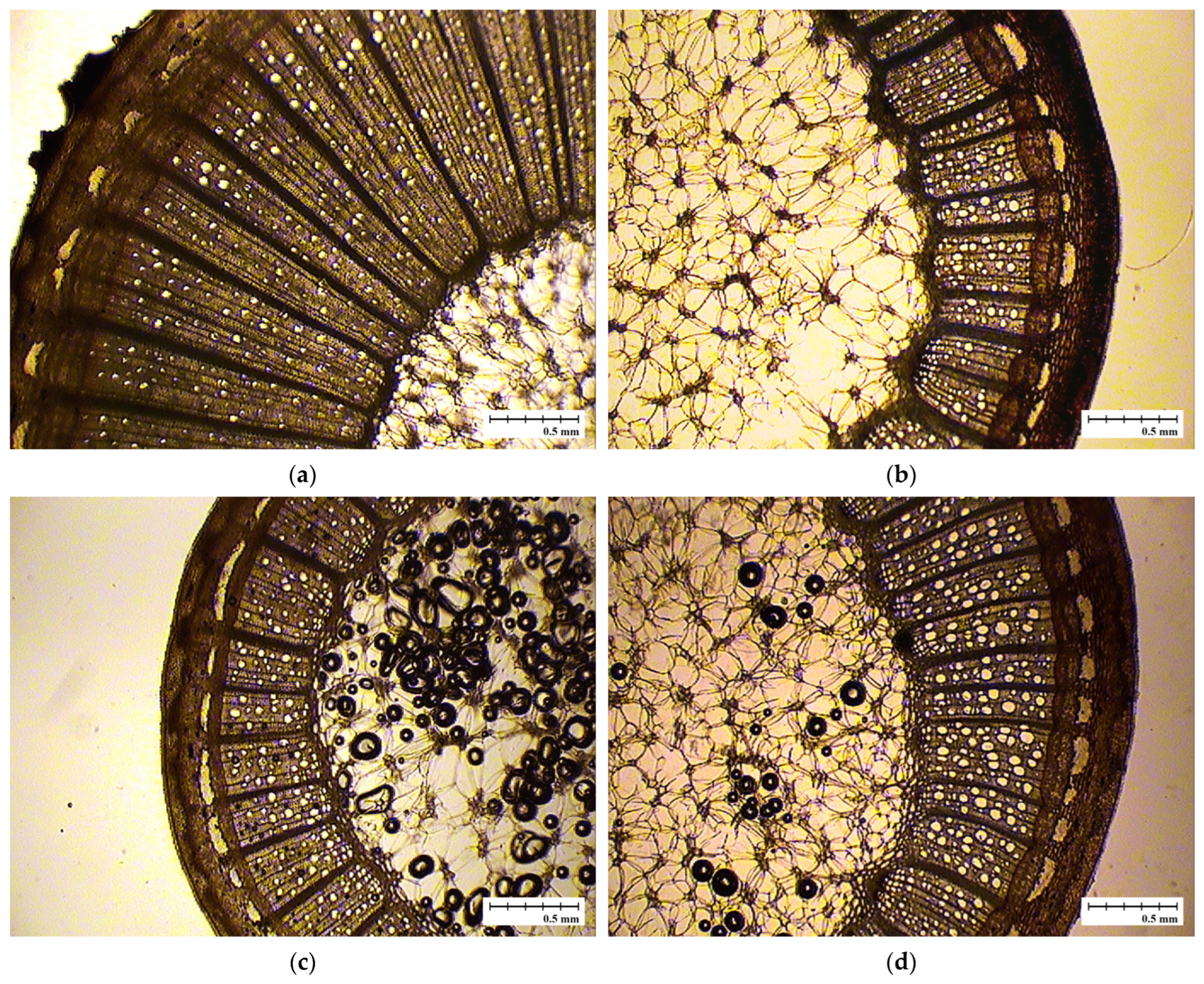


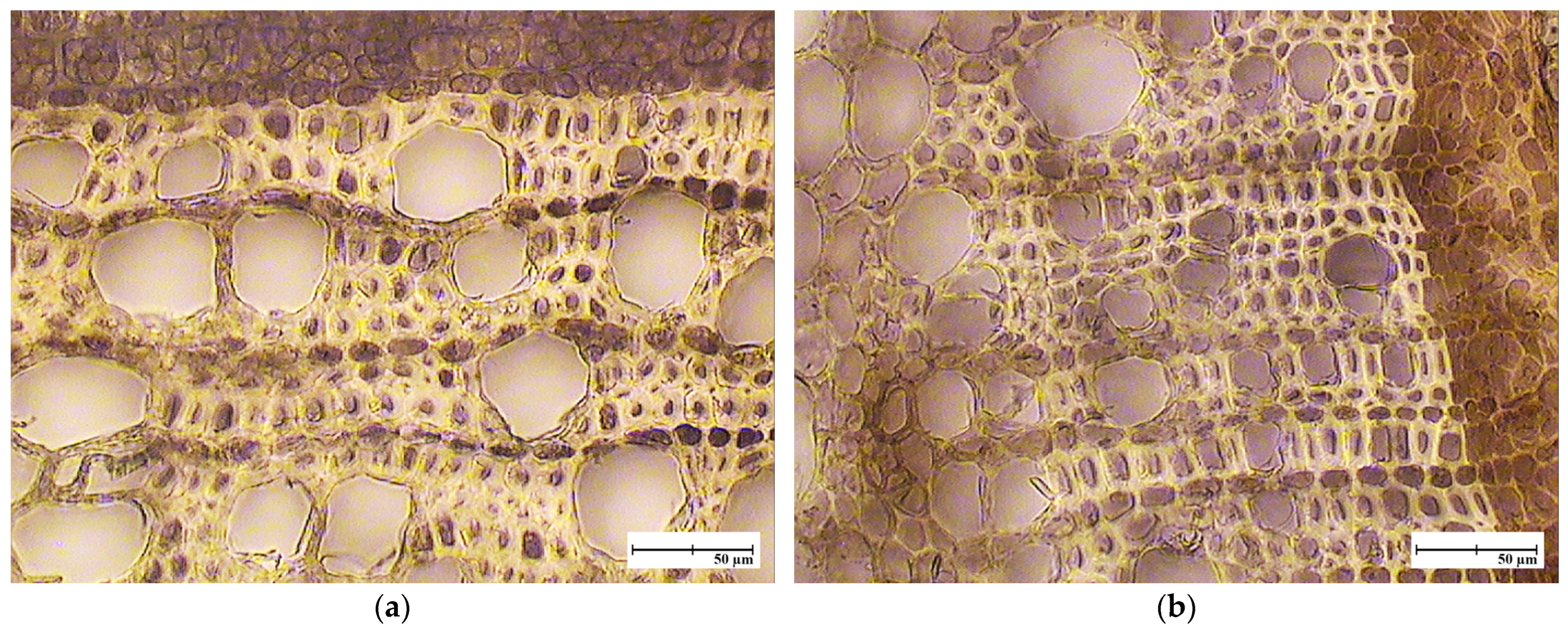
| Cultivar Name | Seed Parent | Pollen Source |
|---|---|---|
| ‘Blush Pixie‘ | ‘Raspberry Royal’ | ‘Pink Tiara’ |
| ‘Coral Pixie’ | ‘Austriana’ | ‘Raspberry Royal’ |
| ‘Gaudi Pixie’ | ‘Winnipeg Parks’ | ‘David Thompson’ |
| ‘Mauve Pixie’ | ‘Violette Parfumee’ | ‘Raspberry Royal’ |
| ‘Milky Pixie’ | ‘Diamond Border’ | ‘Amber Cover’ |
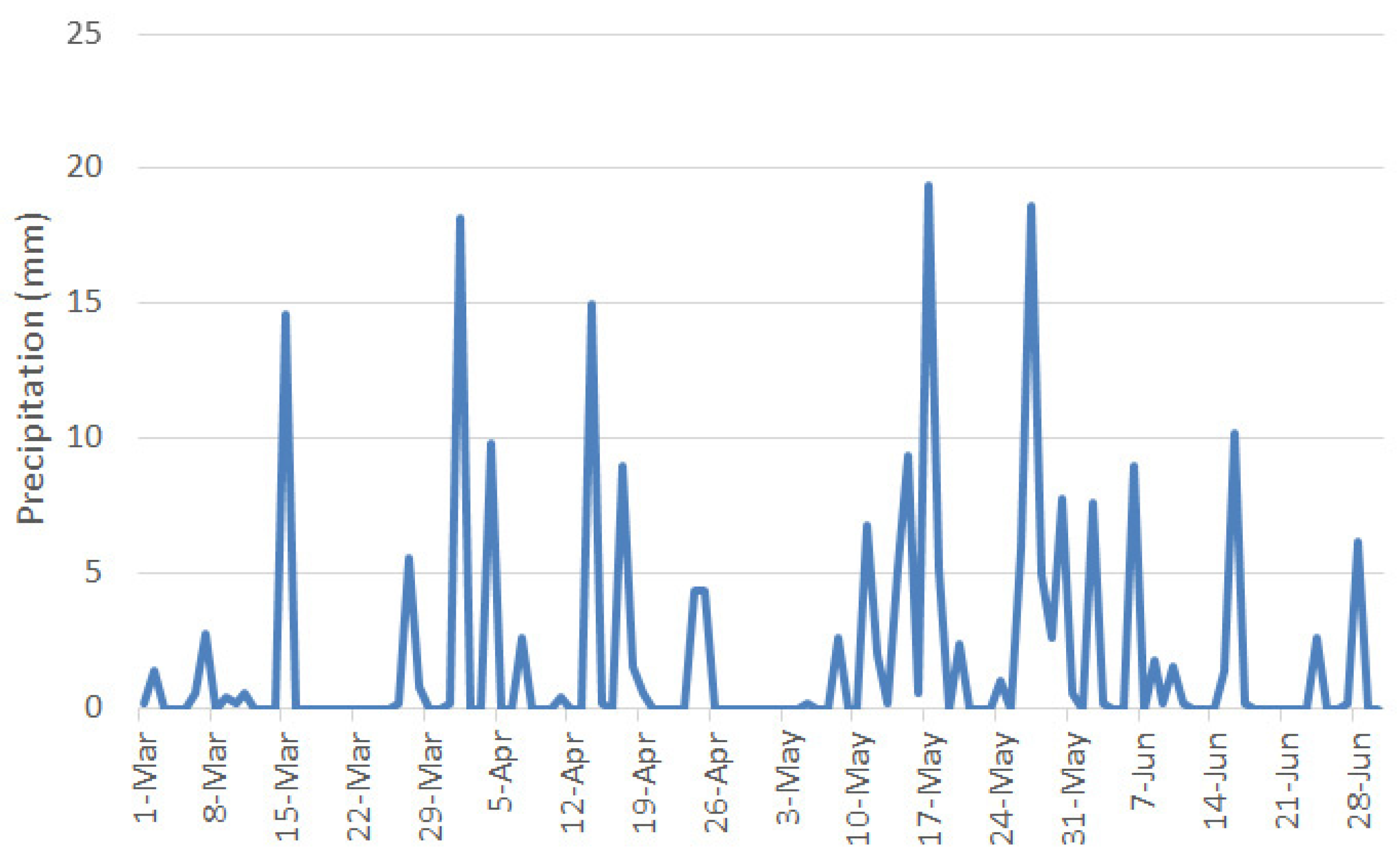
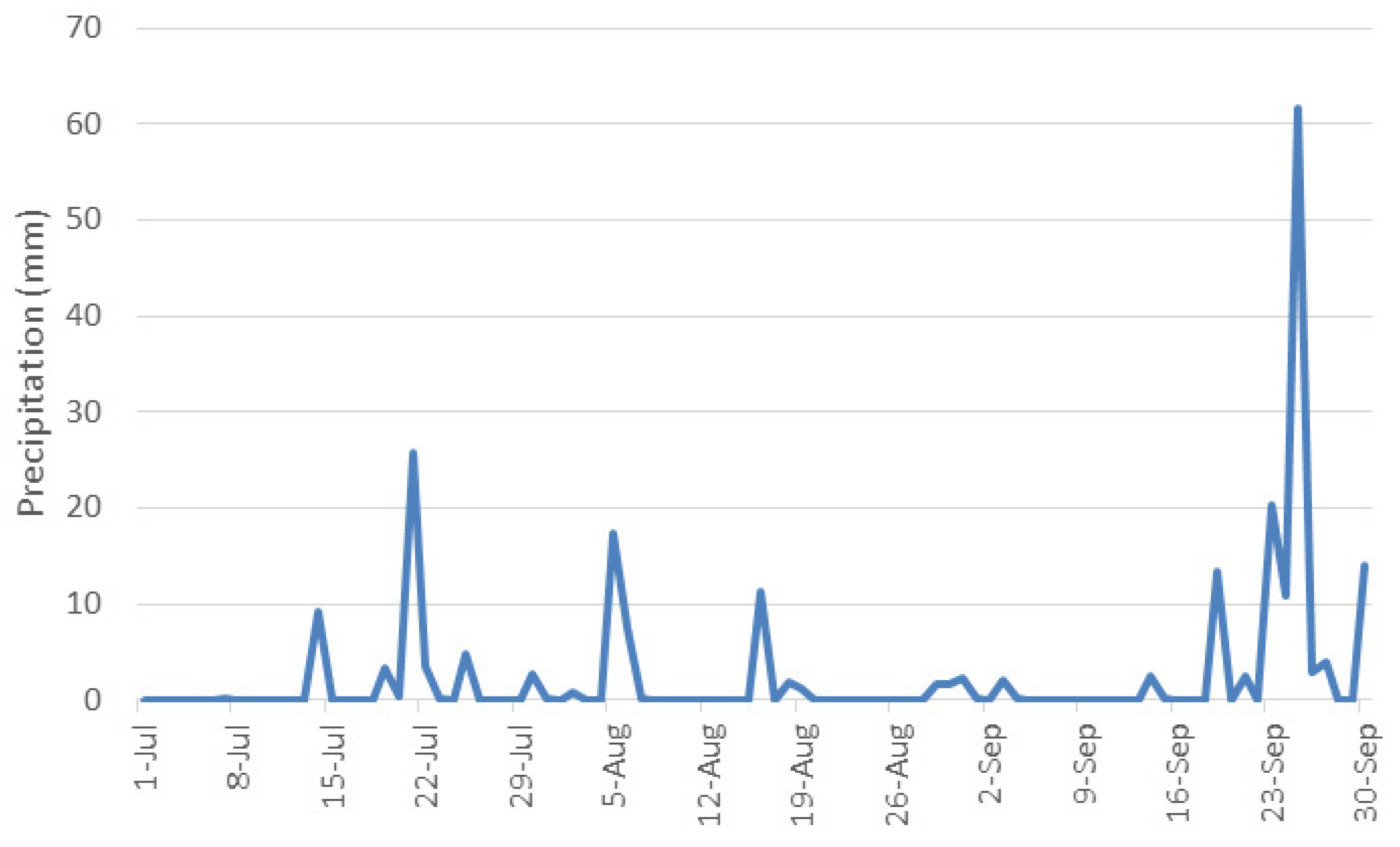
| Air Temperature (°C) | Relative Humidity (%) | Precipitation Sum (mm) | |||
|---|---|---|---|---|---|
| Avg | Max | Min | |||
| January | 4.61 | 18.45 | −1.64 | 15.16 | 77.6 |
| February | 3.61 | 18.59 | −10.25 | 0.04 | 67.6 |
| March | 8.87 | 25.25 | −3.02 | 29.64 | 27.6 |
| April | 10.36 | 23.46 | −1.42 | 73.34 | 66.2 |
| May | 16.78 | 28.84 | 3.69 | 76.38 | 95.6 |
| June | 20.72 | 36.45 | 10.21 | 77.04 | 41.4 |
| July | 24.14 | 37.06 | 10.26 | 69.55 | 50.2 |
| August | 23.11 | 37.96 | 9.41 | 73.97 | 45.6 |
| September | 20.59 | 33.99 | 9.99 | 70.39 | 84.2 |
| October | 16.07 | 28.9 | −1.19 | 70.95 | 88.4 |
| November | 7.87 | 21.76 | −3.56 | 85.83 | 58.8 |
| December | 4.75 | 20.91 | −7.76 | 88.74 | 48.4 |
| ‘Morava Reka’ | ‘Blush Pixie‘ | ‘Coral Pixie’ | ‘Gaudi Pixie’ | ‘Mauve Pixie’ | ‘Milky Pixie’ | |
|---|---|---|---|---|---|---|
| Plant characteristics | ||||||
| Growth type * | shrub | ground cover | ground cover | shrub | shrub | shrub |
| Growth habit | upright | strongly spreading | moderately spreading | intermediate | semi upright | intermediate |
| Height (cm) | 40 c | 52 b | 67 a | 68 a | 50 b | 20 d |
| Flower characteristics | ||||||
| Type | double | double | double | double | double | double |
| Color group | 46A | 068B | 045A | N066B | 061B | NN159 |
| Color of center | red | pink | red | pink | purple | white |
| Shape | star-shaped | rounded | irregularly rounded | rounded | rounded | star-shaped |
| Upper part profile | flat | flat | flat | flattened convex | flat | flattened convex |
| Lower part profile | concave | flat | flat | flat | flat | flat |
| Diameter (cm) | 4.9 b | 2.5 c | 3.9 b | 6.5 a | 4.4 b | 3.8 b |
| Number of petals | 22.2 c | 44.2 b | 41.6 b | 64.4 a | 66.4 a | 60 a |
| Density of petals | medium | dense | loose | medium | loose | medium |
| Fragrance | absent or weak | absent or weak | absent or weak | medium | absent or weak | absent or weak |
| ‘Morava Reka’ | ‘Blush Pixie‘ | ‘Coral Pixie’ | ‘Gaudi Pixie’ | ‘Mauve Pixie’ | ‘Milky Pixie’ | |
|---|---|---|---|---|---|---|
| Stem diameter (mm) | 6.70 ± 0.77 ** a | 5.45 ± 0.59 ab | 5.61 ± 0.67 ab | 5.75 ± 0.27 ab | 5.09 ± 0.06 b | 4.45 ± 0.41 b |
| Cross-section area (CSA, mm2) | 35.62 ± 8.18 a | 23.53 ± 5.12 ab | 25.03 ± 5.93 ab | 26.06 ± 2.46 ab | 20.30 ± 0.52 b | 15.68 ± 2.92 b |
| Pith area (mm2) | 6.88 ± 0.87 a | 10.24 ± 2.99 a | 10.66 ± 2.78 a | 8.01 ± 1.52 a | 10.01 ± 1.58 a | 8.17 ± 1.57 a |
| Xylem area (mm2) | 17.70 ± 6.48 a | 5.94 ± 1.30 b | 7.01 ± 2.66 b | 9.89 ± 0.55 ab | 4.68 ± 1.45 b | 2.55 ± 0.77 b |
| Vascular cambium area (mm2) | 0.59 ± 0.16 a | 0.30 ± 0.05 b | 0.30 ± 0.10 b | 0.29 ± 0.02 b | 0.23 ± 0.02 b | 0.21 ± 0.03 b |
| Phloem area (mm2) | 3.64 ± 0.70 a | 1.67 ± 0.45 bc | 1.11 ± 0.32 bc | 1.84 ± 0.27 b | 0.95 ± 0.14 bc | 0.64 ± 0.24 c |
| Other tissues (mm2) * | 6.80 ± 1.01 a | 5.39 ± 0.61 ab | 5.94 ± 1.51 ab | 6.03 ± 0.50 ab | 4.44 ± 0.37 b | 4.11 ± 0.35 b |
| % of xylem on CSA | 48.52 ± 6.85 a | 25.25 ± 2.32 bc | 28.00 ± 8.53 bc | 37.91 ± 3.22 ab | 22.93 ± 6.59 bc | 16.00 ± 1.77 c |
| % of phloem on CSA | 10.31 ± 1.30 a | 7.06 ± 1.13 b | 4.43 ± 0.42 c | 7.03 ± 0.38 b | 4.69 ± 0.63 bc | 3.95 ± 0.76 c |
| Xylem/phloem ratio | 4.82 ± 1.02 ab | 3.76 ± 0.28 b | 6.32 ± 1.52 a | 5.42 ± 0.72 ab | 4.88 ± 0.86 ab | 4.17 ± 0.40 ab |
| ‘Morava Reka’ | ‘Blush Pixie‘ | ‘Coral Pixie’ | ‘Gaudi Pixie’ | ‘Mauve Pixie’ | ‘Milky Pixie’ | |
|---|---|---|---|---|---|---|
| Vessel area on CSA (%) | 8.01 ± 1.59 * ab | 5.40 ± 0.63 bc | 4.96 ± 1.89 bcd | 10.32 ± 1.28 a | 3.87 ± 0.50 cd | 1.86 ± 0.40 d |
| Ray area on CSA (%) | 9.40 ± 1.10 a | 5.48 ± 1.08 bc | 5.08 ± 0.77 bc | 7.69 ± 0.80 ab | 6.12 ± 2.34 abc | 3.42 ± 1.35 c |
| A(-)VR on CSA (%) | 31.24 ± 4.27 a | 14.39 ± 2.07 b | 18.06 ± 5.66 b | 20.09 ± 1.70 b | 12.98 ± 4.43 b | 10.79 ± 0.11 b |
| Vessel area relative to Xy (%) | 16.38 ± 1.27 bc | 21.51 ± 3.53 ab | 17.27 ± 1.90 bc | 27.03 ± 1.62 a | 17.42 ± 3.16 bc | 11.47 ± 1.25 c |
| Ray area relative to Xy (%) | 19.37 ± 1.18 a | 21.61 ± 3.06 a | 18.74 ± 3.32 a | 20.26 ± 2.30 a | 26.31 ± 6.26 a | 20.88 ± 5.82 a |
| A(-)VR relative to Xy (%) | 64.24 ± 0.75 ab | 56.88 ± 5.58 abc | 63.99 ± 1.42 ab | 52.71 ± 0.69 c | 56.27 ± 5.20 bc | 67.64 ± 6.98 a |
| Vessel lumen area (µm2) | 818.02 ± 65.65 a | 760.37 ± 70.37 ab | 595.63 ± 142.67 bc | 688.93 ± 48.32 ab | 561.68 ± 70.69 bc | 386.14 ± 77.65 c |
| Vessel frequency | 200.43 ± 8.23 c | 282.11 ± 26.83 bc | 297.35 ± 46.84 abc | 393.61 ± 35.96 a | 314.44 ± 71.51 ab | 301.47 ± 32.94 abc |
| Xylem porosity (%) | 16.38 ± 1.27 bc | 21.51 ± 3.53 ab | 17.27 ± 1.90 b | 27.03 ± 1.62 a | 17.42 ± 3.16 b | 11.47 ± 1.25 c |
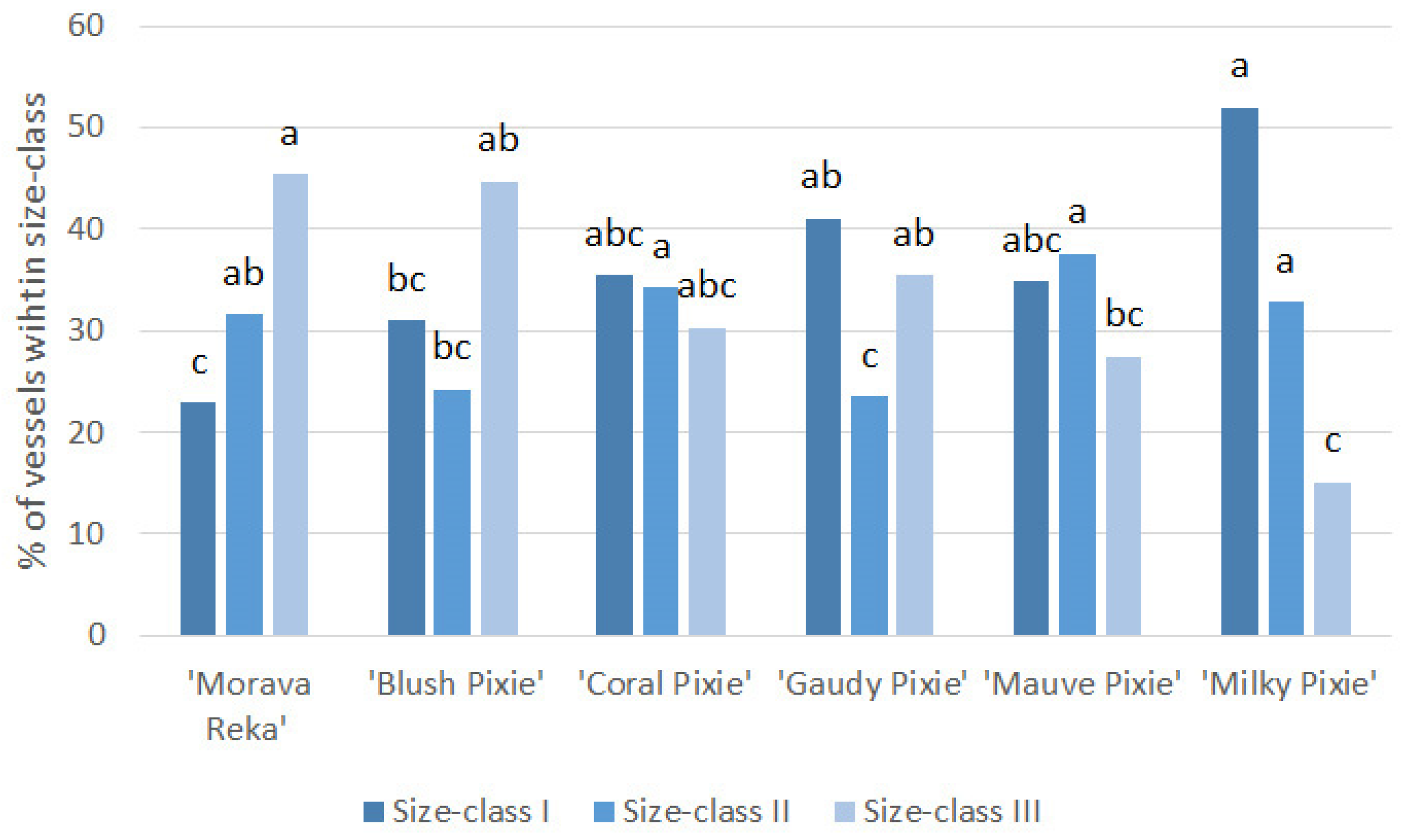
| Vessel Lumen Area (µm2) | Vessel Frequency | % of Vessels < 300 µm2 | % of Vessels from 300 to 700 µm2 | % of Vessels > 700 µm2 | |
|---|---|---|---|---|---|
| Inner xylem zone | 792.77 ± 142.43 * a | 221.11 ± 17.51 a | 24.95 ± 6.47 a | 25.48 ± 6.27 a | 49.58 ± 9.76 a |
| Middle xylem zone | 820.72 ± 96.21 a | 201.85 ± 25.56 a | 18.60 ± 4.85 a | 36.58 ± 8.87 a | 44.82 ± 7.64 a |
| Outer xylem zone | 879.48 ± 23.26 a | 178.31 ± 22.40 a | 24.61 ± 5.83 a | 33.03 ± 7.18 a | 42.36 ± 3.74 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Narandžić, T.; Nikolić, L.; Ljevnaić-Mašić, B.; Božanić Tanjga, B.; Ilić, O.; Čurčić, M.; Ljubojević, M. Protocol for Pre-Selection of Dwarf Garden Rose Varieties. Horticulturae 2024, 10, 996. https://doi.org/10.3390/horticulturae10090996
Narandžić T, Nikolić L, Ljevnaić-Mašić B, Božanić Tanjga B, Ilić O, Čurčić M, Ljubojević M. Protocol for Pre-Selection of Dwarf Garden Rose Varieties. Horticulturae. 2024; 10(9):996. https://doi.org/10.3390/horticulturae10090996
Chicago/Turabian StyleNarandžić, Tijana, Ljiljana Nikolić, Branka Ljevnaić-Mašić, Biljana Božanić Tanjga, Olivera Ilić, Milana Čurčić, and Mirjana Ljubojević. 2024. "Protocol for Pre-Selection of Dwarf Garden Rose Varieties" Horticulturae 10, no. 9: 996. https://doi.org/10.3390/horticulturae10090996
APA StyleNarandžić, T., Nikolić, L., Ljevnaić-Mašić, B., Božanić Tanjga, B., Ilić, O., Čurčić, M., & Ljubojević, M. (2024). Protocol for Pre-Selection of Dwarf Garden Rose Varieties. Horticulturae, 10(9), 996. https://doi.org/10.3390/horticulturae10090996








