Amino Acid Transporters on Amino Acid Absorption, Transport and Distribution in Crops
Abstract
1. Introduction
2. Amino Acid Uptake, Transport, and Distribution
2.1. Amino Acid Uptake
2.2. Root-to-Shoot Transport of Amino Acids
2.3. Amino Acid Transport from Source Leaf to Sink
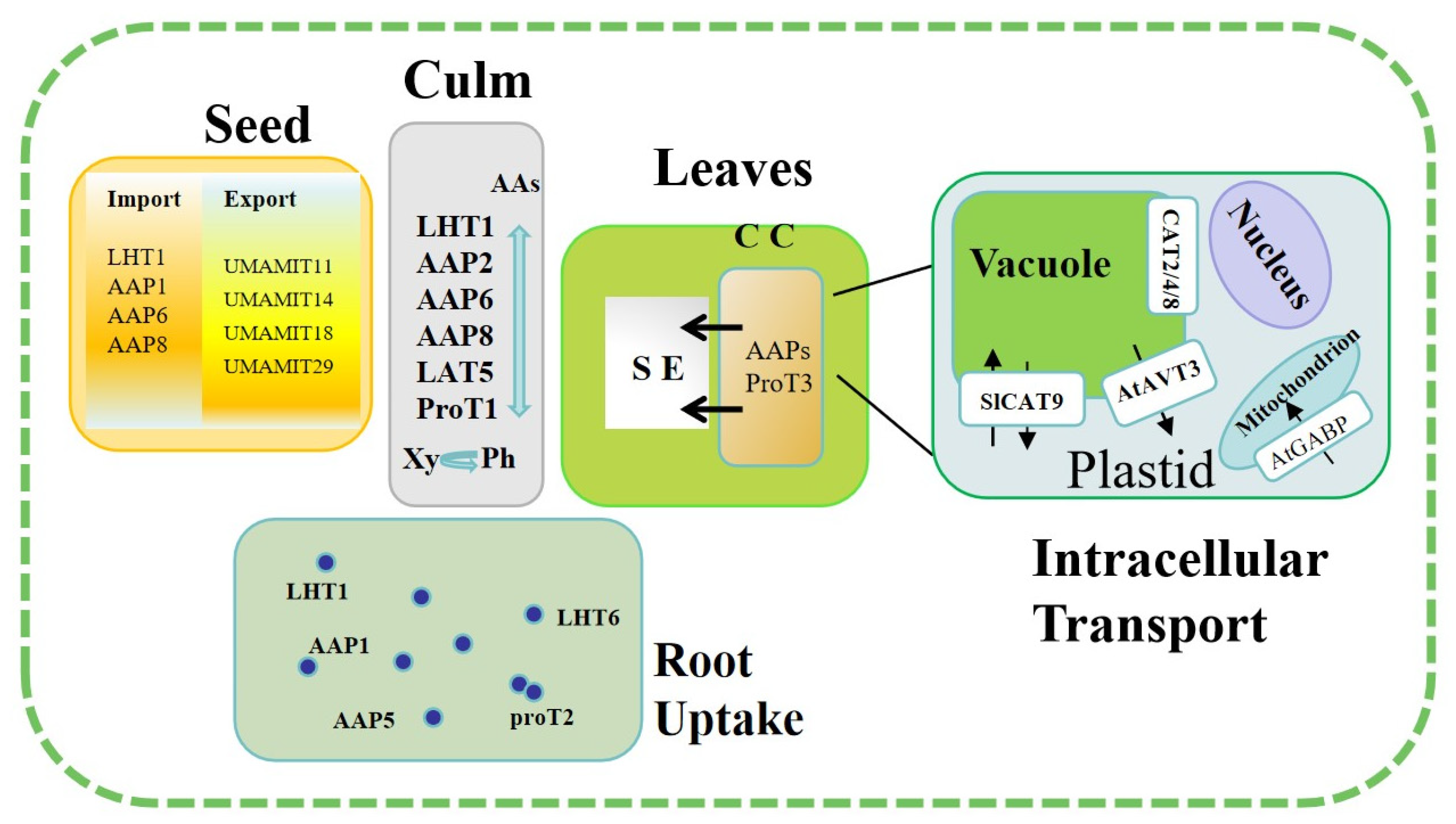
3. Amino Acid Transporter Functions in N and C Metabolism
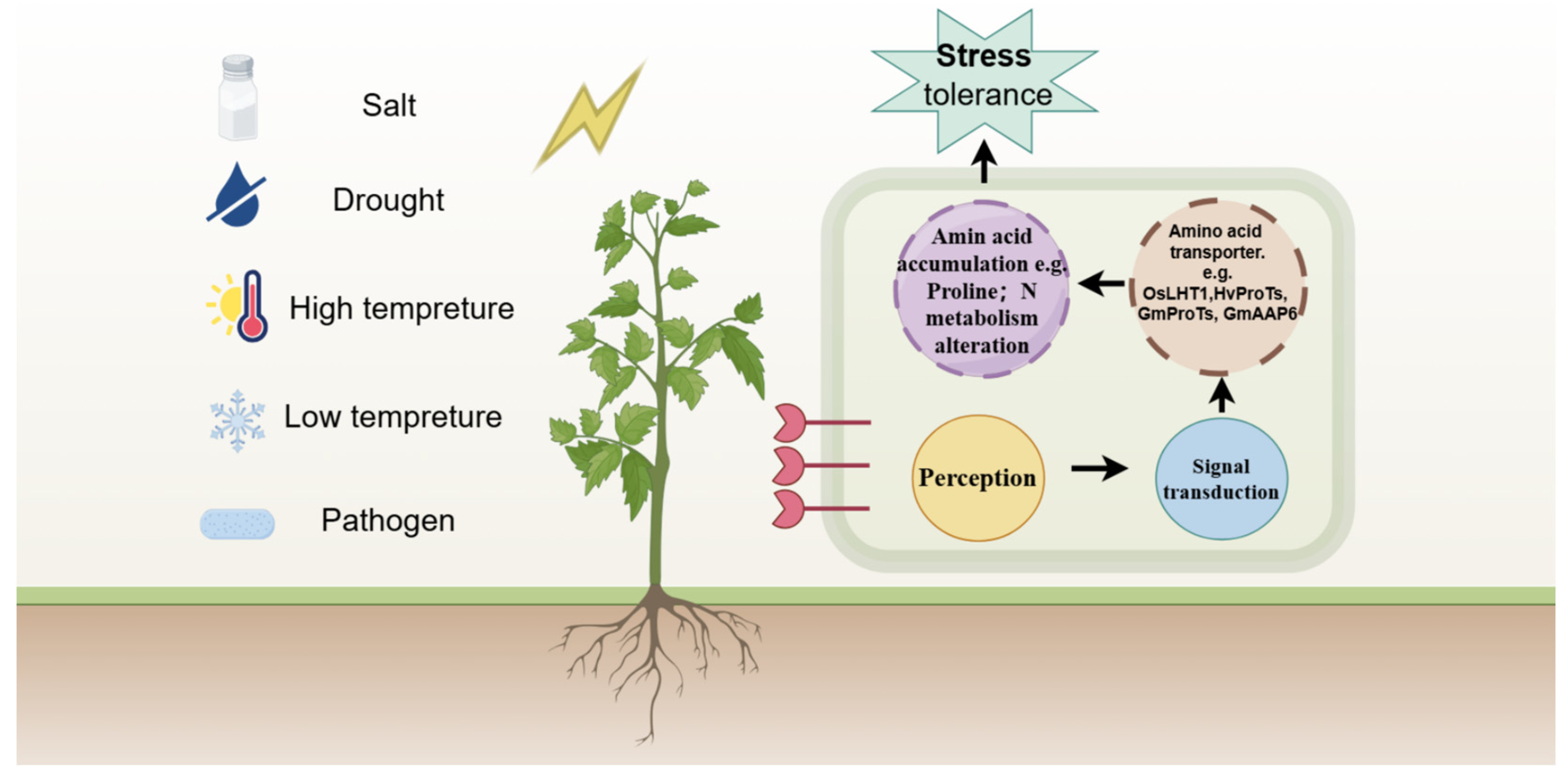
4. Regulation of Amino Acid Transporters in Response to Environmental Stimuli
5. Amino Acid Transporter Functions for Improving Crop Yield and Quality
5.1. Crop Yield Improvement
5.2. Crop Quality Improvement
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bloom, A.J. The increasing importance of distinguishing among plant nitrogen sources. Curr. Opin. Plant Biol. 2015, 25, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Lipson, D.; Näsholm, T. The unexpected versatility of plants: Organic nitrogen use and availability in terrestrial ecosystems. Oecologia 2001, 128, 305–316. [Google Scholar] [CrossRef]
- Jämtgård, S.; Näsholm, T.; Huss-Danell, K. Nitrogen compounds in soil solutions of agricultural land. Soil Biol. Biochem. 2010, 42, 2325–2330. [Google Scholar] [CrossRef]
- Herdiansyah, G.; Farid, M.; Aziz, M.; Sari Dewi, F.; Rahayu, A.S. Nitrogen uptake in fully organic, semi-organic and conventional rice fields in Madiun Regency. IOP Conf. Ser. Earth Environ. Sci. 2022, 1016, 012011. [Google Scholar] [CrossRef]
- Yang, G.; Wei, Q.; Huang, H.; Xia, J. Amino acid transporters in plant cells: A brief review. Plants 2020, 9, 967. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Lv, C.; Zou, Z.; Duan, Y.; Zhou, J.; Zhu, X.; Ma, Y.; Zhang, Z.; Fang, W. CsAAP7.2 is involved in the uptake of amino acids from soil and the long-distance transport of theanine in tea plants (Camellia sinensis L.). Tree Physiol. 2022, 42, 2369–2381. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Garneau, M.G.; Majumdar, R.; Grant, J.; Tegeder, M. Improvement of pea biomass and seed productivity by simultaneous increase of phloem and embryo loading with amino acids. Plant J. Cell Mol. Biol. 2015, 81, 134–146. [Google Scholar] [CrossRef] [PubMed]
- Forsum, O.; Svennerstam, H.; Ganeteg, U.; Näsholm, T. Capacities and constraints of amino acid utilization in Arabidopsis. New Phytol. 2008, 179, 1058–1069. [Google Scholar] [CrossRef]
- Ortiz-Lopez, A.; Chang, H.C.; Bush, D.R. Amino acid transporters in plants. Biochim. Biophys. Acta-Biomembr. 2000, 1465, 275–280. [Google Scholar] [CrossRef]
- Yao, X.; Nie, J.; Bai, R.; Sui, X. Amino Acid Transporters in Plants: Identification and Function. Plants 2020, 9, 972. [Google Scholar] [CrossRef]
- Chang, A.B.; Lin, R.; Studley, W.K.; Tran, C.V.; Saier, M.H., Jr. Phylogeny as a guide to structure and function of membrane transport proteins. Mol. Membr. Biol. 2004, 21, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Dinkeloo, K.; Boyd, S.; Pilot, G. Update on amino acid transporter functions and on possible amino acid sensing mechanisms in plants. Semin. Cell Dev. Biol. 2018, 74, 105–113. [Google Scholar] [CrossRef]
- Wan, Y.; Wang, Y.; Shi, Z.; Rentsch, D.; Ward, J.L.; Hassall, K.; Sparks, C.A.; Huttly, A.K.; Buchner, P.; Powers, S.; et al. Wheat amino acid transporters highly expressed in grain cells regulate amino acid accumulation in grain. PLoS ONE 2021, 16, e0246763. [Google Scholar] [CrossRef]
- Frommer, W.B.; Hummel, S.; Riesmeier, J.W. Expression cloning in yeast of a cDNA encoding a broad specificity amino acid permease from Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 1993, 90, 5944–5948. [Google Scholar] [CrossRef] [PubMed]
- Rentsch, D.; Schmidt, S.; Tegeder, M. Transporters for uptake and allocation of organic nitrogen compounds in plants. FEBS Lett. 2007, 581, 2281–2289. [Google Scholar] [CrossRef]
- Zhang, L.; Tan, Q.; Lee, R.; Trethewy, A.; Lee, Y.H.; Tegeder, M. Altered xylem-phloem transfer of amino acids affects metabolism and leads to increased seed yield and oil content in Arabidopsis. Plant Cell 2010, 22, 3603–3620. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhang, Y.; Zhang, J.; Fan, B.; Zhou, Y.; Cui, X. Expression of AtAAP gene family and endosperm-specific expression of AtAAP1 gene promotes amino acid absorption in Arabidopsis thaliana and maize. Agronomy 2021, 11, 1668. [Google Scholar] [CrossRef]
- Fischer, W.N.; Kwart, M.; Hummel, S.; Frommer, W.B. Substrate specificity and expression profile of amino acid transporters (AAPs) in Arabidopsis. J. Biol. Chem. 1995, 270, 16315–16320. [Google Scholar] [CrossRef]
- Snowden, C.J.; Thomas, B.; Baxter, C.J.; Smith, J.A.C.; Sweetlove, L.J. A tonoplast Glu/Asp/GABA exchanger that affects tomato fruit amino acid composition. Plant J. 2015, 81, 651–660. [Google Scholar] [CrossRef]
- Yang, H.; Krebs, M.; Stierhof, Y.D.; Ludewig, U. Characterization of the putative amino acid transporter genes AtCAT2, 3 & 4: The tonoplast localized AtCAT2 regulates soluble leaf amino acids. J. Plant Physiol. 2014, 171, 594–601. [Google Scholar] [PubMed]
- Su, Y.H.; Frommer, W.B.; Ludewig, U. Molecular and functional characterization of a family of amino acid transporters from Arabidopsis. Plant Physiol. 2004, 136, 3104–3113. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Bogner, M.; Stierhof, Y.D.; Ludewig, U. H+-Independent glutamine transport in plant root tips. PLoS ONE 2010, 5, e8917. [Google Scholar] [CrossRef]
- Lee, Y.H.; Foster, J.; Chen, J.; Voll, L.M.; Weber, A.P.; Tegeder, M. AAP1 transports uncharged amino acids into roots of Arabidopsis. Plant J. 2007, 50, 305–319. [Google Scholar] [CrossRef]
- Chen, L.; Bush, D.R. LHT1, a lysine-and histidine-specific amino acid transporter in Arabidopsis. Plant Physiol. 1997, 115, 1127–1134. [Google Scholar] [CrossRef] [PubMed]
- Ganeteg, U.; Ahmad, I.; Jämtgård, S.; Aguetoni-Cambui, C.; Inselsbacher, E.; Svennerstam, H.; Schmidt, S.; Näsholm, T. Amino acid transporter mutants of Arabidopsis provides evidence that a non-mycorrhizal plant acquires organic nitrogen from agricultural soil. Plant Cell Environ. 2017, 40, 413–423. [Google Scholar] [CrossRef] [PubMed]
- Hirner, A.; Ladwig, F.; Stransky, H.; Okumoto, S.; Keinath, M.; Harms, A.; Frommer, W.B.; Koch, W. Arabidopsis LHT1 is a high-affinity transporter for cellular amino acid uptake in both root epidermis and leaf mesophyll. Plant Cell 2006, 18, 1931–1946. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Dong, C.; Yang, T.; Bao, S.; Fang, W.; Lucas, W.J.; Zhang, Z. The tea plant CsLHT1 and CsLHT6 transporters take up amino acids, as a nitrogen source, from the soil of organic tea plantations. Hortic. Res. 2021, 8, 178. [Google Scholar] [CrossRef] [PubMed]
- Svennerstam, H.; Ganeteg, U.; Näsholm, T. Root uptake of cationic amino acids by Arabidopsis depends on functional expression of amino acid permease 5. New Phytol. 2008, 180, 620–630. [Google Scholar] [CrossRef]
- Perchlik, M.; Foster, J.; Tegeder, M. Different and overlapping functions of Arabidopsis LHT6 and AAP1 transporters in root amino acid uptake. J. Exp. Bot. 2014, 65, 5193–5204. [Google Scholar] [CrossRef]
- Grallath, S.; Weimar, T.; Meyer, A.; Gumy, C.; Suter-Grotemeyer, M.; Neuhaus, J.M.; Rentsch, D. The AtProT family. Compatible solute transporters with similar substrate specificity but differential expression patterns. Plant Physiol. 2005, 137, 117–126. [Google Scholar] [CrossRef]
- Svennerstam, H.; Jämtgård, S.; Ahmad, I.; Huss-Danell, K.; Näsholm, T.; Ganeteg, U. Transporters in Arabidopsis roots mediating uptake of amino acids at naturally occurring concentrations. New Phytol. 2011, 191, 459–467. [Google Scholar] [CrossRef] [PubMed]
- Tegeder, M.; Masclaux-Daubresse, C. Source and sink mechanisms of nitrogen transport and use. New Phytol. 2018, 217, 35–53. [Google Scholar] [CrossRef] [PubMed]
- Santiago, J.P.; Tegeder, M. Connecting source with sink: The role of Arabidopsis AAP8 in phloem loading of amino acids. Plant Physiol. 2016, 171, 508–521. [Google Scholar] [CrossRef] [PubMed]
- Hunt, E.; Gattolin, S.; Newbury, H.J.; Bale, J.S.; Tseng, H.M.; Barrett, D.A.; Pritchard, J. A mutation in amino acid permease AAP6 reduces the amino acid content of the Arabidopsis sieve elements but leaves aphid herbivores unaffected. J. Exp. Bot. 2010, 61, 55–64. [Google Scholar] [CrossRef]
- Perchlik, M.; Tegeder, M. Improving Plant Nitrogen Use Efficiency through Alteration of Amino Acid Transport Processes. Plant Physiol. 2017, 175, 235–247. [Google Scholar] [CrossRef]
- Pereira, E.G.; Santos, L.A.; Chapeta, A.C.O.; de Souza Ribeiro, Y.R.; Santa-Catarina, C.; Bucher, C.P.C.; Bucher, C.A.; García, A.C.; Fernandes, M.S. Disruption of Amino Acid Transporter OsAAP1 Impairs Rice Seedling Establishment and Nitrate Uptake and Assimilation. J. Plant Growth Regul. 2024, 43, 2841–2854. [Google Scholar] [CrossRef]
- Guo, N.; Hu, J.; Yan, M.; Qu, H.; Luo, L.; Tegeder, M. Oryza sativa Lysine-Histidine-type Transporter 1 functions in root uptake and root-to-shoot allocation of amino acids in rice. Plant J. Cell Mol. Biol. 2020, 103, 395–411. [Google Scholar] [CrossRef]
- Santiago, J.P.; Tegeder, M. Implications of nitrogen phloem loading for carbon metabolism and transport during Arabidopsis development. J. Integr. Plant Biol. 2017, 59, 409–421. [Google Scholar] [CrossRef]
- Liu, S.; Wang, D.; Mei, Y.; Xia, T.; Xu, W.; Zhang, Y.; You, X.; Zhang, X.; Li, L.; Wang, N.N. Overexpression of GmAAP6a enhances tolerance to low nitrogen and improves seed nitrogen status by optimizing amino acid partitioning in soybean. Plant Biotechnol. J. 2020, 18, 1749–1762. [Google Scholar] [CrossRef]
- Begam, R.A.; D’Entremont, J.; Good, A. The Arabidopsis L-Type Amino Acid Transporter 5 (LAT5/PUT5) Is Expressed in the Phloem and Alters Seed Nitrogen Content When Knocked Out. Plants 2020, 9, 1519. [Google Scholar] [CrossRef]
- Guo, N.; Gu, M.; Hu, J.; Qu, H.; Xu, G. Rice OsLHT1 Functions in Leaf-to-Panicle Nitrogen Allocation for Grain Yield and Quality. Front. Plant Sci. 2020, 11, 1150. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Yang, G.; Wei, X.; Huang, W.; Fang, Z. OsAAP15, an amino acid transporter in response to nitrogen concentration, mediates panicle branching and grain yield in rice. Plant Sci. 2023, 330, 111640. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yang, G.; Shi, M.; Hao, D.; Wei, Q.; Wang, Z.; Fu, S.; Su, Y.; Xia, J. Disruption of an amino acid transporter LHT1 leads to growth inhibition and low yields in rice. BMC Plant Biol. 2019, 19, 268. [Google Scholar] [CrossRef] [PubMed]
- Müller, B.; Fastner, A.; Karmann, J.; Mansch, V.; Hoffmann, T.K.; Schwab, W.; Suter-Grotemeyer, M.; Rentsch, D.; Truernit, E.; Ladwig, F. Amino acid export in developing Arabidopsis seeds depends on umamit facilitators. Curr. Biol. 2015, 25, 3126–3131. [Google Scholar] [CrossRef] [PubMed]
- Ladwig, F.; Stahl, M.; Ludewig, U.; Hirner, A.A.; Hammes, U.Z.; Stadler, R.; Harter, K.; Koch, W. Siliques Are Red1 from Arabidopsis acts as a bidirectional amino acid transporter that is crucial for the amino acid homeostasis of siliques. Plant Physiol. 2012, 158, 1643–1655. [Google Scholar] [CrossRef]
- Fujiki, Y.; Teshima, H.; Kashiwao, S.; Kawano-Kawada, M.; Ohsumi, Y.; Kakinuma, Y.; Sekito, T. Functional identification of AtAVT3, a family of vacuolar amino acid transporters, in Arabidopsis. FEBS Lett. 2017, 591, 5–15. [Google Scholar] [CrossRef]
- Amir, R.; Galili, G.; Cohen, H. The metabolic roles of free amino acids during seed development. Plant Sci. 2018, 275, 11–18. [Google Scholar] [CrossRef]
- Guo, N.; Qu, H.; Zhi, Y.; Zhang, Y.; Cheng, S.; Chu, J.; Zhang, Z.; Xu, G. Knock out of amino acid transporter gene OsLHT1 accelerates leaf senescence and enhances resistance to rice blast fungus. J. Exp. Bot. 2023, 74, 4143–4157. [Google Scholar] [CrossRef]
- Wang, R.; Okamoto, M.; Xing, X.; Crawford, N.M. Microarray analysis of the nitrate response in Arabidopsis roots and shoots reveals over 1000 rapidly responding genes and new linkages to glucose, trehalose-6-phosphate, iron, and sulfate metabolism. Plant Physiol. 2003, 132, 556–567. [Google Scholar] [CrossRef]
- Nunes-Nesi, A.; Fernie, A.R.; Stitt, M. Metabolic and signaling aspects underpinning the regulation of plant carbon nitrogen interactions. Mol. Plant 2010, 3, 973–996. [Google Scholar] [CrossRef]
- Sahrawy, M.; Avila, C.; Chueca, A.; Cánovas, F.M.; Lopez-Gorgé, J. Increased sucrose level and altered nitrogen metabolism in Arabidopsis thaliana transgenic plants expressing antisense chloroplastic fructose-1,6-bis phosphatase. J. Exp. Bot. 2004, 55, 2495–2503. [Google Scholar] [CrossRef] [PubMed]
- Carter, A.M.; Tegeder, M. Increasing nitrogen fixation and seed development in soybean requires complex adjustments of nodule nitrogen metabolism and partitioning processes. Curr. Biol. 2006, 26, 2044–2051. [Google Scholar] [CrossRef]
- De Groot, C.C.; Marcelis, L.F.; van den Boogaard, R.; Kaiser, W.M.; Lambers, H. Interaction of nitrogen and phosphorus nutrition in determining growth. Plant Soil 2003, 248, 257–268. [Google Scholar] [CrossRef]
- Awasthi, A.; Nain, V.; Srikanth, C.V.; Puria, R. A regulatory circuit between lncRNA and TOR directs amino acid uptake in yeast. Biochim. Biophys. Acta-Mol. Cell Res. 2020, 1867, 118680. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Liu, J.; Li, Z.; Ye, R.; Chen, W.; Huang, Y.; Yuan, Y.; Zhang, Y.; Hu, H.; Zheng, P.; et al. Mitigating growth-stress trade offs via elevated tor signaling in rice. Mol. Plant 2024, 17, 240–257. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.H.; Park, S.J.; Ahn, C.S.; Pai, H.S. MRF Family Genes Are Involved in Translation Control, Especially under Energy-Deficient Conditions, and Their Expression and Functions Are Modulated by the TOR Signaling Pathway. Plant Cell 2017, 29, 2895–2920. [Google Scholar] [CrossRef] [PubMed]
- Scarpin, M.R.; Leiboff, S.; Brunkard, J.O. Parallel global profiling of plant TOR dynamics reveals a conserved role for LARP1 in translation. eLife Sci. 2020, 9, e58795. [Google Scholar] [CrossRef]
- Tünnermann, L.; Colou, J.; Näsholm, T.; Gratz, R. To have or not to have: Expression of amino acid transporters during pathogen infection. Plant Mol. Biol. 2022, 109, 413–425. [Google Scholar] [CrossRef]
- Ding, S.; Shao, X.; Li, J.; Ahammed, G.J.; Yao, Y.; Ding, J.; Hu, Z.; Yu, J.; Shi, K. Nitrogen forms and metabolism affect plant defence to foliar and root pathogens in tomato. Plant Cell Environ. 2021, 44, 1596–1610. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Postel, S.; Kemmerling, B.; Ludewig, U.W.E. Altered growth and improved resistance of A rabidopsis against P seudomonas syringae by overexpression of the basic amino acid transporter AtCAT1. Plant Cell Environ. 2014, 37, 1404–1414. [Google Scholar] [CrossRef]
- Besnard, J.; Sonawala, U.; Maharjan, B.; Collakova, E.; Finlayson, S.A.; Pilot, G.; McDowell, J.; Okumoto, S. Increased Expression of UMAMIT Amino Acid Transporters Results in Activation of Salicylic Acid Dependent Stress Response. Front. Plant Sci. 2021, 11, 606386. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Ji, Y.; Bhuiyan, N.H.; Pilot, G.; Selvaraj, G.; Zou, J.; Wei, Y. Amino acid homeostasis modulates salicylic acid–associated redox status and defense responses in Arabidopsis. Plant Cell 2010, 22, 3845–3863. [Google Scholar] [CrossRef]
- Furlan, A.L.; Bianucci, E.; Giordano, W.; Castro, S.; Becker, D.F. Proline metabolic dynamics and implications in drought tolerance of peanut plants. Plant Physiol. Biochem. 2020, 151, 566–578. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Miao, M.; Meng, Y.; Cao, J.; Fan, T.; Yue, J.; Xiao, F.; Liu, Y.; Cao, S. DFR1-Mediated Inhibition of Proline Degradation Pathway Regulates Drought and Freezing Tolerance in Arabidopsis. Cell Rep. 2018, 23, 3960–3974. [Google Scholar] [CrossRef] [PubMed]
- Fischer, W.N.; Loo, D.D.; Koch, W.; Ludewig, U.; Boorer, K.J.; Tegeder, M.; Rentsch, D.; Wright, E.M.; Frommer, W.B. Low and high affinity amino acid H+-cotransporters for cellular import of neutral and charged amino acids. Plant J. 2002, 29, 717–731. [Google Scholar] [CrossRef]
- Na, G.; Dong, X.; Zhang, W.; Zhao, J.M.; Xue, C.C.; Qiang, Y.; Xue, J.Y.; Wang, H.T.; Zhang, Y.M.; Han, X. Overexpression of GmProT1 and GmProT2 increases tolerance to drought and salt stresses in transgenic Arabidopsis. J. Integr. Agric. 2016, 15, 1727–1743. [Google Scholar]
- Wang, T.; Chen, Y.; Zhang, M.; Chen, J.; Liu, J.; Han, H.; Hua, X. Arabidopsis AMINO ACID PERMEASE1 contributes to salt stress-induced proline uptake from exogenous sources. Front. Plant Sci. 2017, 8, 2182. [Google Scholar] [CrossRef] [PubMed]
- Akbudak, M.A.; Filiz, E. Genome-wide investigation of proline transporter (ProT) gene family in tomato: Bioinformatics and expression analyses in response to drought stress—ScienceDirect. Plant Physiol. Biochem. 2020, 157, 13–22. [Google Scholar] [CrossRef]
- Ueda, A.; Shi, W.; Sanmiya, K.; Shono, M.; Takabe, T. Functional analysis of salt-inducible proline transporter of barley roots. Plant Cell Physiol. 2001, 42, 1282–1289. [Google Scholar] [CrossRef]
- Lu, K.; Wu, B.; Wang, J.; Zhu, W.; Nie, H.; Qian, J.; Huang, W.; Fang, Z. Blocking amino acid transporter OsAAP3 improves grain yield by promoting outgrowth buds and increasing tiller number in rice. Plant Biotechnol. J. 2018, 16, 1710–1722. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wu, B.; Lu, K.; Wei, Q.; Qian, J.; Chen, Y.; Fang, Z. The Amino Acid Permease 5 (OsAAP5) Regulates Tiller Number and Grain Yield in Rice. Plant Physiol. 2019, 180, 1031–1045. [Google Scholar] [CrossRef]
- Jin, F.; Xie, P.; Li, Z.; Wu, B.; Huang, W.; Fang, Z. Blocking of amino acid transporter OsAAP7 promoted tillering and yield by determining basic and neutral amino acids accumulation in rice. BMC Plant Biol. 2024, 24, 447. [Google Scholar] [CrossRef] [PubMed]
- Sanders, A.; Collier, R.; Trethewy, A.; Gould, G.; Sieker, R.; Tegeder, M. AAP1 regulates import of amino acids into developing Arabidopsis embryos. Plant J. 2009, 59, 540–552. [Google Scholar] [CrossRef] [PubMed]
- Perchlik, M.; Tegeder, M. Leaf amino acid supply affects photosynthetic and plant nitrogen use efficiency under nitrogen stress. Plant Physiol. 2018, 178, 174–188. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, R.; Stransky, H.; Koch, W. The amino acid permease AAP8 is important for early seed development in Arabidopsis thaliana. Planta 2007, 226, 805–813. [Google Scholar] [CrossRef]
- Grant, J.E.; Ninan, A.; Cripps-Guazzone, N.; Shaw, M.; Song, J.; Petřík, I.; Novák, O.; Tegeder, M.; Jameson, P.E. Concurrent overexpression of amino acid permease AAP1 (3a) and SUT1 sucrose transporter in pea resulted in increased seed number and changed cytokinin and protein levels. Funct. Plant Biol. 2021, 48, 889–904. [Google Scholar] [CrossRef] [PubMed]
- Peng, B.; Kong, H.; Li, Y.; Wang, L.; Zhong, M.; Sun, L.; Gao, G.; Zhang, Q.; Luo, L.; Wang, G.; et al. OsAAP6 functions as an important regulator of grain protein content and nutritional quality in rice. Nat. Commun. 2014, 5, 4847. [Google Scholar] [CrossRef]
- Fageria, N.K.; Baligar, V.C. Enhancing nitrogen use efficiency in crop plants. Adv. Agron. 2005, 88, 97–185. [Google Scholar]
- Hirel, B.; Le Gouis, J.; Ney, B.; Gallais, A. The challenge of improving nitrogen use efficiency in crop plants: Towards a more central role for genetic variability and quantitative genetics within integrated approaches. J. Exp. Bot. 2007, 58, 2369–2387. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, L.; Yu, X.; Yan, Y.; He, C.; Wang, J.; Sun, M.; Li, Y. Amino Acid Transporters on Amino Acid Absorption, Transport and Distribution in Crops. Horticulturae 2024, 10, 999. https://doi.org/10.3390/horticulturae10090999
Liu L, Yu X, Yan Y, He C, Wang J, Sun M, Li Y. Amino Acid Transporters on Amino Acid Absorption, Transport and Distribution in Crops. Horticulturae. 2024; 10(9):999. https://doi.org/10.3390/horticulturae10090999
Chicago/Turabian StyleLiu, Lu, Xianchang Yu, Yan Yan, Chaoxing He, Jun Wang, Mintao Sun, and Yansu Li. 2024. "Amino Acid Transporters on Amino Acid Absorption, Transport and Distribution in Crops" Horticulturae 10, no. 9: 999. https://doi.org/10.3390/horticulturae10090999
APA StyleLiu, L., Yu, X., Yan, Y., He, C., Wang, J., Sun, M., & Li, Y. (2024). Amino Acid Transporters on Amino Acid Absorption, Transport and Distribution in Crops. Horticulturae, 10(9), 999. https://doi.org/10.3390/horticulturae10090999







